Abstract
The data article refers to the paper “Nutritional, physico-chemical and functional characterization of a global chickpea collection” [1]. The data are referred to a germplasm collection of 57 chickpea accessions from the ex situ repositories of the United States Department of Agriculture (USDA), the Department of Plant, Soil and Food Science of the University of Bari, Italy (DiSSPA), and the Institute of Biosciences and Bioresources of the Italian National Research Council (CNR-IBBR). Thirty-six accessions, belonging to desi and kabuli types, were representative of the geographic distribution of chickpea global cultivation, whereas twenty-one accessions, referable to the Apulian black type, derived from different area of the Apulian region, south of Italy. All the accessions were grown at the experimental farm “P. Martucci” of the University of Bari “Aldo Moro” (41°01′22.1″ N 16°54′21.0″ E) during the growing season 2017–2018, according to a randomized block design with two replicates, each replicate formed by 30 individual plants. This article reports the data of the proximate composition, the total bioactive compounds content, the fatty acid composition and the physico-chemical and functional properties of chickpea flour. Information provided in this article can be used by food industry to develop chickpea-based foods and by geneticists for studies of association mapping aimed at the identification of genomic regions controlling the nutritional and technological traits.
Keywords: Chickpea collection, Chickpea flour, Nutritional composition, Functional properties, Bioactive compounds, Germplasm collection
Specifications Table
| Subject | Agricultural and Biological Sciences |
| Specific subject area | Food Science |
| Type of data | Data, excel files, image |
| How data were acquired | Proximate composition: Official Methods of analysis [2] for protein (total nitrogen × 5.7), moisture and ashes content; Enzymatic-gravimetric method [2] for total dietary fibre content; Solid-liquid extraction by Soxhlet apparatus for lipid content. Fatty Acid composition: Solid-liquid extraction of the lipid fraction, and gas-chromatographic analysis of the fatty acids methyl ester (7890A gas-chromatograph coupled with FID and OpenLAB CDS software C.01.07 - Agilent Technologies, Santa Clara, CA, USA). Bioactive compounds: Solid-liquid extraction followed by spectrophotometric analysis (Cary 60 UV–Vis spectrophotometer -Agilent Technologies, Santa Clara, CA, USA). Antioxidant activity: Solid-liquid extraction with aqueous methanol (20:80 v:v) followed by DPPH assay and spectrophotometric analysis (Cary 60 UV–Vis spectrophotometer - Agilent Technologies, Santa Clara, CA, USA). Physico-chemical and functional properties of the flours: water or oil incorporation followed by mixing and centrifugation (Thermo Fisher Scientific SL16R – Waltham, MA, USA) |
| Data format | Image; raw data; analysed data |
| Parameters for data collection | Fifty-seven accessions of chickpea were grown in the experimental farm “P. Martucci” of the University of Bari (41°01′22.1″ N 16°54′21.0″ E) during the season 2017–2018, with a randomized block design with two replicates, each replicate formed by 30 individual plants. Chickpea seeds were cleaned and milled to obtain a whole meal flour. All determinations were carried out in triplicate with a CV lower than 5%. |
| Description of data collection | The proximate composition of 57 chickpea accessions has been determined, considering the protein, lipid, ashes, total dietary fibre and carbohydrates content, the main fatty acids content, and the bioactive compounds (total phenolic compounds, total anthocyanins, and total carotenoids). The total phytate contents and the antioxidant activities (by means the DPPH assay) were also determined. The physico-chemical (Bulk Density; Water Absorption Index; Water Solubility Index) and functional (Water Absorption Capacity; Oil Absorption Capacity) parameters were also determined. |
| Data source location | Department of Soil, Plant and Food Science (DISSPA), University of Bari Aldo Moro, Italy. |
| Data accessibility | Data is provided with this article |
| Related research article | C. Summo, D. De Angelis, L. Ricciardi, F. Caponio, C. Lotti, S. Pavan, A. Pasqualone, Nutritional, physico-chemical and functional characterization of a global chickpea collection. J. Food Compos. Anal. 84 (2019). https://doi.org/10.1016/j.jfca.2019.103306 |
Value of the Data
|
1. Data
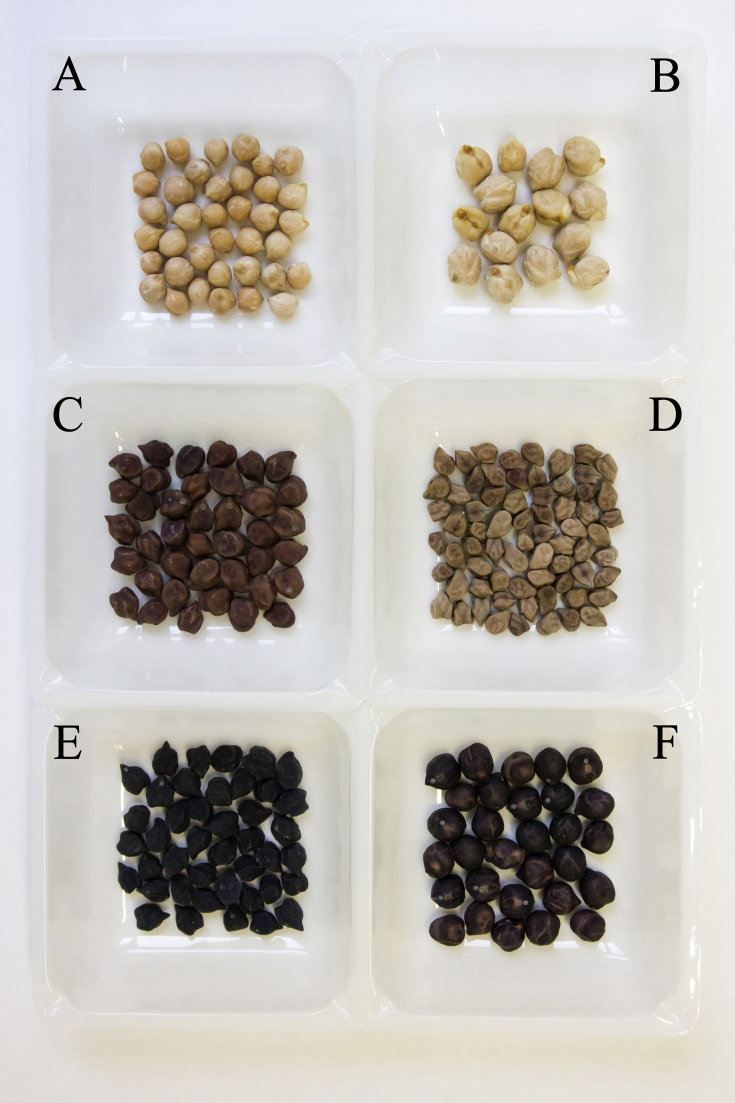
This Data in Brief article provides two figures and the raw data (as supplementary material) regarding the analysis on the chemical composition (S1), bioactive compounds (S2), fatty acid composition (S3), the physicochemical and functional properties (S4) of 57 chickpea accessions. These data are comprehensively discussed in the reference article [1]. The chickpea collection was representative of the geographic distribution of chickpea cultivation in the world. The accessions under investigation were characterized by high phenotypical variability (Fig. 1).
Fig. 1.
Shows the seed phenotype variability of the chickpea accessions investigated. A: small and beige chickpeas (PI343014); B: large and beige chickpeas (PI533681); C: large and brown chickpeas (PI358914); D: small and brown chickpeas (PI359002); E: large and black chickpeas (MG_5); F: large, smooth and black chickpeas (MG_9).
2. Experimental design, materials, and methods
2.1. Plant material and sample preparation
The germplasm collection examined included 57 different chickpea accessions from the ex situ repositories of the United States Department of Agriculture (USDA), the Department of Plant, Soil and Food Science of the University of Bari, Italy (DiSSPA), and the Institute of Biosciences and Bioresources of the Italian National Research Council (CNR-IBBR). All the plants were grown at the experimental farm “P. Martucci” of the University of Bari “Aldo Moro” (41°01′22.1″ N 16°54′21.0″ E) during the growing season 2017–2018, according with a randomized block design with two replicates, each replicate formed by 30 individual plants. In Fig. 1 is shown the variability of seed phenotype of some of the chickpea accessions investigated.
After harvesting at crop maturity, samples were cleaned to remove broken seeds, dust and other undesirable matter. In order to obtain whole meal flour with uniform particle size, the seeds were ground by an electric mill (Model ETA, Vercella Giuseppe, Mercenasco, Italy) equipped with a sieve of 0.6 mm.
2.2. Determination of proximate composition
The protein content (total nitrogen × 5.7), ashes, and moisture content were determined according to the AOAC methods 979.09, 923.03 and 925.10, respectively [2]. The determination of total dietary fiber was carried out by the enzymatic-gravimetric procedure as reported in the AOAC method 991.43 [2]. The lipid content was determined by means of a Soxhlet apparatus using diethyl ether (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) as extracting solvent, as described in AOAC Official Method 945.38F [2]. The carbohydrate content was determined as difference.
2.3. Determination of fatty acid composition
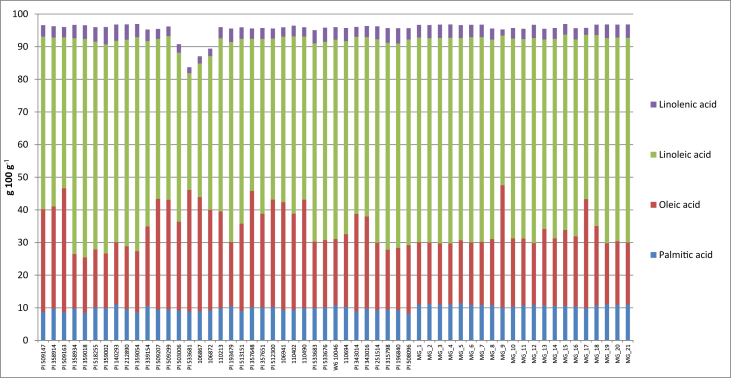
The fatty acid composition, reported in Fig. 2, was determined by gas-chromatographic (GC) analysis of fatty acid methyl esters. Fatty acid preparation and the GC system and conditions were the same as those reported by Summo et al. [3]. The gas-chromatographic system used consisted of a 7890A gas-chromatograph (Agilent Technologies, Salta Clara, CA USA) equipped with a detector FID and an SP2340 fused silica capillary column 60 m × 0.25 mm × 0.2 μm film thickness (Supelco Park, Bellefonte, PA, USA). The temperature of the split injector was 230 °C, with a splitting ratio of 1:50; the detector temperature was 290 °C. The oven temperature was programmed from 60 to 180 °C, with increments of 5 °C min−1, then to 240 °C with increments of 3 °C min−1 and final isothermal of 20 min. Helium was utilized as carrier gas at a constant flow rate of 3 mL min−1. The identification of each fatty acid was carried out by comparing the retention time with that of the corresponding methyl ester standard (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). The results were expressed as percentage.
Fig. 2.
Shows the main fatty acids (expressed as g 100 g−1 of total fatty acids) of the lipid fraction of the 57 chickpea accessions.
2.4. Determination of bioactive compounds
The total carotenoid content (expressed as mg kg −1 of β-carotene on dry matter) was determined using the method reported by Pasqualone et al. [4]. One g of legume flour was added of 5 mL of water-saturated n-butyl alcohol and mixed for 3 h at 260 rpm. Samples were centrifuged for 7 min at 2400×g, and the absorbance of water-saturated n-butyl alcohol extracts was measured at 435.8 nm by a Cary 60 UV–Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA).
The total anthocyanin content was determined using the method reported by Pasqualone et al. [5] with slight modifications. One g of sample was extracted with 10 mL of 85:15 (v/v) methanol/1 M HCl, then stirred for 30 minutes in the dark. The pellet was re-extracted with 10 mL of the solvent at the same conditions and, after centrifugation at 12,000×g for 5 min, the absorbance of the two extracts was determined at 535 nm by a Cary 60 UV–Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Cyanidin 3-O-glucoside standard (Phytoplan, Heidelberg, Germany) was used to prepare the calibration curve in order to express total anthocyanins as mg kg−1 of cyanidin 3-O-glucoside on dry matter.
The total phenolic compound (TPC) content and antioxidant activity (AA) were determined on an aqueous-methanol extract (20:80 v/v) prepared as follows: 1 g of flour was extracted with 10 mL of solvent in a centrifuge tube and stirred for 2h in the dark. Then, the tube was centrifuged at 12,000×g for 3 minutes to recover the supernatant. The determinations were carried out following the procedures proposed by Pasqualone et al. [5]. The total phenolic compounds content was expressed as mg g−1 of ferulic acid on dry matter, considering a calibration curve prepared with ferulic acid at different concentrations. The AA was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity assay and expressed as μmol (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) equivalent g−1 on dry matter.
Total phytate content was measured according to the method reported by Joshi-Saha and Reddy [6] with slight modifications. 0.5 g of chickpea flour was mixed with 10 mL of 2.4% HCl in a centrifuge tube and stirred for 1h. Then, the sample was centrifuged at 10,000×g and 10 °C for 20 minutes. The supernatant was recovered in a centrifuge tube containing 0.5 g of NaCl and stirred for a minute. Then the tube was kept at −20 °C for 20 minutes and the clear supernatant was recovered after centrifugation at 10,000×g at 10 °C for 20 minutes. The extract was diluted 25 times and 1.5 mL of the extract was mixed with 0.5 mL of Wade's reagent (0.03% FeCl3∙6H2O + 0.3% sulfosalicylic acid). The mixture was centrifuged at 6000×g and 10 °C, and the absorbance was then read at 500 nm using a Cary 60 UV–Vis spectrophotometer (Agilent Technologies Inc., Santa Clara, CA, USA). A calibration curve was prepared by different concentrations of phytic acid (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and the results were multiplied by 0.282 (molar ratio of phytate - phosphorus in a molecule of phytate) in order to express the phytate content in the sample as mg g−1 of phytic acid on dry matter [7].
2.5. Physico-chemical and functional properties of flours
Bulk density (BD), water absorption index (WAI), water solubility index (WSI), water absorption capacity (WAC) and oil absorption capacity (OAC) of flours were determined according to the procedures reported by Du et al. [8]. BD (expressed as g mL−1) was determined by weighing the flour into 10 mL pre-weighed cylinder. The cylinder was gently tapped on the laboratory bench until no further reduction of the sample volume was observed and the final volume was registered.
WAI and WSI of chickpea flour were determined by mixing in a pre-weighed centrifuge tubes 1.75 g of flour and 15 mL of distilled water. The mixture was heated at 70 °C for 30 minutes and then centrifuged at 3000×g for 20 minutes. The supernatant was transferred into a pre-weighed porcelain evaporating dish and dried overnight at 105 °C in order to calculate the solid content of the supernatant. Instead, the centrifuge tube with the sediment was weighed. WAI and WSI were calculated as follow:
WAI (g/g) = Weight of sediment in the centrifuge tube/Weight of flour sample.
WSI (%) = Weight of dissolved solids in supernatant × 100/Weight of flour sample.
For WAC 1.20 g of sample were mixed with10 mL of distilled water in pre-weighed centrifuge tubes. Then the mixture was stirred for 30 seconds at 5 min intervals for 30 min. The tubes were centrifuged at 3000×g for 20 minutes. The supernatant was separated and the residual water in the tube was removed by draining for 25 minutes at 50 °C. Finally, the sample was weighed. For OAC 0.75g of chickpea flour were mixed with 9 mL of peanut oil in pre-weighed centrifuge tubes. The mixture was stirred for 1 minute and after a pause of 30 minutes, the tubes were centrifuged at 3000×g for 20 minutes. The supernatant was separated, and the excess of oil was removed by draining, tilting the tubes for 25 minutes. WAC and OAC were expressed as gram of water or oil bound per gram of flour.
Acknowledgments
This research has been performed within the project “LEgume GEnetic REsources as a tool for the development of innovative and sustainable food TEchnological system” supported under the “Thought for Food” Initiative by Agropolis Fondation (through the “Investissements d'avenir” programme with reference number ANR-10-LABX-0001-01”), Fondazione Cariplo, and Daniel & Nina Carasso Foundation. We also acknowledge the farm “Iannone Anna” for technical support in field experiments and for providing germplasm of Apulian Black chickpea.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104612.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary material S1 reports the proximate composition in terms of protein, lipid, ashes, total dietary fibre and carbohydrates (expressed as g 100 g−1 on dry matter) of the 57 chickpea accessions.
Supplementary material S2 reports, for the 57 accessions of chickpeas, the phytate content, (mg phytic acid g−1 d.m.), total phenolic compounds, (mg ferulic acid g−1 d.m.), total carotenoids, (mg β-carotene kg−1d.m.), total anthocyanins (mg cyanidin 3-O-glucoside kg−1 d.m.) and antioxidant activity (μmol Trolox eq g−1 d.m.).
Supplementary material S3 reports the fatty acid composition (expressed as g 100 g−1 of total fatty acids) of the lipid fraction of the 57 chickpea accessions. The trend of the main fatty acids is reported in Fig. 2
Supplementary material S4 reports the physico-chemical (Bulk Density; Water Absorption Index; Water Solubility Index) and functional (Water Absorption Capacity; Oil Absorption Capacity) properties of the whole flours of the 57 chickpeas accessions
References
- 1.Summo C., De Angelis D., Ricciardi L., Caponio F., Lotti C., Pavan S., Pasqualone A. Nutritional, physico-chemical and functional characterization of a global chickpea collection. J. Food Compos. Anal. 2019;84:103306. doi: 10.1016/j.dib.2019.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AOAC International . seventeenth ed. Association of Analytical Communities; Gaithersburg, MD: 2006. Official Methods of Analysis. [Google Scholar]
- 3.Summo C., Palasciano M., De Angelis D., Paradiso V.M., Caponio F., Pasqualone A. Evaluation of the chemical and nutritional characteristics of almonds (Prunus dulcis (Mill). D.A. Webb) as influenced by harvest time and cultivar. J. Sci. Food Agric. 2018;98:5647–5655. doi: 10.1002/jsfa.9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasqualone A., Bianco A.M., Paradiso V.M. Production trials to improve the nutritional quality of biscuits and to enrich them with natural anthocyanins. CyTA - J. Food. 2013;11:301–308. [Google Scholar]
- 5.Pasqualone A., Bianco A.M., Paradiso V.M., Summo C., Gambacorta G., Caponio F., Blanco A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015;180:64–70. doi: 10.1016/j.foodchem.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Joshi-Saha A., Reddy K.S. Repeat length variation in the 5ʹUTR of myo-inositol monophosphatase gene is related to phytic acid content and contributes to drought tolerance in chickpea (Cicer arietinum L.) J. Exp. Bot. 2015;66:5683–5690. doi: 10.1093/jxb/erv156. [DOI] [PubMed] [Google Scholar]
- 7.Naves L.D.P., Rodrigues P.B., Bertechini A.G., Corrêa A.D., de Oliveira D.H., de Oliveira E.C., Duarte W.F., da Cunha M.R.R. Comparison of methodologies to quantify phytate phosphorus in diets containing phytase and excreta from broilers. Asian Austral. J. Anim. Sci. 2014;27:1003–1012. doi: 10.5713/ajas.2013.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du S.K., Jiang H., Yu X., Jane J.L. Physicochemical and functional properties of whole legume flour. LWT-Food Sci. Technol. 2014;55:308–313. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material S1 reports the proximate composition in terms of protein, lipid, ashes, total dietary fibre and carbohydrates (expressed as g 100 g−1 on dry matter) of the 57 chickpea accessions.
Supplementary material S2 reports, for the 57 accessions of chickpeas, the phytate content, (mg phytic acid g−1 d.m.), total phenolic compounds, (mg ferulic acid g−1 d.m.), total carotenoids, (mg β-carotene kg−1d.m.), total anthocyanins (mg cyanidin 3-O-glucoside kg−1 d.m.) and antioxidant activity (μmol Trolox eq g−1 d.m.).
Supplementary material S3 reports the fatty acid composition (expressed as g 100 g−1 of total fatty acids) of the lipid fraction of the 57 chickpea accessions. The trend of the main fatty acids is reported in Fig. 2
Supplementary material S4 reports the physico-chemical (Bulk Density; Water Absorption Index; Water Solubility Index) and functional (Water Absorption Capacity; Oil Absorption Capacity) properties of the whole flours of the 57 chickpeas accessions