Abstract
Background
Malignant giant cell tumor of bone (MGCTB) is extremely rare. Currently, population-based prognosis studies are lacking. This study aimed to determine the impact of demographics, tumor characteristics, and treatment on prognosis among patients with MGCTB.
Methods
The Surveillance, Epidemiology, and End Results database was used to identify patients with MGCTB from 1984 to 2013. Kaplan-Meier analyses were performed to determine the overall survival (OS). Univariable and multivariable Cox analyses were conducted to identify prognostic factors.
Results
There were 250 patients with MGCTB included in our study. The multivariate Cox analysis revealed that age at diagnosis (hazard ratio [HR]: 1.09; 95% confidence interval [CI]: 1.07–1.11; P < 0.001), tumor size (HR: 7.04; 95% CI: 2.38–20.77; P < 0.001), tumor extension (regional vs. localized, HR: 2.64; 95% CI: 1.10–6.34; P = 0.030; distant vs. localized, HR: 6.12; 95% CI: 2.27–16.49; P < 0.001), and radiotherapy (HR: 0.41; 95% CI: 0.18–0.89; P = 0.025) were independent risk factors of OS in patients with MGCTB. Notably, tumor site (HR: 1.98; 95% CI: 0.99–4.00; P = 0.055) exhibited borderline significance. Additionally, we found that patients with tumors measuring >70 mm (P = 0.015), located in the axial skeleton (P < 0.001) and presented with distant metastasis (P < 0.001) tended to receive radiotherapy. Moreover, a nomogram model integrating independent predictors was established to estimate the OS of patients with MGCTB.
Conclusion
This study provides a population-based assessment of the largest number of patients with MGCTB. We found that older age, larger tumor size, regional or distant metastasis, and lack of radiotherapy was associated with poor OS. Surgical methods were not significantly associated with OS. Furthermore, we built a high-quality nomogram to predict 1-, 3-, and 5-year OS for patients with MGCTB. These findings may assist in the clinical diagnosis and treatment of MGCTB.
Keywords: Malignant giant cell tumor of bone, Prognosis, Nomogram
1. Introduction
Giant cell tumor of bone (GCTB) is generally considered to be a benign tumor occurring in the epiphyseal and metaphyseal regions of long bones with a locally aggressive profile [1]. It accounts for approximately 20% and 5% of benign and primary bone tumors, respectively. Very rarely, GCTB undergoes a sarcomatous transformation into a malignant type [2], [3], [4]. According to the definition, malignant giant cell tumor of bone (MGCTB) can be divided into the primary and secondary types [5]. Primary MGCTB is a type of tumor in which high-grade sarcoma components appear simultaneously with GCTB at first diagnosis, Secondary MGCTB is defined as the type in which high-grade sarcoma components occurred in the original treated GCTB. The principal part of MGCTB is secondary, and generally occurs after receiving radiation therapy. Primary MGCTB is relatively rare. A recent analysis involving 2315 GCTB patients revealed that the overall incidence of MGCTB was 4.0%, 1.6% for primary MGCTB, and 2.4% for secondary MGCTB [6].
At present, the diagnosis of MGCTB is mainly based on histological examination because of the limited value of clinical and radiological information. The most common symptoms in patients with MGCTB are local pain and swelling [7]. Local recurrence and distant metastasis are also observed in patients with MGCTB. The lung is the most common site of metastasis, leading to unfavorable outcomes. Currently, there is no consensus regarding the treatment of MGCTB. Conventional treatments include surgery alone or surgery combined with radiotherapy and chemotherapy; nevertheless, the effect is not clear. There are few prognostic studies on MGCTB due to the lack of cases and long-term follow-up data. A study covering 26 cases of primary MGCTB found that the overall mortality rate was 16% and 5-year survival was 87% [8]. However, other studies reported poor prognosis for MGCTB patients, with a short survival period after diagnosis [9], [10].
Owing to the rarity of MGCTB, we can often only investigate it based on single-center experiences or small series studies, which provided inconsistent results. Population-based research for patients with MGCTB is currently lacking. The role of demographics, tumor characteristics, and treatment methods in MGCTB is unclear. This study used the Surveillance, Epidemiology, and End Results (SEER) database to provide current largest sample analysis for identifying predictors of outcome among patients with MGCTB. Furthermore, we constructed a clinical predictive model, termed nomogram, for patients with MGCTB to evaluate the 1-, 3-, and 5-year survival.
2. Materials and methods
2.1. Data source
All data in our study were obtained from the SEER database, which is sponsored by the National Cancer Institute in the USA. The SEER database is a comprehensive resource that captures data regarding patient demographics, clinicopathologic features, and cancer-associated treatment. Currently, the SEER program is composed of population-based cancer registries, accounting for 26% of the USA population [11]. The SEER database contains data without personal identifiers and is accessed publicly; thus, approval from the institutional review board was not required.
2.2. Inclusion criteria
Patients were included in our study according with the following criteria: (1) diagnosed with malignant MGCTB (9250/3) according to the International Classification of Diseases for Oncology Third Edition; (2) diagnosed between 1984 and 2013; (3) the primary site was limited to C40.0–41.9; and (4) MGCTB was the only or the first malignancy.
2.3. Definition of variables
The information related to demographic characteristics, clinicopathologic features, and cancer-associated treatment were extracted from the SEER database. Age at diagnosis and year of diagnosis were considered continuous variables, and race was categorized into the Black, White, and Others. Tumor size, determined by the maximum diameter of the tumor, was divided into three groups, namely ≤70 mm, >70 mm, and unknown. Moreover, tumor extension was categorized into localized, regional, distant, and unknown, according to the SEER Program Coding and Staging Manual. Localized neoplasm was confined to the periosteum, and regional disease was defined as a tumor extending beyond the periosteum without distant metastasis. The distant stage involved distant and further contiguous extension metastasis. For the tumor site, patients coded with C40.0–40.3 and C40.9 were classified as extremity, while those coded with C41.0–41.4 were classified as axial. In addition, the extent of surgical resection was categorized into four groups: no surgery, local excision, gross total resection (GTR), and unknown, as previously described [12]. Considering that the SEER database lacks information regarding the utilization of adjuvant chemotherapy, we only included radiation therapy in our study.
2.4. Statistical analysis
Overall survival (OS) was defined as the primary outcome and a Kaplan-Meier analysis was conducted. Student's t-test and chi-squared test were employed as appropriate. Univariate and multivariate Cox analyses were performed to determine independent predictors of OS. Additionally, we developed a nomogram model to predict the OS of patients with MGCTB, based on the results of the multivariate Cox analysis. The discrimination ability of the model was numerically assessed using the concordance index (C-index). The predictive accuracy of the model was evaluated using calibration curves. Receiver operating characteristic curve analyses were used to compare the prediction performance of the nomogram model with separate clinical features of patients with MGCTB [13]. All statistical analyses were conducted using the SPSS version 24.0 (IBM Corp., Armonk, NY, USA) and R (version 3.5.3) software. A two-sided P-value <0.05 denoted statistical significance.
3. Results
3.1. Demographic data
There were 250 patients with MGCTB included in our study. As summarized in Table 1, the patients were aged 6–87 years (mean age: 36.9). Only 11.8% of the patients were diagnosed before the age of 20 years, and most patients were diagnosed after 2004. Demographically, 47.6% of patients were males and 52.4% were females. The vast majority were White, accounting for approximately 70% of all cases. Regarding tumor characteristics, 70.8% and 27.2% of all patients had primary tumors located in extremity and the axial skeleton, respectively. For cases with known tumor size, the median tumor size at the time of diagnosis was 70 mm. In addition, 38.8% of patients presented with the localized disease, while 28 patients (11.2%) had distant metastasis at presentation. After diagnosis, most patients undwewent surgery. Among these, 90 patients underwent local excision and 83 had gross total resection of the primary lesion. Notably, only 16.4% of the patients received radiotherapy.
Table 1.
Population characteristics.
| Characteristic | Value |
|---|---|
| Total | 250 (100%) |
| Age at diagnosis(continued) | |
| Mean ± sd | 36.9 ± 16.5 |
| Range | 6–87 |
| <20 | 28 (11.2%) |
| 20–39 | 126 (50.4%) |
| 40–59 | 71 (28.4%) |
| ≥60 | 25 (10.0%) |
| Year of dignosis | |
| Range | 1984–2013 |
| 1984–1993 | 42 (16.8%) |
| 1994–2003 | 75 (30.0%) |
| 2004–2013 | 133 (53.2%) |
| Gender | |
| Male | 119 (47.6%) |
| Female | 131 (52.4%) |
| Race | |
| White | 182 (72.8%) |
| Black | 36 (14.4%) |
| Others | 32 (12.8%) |
| Tumor site | |
| Extremity | 177 (70.8%) |
| Axial | 68 (27.2%) |
| Bone,NOS | 5 (2.0%) |
| Tumor size | |
| ≤70 mm | 61 (24.4%) |
| >70 mm | 56 (22.4%) |
| Unknown | 133 (53.2%) |
| Tumor extension | |
| Localized | 97 (38.8%) |
| Regional | 64 (25.6%) |
| Distant | 28 (11.2%) |
| Unknown | 61 (24.4%) |
| Surgery type | |
| No surgery | 50 (20.0%) |
| Local excision | 90 (36.0%) |
| GTR | 83 (33.2%) |
| Unknown | 27 (10.8%) |
| Radiation | |
| No | 200 (80.0%) |
| Yes | 41 (16.4%) |
| Unknown | 9 (3.6%) |
NOS, not otherwise specified; GTR, gross total resection.
3.2. Survival analysis
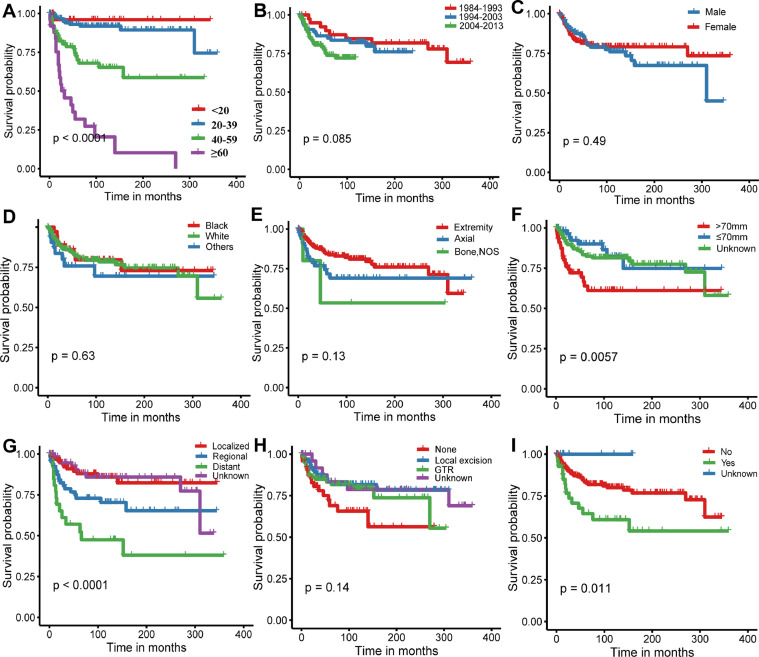
Analyses of Kaplan-Meier curves with log-rank tests demonstrated that age at diagnosis, tumor size (P = 0.0057), tumor extension (P < 0.0001), and radiotherapy (P = 0.011) were associated with OS (Fig. 1). Surprisingly, we found that patients diagnosed after 2004 had no survival advantage over patients diagnosed prior to 2004, which may be partly attributed to our small samples size. This finding suggests that intensive study of this tumor is needed. Subsequently, univariate Cox analyses were conducted, revealing that older age (hazard ration [HR]:1.06; 95% confidence interval [CI]: 1.05–1.08; P < 0.001), tumor size > 70 mm (HR: 2.24; 95% CI: 1.23–4.06; P = 0.008), metastatic disease at presentation (HR: 5.21; 95% CI: 2.41–11.28; P < 0.001), and radiotherapy (HR: 2.25; 95% CI: 1.23–4.09; P = 0.008) were significantly related to decreased OS, whereas local excision (HR: 0.47; 95% CI: 0.23–0.95; P = 0.037) was associated with improved outcome. Multivariate Cox analysis was employed to further determine the independent risk factors of OS in patients with MGCTB. As shown in Table 2, age at diagnosis (HR: 1.09; 95% CI: 1.07–1.11; P < 0.001), tumor size (HR: 7.04; 95% CI: 2.38–20.77; P < 0.001), tumor extension (regional vs. localized, HR: 2.64; 95% CI: 1.10–6.34; P = 0.030; distant vs. localized, HR: 6.12; 95% CI: 2.27–16.49; P < 0.001), and radiotherapy (HR: 0.41; 95% CI: 0.18–0.89; P = 0.025) were confirmed as independent predictors of prognosis, while tumor site (HR: 1.98; 95% CI: 0.99–4.00; P = 0.055) exhibited borderline significance. However, after adjusting for the available clinical variables, there was no association between the type of surgery and OS. Conversely, the multivariate analysis revealed that utilization of adjuvant radiotherapy was correlated to better survival.
Fig. 1.
Kaplan-Meier curves with log-rank tests for patients with MGCTB. (A) Age of diagnosis; (B) year of diagnosis; (C)sex; (D) race; (E) tumor location; (F) tumor size; (G) tumor extension; (H) surgery type; (I) radiation treatment.
Table 2.
Univariate and multivariate cox regression analyses.
| Characteristic | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, per 1year increase | 1.06 (1.05–1.08) | <0.001 | 1.09 (1.07–1.11) | <0.001 |
| Year of diagnosis | 1.05 (1.01–1.10) | 0.025 | 1.07 (1.01–1.13) | 0.017 |
| Gender | ||||
| Male | 1[Reference] | 1[Reference] | ||
| Female | 0.83 (0.48–1.42) | 0.489 | 0.59 (0.31–1.13) | 0.110 |
| Race | ||||
| White | 1[Reference] | 0.189 | 1[Reference] | |
| Black | 0.91 (0.42–1.96) | 0.809 | 0.76 (0.33–1.78) | 0.534 |
| Others | 1.40 (0.65–3.01) | 0.388 | 3.38 (1.31–8.69) | 0.012 |
| Tumor site | ||||
| Extremity | 1[Reference] | 0.179 | 1[Reference] | |
| Axial | 1.65 (0.92–2.95) | 0.094 | 1.98 (0.99–4.00) | 0.055 |
| Bone, NOS | 2.55 (0.61–10.66) | 0.198 | 2.08 (0.40–10.77) | 0.385 |
| Tumor size | ||||
| ≤70 mm | 1[Reference] | 0.144 | 1[Reference] | |
| >70 mm | 2.24 (1.23–4.06) | 0.008 | 7.04 (2.38–20.77) | <0.001 |
| Unknown | 0.76 (0.34–1.68) | 0.496 | 3.43 (1.22–9.62) | 0.019 |
| Tumor extension | ||||
| Localized | 1[Reference] | 1[Reference] | ||
| Regional | 2.39 (1.15–4.96) | 0.020 | 2.64 (1.10–6.34) | 0.030 |
| Distant | 5.21 (2.41–11.28) | <0.001 | 6.12 (2.27–16.49) | <0.001 |
| Unknown | 1.09 (0.46–2.58) | 0.853 | 9.97 (0.33–2.84) | 0.951 |
| Surgery type | ||||
| No surgery | 1[Reference] | 1[Reference] | ||
| Local excision | 0.47 (0.23–0.95) | 0.037 | 0.60 (0.25–1.40) | 0.238 |
| GTR | 0.59 (0.30–1.19) | 0.139 | 0.65 (0.27–1.60) | 0.348 |
| Unknown | 0.44 (0.16–1.21) | 0.112 | 1.52 (0.40–5.80) | 0.544 |
| Radiation | ||||
| No | 1[Reference] | 1[Reference] | ||
| Yes | 2.25 (1.23–4.09) | 0.008 | 0.41 (0.18–0.89) | 0.025 |
| Unknown | ∼ | 0.973 | ∼ | 0.976 |
Values in bold refers to statistical significance.
Abbreviations: HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified; GTR, gross total resection.
3.3. Comparison of demographic and treatment factors by tumor characteristics
Considering the significant associations between survival and tumor characteristics, we attempted to compare of demographic and treatment factors according to tumor site, size, and extension. As presented in Table 3, patients with tumors measuring >70 mm (P = 0.015), located in the axial skeleton (P < 0.001), and presenting with distant metastasis (P < 0.001) tended to receive radiotherapy. Tumors with distant extension were more likely to be treated through conservative management (P = 0.038). Additionally, a significant correlation was observed between the tumor site and tumor extension (P = 0.002). Tumors located in the axial skeleton were more aggressive with a high tendency for distant metastasis compared with those located in an extremity.
Table 3.
Comparison of demographic and treatment factors by tumor characteristics.
| Characteristics | Number of patients (% of patients with specified characteristic)a |
|||||
|---|---|---|---|---|---|---|
| Tumor site |
Tumor size |
Tumor extension |
||||
| Extremity | Axial | ≤70 mm | >70 mm | Localized/regional | Distant | |
| Age at diagnosis | ||||||
| Mean ± SD | 36.1 ± 16.0 | 38.0 ± 17.1 | 40.1 ± 17.0 | 38.6 ± 17.2 | 37.4 ± 16.6 | 40.3 ± 16.3 |
| Gender | ||||||
| Male | 92 (52.0) | 26 (38.2) | 29 (47.5) | 28 (50.0) | 82 (50.9) | 15 (53.6) |
| Female | 85 (48.0) | 42 (61.8) | 32 (52.5) | 28 (50.0) | 79 (49.1) | 13 (46.4) |
| Race | ||||||
| White | 131 (83.4) | 48 (84.2) | 47 (85.5) | 41 (83.7) | 118 (84.9) | 19 (79.2) |
| Black | 26 (16.6) | 9 (15.8) | 8 (14.5) | 8 (16.3) | 21 (15.1) | 5 (20.8) |
| Tumor site | ||||||
| Extremity | NA | NA | 44 (73.3) | 36 (65.5) | 119 (73.9) | 15 (55.6) |
| Axial | NA | NA | 16 (26.7) | 19 (34.5) | 42 (26.1) | 12 (44.4) |
| Tumor size | ||||||
| ≤70 mm | 36 (45.0) | 19 (54.3) | NA | NA | 53 (54.1) | 5 (41.7) |
| >70 mm | 44 (55.0) | 16 (45.7) | NA | NA | 45 (45.9) | 7 (58.3) |
| Tumor extension | ||||||
| Localized | 80 (59.7)b | 17 (31.5)b | 31 (53.4) | 20 (38.5) | NA | NA |
| Regional | 39 (29.1)b | 25 (46.3)b | 22 (37.9) | 25 (48.1) | NA | NA |
| Distant | 15 (11.2)b | 12 (22.2)b | 5 (8.6) | 7 (13.5) | NA | NA |
| Surgery type | ||||||
| No surgery | 30 (18.9) | 17 (28.8) | 6 (10.9) | 8 (14.8) | 20 (13.6)c | 8 (29.6)c |
| Local excision | 67 (42.1) | 22 (37.3) | 22 (40.0) | 12 (22.2) | 65 (44.2)c | 6 (22.2)c |
| GTR | 62 (39.0) | 20 (33.9) | 27 (49.1) | 34 (63.0) | 62 (42.2)c | 13 (48.1)c |
| Radiation | ||||||
| No | 157 (91.3)d | 40 (62.5)d | 53 (91.4)e | 40 (74.1)e | 130 (83.3)f | 15 (53.6)f |
| Yes | 15 (8.7)d | 24 (37.5)d | 5 (8.6)e | 14 (25.9)e | 26 (16.7)f | 13 (46.4)f |
Except for the patients with other races, and unknown tumor site, tumor size, tumor extension, surgery type and radiation.
Pearson □2 test.
P = 0.002.
P = 0.038.
P < 0.001.
P = 0.015.
P < 0.001.
GTR, gross total resection.
3.4. Development and validation of a nomogram model
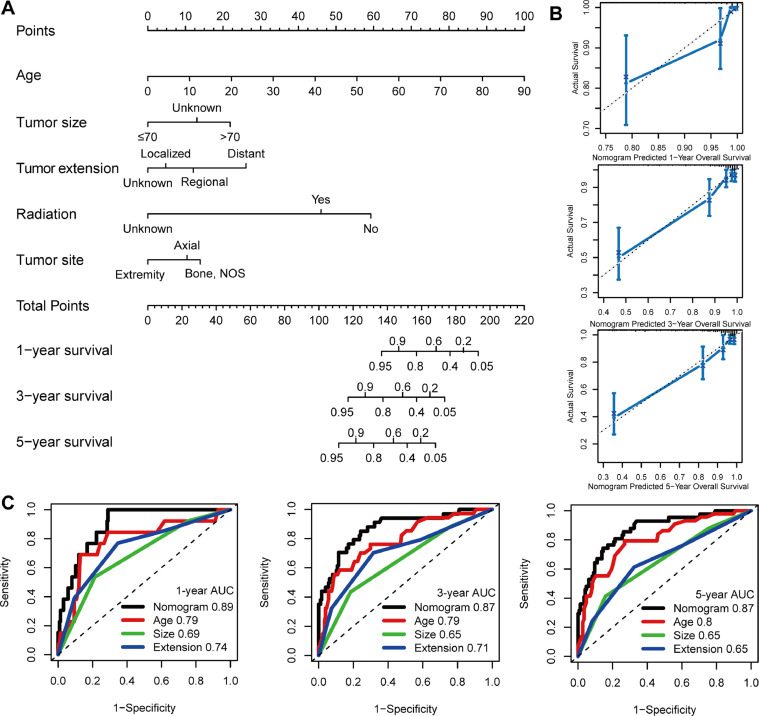
We established a nomogram model integrating independent predictors of OS (e.g., age, tumor site, tumor size, tumor extension, and radiotherapy) to provide a visual statistical predictive tool for the survival of patients with MGCTB (Fig. 2A). The C-index for the nomogram model was 0.851, indicating a favorable discriminative ability. Calibration curves demonstrated excellent accordance between the predictions and observations (Fig. 2B). In addition, the nomogram model revealed a higher predictive accuracy with larger area under the curve compared with individual prognostic factors (e.g., age, tumor size, and tumor extension).
Fig. 2.
Construction and validation of the nomogram model. (A) Nomogram model for predicting the probability of 1-, 3-, and 5-year OS in MGCTBs. (B) Calibration plots of the nomogram for predicting the probability of OS at 1, 3, and 5 years. (C) Time-dependent receiver operating characteristic curve analyses of the nomogram model, age, tumor size, and tumor extension. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
To our knowledge, the current study involved the largest number of cases to evaluate the prognostic factors for MGCTB according to demographics, tumor characteristics at diagnosis, and treatment methods. In addition, we performed a Kaplan-Meier analysis and constructed a nomogram using data from the SEER database. The main findings of the present study was that older age, larger tumor size, regional or distant metastasis, and lack of radiotherapy may be associated with poor OS. Tumors located in the axial skeleton are more aggressive than those in an extremity. Furthermore, patients with tumors measuring >70 mm, located in the axial skeleton, or presenting with distant metastasis tended to receive radiotherapy.
In this population-based analysis, the average age of patients with MGCTB was 36.9 years, and the number of females was slightly higher than that of males. These characteristics were similar to those of benign GCTB, which affects patients in the third and fourth decades of their life, and exhibits a slight predominance in females versus males [14]. Moreover, other studies reported that the skeletal distribution and the radiographic appearance of MGCTB were also similar to those of benign GCTB [15], However, the recurrence rate may be higher for MGCTB than for benign GCTB.
Considering the results of the Kaplan-Meier curves and Cox analyses, smaller tumor size and localized tumor were significantly related to better OS. No relevant prognostic studies involved these findings. Nevertheless, it is reasonable that larger and more aggressive tumors may be more harmful to patients. Furthermore, we found that surgical patients have a lower risk of death than patients without surgery; however, the difference did not reach statistical significance. The main surgical methods used are curettage and amputation. The former is suitable for MGCTB with good radiological performance; however, the recurrence rate linked to this approach is extremely high. Amputation is often used for large tumors with soft tissue involvement, especially secondary MGCTB [10], [16]. Although we have partially balanced the effects of some variables, there was still bias in the selection of surgical approach and we cannot rule out the effect of preoperative or postoperative chemotherapy on prognosis. Considering the results of the previous small-sample studies [8], [10], the choice of surgical approach may be influenced by numerous factors and was differs for each individual. Therefore, our results do not allow us to identify the most beneficial surgical approach for patients with MGCTB. Although early resection or amputation continues to be recommended [17], it is limited by the requirement of a biopsy [15].
The Kaplan–Meier curve and univariate Cox regression analyses also found that patients receiving radiotherapy had a worse OS than patients who do not receive radiotherapy. Interestingly, after adjusting for the effects of other variables, radiotherapy was associated with a better prognosis in the multivariate regression analysis. A reasonable explanation for this phenomenon is that patients with larger or distant metastatic tumors were more likely to receive radiotherapy (Table 3). This results in a poor observed prognosis in the radiotherapy group. After adjusting for these factors, we can conclude that radiotherapy improves the prognosis of MGCTB. Other studies revealed that radiotherapy should be an adjuvant treatment to surgery or an option for cases with unresectable MGCTB [18], [19]. However, radiotherapy alone for MGCTB is linked to a poor prognosis [10]. Another finding was that MGCTB located in the axial skeleton were more aggressive, and these patients often received more radiotherapy.
Various prognostic factors will affect the survival outcome of MGCTB, and we cannot accurately predict clinical outcomes based on a single prognostic factor. The nomogram, a new clinical prediction model integrating all significant variables, calculates and assigns the effect of each variable on the outcome to predict survival over a certain period of time. This was the first study to develop a nomogram based on 250 cases from the SEER database for the prediction of 1-, 3-, and 5-year OS of patients with MGCTB. Several variables (e.g., age, tumor site, tumor size, tumor extension, and use of radiation) were identified as independent prognostic factors for MGCTB through univariate and multivariate Cox analyses. Internal validation also showed that the predicted survival was in good agreement with the actual survival. Through his approach, we can accurately predict the survival rate of each patient at a certain point in time and choose a treatment that is more beneficial to the patient.
We must acknowledge several limitations in our study. Firstly, the SEER database does not include data regarding recurrence. Most cases of MGCTB are secondary, caused by the recurrence of the original benign GCTB. Thus, for the investigation of differences in prognosis, it is meaningful to distinguish the primary and secondary MGCTB cases. Secondly, information concerning the use of chemotherapy is not available in the SEER database. Many studies [10], [17] have reported that the use chemotherapy for the treatment of patients with MGCTB offered some efficacy. However, the impact of chemotherapy on the prognosis is unclear. Furthermore, the SEER database does not contain information regarding targeted therapy, such as bisphosphonates and denosumab. Thirdly, the accurately diagnosis of MGCTB is challenging owing to the lack of a clear definition of the disease. Diagnostic differences between years or registries may affect our results. Finally, owing to the limited number of cases, the constructed nomogram underwent internal validation. Validation of this nomogram using external data is warranted.
5. Conclusion
In this population-based study, age, tumor size, tumor extension, and radiotherapy were independent prognostic factors among patients with MGCTB. Surgical methods were not associated with OS. Tumors located in the axial skeleton were more aggressive than those located in an extremity. Patients with tumors measuring >70 mm, located in the axial skeleton, or presenting with distant metastasis tended to receive radiotherapy. We constructed a nomogram to predict 1-, 3-, and 5-year OS of patients with MGCTB. These findings may assist in the clinical diagnosis and treatment of MGCTB.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China (81871806) and the Major Scientific and Technological Project of Medical and Health in Zhejiang Province (WKJ-ZJ-1527).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100260.
Contributor Information
Jiao-Xiang Chen, Email: chenjiaoxiang@126.com.
Xiang-Yang Wang, Email: xiangyangwang@wmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Turcotte R.E. Giant cell tumor of bone. Orthop. Clin. North. Am. 2006;37:35–51. doi: 10.1016/j.ocl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Marui T. De novo malignant transformation of giant cell tumor of bone. Skeletal Radiol. 2001;30:104–108. doi: 10.1007/s002560000305. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki M., Yamamoto S., Uchio Y. Late malignant transformation of giant cell tumor of bone 41 years after primary surgery. Orthopedics. 2012;35:e1566–e1570. doi: 10.3928/01477447-20120919-32. [DOI] [PubMed] [Google Scholar]
- 4.Grote H.J. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33:169–175. doi: 10.1007/s00256-003-0682-5. [DOI] [PubMed] [Google Scholar]
- 5.Gong L. Histological and clinical characteristics of malignant giant cell tumor of bone. Virchows Archiv. 2012;460:327–334. doi: 10.1007/s00428-012-1198-y. [DOI] [PubMed] [Google Scholar]
- 6.Palmerini E., Picci P., Reichardt P., Downey G. Malignancy in giant cell tumor of bone: a review of the literature. Technol. Cancer Res. Treat. 2019;18 doi: 10.1177/1533033819840000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nascimento A.G., Huvos A.G., Marcove R.C. Primary malignant giant cell tumor of bone: a study of eight cases and review of the literature. Cancer. 1979;44:1393–1402. doi: 10.1002/1097-0142(197910)44:4<1393::aid-cncr2820440433>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Domovitov S.V., Healey J.H. Primary malignant giant-cell tumor of bone has high survival rate. Ann. Surg. Oncol. 2010;17:694–701. doi: 10.1245/s10434-009-0803-z. [DOI] [PubMed] [Google Scholar]
- 9.Mondal A., Kundu B., Gupta S., Biswas J. Secondary malignant giant cell tumour of bone–a study of five cases with short review of literature. Indian J. Pathol. Microbiol. 2002;45:273–275. [PubMed] [Google Scholar]
- 10.Anract P. Malignant giant-cell tumours of bone. clinico-pathological types and prognosis: a review of 29 cases. Int. Orthop. 1998;22:19–26. doi: 10.1007/s002640050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie Z., Lu Q., Peng H. Prognostic factors for patients with chondrosarcoma: a survival analysis based on the surveillance, epidemiology, and end results (SEER) database (1973–2012) J. Bone Oncol. 2018;13:55–61. doi: 10.1016/j.jbo.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchman K.R., Gao Y., Miller B.J. Prognostic factors for survival in patients with Ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39:189–195. doi: 10.1016/j.canep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Heagerty P.J., Lumley T., Pepe M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 14.Sobti A., Agrawal P., Agarwala S., Agarwal M. Giant cell tumor of bone - An Overview. Arch. Bone Joint Surg. 2016;4:2–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor S.K., Jain V., Agrawal M., Singh S., Mandal A.K. Primary malignant giant cell tumor of bone: a series of three rare cases. J. Surg. Orthop. Adv. 2007;16:89–92. [PubMed] [Google Scholar]
- 16.Bertoni F., Bacchini P., Staals E.L. Malignancy in giant cell tumor of bone. Cancer. 2003;97:2520–2529. doi: 10.1002/cncr.11359. [DOI] [PubMed] [Google Scholar]
- 17.Rock M.G. Secondary malignant giant-cell tumor of bone. clinicopathological assessment of nineteen patients. J. Bone Joint. Surg. Am. 1986;68:1073–1079. [PubMed] [Google Scholar]
- 18.Caudell J.J. Radiotherapy in the management of giant cell tumor of bone. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:158–165. doi: 10.1016/s0360-3016(03)00416-4. [DOI] [PubMed] [Google Scholar]
- 19.Rosai A. Bone and joints. In: Rosai J., editor. Rosai and Ackerman's Surgical Pathology. 9th ed. Mosby; London, United Kingdom: 2004. pp. 2169–2172. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.