Abstract
Objective
Prokineticin 2 (PROK2) is a hypothalamic neuropeptide that plays a critical role in the rhythmicity of physiological functions and inhibits food intake. PROK2 is also expressed in the main olfactory bulb (MOB) as an essential factor for neuro-and morphogenesis. Since the MOB was shown to be strongly involved in eating behavior, we hypothesized that PROK2 could be a new target in the regulation of food intake and energy homeostasis, through its effects in the MOB. We also asked whether PROK2 could be associated with the pathophysiology of obesity, the metabolic syndrome (MetS), and type 2 diabetes (T2D) in humans.
Methods
We assessed in wild type mice whether the expression of Prok2 in the MOB is dependent on the nutritional status. We measured the effect of human recombinant PROK2 (rPROK2) acute injection in the MOB on food intake and olfactory behavior. Then, using a lentivirus expressing Prok2-shRNA, we studied the effects of Prok2 underexpression in the MOB on feeding behavior and glucose metabolism. Metabolic parameters and meal pattern were determined using calorimetric cages. In vivo 2-deoxyglucose uptake measurements were performed in mice after intraperitoneally insulin injection. Plasmatic PROK2 dosages and genetic associations studies were carried out respectively on 148 and more than 4000 participants from the D.E.S.I.R. (Data from an Epidemiologic Study on the Insulin Resistance Syndrome) cohort.
Results
Our findings showed that fasting in mice reduced Prok2 expression in the MOB. Acute injection of rPROK2 in the MOB significantly decreased food intake whereas Prok2-shRNA injection resulted in a higher dietary consumption characterized by increased feeding frequency and decreased meal size. Additionally, Prok2 underexpression in the MOB induced insulin resistance compared to scrambled shRNA-injected mice. In the human D.E.S.I.R. cohort, we found a significantly lower mean concentration of plasma PROK2 in people with T2D than in those with normoglycemia. Interestingly, this decrease was no longer significant when adjusted for Body Mass Index (BMI) or calorie intake, suggesting that the association between plasma PROK2 and diabetes is mediated, at least partly, by BMI and feeding behavior in humans. Moreover, common Single Nucleotide Polymorphisms (SNPs) in PROK2 gene were genotyped and associated with incident T2D or impaired fasting glycemia (IFG), MetS, and obesity.
Conclusions
Our data highlight PROK2 as a new target in the MOB that links olfaction with eating behavior and energy homeostasis. In humans, plasma PROK2 is negatively correlated with T2D, BMI, and energy intake, and PROK2 genetic variants are associated with incident hyperglycemia (T2D/IFG), the MetS and obesity.
Keywords: Prokineticin 2, Main olfactory bulb, Food intake, Meal pattern, Insulin resistance, Metabolic syndrome
Highlights
-
•
Fasting alters prokineticin 2 (Prok2) expression in the main olfactory bulb (MOB).
-
•
Acute injection of PROK2 into the MOB diminishes food intake.
-
•
Partial deletion of MOB-Prok2 affects meal pattern and induces insulin resistance.
-
•
Type 2 diabetes (T2D) in humans is correlated with lower plasma PROK2 level.
-
•
Polymorphisms of PROK2 gene associate with incident T2D and the metabolic syndrome.
1. Introduction
Prokineticin 2 (PROK2) is a protein belonging to the prokineticins family [1] which was first identified in the gastrointestinal tract [2]. PROK2 is also expressed in discrete regions of the brain, including the hypothalamus and the main olfactory bulb (MOB) [3]. The MOB is the first central relay of olfactory representation that encodes olfactory stimuli. Once sensory cues integrated in the MOB, olfactory information is directly transmitted to the main centers of the olfactory cortex (e.g., piriform cortex, amygdala) [4] and also to the hypothalamus [5], [6]. Odor detection is one of the most important triggers to initiate food intake for most animals including humans, since olfactory cues are perceived before any food intake and influence behavioral decisions about food choice and consumption. In recent years, robust data have repeatedly highlighted that the olfactory system acts as a main regulator of food intake and energy balance [4], [7], [8]. Olfactory-related brain areas express receptors for metabolic hormones and peptides such as leptin, insulin, glucagon-like peptide-1 (GLP-1), neuropeptide Y (NPY) [7], along with cannabinoid type-1 (CB1) receptors that were found to promote food intake in fasted mice by increasing odor detection [9]. In addition, olfactory performances are dependent on both the nutritional (fasted vs fed) and the metabolic (lean vs obese) status in both humans [10] and rodents [11], and smell disorders have been reported in obese patients [10], [11], [12]. Knocking out the Prok2 pathway in mice damaged the morpho- and neurogenesis of the MOB [13] as well as the rhythmicity of physiological and behavioral functions driven by the main circadian clock located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, including food intake [14], [15]. Moreover, intracerebroventricular injection as well as injection in the arcuate nucleus of the hypothalamus of PROK2 were shown to decrease food intake [1], [16]. PROK2 is also involved in thermoregulation and energy metabolism in rodents [17]. Together, these data suggest that central PROK2 is necessary for the MOB development and function and is involved in the regulation of feeding behavior.
We used a gain/loss of function approach to address the role of PROK2 in the MOB in the regulation of feeding behavior and energy homeostasis in mice. In addition, since a recent study in humans described that plasma PROK2 level was associated with metabolic syndrome (MetS) [18], we also explored whether plasma PROK2 level and PROK2 genetic variants in people from the Data from an Epidemiologic Study on the Insulin Resistance Syndrome (D.E.S.I.R.) cohort could be correlated with metabolic parameters.
Our data highlight for the first time that PROK2 in the MOB is a new link between olfaction, eating behavior, and energy homeostasis. In humans, plasma PROK2 correlated negatively with type 2 diabetes (T2D), Body Mass Index (BMI), and energy intake, and PROK2 genetic variants are associated with incident hyperglycemia (T2D/impaired fasting glycemia (IFG)), MetS, and obesity.
2. Materials and methods
2.1. Mice studies
2.1.1. Animal models
The study was approved by the Institutional Animal Care and Use Committee of Paris Diderot University (#7637 French Ministry of Research). C57Bl/6j 6 week-old male mice were purchased (Janvier, Le Genest Saint Isle, France) and fed a regular chow diet (standard diet 2380 kCal/kg, Safe, Augy, France) for 3 months. Mice were housed under a 12-h/12-h light–dark (L/D) schedule (lights on at 0700 h, defined as Zeitgeber Time 0 (ZT0)).
2.1.2. Surgical and stereotactic procedures
Under isoflurane anesthesia (1.5%, Isoflo, Abbott Laboratories Ltd, Manchester, UK) and after Buprecare administration (180 μg/kg BW i.p.), bilateral injections were performed at eight distinct locations in the MOB (ML: ±0.9 mm from bregma; AP: +5.1, +4.5 mm from bregma and DV: −2, −2.5 mm, according to the coordinates of Paxinos and Franklin's mouse brain atlas [19], at a rate of 0.2 μL/min, with a final volume of 2 μl per MOB lobe to cover the overall volume of the MOB in mice.
2.1.3. Implantation of a guide cannula into the MOB
Mice were anesthetized as described in 2.1.2. and bilaterally implanted with a double stainless steel guide cannula (26 gauge in diameter, PlasticOne, USA) into the near center of the MOB (ML: ±1 mm from bregma; AP: +4.8 mm from bregma and DV: −1.5 mm). The guide cannula was fixed onto the skull with dental cement. A dummy cannula was placed in the guide cannula to reversibly close its tip.
2.1.4. Local injection of human recombinant PROK2 (rPROK2) and subsequent food intake quantification
To acutely stimulate the PROK2 system, rPROK2 (Peprotech, Neuilly sur Seine, France) was injected into the MOB, at a dose of 50 or 500 pmol/mouse following the surgical procedure mentioned in 2.1.2. Injections were performed under two conditions: 1) in ad libitum fed mice at the beginning of the dark phase and 2) in mice fasted for 24 h at the beginning of the light phase. Cumulative food intake was measured 24 h post-injection.
2.1.5. Buried food test
The buried food test is a standard, straight-forward way to test olfactory function. The following protocol was modified from Yang and Crawley [20]. All mice were implanted with a bilateral cannula at least two weeks before the buried food test. The task includes two phases: an habituation phase to prevent any neophobia during the test and the buried food test itself. Habituation: individualized mice implanted with a cannula in the MOB underwent two consecutive days (day 1 and 2) of odorized-food familiarization before the test day. A piece of chocolate-flavored cookie (1 g) was placed in the cage visible to the animal just before the nocturnal shift. Palatability was checked the next morning by the total cookie consumption. During habituation, mice had free access to water and chow diet. The day preceding the test day (day 3), mice were either fed (fed group) or 24 h-fasted (24 h, 24 h-fasted group). Test: on the test day (day 4), each mouse was transferred to the test cage (45 × 24 × 20 cm) containing a layer (5 cm) of clean mouse bedding material and allowed to acclimate for 5 min. Then mice were injected either with saline (fed group and half of the 24 h-fasted group) or with rPROK2 at the dose of 500 pmol/mouse (half of the 24 h-fasted group) through the stainless steel cannula, which was connected to a 10 μL Hamilton syringe by means of a polyethylene tube. An automatic infusion pump was used to microinject 0.5 μL of saline or rPROK2 over 4 min (speed: 0.125 μL/min). Mice were able to move freely during this procedure. They were left to rest for 5 min after the microinjection. In the meantime, a piece of chocolate-flavored cookie (same brand, same weight as during habituation) was randomly placed underneath the bedding (2 cm deep). Then the mouse was re-introduced to the cage and the time to find the buried food (i.e. ability to perceive volatile odors) was measured. The latency time was defined as the time between placement of the mouse in the cage and grasping the cookie with its forepaws or teeth after foraging.
2.1.6. Prok2 underexpression in the MOB
To study the long term effects of Prok2 underexpression in the MOB, mice were injected with a lentivirus expressing Prok2-shRNA (pLKO.1-CMV-TurboGFPTM, 106 TU/ml, Sigma, Lyon, France), following the surgical procedure mentioned in 2.1.2.. Control mice were injected with a non-target scrambled-shRNA. The lentivirus expressing Prok2-shRNA was designed after validation of the Prok2-shRNA efficiency in vitro. To this end, a Prok2 cDNA mouse was subcloned in the PsiCheck TM2 Vector (Promega) and scrambled or Prok2-targeted shRNAs were subcloned in the pSIREN-retroQ-DsRed Express (Clonetech). The vectors were co-transfected into HEK293T cells using jetPRIME (Polyplus) and Dual Glo luciferase assay (Promega). 48 hr later, total RNA were extracted, and Prok2 mRNA expression was determined by qRT-PCR. The selected shRNA has the following sequence: 5′-CTCTGATGATCCTCACCTT-3′.
To validate the injection site and the diffusion of the lentivirus in the MOB, mice were sacrificed 48 h after surgery. Brain was removed after 4% PFA intracardiac perfusion, then post-fixed for 24 h in 4% PFA at 4 °C, before being transferred to 30% sucrose for 48 h. After embedding in OCT, tissues were cryosectioned, and slices were mounted in slides to visualize the GFP fluorescence.
2.1.7. Measurement of food intake and metabolic parameters
24 h cumulative food intake and body weight (BW) were measured weekly, from 1 to 4 weeks post-injection of the lentivirus in the MOB. Body mass composition was analyzed at week 3 using an Echo Medical systems' EchoMRI (Whole Body Composition Analyzers, EchoMRI, Houston, TX, USA), according to the manufacturer's instructions. To study the effects of Prok2 underexpression on metabolic parameters, mice injected in the MOB with the scrambled-shRNA or Prok2-shRNA were placed in calorimetric cage (TSE system, Germany) 3 weeks postsurgery and kept under a 12 h light/12 h dark cycle from 7.00 am to 7:00 pm in a temperature-controlled environment at 22.5 °C with ad libitum access to food and water. Animals were accustomed to daily manipulation. After 48 h of acclimation, feeding behavior, energy expenditure, substrate utilization, and locomotor activity were measured. Ultra structure of meals (meals and bouts number, size and frequency) as defined by Gaetani et al. [21] was performed using in-house analytic sheet under Excel (Microsoft SA, Issy-les-Moulineaux, France). Raw data (mg of change per second resolution) were obtained from automatic food and drink sensors (TSE, Bad Hamburg, Germany). A bout was defined as a change in the amount of food. Each bout was then characterized by the time of its detection, its duration (in seconds), and the amount of food ingested (in mg). A meal was defined as a series of bouts that were separated by less than 300 s. A meal was then characterized by the time of its detection, its duration, the amount of food ingested, and the number of bouts that composed this meal. Meal structures were computed for a time windows of 2 h.
2.1.8. Metabolic tests
Oral Glucose Tolerance Test: after an overnight fast, mice received glucose (3 g/Kg BW) by oral gavage. Glycemia was monitored using a Glucotrend Lector (MenariniDiagnotics, Rungis, France) during 120 min, and blood samples were collected from the tail vein to assay plasma insulin. Insulin Tolerance Test: following a 5 h fast, mice were given a single injection of insulin (ip, 0.75 U/kg BW, Novo-Nordisk, La Défense, France), and glycemia was monitored for 120 min.
2.1.9. Plasma insulin and plasma leptin levels
Plasma insulin and plasma leptin levels were quantified using ELISA assays purchased from ALPCO (Salem, NH, USA).
2.1.10. Plasma free fatty acid and triglyceride levels
Plasma free fatty acid and triglyceride levels were determined using a NEFA C-test (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and a Serum Triglyceride Determination Kit (Sigma–Aldrich), respectively.
2.1.11. In vivo 2-deoxyglucose uptake measurement
Mice were fasted for 5 h then were intraperitoneally injected with insulin at 0.75 U/kg BW (Novo-Nordisk, La Défense, France) and 2-deoxy-D-[1–14C] glucose (2DG) (5 μCi, Amersham). Blood was collected from the tail vein 15, 30, 45, 60, and 90 min post injection. Animals were euthanized subsequently. Plasma D-[14C] 2DG was determined from total blood after deproteinization with a Zn(OH)2 Ba(OH)2. Tissue D-[14C] 2DG and D-[14C] 2DG-6-phosphate content were determined as previously described [22]. Briefly, a piece of each tissue was weighed, dissolved in 1 M NaOH at 55 °C for 60–120 min, and then neutralized with 1 M HCl. D-[14C] 2DG and D-[14C] 2DG-6-phosphate were differentially precipitated by the use of a zinc hydroxide solution (0.3 M) or a perchloric acid solution (6%). Radioactivity was determined with a Packard Tri-Carb 460C liquid scintillation system.
2.1.12. Tissue collection
All animals were sacrificed by cervical dislocation at the beginning of the light phase. The brain was harvested, and the MOB was dissected, snap-frozen in liquid nitrogen and stored at −80 °C. For the study of the molecular circadian rhythm, samples were taken every 4 h for a 24 h period (Zeitgeber time, ZT, ZT0-20) in C57Bl/6j male mice fed ad libitum.
2.1.13. Real time quantitative PCR
Total RNAs were isolated using RNeasy Lipid Tissue mini kit (Qiagen, Courtaboeuf, France) and analyzed by RTqPCR as previously described [19]. The mRNA transcript level for each gene was normalized against the HKG, rpL19. Primers sequences are listed in Table S1.
2.2. Human studies
2.2.1. Population
The D.E.S.I.R. study (Data from an Epidemiological Study on the Insulin-Resistance syndrome) is a prospective study of 5212 participants at inclusion (2,576 men and 2,636 women, aged 30–65 years), recruited from volunteers who were offered periodic health examinations free of charge by the French Social Security system in 10 health examination centers from the western part of France. They were clinically and biologically evaluated every three years, and the final examination was 9 years after inclusion. A detailed description of all clinical and laboratory measurements is reported by Balkau et al. [23]. Dietary intakes were assessed (protein, lipid, carbohydrate, and total energy intake) from a validated questionnaire on eating habits [24]. The socio-economic status was coded as: 1. Agricultural; 2. Craftsmen, tradesmen, head of enterprise - independent, government, scientific, arts, spectacles, executives in enterprise; 3. Executives and professions with superior intellectual; 4. Intermediate professions: teachers, health professions, administrators, technicians, clergy; 5. Employees: governmental, enterprises, commerce, police; 6. Workers: qualified, non-qualified, agricultural; 7. Retired - previously agricultural workers, craftsmen, executives, employees, workers etc.; 8. Without a professional activity (unemployed but has never worked, students, apprentices, home-maker); 9. Unemployed but had previously worked.
To avoid population stratification problems, only individuals born in mainland France were kept for genetic analyses. The study was approved by the ethics committee of the Kremlin Bicêtre Hospital, and all participants signed an informed consent.
T2D was defined as fasting plasma glucose ≥7.0 mmol/l or treatment by glucose-lowering agents. IFG was defined as fasting plasma glucose between 6.1 and 6.9 mmol/l. The MetS was defined according to the NCEP-ATPIII [21] if three of the following factors apply: 1) waist circumference > 102/88 cm for men/women; 2) elevated triglycerides: ≥ 1.70 mmol/l; 3) reduced HDL-C: ≤ 1.03 mmol/l for men and 1.29 mmol/l for women; 4) elevated blood pressure: systolic blood pressure ≥ 130 or diastolic ≥ 85 mmHg; 5) elevated fasting glycemia ≥6.1 mmol/l. Obesity was defined as BMI ≥30 kg/m2.
The 9-year incident cases for any disease were defined in people free of disease at entry who developed the disease at some time during the follow-up.
2.2.2. Genotyping
Seven SNPs spanning the whole PROK2 gene region (chromosome 3p13) were selected as tag SNPs covering 80% of PROK2 allelic variability with a minor allele frequency >5% in European populations using Hapmap (SNPinfo Web Server): rs10865660, rs10779992, rs7634474, rs1320015, rs2322142, rs3796224, rs6782813 (from 5′ to 3’ positions). Genotypes were determined by competitive allele-specific PCR genotyping system assays (KASP, LGC Genomics, Hoddesdon, UK). Genotyping success rate was higher than 97%. Genotypes were in Hardy–Weinberg equilibrium.
2.2.3. Plasma PROK2 levels
Plasma PROK2 levels were quantified using an ELISA assay purchased from SunRed (Shanghai Sunred Biological Technology Co., Ltd) at 6 years of follow-up in a subsample of the cohort, in people not diabetic at inclusion nor during the 3-year follow-up diabetic (n = 77), people not diabetic at the time of blood sampling, but diabetic at the next visit (3 years later) (n = 37), and people with type 2 diabetes (n = 34).
2.3. Statistical analyses
2.3.1. Mice studies
GraphPad Prism 5 was used for all representations and statistical analyses. Results are presented as mean ± SEM. Analyses used either Student's t-test when a normal distribution was validated, or one/two-way(s) ANOVA followed by Bonferroni post hoc test when data were not normally distributed. Rhythmicity was assessed using Circwave v1.4 (free software developed by Roelof A. HUT, University of Groningen, Netherlands). The statistical tests as well as the number of mice used for each experiment are mentioned in the figure caption. Differences between groups were considered significant when P < 0.05.
2.3.2. Human studies
The natural log transformed plasma PROK2 concentration has been used in all analyses. The characteristics of people with and without diabetes were compared by ANCOVA adjusting for sex and age, then sex, age and BMI and/or daily energy intake. Associations between PROK2 and anthropometric, metabolic, and lifestyle variables were first examined using single regression analysis, except socio-economic status by ANOVA; variables associated with P < 0.10 were used as explanatory variables for a stepwise multiple regression (backward) analysis, with PROK2 as the dependent variable.
Associations between SNPs and clinical phenotypes were tested by chi2 and Cochran's trend test (unadjusted tests) and by logistic regression, first adjusted for sex, age, social status, physical activity, smoker status, alcohol consumption, and then adjusted for the same covariates plus BMI. P < 0.05 was considered to be statistically significant. All statistical analyses used SYSTAT 13® software for Windows.
3. Results
3.1. Mice studies
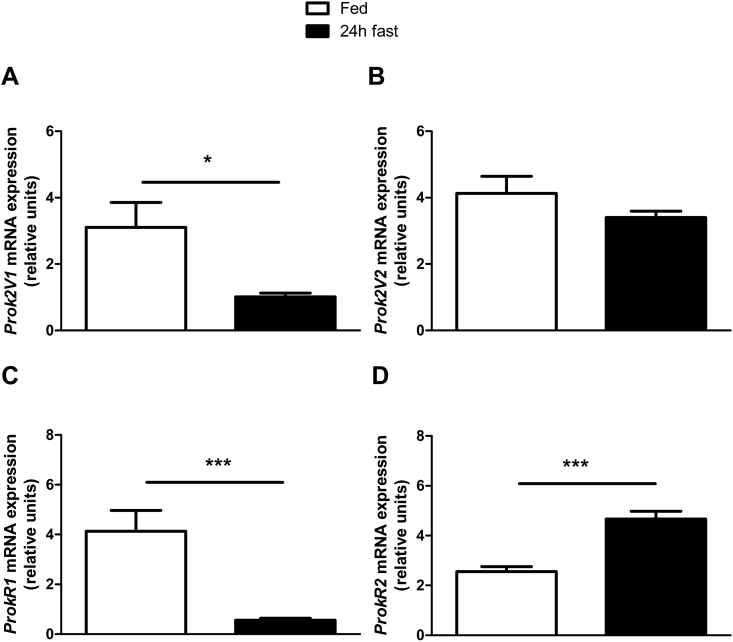
3.1.1. Prok2 mRNA expression in the MOB is modulated by the nutritional status (Figure 1)
Figure 1.
Fasting reduces Prok2V1 and ProkR1 mRNA expression in the MOB. mRNA expression of Prok2V1 (A) and Prok2V2 (B) as well as ProkR1 (C) and ProkR2 (D) were measured in the MOB of 4 month-old C57Bl/6J mice using RT-qPCR. Mice were maintained under a 12-h/12-h light–dark cycle starting at 7.00 am and samples were taken during the early light phase in fed or 24 h fasted mice (A–D). Values represent mean with SEM (n = 4–5/group). *, P < 0.05; and ***, P < 0.001 vs fed, in an unpaired Student's t-test.
The Prok2 primary transcript has two alternative splice variants, Prok2 variant 1 (Prok2V1) and Prok2 variant 2 (Prok2V2) [25]. Fasting induced a 87% decrease of Prok2V1 mRNA expression in the MOB (Figure 1A) but did not affect Prok2V2 expression (p = 0.22, using Students't test, n = 5, Figure 1B). Prok receptor 1 (ProkR1) mRNA expression was 67% lowered by fasting, whereas ProkR2 mRNA expression was increased by 182% (Figure 1C,D).
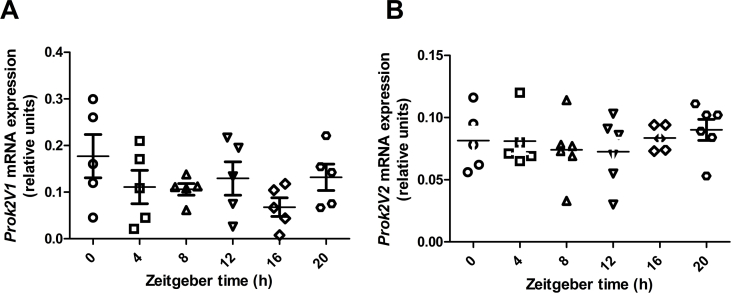
Because Prok2 mRNA expression follows a circadian rhythm in the SCN of the hypothalamus [26] and the MOB contains a robust circadian clock that can cycle independently of the SCN master pacemaker [27], we assessed whether Prok2 is expressed in a rhythmic pattern in the MOB. Neither Prok2V1 nor Prok2V2 mRNA expression in the MOB were significantly modified during the 24 h period of measurement (Figure S1A and B).
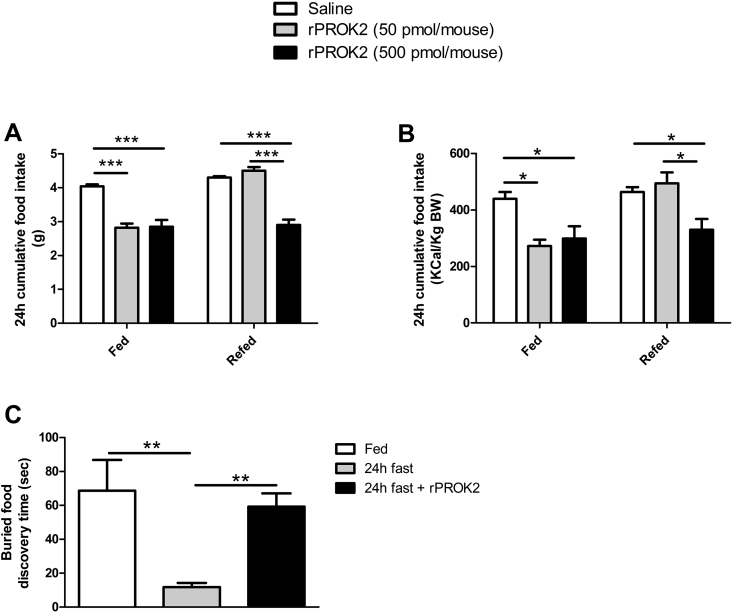
3.1.2. Acute injection of human recombinant PROK2 (rPROK2) in the MOB significantly decreases food intake and impacts olfactory behavior (Figure 2)
Figure 2.
Injection of recombinant PROK2 (rPROK2) in the MOB decreases food intake and impacts olfactory behavior. Cumulative food intake expressed in grams (A) or in Kcal/Kg BW (B) was measured 24 h after acute injection of 50 or 500 pmol of rPROK2 in the MOB. Measurements in ad libitum fed mice were at the beginning of the dark phase (fed), and measurement in fasted animals took place at the beginning of the light phase (refed). Values represent mean with SEM (n = 4–10/group), *, P < 0.05; and ***, P < 0.001 in rPROK2 vs saline or rPROK2 50 pmol vs rPROK2 500 pmol in one-way ANOVA followed by Bonferroni post hoc test. Buried food discovery time expressed in seconds (C) was measured during a buried food test in fed mice or after 24 h of fasting with or without rPROK2 injection (500 pmol/mouse) in the MOB. Values represent mean with SEM (n = 6/group), **, P < 0.01 in fed vs 24 h fast and in 24 h fast vs 24 h fast + rPROK2 500 pmol, in one-way ANOVA followed by Bonferroni post hoc test.
Acute injection of 50 pmol rPROK2 in the MOB of mice fed ad libitum induced a significant, 34% reduction of the cumulative food intake, measured 24 h post injection, compared to saline-injected mice (Figure 2A, fed). Increasing the dose at 500 pmol/mouse had no greater effect (Figure 2A, fed). When administered in the early light phase to 24 h-fasted mice, rPROK2 caused a 31% decrease of food intake (Figure 2A, refed state) but only at the highest dose (Figure 2A, refed). The anorectic effect of rPROK2 remained significant when normalized to animals’ body weight (BW) (Figure 2B).
To assess the effect of rPROK2 injection in the MOB on olfactory behavior, we performed a buried food test which relies on the animal's natural tendency to use olfactory cues for foraging. The main parameter is the latency to find and uncover a small piece of palatable food, hidden beneath a layer of cage bedding. In control mice, 24 h fast significantly decreased the buried food discovery time by 79% when compared to mice fed ad libitum (Figure 2C). Interestingly, rPROK2 injection (500 pmol/mouse) into the MOB of fasted mice resulted in a 3.6-fold increase of the latency to uncover the small piece of buried food compared to the fasted group (Figure 2C). These data show that PROK2 can be a local MOB regulator of olfactory function participating in food intake.
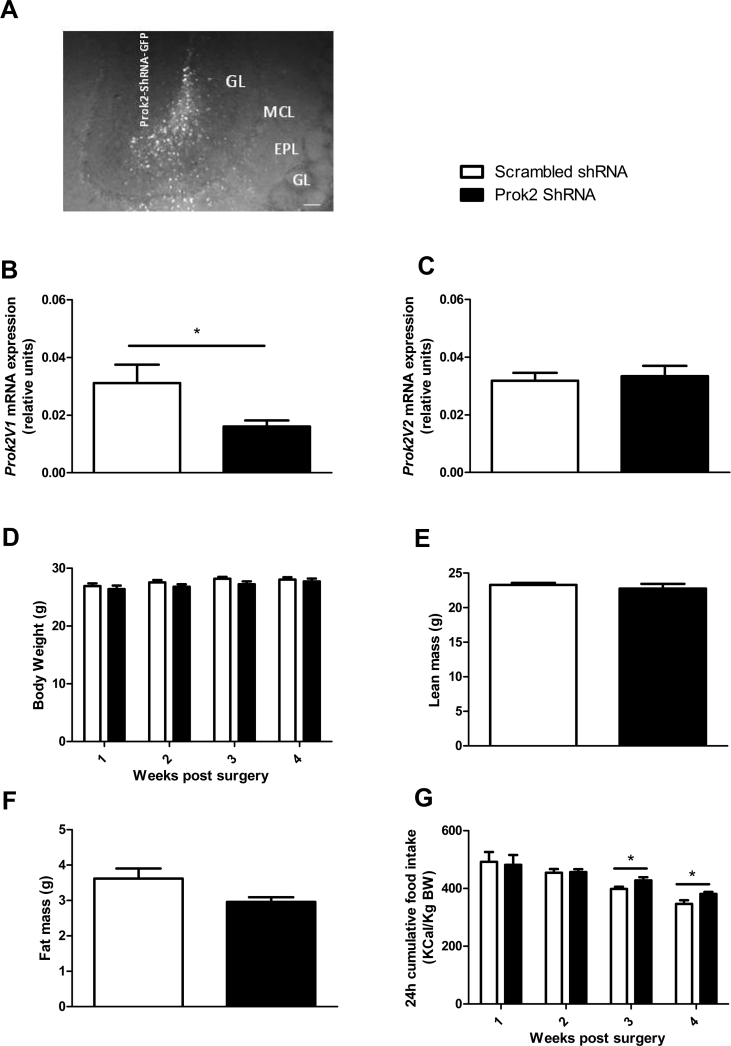
3.1.3. Partial deletion of Prok2 in the MOB has no effect on body weight but alters the food intake microstructure (Figure 3, Figure 4)
Figure 3.
Partial deletion of Prok2 in the MOB has no effect on body weight but increases food intake. Representative brain section showing GFP fluorescence in the ventral MOB (1 of the 4 injection sites), 48 h post injection of lentiviral particles expressing Prok2-shRNA. GL: glomerular layer, EPL, external plexiform layer, MCL, mitral cell layer, GCL, granule cell layer. Scale bar = 100 μm (A). Quantification using RT-qPCR of Prok2V1 (B) and Prok2V2 (C) mRNA expression in the MOB, 6 weeks after the injection of a lentiviral vector expressing Prok2-shRNA or a scrambled-shRNA. Body-weight was measured weekly for 4 weeks postsurgery in Prok2-shRNA and scrambled-shRNA-injected mice (D). Total lean mass (E) and total fat mass (F) were measured 3 weeks post lentiviral injection using EchoMRI. Weekly 24 h cumulative food intake (G) was measured in ad libitum fed mice from 1 to 4 weeks post lentiviral injection. Values represent mean with SEM (n = 6–12/group). *, P < 0.05 vs scrambled-shRNA, in an unpaired Student's t-test.
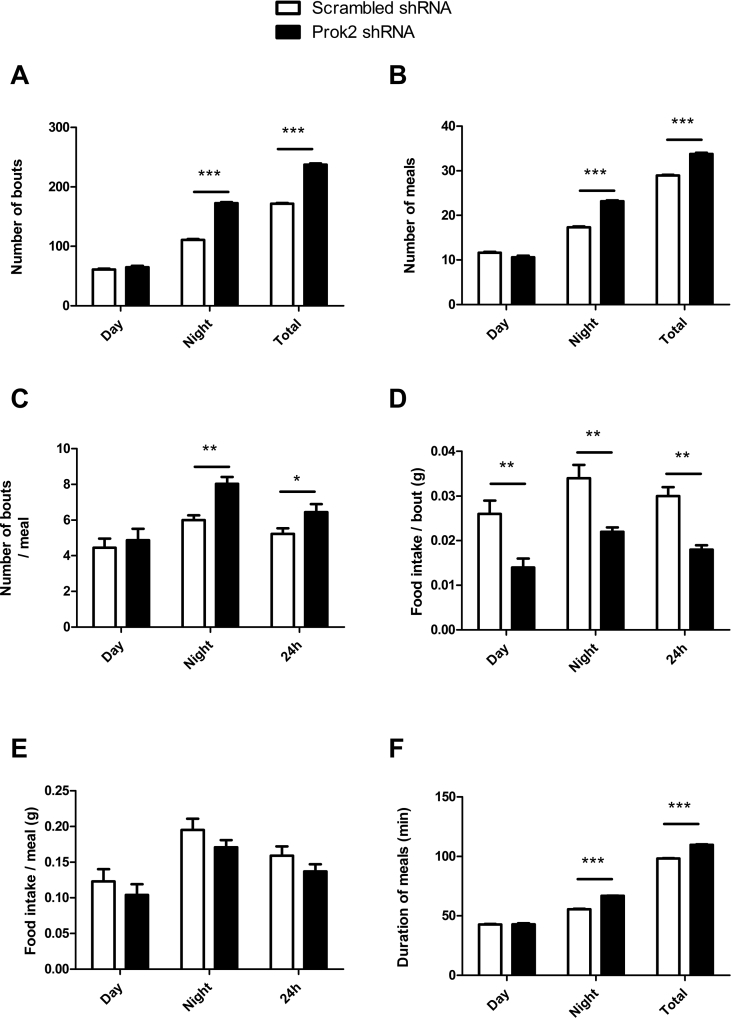
Figure 4.
Partial deletion of Prok2 in the MOB deregulates food intake microstructure. Mice injected in the MOB with the scrambled-shRNA or Prok2-shRNA were placed in calorimetric cages (TSE system, Germany) 3 weeks postsurgery. The food intake pattern including number of bouts (A), number of meals (B), number of bouts per meal (C), food intake per bout (D), and per meal (E), as well as meal duration (F) were assessed. Values represent mean with SEM (n = 6/group), *, P < 0.05, **, P < 0.01 and ***, P < 0.001 vs scrambled-shRNA, using a two-way ANOVA followed by Bonferroni post hoc test.
Injection of the Prok2-shRNA was processed in 8 different locations and restricted to the MOB. Indeed, the GFP staining was localized overall in the MOB, covering the periglomerular, granular, and mitral cells layers, all known to express Prok2 mRNA (Figure 3A) [28]. In Prok2-shRNA-injected mice, Prok2V1 mRNA expression was significantly decreased by 48% compared to scrambled-shRNA-injected mice (Figure 3B). Surprisingly, Prok2V2 mRNA expression was not altered (p = 0.73, using Students’ t test, n = 11, Figure 3C).
Prok2 underexpression in the MOB had no significant effect on BW (Figure 3D). It impacted neither the lean mass (23.29 ± 0.29 g, in scrambled- vs 22.75 ± 0.70 g, in Prok2-shRNA-injected mice, p = 0.49 in an unpaired Student's t-test, n = 6, Figure 3E) nor the fat mass (3.62 ± 0.28 g, in scrambled-vs 2.96 ± 0.13 g, in Prok2-shRNA-injected mice, p = 0.06 in an unpaired Student's t-test, n = 6, Figure 3F). To better understand the role of PROK2-MOB on food intake, we analyzed the quantity and the quality of food consumption. 24 h cumulative food intake was significantly increased in Prok2-shRNA-injected mice compared to scrambled mice, 3 and 4 weeks post-surgery (*p = 0.041 and *p = 0.034 at 3 and 4 weeks postsurgery respectively using Students' t test, n = 9, Figure 3G). To assess more precisely the food intake microstructure, we placed the animals in metabolic cages and monitored the meal pattern (Figure 4).
-
1
Feeding frequency. We first observed that Prok2 underexpression in the MOB resulted in an increased feeding frequency as illustrated by an increased number of bouts (Figure 4A) and meals (Figure 4B), as well as the number of bouts per meal (Figure 4C), compared to scrambled-shRNA-injected mice. Additional analysis revealed that this rise in feeding frequency only occurred during the nocturnal period (Figure 4A–C).
-
2
Meal size. Next, we assessed whether there were any differences in meal size. We observed a significant reduction of the 24 h cumulative food intake per bout in Prok2-shRNA compared to scrambled-shRNA-injected mice, both during the diurnal and nocturnal periods (Figure 4D). This was accompanied by a slight but not significant decrease of the food intake per meal (Figure 4E).
-
3
Meal duration is also an important component of feeding behavior. We observed that the mean meal duration of Prok2-shRNA-injected mice was greater than that of scrambled-shRNA-injected mice (Figure 4F).
3.1.4. Partial deletion of Prok2 in the MOB affects the balance of neuropeptides expression in the hypothalamus (Table 1)
Table 1.
Partial deletion of Prok2 in the MOB affects the balance of AgRP/POMC neuropeptides expression in the hypothalamus.
| A | mRNA relative expression in the MOB |
||||
| NPY | AgRP | POMC | CART | AgRP/POMC | |
| Scrambled-shRNA | 2.56 ± 0.1 | 0.08 ± 0.009 | 0.003 ± 0.009 | 0.27 ± 0.02 | 28.7 ± 7.06 |
| Prok2-shRNA | 2.2 ± 0.6 | 0.06 ± 0.003 | 0.006 ± 0.004 | 0.27 ± 0.06 | 17.0 ± 6.6 |
| p value | 0.5 | 0.09 | 0.7 | 0.99 | 0.7 |
| B | mRNA relative expression in the hypothalamus |
||||
| NPY | AgRP | POMC | CART | AgRP/POMC | |
| Scrambled-shRNA | 0.90 ± 0.3 | 5.2 ± 3.3 | 2.2 ± 1.2 | 4.3 ± 0.7 | 1.8 ± 0.8 |
| Prok2-shRNA | 1.28 ± 0.6 | 5.8 ± 2.7 | 0.32 ± 0.15 | 2.1 ± 0.9 | 21.9 ± 3.7 |
| p value | 0.6 | 0.9 | 0.2 | 0.09 | 0.002** |
Quantification using RT-qPCR of neuropeptide Y (NPY), agouti related peptide (AgRP), proopiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART) mRNA expression in the MOB (A) and in the hypothalamus (B) of mice injected with scrambled-shRNA or Prok2-shRNA. Values represent mean with SEM (n = 5–7/group), **, P < 0.01, vs scrambled-shRNA, in an unpaired Student's t-test.
To elucidate whether MOB and/or hypothalamic neuropeptides expression is associated with the altered meal pattern observed in Prok2-shRNA-injected mice, gene expression analysis of orexigenic and anorexigenic neuropeptides was performed in both structures. As expected [29], we observed a strong expression of NPY mRNA in the MOB while AgRP mRNA expression was low and POMC mRNA expression was at the limit of detection. Prok2 underexpression altered none of the neuropeptides mRNA expression neither in the MOB nor in the hypothalamus. However, the ratio of AgRP/POMC in the hypothalamus was significantly higher in Prok2-shRNA-injected mice compared to scrambled mice. This suggests that underexpression of Prok2 in the MOB can affect the balance of orexigenic/anorexigenic neuropeptides expression in the hypothalamus, leading to an increase in food intake. Prok2 underexpression in the MOB had no effect on ProkR1 and ProkR2 mRNA expression and did not influenced the expression of the main metabolic receptors genes (lepR, insR, GLP-1R, data not shown). Interestingly, Prok2 underexpression in the MOB affected neither Prok2V1 nor Prok2V2 expression in the hypothalamus (V1: 0.040 ± 0.01 relative units (RU), in scrambled-vs 0.029 ± 0.004 RU, in Prok2-shRNA-injected mice, p = 0.27 using Students' t test and V2: 0.010 ± 0.002 RU, in scrambled-vs 0.014 ± 0.002 RU, in Prok2-shRNA-injected mice, p = 0.16 using Students’ t test, n = 9).
3.1.5. Partial deletion of Prok2 in the MOB altered glucose metabolism in vivo (Figure 5)
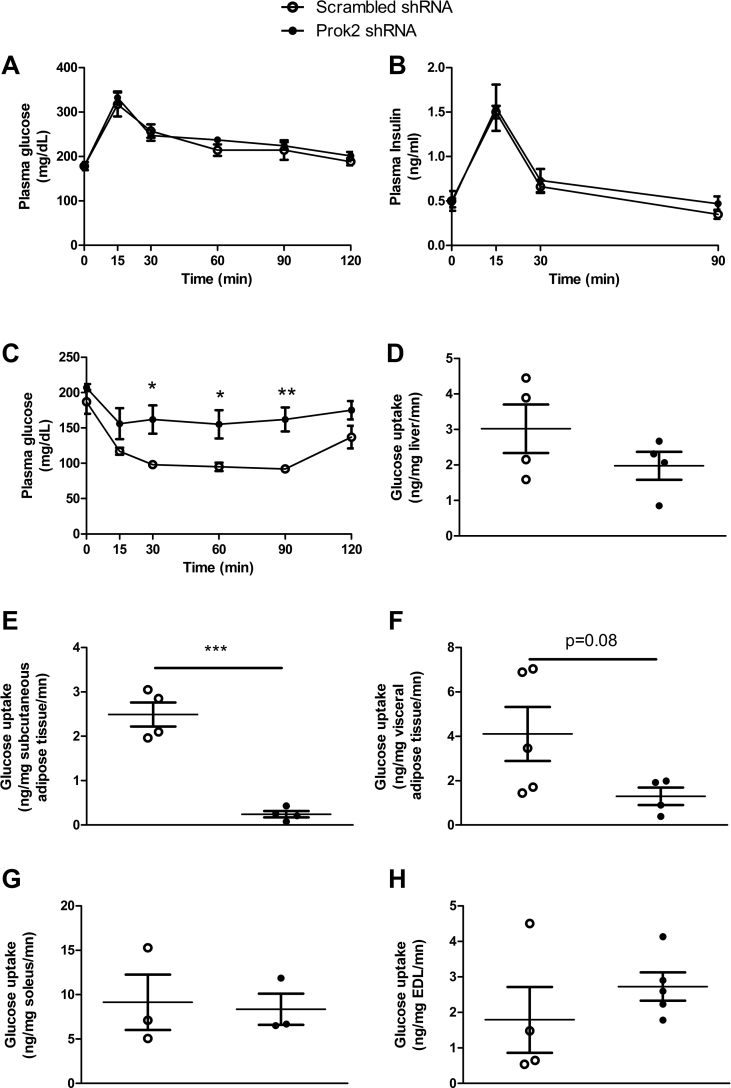
Figure 5.
Partial deletion of Prok2 in the MOB altered glucose metabolism in vivo. Glucose homeostasis studies were performed 4 weeks after injection of Prok2-shRNA or scrambled-shRNA in the MOB. Plasma glucose (A) and insulin (B) were measured during a glucose tolerance test (3 g/kg BW), plasma glucose was measured during an insulin tolerance test (0.75U/kg BW) (C) and glucose uptake was measured after ip insulin (0.75U/kg BW) and 2-deoxy-D-[1–14C] glucose (2DG) (5 μCi) injection, in liver (D), subcutaneous adipose tissue (E), visceral adipose tissue (F), soleus (G), extensor digitorum longus (EDL, H). Values represent mean with SEM (n = 4–6/group), *, P < 0.05 and **, P < 0.01 vs scrambled-shRNA, in two-way ANOVA followed by Bonferroni post hoc test except for glucose uptake that was analyzed using an unpaired Student's t-test.
We measured the effect of Prok2 underexpression in the MOB on glucose tolerance in vivo and found no modification of the glycemic and insulinemic profiles during an oral glucose tolerance test (Figure 5A,B). Neither plasma glucose nor insulin levels were impacted during basal or fasted conditions (data not shown). However, in Prok2-shRNA-injected mice, we were able to show significant lower insulin sensitivity compared to scrambled-shRNA-injected mice during an insulin tolerance test (Figure 5C). By assessing glucose uptake in insulin-responsive tissues (Figure 5D–H), we found that mice that received the Prok2-shRNA in the MOB had a significantly lower glucose uptake exclusively in the subcutaneous adipose tissue compared to scrambled mice (Figure 5D). To further characterize the lipid profile of mice with MOB Prok2 underexpression, we measured plasma leptin, plasma free fatty acids (FFA), and plasma triglycerides (TG) in both scrambled- and Prok2-shRNA-injected mice, 3 weeks post surgery and found no difference between groups (leptin: 2.37 ± 0.49 ng/ml, in scrambled-vs 2.30 ± 0.49 ng/ml, in Prok2-shRNA-injected mice, p = 0.92 using Students' t test, n = 6; FFA: 75.6 ± 11.2 ng/ml, in scrambled-vs 82.4 ± 7.5 ng/ml, in Prok2-shRNA-injected mice, p = 0.55 using Students' t test, n = 6; TG: 43.4 ± 5.5 mg/dl, in scrambled-vs 41.2 ± 9.1 mg/dl, in Prok2-shRNA-injected mice, p = 0.83 using Students’ t test, n = 6).
3.1.6. Partial deletion of Prok2 in the MOB induced changes in respiratory exchange ratio (RER) and fatty acid oxidation in fasted mice (Figures S2 and S3)
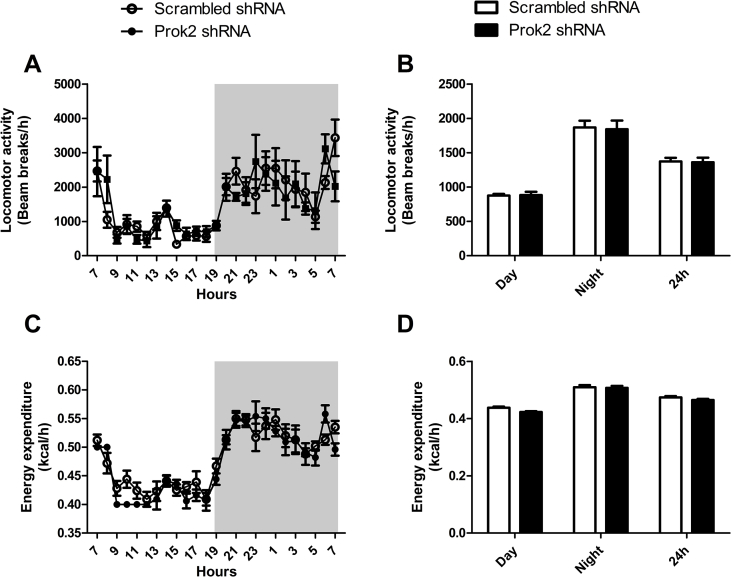
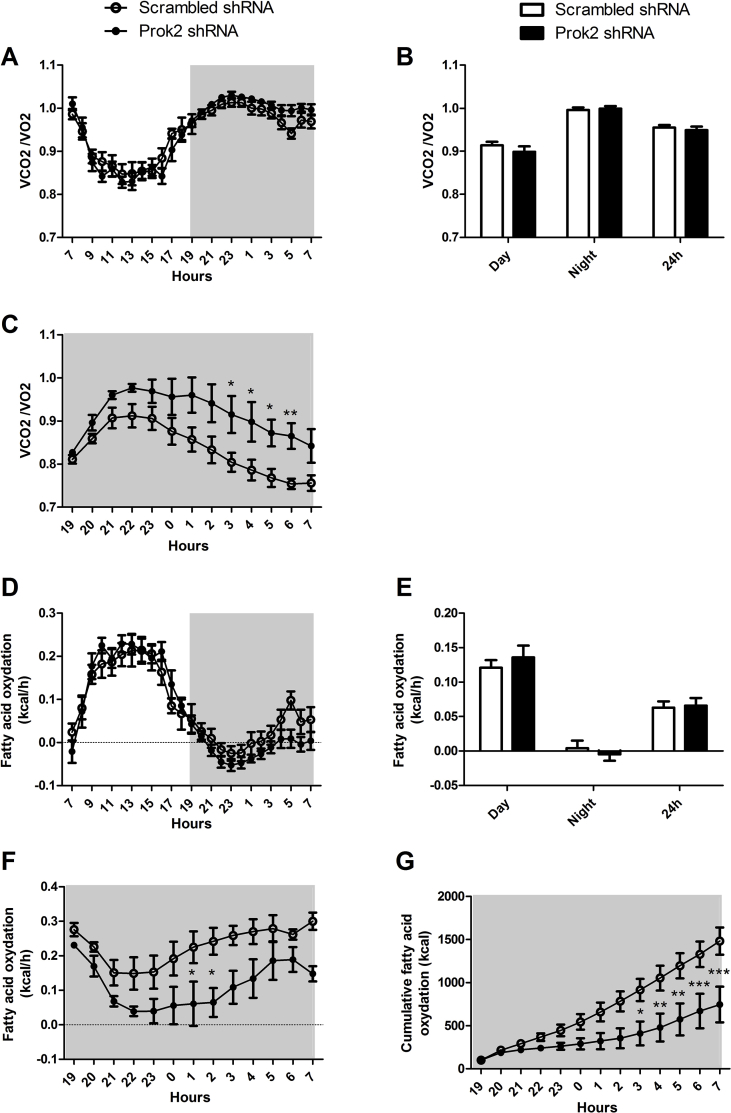
Because Prok2 underexpression in the MOB was associated with a greater food intake without affecting BW, we hypothesized that it may alter energy expenditure. Using metabolic cages, we measured calorimetric parameters and found that injection of Prok2-shRNA in the MOB affected neither locomotor activity (Figure S2A,B) nor energy expenditure (Figure S2C,D) compared to injection of scrambled-shRNA. The RER (=VCO2/VO2) (Figure S3A,B) and the fatty acid oxidation (Figure S3D,E) were also unchanged. Interestingly, adaptation to fasting seemed altered by Prok2 underexpression in the MOB since an overnight fasting induced an increase of the RER (Figure S3C) and a decrease of fatty acid oxidation (Figure S3F and G) in Prok2-shRNA-injected mice compared to scrambled mice. There were no differences of RER and fatty acid oxidation observed during refeeding (not shown).
3.2. Human studies
Because human MOB is rarely affected by epilepsy or tumors [30], biopsies are not available; therefore, it is not possible to make a parallel between MOB-PROK2 in mice and humans. Instead, we were able to quantify PROK2 in the plasma of people from the D.E.S.I.R. cohort and to carry out associations with T2D. We also studied the genetic association between SNPs of PROK2 gene, T2D and the MetS.
3.2.1. Plasma PROK2
Plasma PROK2 was lower in people with T2D compared to the non-diabetic one (Table 2). There was no difference in plasma PROK2 between never diabetic and future diabetic people (data not shown). In univariate regression studies (Table 3), PROK2 was significantly inversely associated with BMI, waist girth, fasting glycemia, HbA1c, total cholesterol, and LDL cholesterol (p < 0.05). Concerning the significant association with socio-economic status, PROK2 was lower in people with lower economic status (codes > 5, including workers, retired people and unemployed) than in people with codes ≤ 5 (including employees, executives, managers, superior intellectual jobs). To perform a stepwise multiple regression, we used all variables with P < 0.10: age, BMI, waist girth, fasting glucose, HbA1c, total and LDL-cholesterol, energy intake, alcohol intake, smoker status, and socio-economic status. The final model included BMI, LDL-cholesterol, energy intake, smoker status, and socio-economic status. When excluding total cholesterol and HbA1c from the initial model due to their strong correlation with LDL-cholesterol and glycemia, respectively, there was no change in the final model.
Table 2.
Characteristics of people with PROK2 plasma levels measured according to whether or not they had T2D at baseline or after 3 years of follow-up, the D.E.S.I.R. study.
| No diabetes | Diabetes | Pa | |
|---|---|---|---|
| n (M/F) | 114 (68/46) | 34 (27/7) | 0.03 |
| Present smokers (n) | 28 | 6 | 0.39 |
| PROK2 (μg/L) | 12.6 (11.5–13.9) | 10.5 (9.2–12.0) | 0.03 |
| Age (years) | 47.8 ± 9.4 | 48.4 ± 7.6 | 0.67 |
| BMI (kg/m2) | 26.6 ± 4.3 | 29.4 ± 4.8 | <0.001 |
| Waist girth (cm) | 89.4 ± 12.0 | 101.0 ± 11.0 | <0.001 |
| Glycemia (mmol/L) | 5.6 ± 0.6 | 7.7 ± 1.2 | <0.001 |
| Energy intake (kcal/day) | 2073 ± 466 | 2270 ± 369 | 0.11 |
Mean ± SD for continuous data, except PROK2 (geometric mean (95%CI)). PROK2 was log transformed for analysis.
ANCOVA adjusted for sex and age (except age) or chi2 when appropriate, n = 148.
Table 3.
Univariate and multivariable regression analysis between log-transformed PROK2 plasma levels and anthropometric, metabolic and lifestyle variables in 148 participants, the D.E.S.I.R. study.
| Single regression analysis |
Stepwise multivariable regression analysis (final parameters) |
|||
|---|---|---|---|---|
| β | P | β | P | |
| Sex | 0.043 | 0.60 | ||
| Age | −0.153 | 0.06 | ||
| BMI | −0.161 | 0.05 | −0.163 | 0.05 |
| Waist girth | −0.196 | 0.02 | ||
| Fasting glucose | −0.176 | 0.03 | ||
| HbA1c | −0.168 | 0.04 | ||
| Systolic blood pressure | −0.051 | 0.54 | ||
| Diastolic blood pressure | 0.034 | 0.68 | ||
| Ln triglycerides | −0.097 | 0.24 | ||
| Total cholesterol | −0.148 | 0.07 | ||
| HDLC | 0.101 | 0.22 | ||
| LDLC | −0.178 | 0.03 | −0.131 | 0.12 |
| Energy intake | −0.144 | 0.08 | −0.182 | 0.05 |
| Alcohol intake | −0.156 | 0.06 | ||
| Smoker status | 0.147 | 0.08 | 0.157 | 0.07 |
| Physical activity | 0.026 | 0.76 | ||
| Socio-economic statusa | F = 2.28 (7df) | 0.03 | NA | 0.04 |
β, standardized partial regression coefficient. In single regression analysis, β is equal to r, Pearson's correlation coefficient.
Sex: man = 1, woman = 2; smoker: yes = 1, no = 2; physical activity index, 3 groups (coding 0–2).
Socio-economic status: 1 = Agricultural (but not represented in the population with Prokineticin 2 measured); 2. Craftsmen, tradesmen, head of enterprise - independent, government, scientific, arts, spectacles, executives in enterprise; 3. Executives and professions with superior intellectual; 4. Intermediate professions: teachers, health professions, administrators, technicians, clergy; 5. Employees: governmental, enterprises, commerce, police; 6. Workers: qualified, non-qualified, agricultural; 7. Retired - previously agricultural workers, craftsmen, executives, employees, workers etc.; 8. Without a professional activity (unemployed, has never worked, students, apprentices, home-maker); 9. Unemployed, has previously worked.
In bold = variables selected for the stepwise analysis for log-transformed serum prokineticin level (all variables with P < 0.10 in single regression analysis). When excluding total cholesterol and HbA1c because of their strong correlation with LDLC and fasting glucose respectively, the final model was not changed.
Since socio-economic status is a categorical variable, a one factor ANOVA was performed instead of single regression analysis. It was entered in the multiple regression model as categorical variable.
3.2.2. PROK2 genotypes
In Table 4, only SNPs with some evidence of association by unadjusted tests are presented. Only marginal associations between some SNPs and incident T2D were found (data not shown). Nevertheless, more consistent associations were found with incident hyperglycemia (T2D/IFG), MetS, and obesity. The minor alleles of rs7634474 and rs3796224 were associated with a lower risk of hyperglycemia (T2D/IFG), while rs1320015 was associated with a higher risk. A minor allele of rs1320015 was associated with a higher risk of MetS, as well as rs2322142. The latter association disappeared after adjustment for baseline BMI. Minor alleles of rs6782813 and rs7634474 were associated with a higher risk of obesity. It can be noted that the association of rs7634474 with obesity was in an opposite direction as compared to its association with hyperglycemia, indicating pleiotropic effects of PROK2 genetic variation. This association of rs7634474 with the incidence of obesity disappeared after adjustment for baseline BMI which indicates that BMI was already higher at baseline in minor allele carriers (BMI = 24.9 ± 3.9 and 24.6 ± 3.8 in minor allele carriers and non carriers respectively, P = 0.04).
Table 4.
Genotype frequencies of PROK2 polymorphisms according to the incidence of type 2 diabetes/impaired fasting glucose (T2D/IFG), the MetS and obesity in more than 4000 participants, the D.E.S.I.R. study.
| Disease | SNP | Gen | Ctrls | Cases | P chi2 2df/trend/dominant/recessive | OR1a | OR2b |
|---|---|---|---|---|---|---|---|
| T2D/IFG | RS7634474 | GG | 2 580 (71.7%) | 381 (75.9%) | 0.07/0.02/0.05/0.08 | A 0.78 (0.63–0.95) P = 0.013 D 0.79 (0.63–0.99) P = 0.040 |
0.76 (0.62–0.94) P = 0.009 0.77 (0.62–0.97) P = 0.028 |
| GA | 920 (25.6%) | 114 (22.7%) | |||||
| AA | 97 (2.7%) | 7 (1.4%) | |||||
| RS1320015 | GG | 1 344 (37.8%) | 154 (31.5%) | 0.02/0.04/0.007/0.77 | A 1.14 (0.99–1.31) P = 0.07 D 1.29 (1.05–1.59) P = 0.016 |
1.14 (0.99–1.31) P = 0.07 1.28 (1.03–1.58) P = 0.024 |
|
| GA | 1 627 (45.8%) | 252 (51.5%) | |||||
| AA | 585 (16.5%) | 83 (17.0%) | |||||
| RS3796224 | GG | 2 878 (81.0%) | 412 (83.7%) | 0.08/0.07/0.14/0.05 | A 0.77 (0.60–0.99) P = 0.041 R 0.15 (0.02–1.11) P = 0.06 |
0.76 (0.59–0.98) P = 0.035 0.17 (0.02–1.23) P = 0.08 |
|
| GA | 635 (17.9%) | 79 (16.1%) | |||||
| AA | 41 (1.2%) | 1 (0.2%) | |||||
| MetS | RS1320015 | GG | 1 333 (37.5%) | 157 (33.2%) | 0.12/0.04/0.07/0.13 | A 1.16 (1.01–1.33) P = 0.037 D 1.21 (0.98–1.49) P = 0.08 |
1.17 (1.01–1.36) P = 0.038 1.36 (0.94–1.46) P = 0.09 |
| GA | 1 635 (46.0%) | 225 (47.6%) | |||||
| AA | 585 (16.5%) | 91 (19.2%) | |||||
| RS2322142 | CC | 2 218 (62.3%) | 269 (57.5%) | 0.09/0.11/0.04/0.78 | A 1.49 (0.98–1.36) P = 0.09 D 1.25 (1.02–1.53) P = 0.030 |
1.14 (0.96–1.36) P = 0.14 1.20 (0.97–1.48) P = 0.09 |
|
| CT | 1 172 (32.9%) | 178 (38.0%) | |||||
| TT | 170 (4.8%) | 21 (4.5%) | |||||
| Obesity | RS6782813 | CC | 3 466 (92.2%) | 296 (87.1%) | 0.004/0.006/0.0009/0.67 | A 1.72 (1.24–2.38) P = 0.0013 D 1.77 (1.26–2.50) P = 0.0011 |
1.78 (1.17–2.71) P = 0.006 1.90 (1.23–2.95) P = 0.004 |
| CT | 285 (7.6%) | 43 (12.6%) | |||||
| TT | 7 (0.2%) | 1 (0.3%) | |||||
| RS7634474 | GG | 2 737 (73.0%) | 230 (67.6%) | 0.10/0.03/0.03/0.34 | A 1.26 (1.02–1.54) P = 0.030 D 1.31 (1.03–1.67) P = 0.028 |
1.17 (0.90–1.51) P = 0.10 1.19 (0.88–1.61) P = 0.12 |
|
| GA | 923 (24.6%) | 99 (29.1%) | |||||
| AA | 90 (2.4%) | 11 (3.2%) |
Considering the risk for the minor allele: A = additive model, D = dominant model, R = recessive model.
Gen = genotype.
OR1 = Odds ratio (95% Confidence interval) for minor allele by logistic regression, additive model, adjusted for sex, age, social status, physical activity, smoker status, alcohol consumption.
OR2 = Odds ratio (95% Confidence interval) by logistic regression, same model as OR1 with additional adjustment for BMI at baseline.
4. Discussion
In the present article, we showed that i) MOB-Prok2V1 expression was reduced with fasting in mice, ii) acute injection of rPROK2 in the MOB significantly decreased food intake whereas Prok2-shRNA injection resulted in an increase of dietary consumption characterized by higher feeding frequency and lower meal size, with no significant repercussion on body weight, iii) Prok2 underexpression in the MOB induced a significant reduction of insulin sensitivity compared to scrambled-shRNA-injected mice, and iv) in the human D.E.S.I.R. cohort, plasma PROK2 levels were negatively related to T2D, BMI, and energy intake, and PROK2 genetic variants were associated with hyperglycemia (T2D/IFG), MetS, and obesity.
Our first important result was that Prok2 mRNAs in the MOB are differentially expressed according to the nutritional status (i.e. fed or fasted). 24 h fasting lowered Prok2V1 mRNA level whereas that of Prok2V2 remained unchanged. This was concomitant with a lower ProkR1 and a higher ProkR2 mRNAs expression. In the literature, Prok2V1, also called Prok2 long form (Prok2 L), encodes the PROK2β isoform [25] that was shown to preferentially bind PROKR1 [31]. These results suggest that, in the MOB, the PROK2β/PROKR1 could be the main couple regulator of feeding behavior. Interestingly, this is in line with data from Beale et al. who showed that the anorectic effects of peripherally administered PROK2 are mediated via the brainstem and require PROKR1 signaling [32]. Previous studies by Gardiner et al. showed that fasting decreased Prok2 expression in the hypothalamus [16], thus emphasizing the similarities between these two key structures involved in the regulation of energy balance. This is a characteristic of neuropeptides that reduce food intake, their expression is often lowered in states of negative energy balance [33]. This is also in agreement with behavioral studies previously demonstrating that fasting increases while feeding decreases the olfactory system responsiveness in rodents [36]. Furthermore, we showed that Prok2 underexpression in the MOB following Prok2 shRNA injection increased food intake with no significant repercussion on body weight. This is consistent with the absence of increased body weight of mice lacking Prok2 compared with their wild-type littermates [13], [14]. Although Prok2 expression in the hypothalamus exhibits a circadian rhythm [3], we did not find such pattern of expression in the MOB. Acute injection of rPROK2 in the MOB significantly decreased food intake -regardless of the animals’ nutritional status-comparable to what was previously reported in the hypothalamus [16]. To our knowledge, this is the first demonstration that PROK2 in the MOB has an anorectic function.
In a complementary way, we showed that the partial decrease of Prok2V1 expression following Prok2-shRNA injection in the MOB induced a mirror phenotype, i.e. an increased food intake. This effect on food intake was quantitatively moderate, probably due to the low amplitude and possibly transient underexpression of Prok2 in the MOB. However, the qualitative effect of the partial decrease of Prok2 expression on food intake microstructure was significant since a change in the pattern of meals accompanied the increase in food intake. Indeed, the partial deletion of Prok2 expression in the MOB was associated with a greater number of bouts and number of meals as well as the number of bouts per meal mainly during the night (active) period. Additionally, meal duration was longer while the quantity of food per bout was shorter. This effect on meal structuration is very interesting, because a growing body of evidence indicate that changes in meal pattern (e.g. meal size and frequency) may have an impact in the progression to insulin resistance and T2D. Indeed, in T2D patients it was shown that eating only two meals a day (breakfast and lunch) reduced body weight and fasting plasma glucose, while it increased oral glucose insulin sensitivity and fasting plasma ghrelin, more than the same caloric restriction split into six meals. These results suggest that, for T2D patients following a hypoenergetic diet, eating larger breakfasts and lunches may be more beneficial than eating six meals throughout the day [34], [35]. In our study, Prok2 underexpression in the MOB resulted in an increased number of meals that could partly explain insulin resistance. One possible explanation is that a higher number of meals could lead to a permanent increase in plasma ghrelin, as described in humans [36]. One can hypothesize that such “chronic” exposure to ghrelin may interfere with inhibiting and stimulating orexigenic pathways, leading to decreased insulin sensitivity. The increased feeding frequency observed in mice with lower Prok2 expression in the MOB is consistent with a role of PROK2 in mediating satiation. It is possible that the increase in both feeding frequency and meal duration induced by Prok2 underexpression in the MOB could be due to a delayed satiation [37]. This would suggest that in the MOB, PROK2 could decrease food intake through a decreased feeding frequency that may have a beneficial effect on weight management and glucose homeostasis. Interestingly, a study from Gill and Panda highlighted that time-restricted feeding may also prevent obesity in humans [38]. How could the deletion of Prok2 in the MOB lead to changes in the meal pattern? It is evident that a decrease of Prok2 expression in the MOB would affect olfaction, since PROK2 is essential for morpho- and neurogenesis of the MOB [13], [15], [28]. Thus, Prok2 underexpression could have a negative impact on olfactory sensitivity, which strongly influences feeding behavior in both rodents and humans [39], [40], [41]. For example, Aschenbrenner et al. described that patients with olfactory loss report alterations of their dietary behaviors [42]. Accordingly, we showed that acute injection of rPROK2 into the MOB impacted olfactory behavior. An additional possibility would be that the disruption of the MOB function caused by Prok2 underexpression could affect the message transmitted to the hypothalamus, thereby altering food intake. Indeed, there is a cross-talk between the MOB and the hypothalamus [4], and sensory detection –including food odors-modulates arcuate circuitry, as shown by Chen et al. [43]. We also found that Prok2-shRNA injected in the MOB resulted in an increase of the AgRP/POMC ratio in the hypothalamus, suggesting that PROK2 in the MOB can alter the balance of orexigenic/anorexigenic neuropeptides expression to regulate food intake. This is consistent with the data of Gardiner et al. who showed that PROK2 increased the release of alpha-melanocyte-stimulating hormone (alpha-MSH) from ex vivo hypothalamic explants [16]. Since Prok2 expression in the hypothalamus is not affected by Prok2 underexpression in the MOB, we can exclude an effect on hypothalamic neuropeptides caused by local Prok2 knockdown. We can postulate that this PROK2-dependent regulation of MOB output would result in the adjustment of hypothalamic neuropeptides activity in addition to other synaptic inputs from other sensory modalities for instance, thus contributing to the regulation of food intake.
Finally, partial decrease of Prok2 expression in the MOB also caused insulin resistance with no change in glucose tolerance. This was due, at least in part, to a decreased glucose uptake in the subcutaneous adipose tissue, with no modification of plasma leptin, free fatty acids and triglycerides. As mentioned previously, the induction of insulin resistance could be related to changes in meal pattern and consequent energy fluxes in the organism that deregulate the overall energy homeostasis [36]. Changes in insulin sensitivity could also be related to a dysregulation of the autonomic nervous system activity that is controlled, at least in part, by the hypothalamus [44], [45], [46]. Thus, defects in the crosstalk between the MOB and the hypothalamus may induce a sympathetic/parasympathetic imbalance and consequently may affect insulin sensitivity. Our data are in agreement with Riera et al., in that a disruption of the MOB function induced changes in insulin sensitivity through modulations of the autonomic nervous system activity [8]. Taken together these data suggest the existence of a neuronal network that would relay information from sensory cues to autonomic neurons and peripheral metabolism. It would require integration of olfactory signals in the MOB, relayed in the olfactory cortex and – among other brain areas - central neurons in the hypothalamus (including POMC and NPY/AgRP neurons in the arcuate nucleus) leading to activation/inhibition of the autonomic nervous system and regulation of insulin sensitivity. Several articles also emphasized the role of prokineticin signaling in the regulation of glucose homeostasis. Indeed, ProkR1 null mice display an impaired glucose tolerance and insulin sensitivity [47], while genetically induced ProkR1 loss in the endothelial cells (ec-ProkR1−/−) led to an impaired capillary formation and transendothelial insulin delivery, thus leading to insulin resistance [48].
Outside of the central nervous system, PROK2 is also expressed in the gastrointestinal tract and can be detected in plasma [49]. However, the dearth of a good antibody in rodents that would be sensitive enough to detect properly the protein makes it difficult to measure plasma PROK2 level. This explains why the literature on Prok2 in mice refers to mRNA expression instead of protein level. Fortunately, in humans the low sensitivity of the PROK2 antibody is less problematic since it is easier to collect large plasma volumes. Thus, we were able to measure plasma PROK2 levels in a subset of people from the D.E.S.I.R. cohort to determine whether they correlate with clinical parameters. Our analysis of a subset of the D.E.S.I.R. cohort showed that plasma PROK2 was associated with T2D, but this association disappeared after adjustment for BMI and/or caloric intake. Using single regression analysis, plasma PROK2 was mainly associated with anthropometric parameters such as BMI and waist girth, metabolic variables such as plasma glucose, HbA1c, and LDLC, and with a lifestyle/environmental factor, the socio-economic status. In a multivariable model, BMI, energy intake, plasma LDLC, smoker and socio-economic status remained associated with PROK2 levels.
Finally, our genetic association study performed on the whole cohort D.E.S.I.R. highlighted associations with incident hyperglycemia and incident MetS, even after adjustment for BMI and with obesity incidence. These associations are compatible with the additive model, showing an allele dose effect, but also most of the times with the dominant model meaning that carrying one or two alleles makes no difference. Actually, these results indicate associations between phenotypes and genetic variation but cannot allow to conclude firmly on the genetic model.
Since the polymorphisms were not associated with plasma PROK2 levels, which might be due to the small sample of people in whom PROK2 was measured, it cannot be inferred that the associations with metabolic phenotypes are mediated by such an effect. None of the tested polymorphisms is coding. Nevertheless, the genetic variation may modify PROK2 expression, even without modifying plasma PROK2 levels. These changes in expression may occur at the hypothalamus or at the MOB level, as well as in the gastro-intestinal tract, since all these organs may be involved in insulin resistance and/or body weight regulation. For instance, the associations – opposite in direction - found with rs7634474 for insulin resistance and obesity could indicate different regulations in different organs. Whether the SNPs modify PROK2 expression is not known; however, the rs6782813 which shows a strong association with obesity is located in the 3′UTR region and therefore may play a role in mRNA stability and PROK2 expression. Also, because we chose tag SNPs covering nearly all the genetic variability at the PROK2 locus, the SNPs may not be functional per se but in linkage disequilibrium with functional variants.
These results in humans are described here for the first time and need to be replicated.
In conclusion, our data highlight MOB-PROK2 as a new actor in the relationship between olfaction, feeding behavior, and energy homeostasis. Although the specific deficiency of Prok2 in the MOB has only a moderate effect on the control of the energy balance, our data should be placed in a physiological context and compared with those showing a role of PROK2 in the hypothalamus [16]. Thus, we assume that this system of regulating food intake, which is present in two key brain areas of energy balance control, works in synergy and in a complementary way in a physiological situation. Finally, our study also provides evidence that plasma PROK2 in humans is negatively correlated to T2D, BMI, and energy intake and that PROK2 genetic variants are associated with incident hyperglycemia (T2D/IFG), the MetS, and obesity.
Acknowledgements
This work was supported by grants from CORDDIM Ile-de-France, Société Francophone du Diabète (SFD, grant SFD-BD) and Fondation pour la Recherche Médicale (FRM, grant FDT20150532232).
We also acknowledge the animal core facility “Buffon” of the University Paris Diderot-Paris 7/Institut Jacques Monod, Paris for animal husbandry and breeding. We acknowledge the technical platform Functional and Physiological Exploration platform (FPE) of the Unit “Biologie Fonctionnelle et Adaptative,” (Université de Paris, BFA, UMR 8251, CNRS, F-75013 Paris, France) for metabolic and food behavior analysis.
The D.E.S.I.R. study has been funded by INSERM contracts with Caisse nationale de l'assurance Maladie des Travailleurs Salariés (CNAMTS), Lilly, Novartis Pharma, and Sanofi-Aventis; INSERM (Réseaux en Santé Publique, Interactions entre les déterminants de la santé, Cohortes Santé TGIR 2008); the Association Diabète Risque Vasculaire; the Fédération Française de Cardiologie; La Fondation de France; Association de Langue Française pour l'Etude du Diabète et des Maladies Métaboliques (ALFEDIAM)/Société Francophone de Diabétologie (SFD); l'Office National Interprofessionnel des Vins (ONIVINS); Ardix Medical; Bayer Diagnostics; Becton Dickinson; Cardionics; Merck Santé; Novo Nordisk; Pierre Fabre; Roche; Topcon.
The D.E.S.I.R. Study Group:
CESP, Inserm U1018: B. Balkau, P. Ducimetière, E. Eschwège; Univ Paris Descartes: F. Rancière; Inserm U367: F. Alhenc-Gelas; CHU d’Angers: A. Girault; Bichat Hospital: F. Fumeron, M. Marre, R Roussel; CHU de Rennes: F. Bonnet; CNRS UMR8090, Lille: A Bonnefond, S. Cauchi, P. Froguel; Centres d'examens de santé de l’Assurance Maladie: Alençon, Angers, Blois, Caen, Chateauroux, Chartres, Cholet, Le Mans, Orléans, Tours; Institut de Recherche en Médecine Générale: J. Cogneau; General practitioners of the Region; Institut inter-Régional pour la Santé (IRSA): C. Born, E. Caces, M. Cailleau, O Lantieri, J.G. Moreau, F. Rakotozafy, J. Tichet, S. Vol.
Footnotes
Table S1.
| Gene | Primer sequences |
|
|---|---|---|
| Forward | Reverse | |
| Prok2V1 | gctgctaccgctgctgttca | cctgccttccatttgcaaca |
| Prok2V2 | ccccctgactcggaaagt | agtccttaaacacgccaagc |
| ProkR1 | acctcgacctcaggaccac | ttgcttatttcagtcggatgc |
| ProkR2 | cctccgtcaactaccttcgt | gggtggacaatagcgaggt |
| LepR | gatgttccaaaccccaagaa | ttctgcatgcttggtaaaaaga |
| InsR | tctttcttcaggaagctacatctg | tctttcttcaggaagctacatctg |
| GLP-1R | ctgcccagcaacaccagt | cagtcggcagcctagagagt |
| NPY | ccgctctgcgacactacat | tgtctcagggctggatctct |
| AgRP | tttgtcctctgaagctgtatgc | gcatgaggtgcctcccta |
| POMC | agtgccaggacctcacca | cagcgagaggtcgagtttg |
| CART | cgagaagaagtacggccaag | ctggcccctttcctcact |
| RPL19 | gggcaggcatatgggcata | ggcggtcaatcttcttggatt |
Conflict of interest
All authors declare no conflict of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figure S1.
Prok2 mRNA expression in the MOB is not circadian. Expression in the MOB of Prok2 variants 1 (A) and 2 (B) mRNA were measured in 4 month-old C57Bl/6J mice using RT-qPCR. Mice were maintained under a dark: light cycle of 12 h starting at 7.00 am (ZT0) and samples were taken every 4 h (Zeitgeber time, ZT). Rhythmicity was assessed using Circwave v1.4. Values represent mean with SEM (n = 4–5/group). Data were analyzed using a one way ANOVA, followed by post hoc Bonferroni test.
Figure S2.
Partial deletion of Prok2 in the MOB affected neither locomotor activity nor energy expenditure. Mice injected in the MOB with the scrambled-shRNA or Prok2-shRNA were placed in calorimetric cages (TSE system, Germany) 3 weeks postsurgery. Locomotor activity (A, B) and energy expenditure (C, D) were measured in fed conditions. Values represent mean with SEM (n = 6/group). The 24h studies (A, C) were analyzed using a two-way ANOVA, followed by post hoc Bonferroni test and the cumulative studies (B, D) were analyzed using an unpaired Student's t-test.
Figure S3.
Partial deletion of Prok2 in the MOB induced changes in respiratory exchange ratio (RER) and fatty acid oxidation in fasted mice. Mice injected in the MOB with the scrambled-shRNA or Prok2-shRNA were placed in calorimetric cages (TSE system, Germany) 3 weeks postsurgery. RER (RER = VCO2/VO2, A-C) and fatty acid oxidation (D-G) were measured in fed conditions (A, B, D, E) or during an overnight fasting (C, F, G). Values represent mean with SEM (n = 6/group). The 24h- and the overnight fasting studies (A, C, D, F, G) were analyzed using 2-way ANOVA followed by post hoc Bonferroni test and the cumulative studies (B, E) were analyzed using an unpaired Student's t-test.
References
- 1.Negri L., Lattanzi R., Giannini E., De Felice M., Colucci A., Melchiorri P. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. British Journal of Pharmacology. 2004;142(1):181–191. doi: 10.1038/sj.bjp.0705686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M., Bullock C.M., Knauer D.J., Ehlert F.J., Zhou Q.Y. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Molecular Pharmacology. 2001;59(4):692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 3.Cheng M.Y., Leslie F.M., Zhou Q.-Y. Expression of prokineticins and their receptors in the adult mouse brain. The Journal of Comparative Neurology. 2006;498(6):796–809. doi: 10.1002/cne.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gascuel J., Lemoine A., Rigault C., Datiche F., Benani A., Penicaud L. Hypothalamus-olfactory system crosstalk: orexin a immunostaining in mice. Frontiers in Neuroanatomy. 2012;6:44. doi: 10.3389/fnana.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price J.L., Slotnick B.M., Revial M.F. Olfactory projections to the hypothalamus. The Journal of Comparative Neurology. 1991;306(3):447–461. doi: 10.1002/cne.903060309. [DOI] [PubMed] [Google Scholar]
- 6.Russo C., Russo A., Pellitteri R., Stanzani S. Ghrelin-containing neurons in the olfactory bulb send collateralized projections into medial amygdaloid and arcuate hypothalamic nuclei: neuroanatomical study. Experimental Brain Research. 2018;236(8):2223–2229. doi: 10.1007/s00221-018-5298-z. [DOI] [PubMed] [Google Scholar]
- 7.Palouzier-Paulignan B., Lacroix M.-C., Aimé P., Baly C., Caillol M., Congar P. Olfaction under metabolic influences. Chemical Senses. 2012;37(9):769–797. doi: 10.1093/chemse/bjs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riera C.E., Tsaousidou E., Halloran J., Follett P., Hahn O., Pereira M.M.A. The sense of smell impacts metabolic health and obesity. Cell Metabolism. 2017;26(1):198–211. doi: 10.1016/j.cmet.2017.06.015. e5. [DOI] [PubMed] [Google Scholar]
- 9.Soria-Gomez E., Bellocchio L., Marsicano G. New insights on food intake control by olfactory processes: the emerging role of the endocannabinoid system. Molecular and Cellular Endocrinology. 2014;397(1–2):59–66. doi: 10.1016/j.mce.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Richardson B.E., Vander Woude E.A., Sudan R., Thompson J.S., Leopold D.A. Altered olfactory acuity in the morbidly obese. Obesity Surgery. 2004;14(7):967–969. doi: 10.1381/0960892041719617. [DOI] [PubMed] [Google Scholar]
- 11.Thiebaud N., Johnson M.C., Butler J.L., Bell G.A., Ferguson K.L., Fadool A.R. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. Journal of Neuroscience – the Official Journal of the Society for Neuroscience. 2014;34(20):6970–6984. doi: 10.1523/JNEUROSCI.3366-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obrebowski A., Obrebowska-Karsznia Z., Gawliński M. Smell and taste in children with simple obesity. International Journal of Pediatric Otorhinolaryngology. 2000;55(3):191–196. doi: 10.1016/s0165-5876(00)00397-9. [DOI] [PubMed] [Google Scholar]
- 13.Ng K.L., Li J.-D., Cheng M.Y., Leslie F.M., Lee A.G., Zhou Q.-Y. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science (New York, N.Y.) 2005;308(5730):1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 14.Li J.-D., Hu W.-P., Boehmer L., Cheng M.Y., Lee A.G., Jilek A. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. Journal of Neuroscience – the Official Journal of the Society for Neuroscience. 2006;26(45):11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prosser H.M., Bradley A., Chesham J.E., Ebling F.J.P., Hastings M.H., Maywood E.S. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(2):648–653. doi: 10.1073/pnas.0606884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardiner J.V., Bataveljic A., Patel N.A., Bewick G.A., Roy D., Campbell D. Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes. 2010;59(2):397–406. doi: 10.2337/db09-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W., Li J.-D., Hu W.-P., Cheng M.Y., Zhou Q.-Y. Prokineticin 2 is involved in the thermoregulation and energy expenditure. Regulatory Peptides. 2012;179(1–3):84–90. doi: 10.1016/j.regpep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Guo X., Ma H., Lu L., Zhang R. Prokineticin-2 is associated with metabolic syndrome in a middle-aged and elderly Chinese population. Lipids in Health and Disease. 2016;15:1. doi: 10.1186/s12944-015-0172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G., Franklin K. 4th ed. Academic Press; 2012. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 20.Yang M., Crawley J.N. Simple behavioral assessment of mouse olfaction. Current Protocols in Neuroscience. 2009 doi: 10.1002/0471142301.ns0824s48. Chapter 8: Unit 8.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaetani S., Oveisi F., Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology – Official Publication of the American College of Neuropsychopharmacology. 2003;28(7):1311–1316. doi: 10.1038/sj.npp.1300166. [DOI] [PubMed] [Google Scholar]
- 22.Lerat H., Imache M.R., Polyte J., Gaudin A., Mercey M., Donati F. Hepatitis C virus induces a prediabetic state by directly impairing hepatic glucose metabolism in mice. Journal of Biological Chemistry. 2017;292(31):12860–12873. doi: 10.1074/jbc.M117.785030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balkau B., Lange C., Fezeu L., Tichet J., de Lauzon-Guillain B., Czernichow S. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2008;31(10):2056–2061. doi: 10.2337/dc08-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lasfargues G., Vol S., Le Clésiau H., Bedouet M., Hagel L., Constans T. Validity of a short self-administered dietary questionnaire compared with a dietetic interview. Presse Medicale (Paris, France 1983) 1990;19(20):953–957. [PubMed] [Google Scholar]
- 25.Chen J., Kuei C., Sutton S., Wilson S., Yu J., Kamme F. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Molecular Pharmacology. 2005;67(6):2070–2076. doi: 10.1124/mol.105.011619. [DOI] [PubMed] [Google Scholar]
- 26.Cheng M.Y., Bullock C.M., Li C., Lee A.G., Bermak J.C., Belluzzi J. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417(6887):405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 27.Granados-Fuentes D., Tseng A., Herzog E.D. A circadian clock in the olfactory bulb controls olfactory responsivity. Journal of Neuroscience – the Official Journal of the Society for Neuroscience. 2006;26(47):12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen Y., Zhang Z., Li Z., Liu G., Tao G., Song X. The PROK2/PROKR2 signaling pathway is required for the migration of most olfactory bulb interneurons. The Journal of Comparative Neurology. 2019 doi: 10.1002/cne.24719. [DOI] [PubMed] [Google Scholar]
- 29.Won M.H., Kang T.C., Lee J.C., Choi K.Y., Park S.K., Jeong Y.G. Age-related change of neuropeptide Y-immunoreactive neurons in the rat main olfactory bulb. Neuroscience Letters. 2000;289(2):119–122. doi: 10.1016/s0304-3940(00)01282-9. [DOI] [PubMed] [Google Scholar]
- 30.Wolf P. Epilepsy and the sensory systems. Epilepsy Currents. 2016;16(6):369–372. doi: 10.5698/1535-7511-16.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lattanzi R., Maftei D., Negri L., Fusco I., Miele R. PK2β ligand, a splice variant of prokineticin 2, is able to modulate and drive signaling through PKR1 receptor. Neuropeptides. 2018;71:32–42. doi: 10.1016/j.npep.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Beale K., Gardiner J.V., Bewick G.A., Hostomska K., Patel N.A., Hussain S.S. Peripheral administration of prokineticin 2 potently reduces food intake and body weight in mice via the brainstem. British Journal of Pharmacology. 2013;168(2):403–410. doi: 10.1111/j.1476-5381.2012.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno T.M., Kleopoulos S.P., Bergen H.T., Roberts J.L., Priest C.A., Mobbs C.V. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47(2):294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 34.Kahleova H., Belinova L., Malinska H., Oliyarnyk O., Trnovska J., Skop V. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia. 2014;57(8):1552–1560. doi: 10.1007/s00125-014-3253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahleova H., Malinska H., Kazdova L., Belinova L., Tura A., Hill M. The effect of meal frequency on the fatty acid composition of serum phospholipids in patients with type 2 diabetes. Journal of the American College of Nutrition. 2016;35(4):317–325. doi: 10.1080/07315724.2015.1046197. [DOI] [PubMed] [Google Scholar]
- 36.Belinova L., Kahleova H., Malinska H., Topolcan O., Windrichova J., Oliyarnyk O. The effect of meal frequency in a reduced-energy regimen on the gastrointestinal and appetite hormones in patients with type 2 diabetes: a randomised crossover study. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degen L., Matzinger D., Drewe J., Beglinger C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides. 2001;22(8):1265–1269. doi: 10.1016/s0196-9781(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 38.Gill S., Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can Be modulated for health benefits. Cell Metabolism. 2015;22(5):789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg H.W., Pangborn R.M., Roessler E.B., Webb A.D. Influence of hunger on olfactory acuity. Nature. 1963;197:108. doi: 10.1038/197108a0. [DOI] [PubMed] [Google Scholar]
- 40.Aimé P., Duchamp-Viret P., Chaput M.A., Savigner A., Mahfouz M., Julliard A.K. Fasting increases and satiation decreases olfactory detection for a neutral odor in rats. Behavioural Brain Research. 2007;179(2):258–264. doi: 10.1016/j.bbr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Albrecht J., Schreder T., Kleemann A.M., Schöpf V., Kopietz R., Anzinger A. Olfactory detection thresholds and pleasantness of a food-related and a non-food odour in hunger and satiety. Rhinology. 2009;47(2):160–165. [PubMed] [Google Scholar]
- 42.Aschenbrenner K., Hummel C., Teszmer K., Krone F., Ishimaru T., Seo H.-S. The influence of olfactory loss on dietary behaviors. The Laryngoscope. 2008;118(1):135–144. doi: 10.1097/MLG.0b013e318155a4b9. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y., Lin Y.-C., Kuo T.-W., Knight Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160(5):829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esler M., Rumantir M., Wiesner G., Kaye D., Hastings J., Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. American Journal of Hypertension. 2001;14(11 Pt 2):304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- 45.Karlsson A.K., Attvall S., Jansson P.A., Sullivan L., Lönnroth P. Influence of the sympathetic nervous system on insulin sensitivity and adipose tissue metabolism: a study in spinal cord-injured subjects. Metabolism Clinical and Experimental. 1995;44(1):52–58. doi: 10.1016/0026-0495(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 46.Parlevliet E.T., Coomans C.P., Rensen P.C.N., Romijn J.A. The brain modulates insulin sensitivity in multiple tissues. Frontiers of Hormone Research. 2014;42:50–58. doi: 10.1159/000358314. [DOI] [PubMed] [Google Scholar]
- 47.Szatkowski C., Vallet J., Dormishian M., Messaddeq N., Valet P., Boulberdaa M. Prokineticin receptor 1 as a novel suppressor of preadipocyte proliferation and differentiation to control obesity. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dormishian M., Turkeri G., Urayama K., Nguyen T.L., Boulberdaa M., Messaddeq N. Prokineticin receptor-1 is a new regulator of endothelial insulin uptake and capillary formation to control insulin sensitivity and cardiovascular and kidney functions. Journal of the American Heart Association. 2013;2(5) doi: 10.1161/JAHA.113.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Von Hunolstein J.-J., Nebigil C.G. Can prokineticin prevent obesity and insulin resistance? Current Opinion in Endocrinology Diabetes and Obesity. 2015;22(5):367–373. doi: 10.1097/MED.0000000000000185. [DOI] [PubMed] [Google Scholar]