Abstract

A series of dihydropyrrol-2-ones (DHPs) were designed and synthesized via an efficient multicomponent reaction at room temperature for evaluation of their bioactivities against four human cancer lines (MCF-7, RKO, HeLa, and A549) in vitro. Preliminary structure–activity relationship studies showed that R4 = 3-MeO-4-OH-Ph is a crucial group for increasing cytotoxicities against RKO cells and the influences of R1–R3 depend on their combination. It was found that DHPs 5a, 5q, and 5s showed the best antiproliferative activities against A549, RKO, and all four studied cell lines, respectively (IC50 = 1.9, 0.8, and 0.9–2.4 μM). They can be used as new lead compounds for developing potentially selective or broad spectrum anticancer agents. 5q proves as a potent G0/G1-phase arresting agent inducing cell apoptosis by increasing/decreasing the levels of p53 and p21/cyclin D1.
Introduction
Cancer, the second leading cause of global death, was responsible for 8.8 million deaths in 2015, amounting to nearly 1 in 6 of all deaths in the world.1 Therefore, many different fields have devoted much effort to exploring anticancer therapeutics. In cell cycles, there are two important transitions, the transitions of the G1 to S phase and G2 to M phase. These transitions are very important for regulating cellular multiplication processes and cell apoptosis, and are easily affected by environmental conditions. The tumorigenesis of many cancers is closely related to the improper signaling of cell cycle regulators. G0/G1-phase arresting agents can arrest the cellular proliferating cycle progressing from the G0/G1 to S phase. Palbociclib (I, Figure 1),2 first approved as a CDK4/6 inhibitor for breast cancer in 2015, arrests tumorigenesis by interfering with S and M phases of the cell cycle and withdraws the cell cycle back into the G0/G1-phase. Two other CDK4/6 inhibitors, abemaciclib (Lilly) (II)3 and ribociclib (III),4 are approved for the treatment of multiple types of cancers. Compound IV exhibits potent activity against breast cancer cells, arresting cells in the G0/G1-phase and decreasing the cellular levels of cyclin D1.5 G0/G1-phase arresting agents V(6) and VI(7) show good anticancer activity against SH-SY5Y cells and HCT-116 colon cancer cells, respectively.
Figure 1.
Known G0/G1-phase arresting agents.
Multicomponent reaction (MCR) has advantages of atom economy, high efficiency, and fast building structural diversity and complexity of compound libraries8−10 and becomes a powerful tool in drug synthesis and discovery.9,11,12 Considering that heterocycles have diverse bioactivities, we are interested in their MCR synthesis13−17 and bioactivities.18 Pyrrolidone rings are important heterocycles with a wide range of pharmacological activities, such as G0/G1-phase arresting agents VI. In this work, we report novel G0/G1-phase arresting agents pentasubstituted dihydropyrrol-2-ones (DHPs) (Table 1), the products of a convenient MCR that we developed.13
Table 1. Antiproliferative Activities of DHPs 5a–5ta.
| IC50 (μM)b |
||||||||
|---|---|---|---|---|---|---|---|---|
| DHP | R1 | R2 | R3 | R4 | MCF-7 | RKO | HeLa | A549 |
| 5a | Et | Ph | Ph | 4-ClPh | 31.2 ± 2.9 | 21.2 ± 7.3 | 30.6 ± 2.6 | 1.9 ± 1.2 |
| 5b | Et | Ph | Ph | 4-MePh | 32.9 ± 0.9 | 3.0 ± 0.2 | 2.4 ± 0.6 | 7.0 ± 2.4 |
| 5c | Et | Ph | Ph | 4-CF3Ph | 3.1 ± 0.05 | 3.1 ± 0.1 | 4.6 ± 1.7 | 2.7 ± 0.8 |
| 5d | Et | Ph | Ph | 4-OHPh | 98.7 ± 24.3 | 91.5 ± 16.2 | >100 | >100 |
| 5e | Et | Ph | Ph | 3-MeOPh | >100 | >100 | >100 | >100 |
| 5f | Et | Ph | Ph | 4-MeOPh | 25.6 ± 6.0 | 34.6 ± 1.6 | 12.2 ± 1.9 | 8.4 ± 1.2 |
| 5g | Et | Ph | Ph | 3-OH-4-MeOPh | 86.8 ± 30.5 | 60.7 ± 12.9 | >100 | >100 |
| 5h | Et | Ph | Ph | 3-MeO-4-OHPh | 18.9 ± 1.5 | 2.4 ± 0.2 | 14.1 ± 2.9 | 25.0 ± 3.7 |
| 5i | Et | 4-ClPh | 4-ClPh | 3-MeO-4-OHPh | 35.0 ± 4.9 | 6.6 ± 1.3 | 83.0 ± 10.2 | 34.4 ± 3.8 |
| 5j | Et | 4-ClPh | 3-ClPh | 3-MeO-4-OHPh | 40.8 ± 8.3 | 5.9 ± 1.1 | 90.5 ± 13.5 | 34.3 ± 2.3 |
| 5k | Et | 3-ClPh | 3-ClPh | 3-MeO-4-OHPh | 20.3 ± 5.4 | 1.3 ± 0.1 | 85.6 ± 19.6 | 24.5 ± 1.4 |
| 5l | Et | 4-BrPh | 4-BrPh | 3-MeO-4-OHPh | 37.9 ± 4.4 | 4.9 ± 1.2 | 66.2 ± 9.0 | 30.8 ± 5.6 |
| 5m | Et | 4-BrPh | 3-ClPh | 3-MeO-4-OHPh | 7.6 ± 2.5 | 1.3 ± 0.1 | 14.7 ± 5.9 | 4.4 ± 0.3 |
| 5n | Et | 3-CF3Ph | 3-CF3Ph | 3-MeO-4-OHPh | 34.7 ± 8.6 | 12.8 ± 1.3 | 68.8 ± 10.6 | 43.9 ± 5.0 |
| 5oc | Et | n-butyl | n-butyl | 3-MeO-4-OHPh | 32.7 ± 7.1 | 24.8 ± 4.0 | 32.4 ± 4.2 | >100 |
| 5pc | Et | cyclohexyl | cyclohexyl | 3-MeO-4-OHPh | 12.9 ± 5.3 | 15.3 ± 1.0 | 16.7 ± 2.9 | 18.9 ± 1.2 |
| 5q | Et | Ph | 3-ClPh | 3-MeO-4-OHPh | 8.7 ± 1.7 | 0.8 ± 0.1 | 10.6 ± 2.3 | 5.6 ± 1.7 |
| 5r | Me | 4-ClPh | 4-ClPh | 3-MeO-4-OHPh | 3.8 ± 1.3 | 0.9 ± 0.05 | 1.2 ± 0.1 | 2.6 ± 0.3 |
| 5s | Me | 4-BrPh | 4-BrPh | 3-MeO-4-OHPh | 1.0 ± 0.3 | 0.9 ± 0.02 | 1.0 ± 0.04 | 2.4 ± 0.6 |
| 5t | Me | 3-CF3Ph | 3-CF3Ph | 3-MeO-4-OHPh | 2.4 ± 0.6 | 1.4 ± 0.2 | 2.1 ± 0.7 | 3.9 ± 0.6 |
| 5-Fu | 24.4 ± 3.9 | 3.0 ± 0.2 | 14.5 ± 3.0 | 14.7 ± 5.3 | ||||
DHPs were synthesized by the MCR that we developed,13 and all are new compounds except 5a.
Antiproliferative activity (half maximal inhibitory concentration (IC50)) determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay after 72 h of incubation. Data expressed as mean ± standard deviation from at least three independent experiments.
Synthesized as other DHPs except using ethanol as solvent and under 70 °C.
Results and Discussion
In the experimental screening of the bioactivities against MCF-7 (human breast cancer), RKO (human colon cancer), HeLa (human cervical cancer), and A549 (human lung cancer) cell lines of small organic compounds, it was found that DHP 5a has good activity against the four cell lines, especially A549. Then, 19 DHPs (5b–5t) were designed and synthesized to further study their bioactivities using 5-fluorouracil (5-Fu) as a positive control. Their antiproliferative activities were expressed as IC50 values and are summarized in Table 1. As shown in Table 1, most of the DHPs exhibited moderate to satisfactory antiproliferative activities against the four human cancer cell lines. Among these DHPs, 5a displayed higher cytotoxicities against A549 cells (IC50 = 1.9 μM) than the other three human cancer cells, with the IC50 value being 7.7-fold higher than that of 5-Fu (IC50 =14.7 μM). DHP 5b showed higher cytotoxicities against RKO, HeLa, and A549 cells than MCF-7, with IC50 values one to 6-fold higher than that of 5-Fu. Moreover, DHP 5q demonstrated the best antiproliferative activity (IC50 = 0.8 μM) against the RKO cell line. DHPs 5c and 5r–5t showed satisfactory antiproliferative activities against all of the tested cancer cells.
Based on the above primary antiproliferative activities, the structure–activity relationship of 5 was discussed. As for R4, 4-CF3Ph is in favor of the antiproliferative activities against the four cell lines (2.7–4.6 μM) than other groups (comparing 5c and 5a–5h except 5c). Very interestingly, the combination of 3-MeO and 4-OH functional groups at phenyl shows excellent selectivity to the RKO cell line (5h, 2.4 μM). However, 4-OH (5d, 91.5 μM), 3-MeO (5e, >100 μM), 4-MeO (5f, 34.6 μM), and the combination of 4-MeO and 3-OH (5g, 60.7 μM) show much lower or almost no activities to the RKO cell line. Therefore, using R4 = 3-MeO-4-OHPh and R1 = Et, the influences of R2 and R3 on the selectivity were investigated (5i–5q). It can be seen that except 5o and 5p with alkyl R2 and R3, others with aryl R2 and R3 show much higher activity against the RKO cell line than the other three cell lines, with the combination of R2 = Ph and R3 = 3-ClPh optimal (5q) against RKO cells. Unexpectedly, only changing R1 from Et (5i, 5l, and 5n) to Me (5r–5t) leads to a significant decrease of the selectivity with the increase of the activities against all cell lines. This means that R1 is also a very important group. The obtained experimental results indicate that R4 = 3-MeO-4-OHPh is crucial for the antiproliferative activities against RKO cells and the influences of R1–R3 depend on their combinations.
To understand the primary mechanism of action, the most potent DHP 5q against RKO cells was used to investigate the effects on the cell cycle distribution and apoptosis in RKO cells via flow cytometry. As shown in Figure 2A, DHP 5q arrested the RKO cells at the G0/G1 phase in a dose-dependent manner and induced the increase of RKO cells at the G0/G1 phase from 31.33% (the blank control group) to 59.81% (5 μM). Concurrently, a reduction of RKO cells at G2/M and S phases was observed. The apoptotic effect of DHP 5q was investigated by Annexin V-FITC/propidium iodide analyses, using dimethyl sulfoxide (DMSO) as the control. As shown in Figure 2B, DHP 5q induced 30% apoptosis at higher concentrations.
Figure 2.
(A) Effects of 5q on cell cycle distribution in RKO cells. DMSO (0.1%) was used as the control. (B) Annexin V-FITC/propidium iodide analyses on the apoptosis of RKO cells 48 h after co-culturing with compound 5q. The four quadrants were identified as follows: viable (Q4), early apoptotic (Q3), late apoptotic (Q2), and necrosis (Q1). DMSO (0.1%) was used as the control.
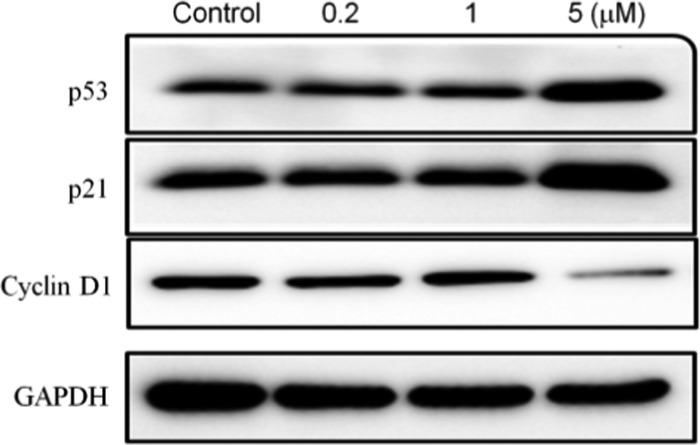
It is well known that D-type cyclins (cyclins D1, D2, and D3) are involved in the regulation of apoptosis and cell cycle progression from the G0/G1-phase into the S phase.19 Overexpression of Cyclin D1 has been reported to be associated with shorter survival in patients, and its high expression generally increases migration and invasion of tumors.20 The tumor suppressor, p53 protein, could induce tumor apoptosis and cell cycle arrest.21 p21 is considered as an independent prognostic parameter for colorectal cancer, and its overexpression is positively correlated with the survival time of patients, which may be related to the inhibition of tumor cell proliferation by blocking the cell cycle in the G1 phase.22 Therefore, the effect of DHP 5q on p53, p21, and cyclin D1 was investigated to explore the mechanism of 5q that induced the G0/G1-phase arrest. As shown in Figure 3, the expression of p53 and p21 proteins was increased by the presence of DHP 5q (5 μM), while the expression of cyclin D1 was inhibited (5 μM), which is consistent with the results of the cell cycle and apoptosis assay.
Figure 3.
Effect on the expression of cell cycle protein (p53, p21, and cyclin D1) in RKO cells after treatment with compound 5q for 48 h.
Conclusions
Twenty DHPs 5a–5t (all are new compounds except 5a) were designed and synthesized for their bioactivity evaluation against four human cancer cell lines (MCF-7, RKO, HeLa, and A549). It was found that R4 = 3-MeO-4-OH-Ph is a crucial group for increasing cytotoxicities against RKO cells and the influences of R1–R3 depend on their combination, with the most potent DHP 5q against RKO cells (IC50 = 0.8 μM) and three potent DHPs (5r–5t) against four studied cancer cell lines (IC50 = 0.9–3.9 μM) containing 3-MeO-4-OH-Ph as the R4 group. Interestingly, the study on the primary mechanism of 5q against RKO cells proves 5q as a G0/G1-phase arresting agent inducing cell apoptosis by decreasing the levels of cyclin D1 but increasing the levels of p53 and p21 in RKO cells. The advantages of an efficient synthetic method, simple molecular structure, and potential antiproliferative activity against multiple cancer cell lines are expected to make the most potent anticancer DHPs 5a (1.9 μM), 5q (0.8 μM), and 5s (0.9–2.4 μM) against A549, RKO, and all studied four cell lines, respectively, attractive lead compounds for developing potential selective or broad spectrum anticancer agents.
Experimental Section
General Procedures for the Synthesis of Pentasubstituted 2,5-Dihydro-1H-pyrrol-2-ones 5a–5t
Pentasubstituted 2,5-dihydro-1H-pyrrol-2-ones 5a–5t were synthesized as reported in our previous work.13 The reactions were run with the following steps: (a) but-2-ynedioates (1 mmol) and amines (R2NH2, 1 mmol) were added into test tube A with 3 mL of MeOH and kept at room temperature for 30 min; (b) amines (R3NH2, 1.6 mmol), aldehydes (3.5 mmol), salicylic acid (0.3 mmol), and Cu(OAc)2·H2O (0.4 mmol) were added into test tube B with 3 mL of MeOH, and then the mixture in tube B was added into the above mixture in tube A and stirred at room temperature for desired time. After completion of the reactions, the product mixture was purified by preparative thin layer chromatography with petroleum ether/ethyl acetate (15:1–3:1) as the eluent to afford the desired products. It is worth mentioning that the procedures for the synthesis of 5o and 5p are the same as those mentioned above except EtOH and 70 °C, instead of MeOH and room temperature, were used as solvent and temperature, respectively.
Cell Culture
The human breast cancer MCF-7, colon cancer RKO, cervical cancer HeLa, and nonsmall cell lung cancer A549 were obtained from American Type Culture Collection (ATCC). All cells were maintained in RPMI-1640 medium (Gibco) with 10% fetal bovine serum (Capricorn Scientific GmbH, Germany) at 37 °C in a humidified incubator with 5% CO2.
Cell Proliferation Assay
MTT assay is a quantitative colorimetric method for the determination of cell survival and proliferation.23,24 The assessed parameter is the metabolic activity of viable cells. Metabolically active cells reduce pale yellow tetrazolium salt (MTT) (Sigma) to a dark blue water-insoluble formazan, which can be directly quantified after solubilization with DMSO. The absorbance of the formazan directly correlates with the number of viable cells. Cells were plated at a density of 2 × 103 cells/well in 96-well plates. On the following day, the cells were incubated with tested compounds for at least three cell doublings. At the end of the treatment period, 10 μL of MTT solution (5 mg/mL) was added and incubated for another 4 h. After the supernatant was carefully removed, 150 μL of DMSO was added for solubilization for about 10 min and the absorbance at 570 nm was measured using a microplate spectrophotometer (Benchmark Plus, Bio-Rad Laboratories, CA).25 5-Fluorouracil was used as a reference drug and tested under similar conditions. The inhibition percentage was calculated using the formula: Inhibition% = [(Ac – As)/(Ac – Ab)] × 100%. (As: absorbance of experimental wells containing cells, MTT, and compounds; Ac: control wells containing cells and MTT but no compound; Ab: blank wells containing only MTT). The IC50 values were calculated by use of the PRISM statistical package (GraphPad Software, San Diego, CA).25
Cell Cycle Analysis
Flow cytometry analysis was performed as a reported method.26 Generally, after 24 h incubation, the cells were treated with compound 5q for different concentrations, respectively. Following treatment, the cells were harvested, washed with cold phosphate-buffered saline (PBS) twice, and fixed in 70% ethanol at −20 °C for appropriate time. The cells were then washed twice with cold PBS and incubated with RNAase and propidium iodide (50 μg/mL) for 30 min at 37 °C in the dark. The percentages of cell cycle distribution were analyzed on a BD LSRFortessa Flow Cytometer (BD Accuri Cytometers Inc.; Ann Arbor, MI).
Western Blot Analysis
Protein concentrations of cell lysates were determined using the BCA Protein Assay Kit (Thermo Fisher). Equal amounts of protein (30 μg) from each cell lysate were resolved on Criterion TGX precast gels and transferred onto 0.45 μm nitrocellulose membranes (Bio-Rad). Membranes were blocked and incubated overnight at 4 °C with primary antibodies: anti-p53 (#2527S, Cell Signaling), anti-p21 (#2947S, Cell Signaling), anti-Cyclin D1 (#2978S, Cell Signaling), and anti-GAPDH (#5174, Cell Signaling). After TBST washes (1× Tris-buffered saline, 0.1% Tween-20), the membranes were incubated with the corresponding horseradish-peroxidase-conjugated secondary antibodies (Bio-Rad) for 1 h at room temperature. Proteins were detected with an enhanced chemiluminescence method (SuperSignal West Pico substrate; Pierce; Rockford, IL).
Glossary
Abbreviations
- DHPs
dihydropyrroles
- MCRs
multicomponent reactions
- 5-Fu
5-fluorouracil
- IC50
half maximal inhibitory concentration
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02543.
Materials and general methods, the structural characteristics of pentasubstituted 2,5-dihydro-1H-pyrrol-2-ones 5a–5t; NMR spectra of DHPs 5a–5t (PDF)
Author Contributions
† D.Y., C.H., and H.L. contributed equally.
This work was supported by the Science and Technology Program of Guangdong Province (2015A010105015 and 2016A020217008), National Natural Science Foundation of China (21272111 and 81872735), Guangdong Natural Science Foundation (no. 2018A030313046), Pearl River S&T Nova Program of Guangzhou (201506010064), Science Foundation for Excellent Youth Scholars of Guangdong Province (2014018), Guangdong Distinguished Young Teachers in Higher Education of China (Yue Teacher (2014145) and Science and Technology Planning Project of Guangdong Province (2017A050501020).
The authors declare no competing financial interest.
Supplementary Material
References
- Cancer; World Health Organization. http://www.who.int/mediacentre/factsheets/fs297/en/.
- Rocca A.; Schirone A.; Maltoni R.; Bravaccini S.; Cecconetto L.; Farolfi A.; Bronte G.; Andreis D. Progress with palbociclib in breast cancer: Latest evidence and clinical considerations. Ther. Adv. Med. Oncol. 2017, 9, 83–105. 10.1177/1758834016677961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J. A new cell-cycle target in cancer - inhibiting cyclin d-dependent kinases 4 and 6. N. Engl. J. Med. 2016, 375, 1920–1923. 10.1056/NEJMp1612343. [DOI] [PubMed] [Google Scholar]
- Tripathy D.; Bardia A.; Sellers W. R. Ribociclib (lee011): Mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin. Cancer Res. 2017, 23, 3251–3262. 10.1158/1078-0432.CCR-16-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S.; Zhong H.; Zhao X.; Yang J.; Jing Y.; Liu D.; Zhao L. Synthesis, anticancer evaluation and pharmacokinetic study of novel 10-o-phenyl ethers of dihydroartemisinin. Eur. J. Med. Chem. 2017, 141, 584–595. 10.1016/j.ejmech.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Ge B.-C.; Feng H.-F.; Cheng Y.-F.; Wang H.-T.; Xi B.-M.; Yang X.-M.; Xu J.-P.; Zhou Z.-Z. Design, synthesis and biological evaluation of substituted aminopyridazin-3(2 h)-ones as G0/G1-phase arresting agents with apoptosis-inducing activities. Eur. J. Med. Chem. 2017, 141, 440–445. 10.1016/j.ejmech.2017.09.077. [DOI] [PubMed] [Google Scholar]
- Nunes R. C.; Ribeiro C. J. A.; Monteiro Â.; Rodrigues C. M. P.; Amaral J. D.; Santos M. M. M. In vitro targeting of colon cancer cells using spiropyrazoline oxindoles. Eur. J. Med. Chem. 2017, 139, 168–179. 10.1016/j.ejmech.2017.07.057. [DOI] [PubMed] [Google Scholar]
- Neochoritis C. G.; Zhao T.; Domling A. Tetrazoles via multicomponent reactions. Chem. Rev. 2019, 119, 1970–2042. 10.1021/acs.chemrev.8b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera L.; Rivera D. G. Multicomponent reaction toolbox for peptide macrocyclization and stapling. Chem. Rev. 2019, 119, 9836–9860. 10.1021/acs.chemrev.8b00744. [DOI] [PubMed] [Google Scholar]
- Hall D. G.; Rybak T.; Verdelet T. Multicomponent hetero- 4+2 cycloaddition/allylboration reaction: From natural product synthesis to drug discovery. Acc. Chem. Res. 2016, 49, 2489–2500. 10.1021/acs.accounts.6b00403. [DOI] [PubMed] [Google Scholar]
- de Koning M. C.; Joosen M. J. A.; Worek F.; Nachon F.; van Grol M.; Klaassen S. D.; Alkema D. P. W.; Wille T.; de Bruijn H. M. Application of the ugi multicomponent reaction in the synthesis of reactivators of nerve agent inhibited acetylcholinesterase. J. Med. Chem. 2017, 60, 9376–9392. 10.1021/acs.jmedchem.7b01083. [DOI] [PubMed] [Google Scholar]
- Upadhyay K. D.; Dodia N. M.; Khunt R. C.; Chaniara R. S.; Shah A. K. Synthesis and biological screening of pyrano[3,2-c]quinoline analogues as anti-inflammatory and anticancer agents. ACS Med. Chem. Lett. 2018, 9, 283–288. 10.1021/acsmedchemlett.7b00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L.; Zheng S.; Cai X.; Chen Z.; Zhu Q.; Liu S. Development of four-component synthesis of tetra- and pentasubstituted polyfunctional dihydropyrroles: Free permutation and combination of aromatic and aliphatic amines. ACS Comb. Sci. 2013, 15, 183–192. 10.1021/co300148c. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Huang L.; Chen Z.; Zheng S.; Lv L.; Zhu Z.; Cao D.; Jiang H.; Liu S. A new series of C-6 unsubstituted tetrahydropyrimidines: Convenient one-pot chemoselective synthesis, aggregation-induced and size-independent emission characteristics. Chem. Eur. J. 2013, 19, 1268–1280. 10.1002/chem.201203012. [DOI] [PubMed] [Google Scholar]
- Chen Z.-P.; Wang H.-B.; Wang Y.-Q.; Zhu Q.-H.; Xie Y.; Liu S.-W. Synthesis of fused pyrrolo[3,4-d]tetrahydropyrimidine derivatives by proline-catalyzed multicomponent reaction. Tetrahedron 2014, 70, 4379–4385. 10.1016/j.tet.2014.04.075. [DOI] [Google Scholar]
- Zheng S.; Zhong S.; Chen Z.; Chen W.; Zhu Q. Efficient synthesis of a series of novel octahydroquinazoline-5-ones via a simple on-water urea-catalyzed chemoselective five-component reaction. ACS Comb. Sci. 2016, 18, 475–481. 10.1021/acscombsci.6b00038. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Ye Z.; Yang W.; Cai X.; Tang B. Z. One-pot synthesis and structure-property-relationship of aminomaleimides: Fluorescence efficiencies in monomers and aggregates easily tuned by switch of aryl and alkyl. J. Org. Chem. 2017, 82, 1096–1104. 10.1021/acs.joc.6b02706. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Gao L.; Chen Z.; Zheng S.; Shu H.; Li J.; Jiang H.; Liu S. A novel class of small-molecule caspase-3 inhibitors prepared by multicomponent reactions. Eur. J. Med. Chem. 2012, 54, 232–238. 10.1016/j.ejmech.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Asghar U.; Witkiewicz A. K.; Turner N. C.; Knudsen E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E. A.; Caldon C. E.; Barraclough J.; Stone A.; Sutherland R. L. Cyclin d as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- Engeland K. Cell cycle arrest through indirect transcriptional repression by p53: I have a dream. Cell Death Differ. 2018, 25, 114–132. 10.1038/cdd.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirbes T. K.; Baldus S. E.; Moenig S. P.; Nolden S.; Kunze D.; Shafizadeh S. T.; Schneider P. M.; Thiele J.; Hoelscher A. H.; Dienes H. P. Prognostic impact of p21/waf1/cip1 in colorectal cancer. Int. J. Cancer 2000, 89, 14–18. . [DOI] [PubMed] [Google Scholar]
- Hansen J.; Bross P.. A Cellular Viability Assay to Monitor Drug Toxicity. In Methods in Molecular Biology, Bross P., Gregersen N., Eds.; Humana Press: New Jersey, 2010; pp 303–311. [DOI] [PubMed] [Google Scholar]
- Sylvester P. W.Optimization of the Tetrazolium Dye (MTT) Colorimetric Assay for Cellular Growth and Viability. In Methods in Molecular Biology, Satyanarayanajois S. D., Ed.; Humana Press, 2011; pp 157–168. [DOI] [PubMed] [Google Scholar]
- van Meerloo J.; Kaspers G. J. L.; Cloos J.. Cell sensitivity Assays: The MTT Assay. In Methods in Molecular Biology, 2nd ed.; Cree I. A., Ed.; Humana Press, 2011; pp 237–245. [DOI] [PubMed] [Google Scholar]
- Pozarowski P.; Darzynkiewicz Z.. Analysis of Cell Cycle by Flow Cytometry. In Methods in Molecular Biology, Schönthal A. H., Ed.; Humana Press: New Jersey, 2004; pp 301–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.