Abstract
Background
Obesity, Type 2 diabetes (T2D) as well as stress-related disorders are rising public health threats and major burdens for modern society. Chronic stress and depression are highly associated with symptoms of the metabolic syndrome, but the molecular link is still not fully understood. Furthermore, therapies tackling these biological disorders are still lacking. The identification of shared molecular targets underlying both pathophysiologies may lead to the development of new treatments. The FK506 binding protein 51 (FKBP51) has recently been identified as a promising therapeutic target for stress-related psychiatric disorders and obesity-related metabolic outcomes.
Scope of the review
The aim of this review is to summarize current evidence of in vitro, preclinical, and human studies on the stress responsive protein FKBP51, focusing on its newly discovered role in metabolism. Also, we highlight the therapeutic potential of FKBP51 as a new treatment target for symptoms of the metabolic syndrome.
Major conclusions
We conclude the review by emphasizing missing knowledge gaps that remain and future research opportunities needed to implement FKBP51 as a drug target for stress-related obesity or T2D.
Keywords: FKBP51, SAFit2, Adipogensis, Glucose uptake, Obesity, Stress, Type 2 diabetes
1. Introduction
Homeostatic mechanisms govern the stress response, energy balance, and glucose homeostasis in order to maintain a dynamic equilibrium following internal or external challenges [1]. This requires a complex physiological response (involving multiple organ systems) to sense, integrate, and respond to changes in the environment. Interestingly, regulation of these homeostatic systems relies on many shared environmental and genetic factors, whereby manipulation of one factor can simultaneously influence stress-coping behaviors, body weight, and blood glucose. Identification of such shared factors may prove beneficial in treating stress-related comorbidities such as psychiatric disorders, obesity, and T2D. In this context, FKBP51 has recently been identified as a promising therapeutic target for the treatment of stress-related psychiatric disorders [2], [3] and obesity-related metabolic outcomes [4]. In this review, we first summarize key physiological mechanisms orchestrating the interplay of the body's stress response, energy balance, and glucose homeostasis, without giving an exhaustive overview (the reader is referred to in-depth reviews at each section). In the main part, we summarize and discuss the newly discovered role of FKBP51 in metabolism and highlighting its therapeutic potential for metabolic diseases.
1.1. The stress response
The stress response refers to the repertoire of physiological and behavioral reactions that arise in response to a stressor [5]. By definition, a stressor is any threat, real or perceived, to homeostasis. Therefore, stressors can either be physical in nature, such as metabolic stressors (fasting, physical activity) or psychogenic in nature, such as social stress or predator exposure. Although different types of stressors activate different brain networks, they all converge to stimulate the sympathoadrenomedullary (SAM) system and the hypothalamic-pituitary-adrenal (HPA) axis. The sympathetic nervous system comprises the most immediate physiological response involving direct catecholaminergic innervation of peripheral organs, including the adrenal medulla, which releases catecholamines into systemic circulation. Activation of the SAM system represents the “fight or flight” response, characterized by increased heart rate and respiration, redirection of blood flow away from digestive and reproductive organs, and mobilization of energy stores. Indeed, activation of the sympathetic nervous system has important metabolic effects. For example, increased sympathetic drive to white adipose tissue (WAT) and brown adipose tissue (BAT) recruits brown adipocytes and furthermore mobilizes free fatty acids [6], [7], [8], [9]. Similarly, increased sympathetic drive enhances glycogenolysis and glucose output in the liver [10].
The HPA axis mediates the slower, sustained response to a certain stressor. Activation of the HPA axis involves the release of corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP) from parvocellular neurons within the hypothalamic paraventricular nucleus (PVN) into the hypophyseal portal blood system, which bridges the hypothalamus and anterior part of the pituitary gland. At the pituitary gland, CRH and AVP stimulate the release of adrenocorticotropic hormone (ACTH) into systemic circulation (reviewed by [5]). In turn, ACTH stimulates the secretion of glucocorticoids (GCs) from the adrenal cortex. GCs (cortisol in humans or corticosterone in rodents) are recognized as the major end products of the HPA axis, which subsequently act on multiple organs to modulate the effects of a wide range of physiological processes. GCs exert their effects through type I mineralocorticoid receptors (MRs) and type II glucocorticoid receptors (GRs), which present distinct binding affinities for GCs and distinct distribution profiles [11]. MRs have a higher affinity for GCs than GRs, and as a consequence GRs are only activated in response to stress or at the GC circadian peak [12]. Through GRs, GCs are involved in a negative feedback circuit whereby they operate at different levels of the HPA axis and at higher brain centers to terminate the stress response [5]. Furthermore, in terms of metabolic regulation, GR signaling is known to favor food intake, promote gluconeogenesis in the liver, protein degradation and amino acid mobilization in muscle, and lipolysis in fat [13], [14], [15], [16]. Taken together, SAM activation coupled to GC actions favors processes that increase the availability of circulating energy stores.
1.2. Energy balance
Energy balance refers to the dynamic equilibrium between energy input and output. Body weight maintenance is a tightly regulated homeostatic system balancing energy input and output. This balance is subject to multiple levels of regulation involving complex, redundant mechanisms comprising thousands of genes and multiple organs and involving both hormonal and neuronal signaling networks. Especially, the proper communication between the brain, adipose tissue, and muscle tissue via hormones, like insulin and leptin, is essential for a healthy energy status. Further, interactions between environmental cues (diet, physical activity, stress exposure) and genetic factors determine individual susceptibility to gain weight as a result of diverging changes to components of energy intake or expenditure.
1.2.1. Energy intake
Energy intake refers to the caloric gain through ingestion of carbohydrates, fat, and protein. Two complementary drives regulate energy input: homeostatic and non-homeostatic pathways [17]. Whereas homeostatic pathways increase the motivation to eat in response to energy deficits, non-homeostatic pathways are able to override homeostatic pathways to favor consumption beyond metabolic needs. Non-homeostatic feeding relates to the rewarding properties of food. As a natural reward, palatable foods activate the brain's reward system, notably the mesocorticolimbic circuit, in which dopaminergic neurons originating in the ventral tegmental area (VTA) send projections to various regions including the nucleus accumbens (NAc). Activation of mesolimbic dopamine neurons is associated with increased motivation to obtain not only food rewards but also drugs of abuse. For homeostatic control of feeding, primary central pathways interact with peripheral pathways via metabolic signaling molecules. Several nuclei in the brain, primarily situated in the hypothalamus and the brainstem integrate information from circulating hormones about peripheral energy levels [18]. Leptin and insulin are two major hormones which inform the brain about recent changes in the metabolic status [19]. Leptin is secreted proportional to body fat mass from adipocytes and reduces food intake and increases energy expenditure [20]. Insulin, secreted from the pancreas, also correlates with body weight and adiposity and acts as a negative feedback control for adiposity [21], [22], [23]. Both hormones reflect the energy status within the periphery, subsequently signaling to the brain to mount an appropriate response. In particular, the arcuate nucleus of the hypothalamus (ARC) is a key region to translate the hormonal signals into behavioral responses (i.e., eating). The ARC contains two main neuronal populations regulating feeding, the neuropeptide Y (NPY)/agouti related peptide (AGRP) expressing neurons and the proopiomelanocortin (POMC)/cocaine and amphetamine related transcript (CART) neurons. These neurons are able to sense a broad range of nutrient and hormonal signals (nutrients, insulin, and leptin), and their responses change according to the energy state [24], [25].
1.2.2. Energy expenditure
Energy expenditure comprises the energy needed to maintain normal body functions and consists of obligatory energy expenditure, physical activity, and adaptive thermogenesis [26]. While obligatory energy expenditure (referring to the energy required for core body functions) is relatively fixed, adaptive thermogenesis (processes that dissipate energy as heat to maintain body temperature) is highly variable and is sensitive to environmental (e.g. cold temperature exposure and persistent organic pollutants [27]) and genetic factors, like mutations in genes sequences (e.g. leptin or the leptin receptor [28]). In mammals, there are two major types of adipose tissue, BAT and WAT, which are both structurally and functionally distinct [29]. Whereas WAT primarily acts as a storage site for lipids, BAT functions as a thermogenic tissue, dissipating energy as heat to mediate non-shivering thermogenesis. Although traditionally viewed as a function of BAT, adaptive thermogenesis is additionally governed by white adipocyte transdifferentiation into beige adipocytes, in a process referred to as ‘browning.’ The expression of UCP1 (uncoupling protein 1) in BAT mediates non-shivering thermogenesis through its ability to separate fatty acid oxidation from ATP synthesis [30]. Consequently, adipocytes in BAT have a relatively high metabolic rate. Inducible ‘brown-like’ adipocytes (beige cells) can be formed in WAT in response to various stimuli. Since there is a negative correlation between body mass index (BMI) and the activities of brown and beige cells, recruitment and/or activation of BAT holds promise for the treatment of metabolic diseases.
1.3. Glucose homeostasis
Glucose homeostasis refers to the hormonal and neural regulatory mechanisms that maintain blood glucose levels within a very narrow range. In healthy individuals, the body regulates glucose release and production in order to ensures sufficient glucose flux to meet the demands of the body [23]. The proper control of glucose homeostasis requires the synchronized actions of several organ systems, including but not limited to, the brain, liver, skeletal muscle, and adipose tissue [23], [31]. The multiple mechanism regulating glucose metabolism are complex and tightly regulated by hormones, like insulin and leptin, and their impact on glucose homeostasis are in detail reviewed elsewhere [31]. Interestingly, blood glucose levels are highly influenced by GCs the main hormones released after a stressful event. For instance, GCs increase glucose production in the liver by stimulating hepatic gluconeogenesis [14]. Additionally, GCs decrease glucose utilization and uptake in skeletal muscle and WAT [32]. Indeed, energy and glucose homeostasis are intimately connected since both systems respond to changes in energy stores and availability. Accordingly, they share many common regulatory pathways.
1.4. Interplay between stress and metabolic regulation
Chronic stress is a major risk factor for obesity and metabolic-related diseases, highlighting the complementary biology between stress and metabolic regulation [33]. Yet the relationship between stress and energy metabolism is highly complex, exemplified by diverging metabolic outcomes in response to stress. For example, in response to stress, some individuals increase feeding and body weight whereas others decrease feeding and lose weight. Moreover, stress-induced hyperphagia is not necessarily followed by an increase in body weight, suggesting that mechanisms regulating energy expenditure are activated simultaneously. Such conflicting responses to stress indicate that opposing metabolic drives respond to stress. Specifically, GCs, the end products of the HPA axis, affect energy intake and expenditure to favor a positive energy balance [34]. In contrast, sympathetically-activated β-adrenergic receptors increase energy expenditure via activation of thermogenesis in BAT in order to favor a negative energy balance [26], [35], a process that is suppressed by GCs [36], [37]. Therefore, stress promotes body weight gain when hyperphagia prevails. However, in the presence of stress-induced hypophagia or when BAT recruitment dominates, weight loss results (reviewed by [38]). Despite clear effects of stress on metabolic outcomes, only a few molecular mediators at the interface between stress and metabolic regulation are yet discovered [39], [40]. However, the complex interactions remain poorly defined. Here, we suggest that FKBP51 may also represent a molecular link between stress and metabolic pathways.
2. The FK506 binding protein 51 is a co-chaperone with multiple interaction sites
FKBP51 (encoded by the FKBP5 gene) is a 51-kDa protein and a member of the immunophilin family, which is able to bind the immunosuppressants rapamycin and FK506 [41]. Unlike the lower molecular weight members, FKBP51 does not initiate the immunosuppression activity of FK506 [42], [43]. Rather, FKBP51 is well established as a heatshock protein 90 kDa (HSP90)-associated co-chaperone, regulating steroid hormone receptor signaling. FKBP51 negatively regulates the GR by reducing GC-binding, delaying nuclear translocation, and thereby decreasing GR-dependent transcriptional activity [44], [45], [46], [47]. Its effects on GR signaling have important implications for the regulation of the stress response since GRs mediate the termination of the stress response. In fact, higher levels of FKBP5 mRNA are associated with higher levels of circulating cortisol and reduced negative feedback inhibition of the stress response [44], [46], [48], [49], [50]. Through its regulation of GR sensitivity for hormone binding, FKBP51 is perfectly positioned to modulate stress-related metabolic outcomes that are mediated through GCs. Equally important, however, is that FKBP51 expression in turn is induced by GR activation itself, representing an ultra-short, negative feedback loop regulating GR sensitivity [51].
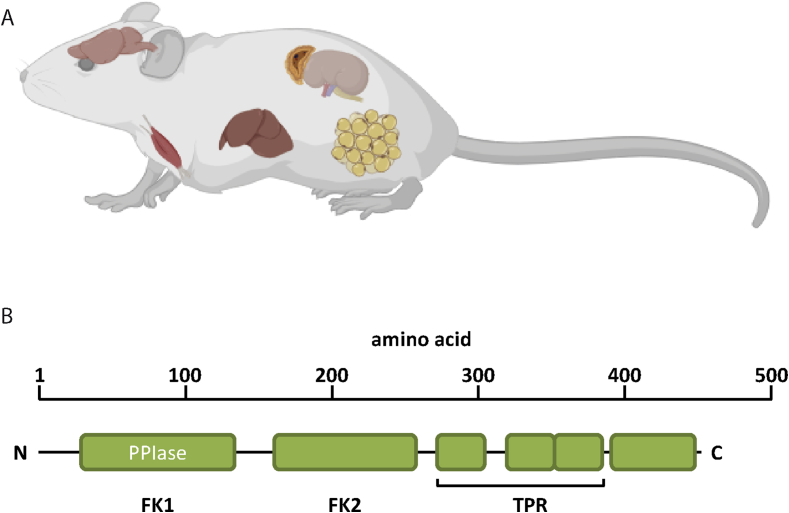
FKBP51 shares high protein domain structure homology to FKBP52 [43]. Both proteins contain two domains located N-terminally (FK1, FK2) with homology to FKBP12 (Figure 1). Only the FK1-domain (FK506 binding domain) interacts with the immunosuppressant drug tacrolimus (FK506). The FK1-domain of FKBP51 and FKBP52 is enzymatically active in catalyzing the isomerization of peptidyl-prolyl bonds of model peptides [52]. This domain has been shown to be the main determinant for the divergent impact of FKBP51 and FKBP52 on GR function [47], [53]. While the FK1 domain is important for GR regulation, its biochemical activity is not [54]. To modulate GR function, the FK1-domain as well as the HSP90-binding TPR (tetratricopeptide repeat) domain are essential. The C-terminal TPR-domain is conserved in both FKBP51 and FKBP52 and enables binding to the EEVD motif at the C-terminus of HSP90 [55]. Moreover, through its scaffolding function, FKBP51 is known to regulate NF-κB, Akt1&2, GSK3β, calcineurin/NFAT, DNMT1, and autophagic signaling pathways [4], [56], [57], [58], [59], [60], [61]. FKBP51 and FKBP52 have distinct expression profiles and may therefore exert tissue- and cell type-specific effects [62, www.proteinatlas.org]. Importantly, when both proteins are expressed in the same cells they may have opposite functions, as already shown in the context of GR signalling [63], [64]. It is therefore of high importance to differentiate between FKBP51- and FKBP52-mediated effects, an issue that is most crucial when it comes to pharmacological manipulations (see section 4).
Figure 1.
(A) Overview of important sites of metabolism related FKBP51 expression, including brain, adrenals, muscle, and fat tissue. (B) Schematic representation of the protein domain structure of the large immunophilins FKBP51 and FKBP52. FK1 and FK2 = FK506 binding domain. TPR = tetratricopeptide repeat domain.
Given the significant interplay between diverse signaling pathways involved in the regulation of homeostatic systems, FKBP51 may be well positioned to mediate the crosstalk between stress and metabolic systems. As a stress-responsive gene, FKBP5 is able to sense changes in the environment and respond accordingly, which is a defining feature of any metabolic regulatory pathway. In the following sections, we provide accumulated evidence that FKBP51 is an important regulator of whole-body energy and glucose homeostasis through its regulation of diverse signaling modalities. Further, we discuss the possible relevance of targeting FKBP51 for the treatment of stress-mediated pathophysiology.
2.1. FKBP51 shows its highest expression in muscle and adipose tissue
FKBP51 is broadly expressed in the mammalian body (Figure 1). Nevertheless, there are tremendous differences in FKBP51 expression across various tissues, with a high expression in metabolically relevant tissues in the periphery [65]. According to online gene banks and recent publications, FKBP51 shows its strongest expression in human adipocytes, skeletal muscle and lymphocytes [66]. The hippocampus and the amygdala, two central regions controlling the stress response and anxiety-related behaviors, show the highest expression of FKBP51 in the brain, especially after acute stress exposure [67]. Interestingly, FKBP51 is also highly expressed and regulated in control centers of whole-body metabolism, namely the ventromedial hypothalamic nuclei, ARC, PVN, and the nucleus of the solitary tract. Although the importance of tissue and nuclei specific actions of FKBP51 is increasingly recognized, to-date only limited data are available.
2.2. Human FKBP51 is associated with T2D and markers of insulin resistance
In humans, the FKBP5 gene is mostly associated with gene x early life interactions [68] that are described to predict the adult risk to develop psychiatric disorders, such as depression and posttraumatic stress disorders [69], [70]. Currently, there are only a few studies focusing on the link between FKBP51 and metabolic disorders. However, recent studies revealed new data on the co-chaperone's function in metabolism. The first study investigating the effects of FKBP51 expression in adipose tissue on metabolism was led by Eriksson and colleagues in 2014 [66]. The authors nicely showed that dexamethasone, a potent GR agonist, acts as a direct regulator of FKBP51 in subcutaneous and omental adipose tissue. Furthermore, they identified SNPs within the human FKBP5 gene that were associated with T2D. They further proposed that the endogenous expression of FKBP5 in adipose tissue correlates positively with markers of insulin resistance. Finally, the authors suggest that SNPs within the FKBP5 gene may be linked to the susceptibility to develop insulin resistance and dyslipidemia. In a follow-up study with a larger and more diverse cohort, Sidibeh and colleagues provided further evidence that FKBP5 gene expression is linked to insulin resistance [71]. They revealed that FKBP5 negatively correlates with genes regulating adipogenesis, suggesting that human FKBP51 might be involved in adipocyte differentiation. These results are in line with preclinical results underpinning a regulatory role of FKBP51 in adipogenesis [72]. However, in humans it is not yet known whether this link is caused by changes in FKBP51 protein levels. Hence, it would be very interesting to include the changes in FKBP51 protein level as a parameter in future studies. In fact, results from animal studies suggest that loss of FKBP51 function leads to a better health status under high-fat diet conditions [4], [73].
Also, a study by Ortiz and colleagues reported an association between FKBP5 intronic methylation and a risk of cardiovascular disease in T2D patients [68]. In this study, the authors investigated the methylation of FKBP5 at intron 2 in T2D patients only. Despite the limitations of a small cohort size and the lack of a control group, the results suggest that FKBP5 methylation at intron 2 is a marker for increased cardiovascular risk in T2D [68]. Another study demonstrated that intronic DNA methylation of FKBP5 at intron 2 and 7 is significantly lower in patients suffering from Cushing's Syndrome compared to the controls, which in turn leads to a higher gene expression and subsequently results in GC resistance [74].
Whereas the above mentioned studies could not find any correlation of FKBP5 and body weight, a study by Hartmann and colleagues showed that the SNP rs1360780 within the FKBP5 gene is associated with reduced weight loss following bariatric surgery [75]. Taken together, the few existing studies in human cohorts suggest a role of FKBP5 in the development of metabolic disorders. However, additional clinical studies, with greater sample sizes, are required to solidify the current findings. Moreover, it is necessary to study broader population groups in order to characterize the association between stress and metabolic disorders.
2.3. Preclinical studies show a beneficial effect of FKBP51 loss in mice
In parallel to human studies of SNPs within the FKBP5 gene, FKBP51 has been heavily researched in preclinical studies. Until now, the main focus of FKBP51 research in vivo has primarily examined the stress response, stress-related disorders, and cancer. Yet as early as 2012 two papers had reported that FKBP51 knockout (KO) mice are leaner than their littermates under normal chow diet [76], [77]. These findings initiated the first studies examining FKBP51 within the context of metabolism. In 2014, a study from the Schmidt lab examined the interaction between chronic stress and obesity [78]. Despite the findings that chronic stress induces hyperphagia and weight loss, the results showed a positive correlation between FKBP5 mRNA and body weight gain as well as food intake. Thus, the study was the first to suggest FKBP51 as a link between stress-related disorders and the metabolic syndrome. A few years later, two independent research groups showed that FKBP51 null mice are resistant against high fat diet-induced weight gain and adiposity and showed improved glucose tolerance and increased energy expenditure [4], [73]. In both cases, genetic deletion of FKBP51 had no effect on food intake. Interestingly, while both studies observed the same body weight phenotype, they discovered independent pathways through which FKBP51 influences body weight. Stechschulte and colleagues identified FKBP51 as a regulator of adipocyte differentiation, in which loss of FKBP51 triggers browning in white adipose tissue. They showed that FKBP51 KO animals have a reduced PPARγ activity and increased expression of markers of browning, (i.e. UCP-1 and PRDM16) in WAT [73]. Alternately, our own study demonstrated that FKBP51 acts through AKT2-AS160 signaling to regulate glucose uptake specifically in muscle tissue. Furthermore, our study was the first to present that treatment with a selective FKBP51 antagonist, SAFit2, improves the metabolic health of obese mice. Interestingly, while FKBP51 is also expressed and regulated in metabolic brain centers, its role in those centers is so far unexplored, leaving many directions for researchers to pursue.
3. Molecular regulation of metabolic pathways by FKBP51
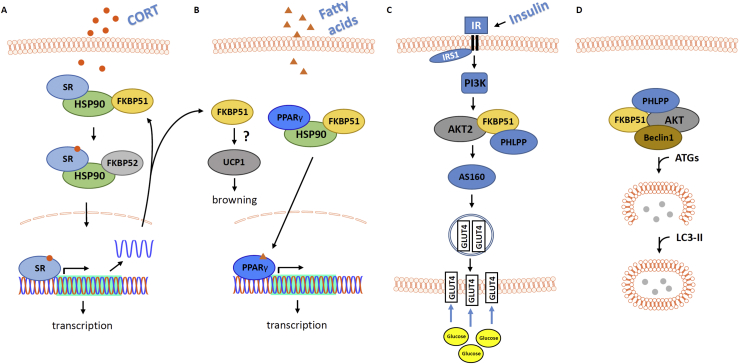
As introduced above, FKBP51 is mainly characterized as a co-chaperone of the HSP90 complex in order to regulate the ultra-short negative feedback loop involved in terminating the stress response. However, FKBP51 has many more interaction partners like AKT, Beclin1 and NF-κB. In the following paragraphs, we will show that many of FKBP51's interacting partners are involved in essential metabolic pathways, underpinning FKBP51 as a potential new therapeutic target for metabolic diseases (Figure 2).
Figure 2.
Schematic representation of important metabolism-related cellular signaling cascades where FKBP51 was shown to play a decisive role (see main text for further details). (A) FKBP51 interacts with HSP90 and several steroid receptors (SR), including the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) and thereby modulates SR ligand sensitivity, translocation and function. Among the genes that are regulated by glucocorticoids (GCs) via MR/GR activation is Fkbp5, thereby forming an ultra-short feedback loop. At the same time, FKBP5 has been shown to interact with other signaling pathways, thereby affecting cellular function in a cell-type specific manner [132], [133], [134]. (B) In fat tissue, FKBP51 was shown to affect PPARγ signaling and adipogenesis (not depicted) [73], [105]. In addition, an effect of UCP1 and consequently browning of white adipose tissue (WAT) has been postulated [4], [73]. (C) In muscle cells, FKBP51 interacts with AKT2 in the insulin signaling pathway, ultimately modulating cellular glucose uptake [4]. (D) In the brain, FKBP51 was shown to regulate autophagy via interaction with Beclin1 [56].
3.1. FKBP51 regulates glucose uptake in muscle tissue
The AKT protein family consists of three related isoforms, AKT1, AKT2, and AKT3. All members share a high degree of homology, each containing a N-terminal pleckstrin homology (PH) domain, a kinase domain, and a hydrophobic motif at the C-terminus [79]. However, each isoform differs in its tissue expression levels. Whereas AKT1 is widely distributed across tissues and recognized for its role in cell growth and survival [80], [81], AKT2 is largely restricted to insulin sensitive tissues, fat and muscle, where it contributes to the regulation of glucose homeostasis [82], [83]. AKT3 expression is mainly limited to the testis and brain [84].
As a key downstream target of phosphoinositide-3 kinase (PI3K), many cytokines and growth factors, including insulin, activate AKT signaling. Briefly, PI3K converts phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-trisphosphate (PIP3), which subsequently acts as a binding site for PH domain proteins, including AKT and PDK1 (3-phosphoinositide-dependent protein kinase 1). At the plasma-membrane, PDK1 phosphorylates the activation loop of Akt at Thr308 [85]. For maximal activation, AKT is also phosphorylated at Ser473 (AKT2 Ser474) in the hydrophobic motif [86] by mTORC2 [87]. Once activated, AKT phosphorylates a plethora of downstream targets to regulate metabolism, cell proliferation, and cell survival [79]. To inactivate AKT signaling, protein phosphatase 2 (PP2) and PH domain leucine-rich repeat phosphatase (PHLPP) dephosphorylate Thr308 and Ser473, respectively [88], [89]. Two isoforms of PHLPP (PHLPP1 and PHLPP2) exist, and while both dephosphorylate Ser473 (or Ser474 at Akt2), PHLPP1 specifically acts on AKT2 and AKT3 whereas PHLPP2 acts on AKT1 and AKT3 providing specificity for the termination of AKT signaling [90].
FKBP51 has been shown to regulate AKT signaling through its role as a scaffolding protein. The first study to establish a link between FKBP51 and AKT demonstrated that overexpression of FKBP51 reduces phosphorylation of AKT1 at Ser473, but has no effect on the phosphorylation of Thr308 in a pancreatic cancer cell line [59]. Accordingly, siRNA downregulation or genetic deletion of FKBP51 increased Ser473 phosphorylation, with no effect on Thr308 phosphorylation. The authors demonstrated that FKBP51 regulates AKT1-Ser473 phosphorylation through its ability to interact with both PHLPP and AKT. Specifically, PHLPP and AKT co-immunoprecipitated with FKBP51, and in turn FKBP51 overexpression led to an increased interaction between PHLPP and AKT across all AKT isoforms and corresponding PHLPP isoforms. Importantly, decreased Ser473 phosphorylation resulted from FKBP51 overexpression was prevented by knockdown of PHLPP. At a functional level, FKBP51 expression is downregulated or lost in pancreatic cancer and breast cancer cell lines [59], which agrees with the observed AKT hyperactivation in many cancers. Reconstitution of FKBP51 in cancer cells decreased Akt phosphorylation at Ser473 and sensitized the cells to chemotherapeutic agents, supporting the earlier findings that loss of FKBP51 expression is associated with chemotherapy resistance [91].
Very recently, we demonstrated that FKBP51 is involved in the regulation of glucose homeostasis through its regulation of AKT2 signaling. We found that AKT2 signaling, as determined through the phosphorylation of AKT2 and downstream effectors (AKT substrate 160 (AS160) and p70S6K), is increased in skeletal muscle (soleus and extensor digitorum longus muscles) of FKBP51 KO mice and of mice treated with the FKBP51 antagonist SAFit2. This agrees with the relatively high expression level of FKBP51 and low expression level of its functional counterpart, FKBP52, detected in skeletal muscle. Given the importance of skeletal muscle AKT signaling in the maintenance of glucose homeostasis [92], [93], we examined molecular markers and functional readouts of glucose uptake. Briefly, the glucose transporter protein 4 (GLUT4) is responsible for insulin-stimulated glucose uptake in skeletal muscle. In an unstimulated state, GLUT4 is localized to specialized intracellular structures that consist of GLUT4 storage vesicles [94]. Upon insulin stimulation, AKT signaling is activated leading to the phosphorylation of AS160 and the translocation of GLUT4 to the plasma membrane, which facilitates increased glucose uptake [95]. Both pharmacological antagonism and genetic deletion of FKBP51 increase the expression of GLUT4 at the plasma membrane and increase 2-deoxyglucose uptake in primary myotubes. Meanwhile, simultaneous overexpression of AKT2 and FKBP51 prevented AKT2-induced increases in glucose uptake. Co-immunoprecipitation experiments revealed that FKBP51 not only interacts with AKT2 and PHLPP but interacts with the downstream effector AS160. Taken together, AKT2 signaling is an important regulator of cellular survival and metabolism. Through its function as regulator of AKT signaling, FKBP51 has been implicated in cellular survival in cancer and glucose uptake in obesity and diabetes. Whether FKBP51-AKT signaling is involved in additional metabolic functions beyond its regulation of glucose uptake is an open area of research.
3.2. FKBP51 regulates adipocyte differentiation in fat tissue
The pathophysiology of obesity is associated with the massive expansion of visceral and subcutaneous fat depots. Adipose tissue is a remarkable organ with fundamental effects on whole body metabolism. With its function as an energy storage site, a source of circulating free fatty acids, and a hormone secretion site, adipose tissue plays a major role in regulation and dysregulation of nutrient homeostasis [96], [97]. Adipocytes are the major cell type of fat tissue. The accurate transformation of mesenchymal stem cells to mature adipocytes, so called adipogenesis, is crucial for proper functionality. Adipogenesis consists of 2 main phases, the determination of mesenchymal stem cells to pre-adipocytes and the final differentiation of pre-adipocytes to mature adipocytes. It is activated by multiple signaling cascades within the mitogen-activated protein (MAPK) family in response to a plethora of stimuli [98]. The so called ‘master’ regulator of adipogenesis is peroxisome-activated receptor γ (PPARγ). PPARγ acts as a pro-adipogenic factor, regulating the terminal differentiation phase [96]. The activation of PPARγ by the AKT-p38/MAPK pathway was initially reported by Aoudi and colleagues [99], [100]. Phosphorylation of AKT leads to an activation of p38 kinase, which phosphorylates the transcription factors GRα (at serine 220 and 234) [101] and PPARγ (at serine 112) [102] to stimulate lipolysis and to reduce adipogenesis, respectively. Interestingly, recent data suggests that FKBP51 is one of the most highly induced proteins during WAT adipocyte differentiation [72], [103]. In vitro studies in 3T3-L1 pre-adipocytes have shown that FKBP51 is an important regulator of adipocyte maturation [104]. In fact, loss of FKBP51 in pre-adipocytes prevents adipocyte differentiation and accumulation of lipid droplets [101]. Furthermore, FKBP51 KO cells tend to have an increased resistance to lipid accumulation and a decrease in the expression of lipogenic genes, such as CD36, in mature adipocytes [105]. The authors discovered that FKBP51 scaffolds PHLPP to inhibit AKT activity, through dephosphorylation, and thereby negatively regulates p38 kinase, which in turn reduces GRα activity to repress lipolysis and induces PPARγ to increase adipogenesis [101], [105]. These studies indicate that FKBP51 is an important regulator of the balance between lipolysis and lipogenesis in adipocytes.
A few years later, Stechschulte and colleagues confirmed their reduced PPARγ and increased GRα activity in FKBP51 deficient mice in vivo [73]. Interestingly, they showed that FKBP51 null mice were resistant to the PPARγ agonist rosiglitazone in WAT. These findings replicate the in vitro data and further support the notion that the FKBP51-Akt/p38 MAPK cascade is, in part, responsible for the reduced WAT mass in FKBP51 KO mice [73]. Surprisingly, the resistance to rosiglitazone was only observed in WAT. BAT of FKBP51 KO mice stayed responsive to the PPARγ agonist.
The diverse effects of rosiglitazone in FKBP51 KO mice could be due to differences in FKBP51 expression in white and brown adipocytes, which derive from different adipocyte precursor lineages [106]. In fact, FKBP51 shows a lower expression in BAT compared to WAT [73]. Despite the expression differences of FKBP51, the levels of FKBP52 might be as important. FKBP52 competes for binding partners with FKBP51, thereby affecting downstream signaling pathways differently [43], [63]. For instance, FKBP52 is minimally expressed in skeletal muscle and highly expressed in WAT. Consequently, FKBP52 does not compete with FKBP51 for the binding site with AKT2 in muscle, but interferes with its binding in WAT, thereby altering functional implications [4].
Next to its regulatory function on PPARy activity, FBKP51 also interacts in complex with Hsp90 with steroid receptors, like the GR, MR, AR, and PR [55]. So far, there are no conclusive data on a function of FKBP51 in modulating MR, AR, or PR function in adipocytes. Interestingly, especially adipocyte GRs are activated by glucocorticoids and are associated with adipogenesis [107] (the interplay between FKBP51, Hsp90 and GR are reviewed in detail elsewhere [64], [104]). Within the first hours of adipocyte differentiation, FKBP51 rapidly translocates from the mitochondria to the nucleus. This shuttling of FKBP51 results in an increased interaction with GR and thereby a decrease of transcriptional activity of GR [72]. Whether or not the activation of PPARy and GR lead to nuclear shuttling of FKBP51 via differential mechanisms is so far not clear.
Mounting evidence suggests that FKBP51 is also important in the browning of WAT. Elevated levels of various thermogenic genes, such as PGC-1a, UCP-1 and PRDM16 has been observed in WAT of FKBP51 KO mice. An upregulation of thermogenic genes is associated with increased energy expenditure and heat production, explaining the lean phenotype of FKBP51 KO mice [4], [73]. However, the detailed molecular mechanism for the elevated energy expenditure and increased expression of browning markers in FKBP51 KO mice is still unclear. It is worth speculating that the observed effect of the UCP-1 upregulation in WAT of FKBP51 KO mice might not be mediated directly by FKBP51 but rather indirectly via sympathetic or parasympathetic innervation. Considering the distinct molecular and physiological properties of various fat depots within the body, specific manipulations of different adipose depots would be necessary to fully unravel the direct or indirect role of FKBP51 in the regulation of adipogenesis and browning in vivo.
3.3. Role of FKBP51 in modulating autophagy
Autophagy is an important catabolic process to maintain cellular homeostasis and cellular function. It is tightly regulated and crucial for targeting damaged cytosolic macromolecules such as organelles, proteins, glycogens, and lipids to lysosomes for degradation [108]. Recent data demonstrated an important role of autophagy in the regulation of metabolic processes such as, food intake, adipose tissue development, liver complications, and insulin resistance [109], [110], [111]. For instance, defects in autophagy signaling have been implicated in the development of obesity and T2D [112]. Moreover, it has been shown that hypothalamic autophagy is increased during starvation to supply cells with sufficient nutrients [113]. Additionally, a regulatory role of autophagy in adipocyte mass development and differentiation has been reported [110], [114]. Intriguingly, FKBP51 acts as a regulatory molecule of both processes as well [67], [72], which indicates converging pathways of FKBP51 and autophagy. Indeed, in 2010, Romano et al. initially described a decisive role for FKBP51 in the cellular response to irradiation resulting in a shift from apoptosis to autophagy [115]. More recent studies have highlighted the mechanistic impact of FKBP51 on the regulation of autophagy and related processes. Furthermore, autophagy can be induced through GCs, and Hsp90 and its co-chaperone FKBP51 are key modulators of autophagy function [59], [116], [117], [118]. The initiation and regulation of autophagy involves complex signaling pathways, which are not focus of this review, but are reviewed in depth elsewhere [119], [120], [121]. However, one key molecule, Beclin1, is of particular interest. Beclin1 interacts with several other proteins to induce the initiation of autophagy signaling. Interestingly, it was demonstrated that FKBP51 promotes the induction of autophagic signaling by phosphorylating Beclin1 at serine 234 and serine 295. In parallel, the Beclin1-phosphorylating kinase, AKT, is dephosphorylated at serine 473 by the FKBP51-mediated recruitment of PHLPP, which promotes the induction of autophagy. Furthermore, synthetic GCs (i.e. dexamethasone) and antidepressants act synergistically with FKBP51 in the induction of autophagy [56], [122]. Despite the emerging roles of autophagy and FKBP51 in energy metabolism, no study has systemically investigated the FKBP51-Beclin1-Autophagy-axis in metabolic control. Indeed, in future studies, it will be important to delineate the regulatory action of FKBP51 on Beclin1 in the context of whole-body metabolism.
4. FKBP51 as a therapeutic target
FKBPs bind the immunosuppressive compounds FK506 and rapamycin [123], [124]. These natural compounds, which were first isolated from bacterial Streptomyces strains, have been shown to bind to the peptidyl-prolyl-isomerase pocket of the FK1 domain, thereby inducing a complex with calcineurin (in the case of FK506) or mTOR (in the case of rapamycin) [125], [126]. As FKBPs are also implicated in a wide range of intracellular signaling pathways that are independent of immune suppression [127], [128], non-immunosuppressant FK506-derived ligands were developed (e.g. FK1706), which had neuroprotective properties [125]. However, none of these ligands could discriminate the different FKBPs, especially not between FKBP12 (with immunosuppressant properties) and the larger molecular weight FKBPs FKBP51 and FKBP52 (with non-immunosuppressant properties). Given the different and often opposing functions of the different FKBPs, selectivity of novel ligands is of utmost importance. As mentioned previously, while FKBP51 and FKBP52 share 70% sequence homology, they have diverging effects on many signaling pathways, including steroid hormone receptor signaling and AKT signaling pathways. Therefore, for the therapeutic potential of FKBP51 to be realized, agents must be able to select between FKBP51 and its often functional opposing homolog FKBP52. It soon became clear that the specific chemical targeting of FKBP51 is challenging, as large-scale screening assays for novel FKBP51 ligands did not reveal any new hits, other than the already known FK506 and rapamycin. The eventual breakthrough was achieved by Hausch and colleagues, using structure-based rational design [129]. In a stepwise approach guided by co-crystal structures a ligand-induced conformational change was observed that favored FKBP51 over FKBP52. Further development of the prototype compounds eventually resulted in the first selective FKBP51 inhibitors termed SAFit1 (abbreviated for selective antagonists of the FK506-binding protein 51 by induced fit) and SAFit2 [2]. Both ligands have Ki values of less than 10 nM and show more than 10,000-fold selectivity of FKBP51 over FKBP52. These new compounds are non-immunosuppressive, and they selectively stimulate neurite outgrowth in vitro. For in vivo applications SAFit2 shows the better pharmacokinetic properties and crosses the blood brain barrier. As expected from the well-described function of FKBP51 in reducing the sensitivity of the GR to its ligand, treatment with SAFit2 enhanced GR-mediated GC feedback, as indicated by lower circadian peak corticosterone levels and an enhanced dexamethasone-mediated suppression of the HPA axis [2].
Since their first description, the selective FKBP51 ligands SAFit1 and SAFit2 have been tested in a number of in vivo disease models, underlining the versatile applicability of a selective pharmacological FKBP51 inhibition. As psychiatric disorders are closely linked to FKBP51 function, the brain-permeable SAFit2 was tested for effects on anxiety and depression-like behavior. Intriguingly, FKBP51 inhibition was shown to reduce passive stress coping behavior in the forced swim test and exploration anxiety in the elevated plus maze and the dark-light box after only a few hours post-administration [2], [3]. The effect of FKBP51 antagonism on anxiety was specific to the amygdala, as the anxiolytic effect could be mimicked by injecting SAFit2 directly into this brain region. Similarly, FKBP51 inhibition was shown to reduce chronic pain [130], [131]. When applied as treatment of metabolic disorders, we could recently show that prolonged SAFit2 treatment reduces body weight gain and reverses high-fat diet-induced glucose intolerance [4]. The effect mimicked the metabolic phenotype of FKBP51 KO mice, and no SAFit2 effect was observed when FKBP51 KO mice were treated, highlighting the specificity of the antagonist. Importantly, the improvement of glucose tolerance was already observed just 48 h after beginning treatment and preceded the body weight phenotype. This suggests that the effects of FKBP51 inhibition on glucose uptake are the most proximal beneficial treatment effects and independent of the improved body weight phenotype. Notably, pharmacological antagonism via SAFit2 disrupts the scaffolding function of FKBP51 by weakening the interaction between FKBP51 and AS160 while strengthening the interaction between AKT2 and AS160 to ultimately promote a steric arrangement that favors glucose uptake.
Indeed, the first results of selective FKBP51 inhibition in relation to metabolism and metabolic disorders are highly promising and are a starting point for further investigations. Further improvement of the current inhibitors SAFit1 and SAFit2 to enhance their drug-like properties, including a lower molecular weight, will be important. Likewise, further improvement of the pharmacokinetic properties of SAFit1 would enable the pharmacological blockade of FKBP51 only in the periphery, as SAFit1 does not cross the blood brain barrier. Yet much work is still needed to disentangle the mechanism of action by which FKBP51 ligands work on the molecular level in the different tissues expressing FKBP51 in order to optimize the therapeutic effects of FKBP51 antagonists.
5. Conclusion and future directions
This review's intent was to highlight the accumulating evidence that FKBP51 plays an important role in the regulation of whole-body energy and glucose metabolism, presenting FKBP51 as a complex co-chaperone beyond the well-established function as a negative GR regulator. The recent insights highlight FKBP51 as a potential drug target for obesity and its associated comorbidities. However, a lot of research is needed to advance the field. Below, we list a few future directions, which we believe are crucial to advance the knowledge about FKBP51's metabolic action:
-
1.
Tissue-specific manipulation of FKBP51 in muscle, adipose and brain tissue will be important to disentangle the differential functions of FKBP51 in specific cells-types.
-
2.
Better insight into the specific actions of FKBP51 inhibitors at the molecular level will be necessary.
-
3.
Development of pathway specific FKBP51 antagonists will be key for symptom tailored treatment.
-
4.
Clinical studies examining FKBP51 in human cohorts in the context of metabolism will further the therapeutic development of FKBP51 antagonists
We hope that future preclinical and clinical studies will fill the knowledge gap to fully disentangle the molecular mechanism of FKBP51 in metabolism and help to implement FKBP51 as drug target for the treatment of metabolic disorders.
Acknowledgements
The current work was supported by the BioM M4 award “PROCERA” of the Bavarian State Ministry (Schmidt), the “OptiMD” grant of the Federal Ministry of Education and Research (01EE1401D; Schmidt) and the “GUTMOM” grant of the Federal Ministry of Education and Research (01EA1805; Schmidt).
Contributor Information
Alexander S. Häusl, Email: alexander.haeusl@biophyll.com.
Mathias V. Schmidt, Email: mschmidt@psych.mpg.de.
Conflict of interests
The authors declare conflict of interest.
References
- 1.Chrousos G.P. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 2.Gaali S., Kirschner A., Cuboni S., Hartmann J., Kozany C., Balsevich G. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nature Chemical Biology. 2015;11(1):33–37. doi: 10.1038/nchembio.1699. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann J., Wagner K.V., Gaali S., Kirschner A., Kozany C., Ruhter G. Pharmacological inhibition of the psychiatric risk factor FKBP51 has anxiolytic properties. Journal of Neuroscience. 2015;35:1529–2401. doi: 10.1523/JNEUROSCI.4024-14.2015. (Electronic): 9007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsevich G., Häusl A.S., Meyer C.W., Karamihalev S., Feng X., Pöhlmann M.L. Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nature Communications. 2017;8(1):1725. doi: 10.1038/s41467-017-01783-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousin B., Cinti S., Morroni M., Raimbault S., Ricquier D., Penicaud L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. Journal of Cell Science. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. ( Pt 4(0021-9533 (Print)) [DOI] [PubMed] [Google Scholar]
- 7.Gilgen A., Maickel R.P., Nikodijevic O., Brodie B.B. Essential role of catecholamines in the mobilization of free fatty acids and glucose after exposure to cold. Life Sciences. 1962;1(1962):709–715. doi: 10.1016/0024-3205(62)90138-8. [DOI] [PubMed] [Google Scholar]
- 8.Giordano A., Morroni M., Santone G., Marchesi G.F., Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. Journal of Neurocytology. 1996;25(2):125–136. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- 9.Young P., Arch J.R., Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Letters. 1984;167(1):10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 10.Halter J.B., Beard J.C., Porte D. Islet function and stress hyperglycemia: plasma glucose and epinephrine interaction. American Journal of Physiology. 1984;247(1 Pt 1):E47–E52. doi: 10.1152/ajpendo.1984.247.1.E47. [DOI] [PubMed] [Google Scholar]
- 11.de Kloet E.R. Functional profile of the binary brain corticosteroid receptor system: mediating, multitasking, coordinating, integrating. European Journal of Pharmacology. 2013;719(1–3):53–62. doi: 10.1016/j.ejphar.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 12.De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 13.Coderre L., Srivastava A.K., Chiasson J.L. Role of glucocorticoid in the regulation of glycogen metabolism in skeletal muscle. American Journal of Physiology. 1991;260(6 Pt 1):E927–E932. doi: 10.1152/ajpendo.1991.260.6.E927. [DOI] [PubMed] [Google Scholar]
- 14.Exton J.H. Regulation of gluconeogenesis by glucocorticoids. Monographs on Endocrinology. 1979;12:535–546. doi: 10.1007/978-3-642-81265-1_28. [DOI] [PubMed] [Google Scholar]
- 15.Munck A., Guyre P.M., Holbrook N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 16.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. Journal of Biological Chemistry. 2006;281(52):40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 17.Lutter M., Nestler E.J. Homeostatic and hedonic signals interact in the regulation of food intake. Journal of Nutrition. 2009;139(3):629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 19.Benoit S.C., Clegg D.J., Seeley R.J., Woods S.C. Insulin and leptin as adiposity signals. Recent Progress in Hormone Research. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 20.Cota D., Marsicano G., Tschöp M., Grübler Y., Flachskamm C., Schubert M. The endogenous cannabinoid. Journal of Clinical Investigation. 2003;112(3):423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 22.White M.F., Kahn C.R. The insulin signaling system. Journal of Biological Chemistry. 1994;269:1–4. (0021-9258 (Print)) [PubMed] [Google Scholar]
- 23.Saltiel A.R., Kahn C.R. Glucose and Lipid Metabolism. 2001;414(December):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 24.Morton G.J., Meek T.H., Schwartz M.W. Neurobiology of food intake in health and disease. Nature Reviews Neuroscience. 2014;15(6):367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cone R.D. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8(5):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 26.Lowell B.B., Spiegelman B.M. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. 0028-0836 (Print) [DOI] [PubMed] [Google Scholar]
- 27.Tremblay A., Royer M.-M., Chaput J.-P., Doucet E.É. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. International Journal of Obesity. 2012;37:759–764. doi: 10.1038/ijo.2012.124. [DOI] [PubMed] [Google Scholar]
- 28.Mcpherson R. vol. 23. 2007. (Genetic contributors to obesity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saely C.H., Geiger K., Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58:15–23. doi: 10.1159/000321319. 1423-0003 (Electronic)) [DOI] [PubMed] [Google Scholar]
- 30.Nicholls D.G. The physiological regulation of uncoupling proteins. Biochimica et Biophysica Acta. 2006;1757:459–466. doi: 10.1016/j.bbabio.2006.02.005. 0006-3002 (Print) [DOI] [PubMed] [Google Scholar]
- 31.Morton G., Schwartz M. Leptin and the central nervous system control of glucose metabolism. Physiological Reviews. 2011;(8):389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero L.M., Butler L.K. vol. 20. 2007. (Endocrinology of stress). [Google Scholar]
- 33.Ulrich-Lai Y.M., Ryan K.K. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metabolism. 2014;19(6):910–925. doi: 10.1016/j.cmet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dallman M.F. Stress-induced obesity and the emotional nervous system. Trends in Endocrinology and Metabolism. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. 1879-3061 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. 0031-9333 (Print) [DOI] [PubMed] [Google Scholar]
- 36.Soumano K., Desbiens S., Rabelo R., Bakopanos E., Camirand A., Silva J.E. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Molecular and Cellular Endocrinology. 2000;165(1–2):7–15. doi: 10.1016/s0303-7207(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 37.Van Den Beukel J.C., Grefhorst A., Quarta C., Steenbergen J., Mastroberardino P.G., Lombés M. Direct activating effects of adrenocorticotropic hormone (ACTH) on brown adipose tissue are attenuated by corticosterone. Federation of American Societies for Experimental Biology Journal. 2014;28(11):4857–4867. doi: 10.1096/fj.14-254839. [DOI] [PubMed] [Google Scholar]
- 38.Razzoli M., Bartolomucci A. The dichotomous effect of chronic stress on obesity. Trends in Endocrinology and Metabolism. 2016;27(7):504–515. doi: 10.1016/j.tem.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuperman Y., Issler O., Regev L., Musseri I., Navon I., Neufeld-Cohen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(18):8393–8398. doi: 10.1073/pnas.1003969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuperman Y., Weiss M., Dine J., Staikin K., Golani O., Ramot A. CRFR1 in AgRP neurons modulates sympathetic nervous system Activity to adapt to cold stress and fasting. Cell Metabolism. 2016;23(6):1185–1199. doi: 10.1016/j.cmet.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinars C.R., Cheung-Flynn J., Rimerman R.A., Scammell J.G., Smith D.F., Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:868–873. doi: 10.1073/pnas.0231020100. 0027-8424 (Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galigniana N.M., Ballmer L.T., Toneatto J., Erlejman A.G., Lagadari M., Galigniana M.D. Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51. Journal of Neurochemistry. 2012;122(1):4–18. doi: 10.1111/j.1471-4159.2012.07775.x. [DOI] [PubMed] [Google Scholar]
- 43.Storer C.L., Dickey C.A., Galigniana M.D., Rein T., Cox M.B. FKBP51 and FKBP52 in signaling and disease. Trends in Endocrinology and Metabolism. 2011;22(12):481–490. doi: 10.1016/j.tem.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denny W.B., Valentine D.L., Reynolds P.D., Smith D.F., Scammell J.G. Squirrel monkey immunophilin {FKBP}51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141 doi: 10.1210/endo.141.11.7785. (0013-7227 (Print)): 4107–13. [DOI] [PubMed] [Google Scholar]
- 45.Scammell J.G., Denny W.B., Valentine D.L., Smith D.F. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three new world primates. General and Comparative Endocrinology. 2001;124(2):152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 46.Westberry J.M., Sadosky P.W., Hubler T.R., Gross K.L., Scammell J.G. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone {FKBP}51. The Journal of Steroid Biochemistry and Molecular Biology. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. 0960-0760 (Print) [DOI] [PubMed] [Google Scholar]
- 47.Wochnik G.M., Rüegg J., Abel G.A., Schmidt U., Holsboer F., Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. Journal of Biological Chemistry. 2005;280(6):4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 48.Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299 doi: 10.1001/jama.299.11.1291. (1538-3598 (Electronic)): 1291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Touma C., Gassen N.C., Herrmann L., Cheung-Flynn J., Bll D.R., Ionescu I.A. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biological Psychiatry. 2011;70(10):928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Ising M., Depping A.M., Siebertz A., Lucae S., Unschuld P.G., Kloiber S. Polymorphisms in the {FKBP}5 gene region modulate recovery from psychosocial stress in healthy controls. European Journal of Neuroscience. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. 1460-9568 (Electronic) [DOI] [PubMed] [Google Scholar]
- 51.Vermeer H., Hendriks-Stegeman B.I., van der Burg B., van Buul-Offers S.C., Jansen M. Glucocorticoid-induced increase in lymphocytic {FKBP}51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. Journal of Clinical Endocrinology & Metabolism. 2003;88 doi: 10.1210/jc.2002-020354. (0021-972X (Print)): 277–84. [DOI] [PubMed] [Google Scholar]
- 52.Pirkl F., Buchner J. Functional analysis of the hsp90-associated human peptidyl prolyl Cis/Trans isomerases FKBP51, FKBP52 and cyp40. Journal of Molecular Biology. 2001;308(4):795–806. doi: 10.1006/jmbi.2001.4595. [DOI] [PubMed] [Google Scholar]
- 53.Riggs D.L., Roberts P.J., Chirillo S.C., Cheung-Flynn J., Prapapanich V., Ratajczak T. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. The EMBO Journal. 2003;22(5):1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riggs D.L., Cox M.B., Tardif H.L., Hessling M., Buchner J., Smith D.F. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Molecular and Cellular Biology. 2007;27(24):8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schülke J.-P., Wochnik G.M., Lang-Rollin I., Gassen N.C., Knapp R.T., Berning B. Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gassen N.C., Hartmann J., Zschocke J., Stepan J., Hafner K., Zellner A. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Medicine. 2014;11(11) doi: 10.1371/journal.pmed.1001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gassen N.C., Hartmann J., Zannas a. S., Kretzschmar A., Zschocke J., Maccarrone G. FKBP51 inhibits GSK3β and augments the effects of distinct psychotropic medications. Molecular Psychiatry. 2016;21(2):277–289. doi: 10.1038/mp.2015.38. [DOI] [PubMed] [Google Scholar]
- 58.Jiang W., Cazacu S., Xiang C., Zenklusen J.C., Fine H.A., Berens M. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-kappaB signaling pathway. Neoplasia. 2008;10:235–243. doi: 10.1593/neo.07929. 1476-5586 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pei H., Li L., Fridley B.L., Jenkins G.D., Kalari K.R., Lingle W. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16(3):259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romano S., Xiao Y., Nakaya M., D'Angelillo A., Chang M., Jin J. FKBP51 employs both scaffold and isomerase functions to promote NF-kappaB activation in melanoma. Nucleic Acids Research. 2015;43(14):6983–6993. doi: 10.1093/nar/gkv615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zannas A.S., Jia M., Hafner K., Baumert J., Wiechmann T., Pape J.C. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB-driven inflammation and cardiovascular risk. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(23):11370–11379. doi: 10.1073/pnas.1816847116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A. Tissue-based map of the human proteome. Science. 2015;347(6220) doi: 10.1126/science.1260419. 1260419–1260419. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt M.V., Paez-Pereda M., Holsboer F., Hausch F. The prospect of FKBP51 as a drug target. ChemMedChem. 2012;7(8):1351–1359. doi: 10.1002/cmdc.201200137. [DOI] [PubMed] [Google Scholar]
- 64.Zgajnar N.R., Leo S.A. De., Lotufo C.M., Erlejman A.G., Piwien-Pilipuk G., Galigniana M.D. Biological actions of the hsp90-binding immunophilins FKBP51 and FKBP52. Biomolecules. 2019;9(2) doi: 10.3390/biom9020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baughman G., Widerrecht G.J., Chang F., Martin M.M., Bourgeois S. Tissue distribution and abundance of human FKBP51, and FK506-binding protein that can mediate calcineruin inhibition. Biochemical and Biophysical Research Communications. 1997;232(2):437–443. doi: 10.1006/bbrc.1997.6307. [DOI] [PubMed] [Google Scholar]
- 66.Pereira M.J., Palming J., Svensson M.K., Rizell M., Dalenbäck J., Hammar M. FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metabolism. 2014;63:1198–1208. doi: 10.1016/j.metabol.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Scharf S.H., Liebl C., Binder E.B., Schmidt M.V., Müller M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS One. 2011;6(2):1–10. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ortiz R., Joseph J.J., Lee R., Wand G.S., Golden S.H. Type 2 diabetes and cardiometabolic risk may be associated with increase in DNA methylation of FKBP5. Clinical Epigenetics. 2018;10(1):82. doi: 10.1186/s13148-018-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zannas A.S., Binder E.B. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes, Brain and Behavior. 2014;13(1):25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- 71.Sidibeh C.O., Pereira M.J., Abalo X.M., J Boersma G., Skrtic S., Lundkvist P. FKBP5 expression in human adipose tissue: potential role in glucose and lipid metabolism, adipogenesis and type 2 diabetes. Endocrine. 2018;62(1):116–128. doi: 10.1007/s12020-018-1674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toneatto J., Guber S., Charo N.L., Susperreguy S., Schwartz J., Galigniana M.D. Dynamic mitochondrial-nuclear redistribution of the immunophilin FKBP51 is regulated by the PKA signaling pathway to control gene expression during adipocyte differentiation. Journal of Cell Science. 2013;126(23):5357–5368. doi: 10.1242/jcs.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stechschulte L.A., Qiu B., Warrier M., Hinds T.D., Zhang M., Gu H. FKBP51 null mice are resistant to diet-induced obesity and the PPARγ agonist rosiglitazone. Endocrinology. 2016;157(10):3888–3900. doi: 10.1210/en.2015-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Resmini E., Santos A., Aulinas A., Webb S.M., Vives-Gilabert Y., Cox O. Reduced DNA methylation of FKBP5 in Cushing's syndrome. Endocrine. 2016;54(3):768–777. doi: 10.1007/s12020-016-1083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hartmann I.B., Fries G.R., Bücker J., Scotton E., von Diemen L., Kauer-Sant’Anna M. The FKBP5 polymorphism rs1360780 is associated with lower weight loss after bariatric surgery: 26 months of follow-up. Surgery for Obesity and Related Diseases. 2016;12(8):1554–1560. doi: 10.1016/j.soard.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 76.Hartmann J., Wagner K.V., Liebl C., Scharf S.H., Wang X.D., Wolf M. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62(1):332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez E.R. Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2012;1823(3):722–729. doi: 10.1016/j.bbamcr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balsevich G., Uribe A., Wagner K.V., Hartmann J., Santarelli S., Labermaier C. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. Journal of Endocrinology. 2014;222(1):15–26. doi: 10.1530/JOE-14-0129. [DOI] [PubMed] [Google Scholar]
- 79.Hers I., Vincent E.E., Tavaré J.M. Akt signalling in health and disease. Cellular Signalling. 2011;23(10):1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Chen W.S., Xu P.Z., Gottlob K., Chen M.L., Sokol K., Shiyanova T. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes & Development. 2001;15(17):2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho H., Thorvaldsen J.L., Chu Q., Feng F., Birnbaum M.J. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. Journal of Biological Chemistry. 2001;276(42):38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 82.Garofalo R.S., Orena S.J., Rafidi K., Torchia A.J., Stock J.L., Hildebrant A.L. Sever diabetes, age-dependent loss of adipose tissue, and ild growth deficiency in mice lacking Akt2/PKBb. Journal of Clinical Investigation. 2003;112(2):197–208. doi: 10.1172/JCI16885. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho H., Mu J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 84.Yang Z.-Z., Tschopp O., Hemmings-Mieszczak M., Feng J., Brodbeck D., Perentes E. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. Journal of Biological Chemistry. 2003;278(34):32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 85.Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R., Reese C.B. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Current Biology: CB. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 86.Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P. Mechanism of activation of protein kinase B by insulin and IGF-1. The EMBO Journal. 1996;15(23):6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 87.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, N.Y.) 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 88.Andjelković M., Jakubowicz T., Cron P., Ming X.F., Han J.W., Hemmings B.A. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(12):5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao T., Furnari F., Newton A.C. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Molecular Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Brognard J., Sierecki E., Gao T., Newton A.C. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Molecular Cell. 2007;25(6):917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 91.Li L., Fridley B., Kalari K., Jenkins G., Batzler A., Safgren S. Gemcitabine and cytosine arabinoside cytotoxicity: association with lymphoblastoid cell expression. Cancer Research. 2008;68(17):7050–7058. doi: 10.1158/0008-5472.CAN-08-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl. 2):S157–S163. doi: 10.2337/dc09-S302. (1935-5548 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zierath J.R., Wallberg-Henriksson H. From receptor to effector: insulin signal transduction in skeletal muscle from type {II} diabetic patients. Annals of the New York Academy of Sciences. 2002;967 doi: 10.1111/j.1749-6632.2002.tb04270.x. (0077-8923 (Print)): 120–34. [DOI] [PubMed] [Google Scholar]
- 94.Leto D., Saltiel A.R. Regulation of glucose transport by insulin: traffic control of {GLUT}4. Nature Reviews Molecular Cell Biology. 2012;13 doi: 10.1038/nrm3351. (1471-0080 (Electronic)): 383–96. [DOI] [PubMed] [Google Scholar]
- 95.Sano H., Kane S., Sano E., Miinea C.P., Asara J.M., Lane W.S. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. Journal of Biological Chemistry. 2003;278 doi: 10.1074/jbc.C300063200. (0021-9258 (Print)): 14599–602. [DOI] [PubMed] [Google Scholar]
- 96.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.MacDougald O.A., Mandrup S. Adipogenesis: forces that tip the scales. Trends in Endocrinology and Metabolism. 2002;13(1):5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- 98.Pearson G., Robinson F., Gibson T.B., Xu B.E., Karandikar M., Berman K. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews. 2001 doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 99.Aouadi M., Laurent K., Prot M., Le Marchand-Brustel Y., Binétruy B., Bost F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes. 2006 doi: 10.2337/diabetes.55.02.06.db05-0963. [DOI] [PubMed] [Google Scholar]
- 100.Aouadi M., Jager J., Laurent K., Gonzalez T., Cormont M., Binétruy B. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Letters. 2007 doi: 10.1016/j.febslet.2007.10.064. [DOI] [PubMed] [Google Scholar]
- 101.Stechschulte L.A., Hinds T.D., Khuder S.S., Shou W., Najjar S.M., Sanchez E.R. FKBP51 controls cellular adipogenesis through p38 kinase-mediated phosphorylation of GRα and PPARγ. Molecular Endocrinology. 2014 doi: 10.1210/me.2014-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu E., Kim J.B., Sarraf P., Spiegelman B.M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996 doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 103.Yeh W.C., Li T.K., Bierer B.E., McKnight S.L., Smith D.F., Clardy J. Identification and characterization of an immunophilin expressed during the clonal expansion phase of adipocyte differentiation. Proceedings of the National Academy of Sciences. 1995;92(24):11081–11085. doi: 10.1073/pnas.92.24.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toneatto J., Charó N.L., Galigniana N.M., Piwien-Pilipuk G. Adipogenesis is under surveillance of Hsp90 and the high molecular weight immunophilin FKBP51. Adipocyte. 2015 doi: 10.1080/21623945.2015.1049401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stechschulte L.A., Hinds T.D., Ghanem S.S., Shou W., Najjar S.M., Sanchez E.R. FKBP51 reciprocally regulates GRα and PPARγ activation via the Akt-p38 pathway. Molecular Endocrinology. 2014 doi: 10.1210/me.2014-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inagaki T., Sakai J., Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nature Reviews Molecular Cell Biology. 2016;17(8):480–495. doi: 10.1038/nrm.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marzolla V., Armani A., Zennaro M.C., Cinti F., Mammi C., Fabbri A. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Molecular and Cellular Endocrinology. 2012;350(2):281–288. doi: 10.1016/j.mce.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 108.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008 doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh R. Autophagy in the control of food intake. Adipocyte. 2012;1(2):75–79. doi: 10.4161/adip.18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamamoto S., Kuramoto K., Wang N., Situ X., Priyadarshini M., Zhang W. Autophagy differentially regulates insulin production and insulin sensitivity. Cell Reports. 2018;23(11):3286–3299. doi: 10.1016/j.celrep.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y., Sowers J.R., Ren J. Targeting autophagy in obesity: from pathophysiology to management. Nature Reviews Endocrinology. 2018;14(6):356–376. doi: 10.1038/s41574-018-0009-1. [DOI] [PubMed] [Google Scholar]
- 113.Kaushik S., Rodriguez-Navarro J.A., Arias E., Kiffin R., Sahu S., Schwartz G.J. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metabolism. 2011;14(2):173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.An L., Zhang Y.Z., Yu N.J., Liu X.M., Zhao N., Yuan L. Role for serotonin in the antidepressant-like effect of a flavonoid extract of Xiaobuxin-Tang. Pharmacology Biochemistry and Behavior. 2008;89(4):572–580. doi: 10.1016/j.pbb.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 115.Romano S., D'Angelillo A., Pacelli R., Staibano S., De Luna E., Bisogni R. Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death & Differentiation. 2010;17(1):145–157. doi: 10.1038/cdd.2009.115. [DOI] [PubMed] [Google Scholar]
- 116.Marz A.M., Fabian A.-K., Kozany C., Bracher A., Hausch F. Large FK506-binding proteins shape the pharmacology of rapamycin. Molecular and Cellular Biology. 2013;33(7):1357–1367. doi: 10.1128/MCB.00678-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang R.C., Wei Y., An Z., Zou Z., Xiao G., Bhagat G. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science (New York, N.Y.) 2012;338(6109):956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laane E., Tamm K.P., Buentke E., Ito K., Khahariza P., Oscarsson J. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death & Differentiation. 2009;16(7):1018–1029. doi: 10.1038/cdd.2009.46. [DOI] [PubMed] [Google Scholar]
- 119.Mizushima N., Levine B., Cuervo A.M., Klionsky D.J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levine B., Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature Reviews Molecular Cell Biology. 2007 doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 122.Gassen N.C., Hartmann J., Schmidt M.V., Rein T. FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy. 2015;11(3):578–580. doi: 10.1080/15548627.2015.1017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bierer B.E., Mattila P.S., Standaert R.F., Herzenberg L.A., Burakoff S.J., Crabtree G. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blackburn E.A., Walkinshaw M.D. Targeting FKBP isoforms with small-molecule ligands. Current Opinion in Pharmacology. 2011;11(4):365–371. doi: 10.1016/j.coph.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 125.Dunyak B.M., Gestwicki J.E. Peptidyl-proline isomerases (PPIases): targets for natural products and natural product-inspired compounds. Journal of Medicinal Chemistry. 2016;59(21):9622–9644. doi: 10.1021/acs.jmedchem.6b00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schreiber S.L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science (New York, N.Y.) 1991;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 127.Bonner J.M., Boulianne G.L. Diverse structures, functions and uses of FK506 binding proteins. Cellular Signalling. 2017;38:97–105. doi: 10.1016/j.cellsig.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 128.Ratajczak T., Cluning C., Ward B.K. Steroid receptor-associated immunophilins: a gateway to steroid signalling. Clinical Biochemist Reviews. 2015;36(2):31–52. [PMC free article] [PubMed] [Google Scholar]
- 129.Feng X., Pomplun S., Hausch F. Recent progress in FKBP ligand development. Current Molecular Pharmacology. 2015;9(1):27–36. doi: 10.2174/1874467208666150519113313. [DOI] [PubMed] [Google Scholar]
- 130.Maiarù M., Morgan O.B., Mao T., Breitsamer M., Bamber H., Pöhlmann M. The stress regulator Fkbp51: a novel and promising druggable target for the treatment of persistent pain states across sexes. Pain. 2018 doi: 10.1097/j.pain.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maiarù M., Tochiki K.K., Cox M.B., Annan L.V., Bell C.G., Feng X. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Science Translational Medicine. 2016;8(325):325ra19. doi: 10.1126/scitranslmed.aab3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Criado-Marrero M., Rein T., Binder E.B., Porter J.T., Koren J., Blair L.J. Hsp90 and FKBP51: complex regulators of psychiatric diseases. Philosophical Transactions of the Royal Society B: Biological Sciences. 2018 doi: 10.1098/rstb.2016.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davies T.H., Ning Y.M., Sánchez E.R. A new first step in activation of steroid receptors. Hormone-induced switching of FKBP51 and FKBP52 immunophilins. Journal of Biological Chemistry. 2002 doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 134.Hinds T.D., Stechschulte L.A., Elkhairi F., Sanchez E.R. Analysis of FK506, timcodar (VX-853) and FKBP51 and FKBP52 chaperones in control of glucocorticoid receptor activity and phosphorylation. Pharmacology Research and Perspectives. 2014 doi: 10.1002/prp2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]