Abstract
The neural underpinnings of subjective experience during resting state remain elusive. Dynamic features of EEG oscillations may provide more understanding of the relationship between the content of inner conscious experience and electrical brain activity. We tested a correlation of rating on the Amsterdam Resting-State Questionnaire (ARSQ) with dynamic parameters of EEG recorded in 49 healthy volunteers during the 10-min resting session. The participants filled ARSQ immediately after the rest. We investigated both linear (1 Hz-band power spectral density - PSD) and dynamic features (standard deviation and frequency of Hilbert envelope) of EEG averaged for the whole resting-state segment. Besides, we conducted a procedure of k-mean clustering based on PSD, localization of components retrieved by independent component analysis for 10-sec EEG epochs to assess spectral and temporal variability of EEG. The correlation analysis showed that the increase of PSD and cluster duration of the high-frequency alpha rhythm (12–13 Hz) in central and frontal areas was positively associated with the rating of experienced thoughts related to Planning (r = 0.44). The time of the presence of low amplitude delta oscillations correlated negatively with Planning (r = -0.52). The participants with higher ARSQ scores of Visual Thoughts had a higher standard deviation of the wideband (1–30 Hz) Hilbert envelope. Our data suggest that the dynamic properties of EEG reflect cognitive states assessed by ARSQ.
Keywords: Neuroscience, Psychology, Behavioral neuroscience, Cognitive psychology, Signal processing, EEG, Resting state, Power spectral density, K-mean clustering, Hilbert envelope, Amsterdam resting-state questionnaire
1. Introduction
A background neural activity or "resting state" has attracted the attention of human neuroscience only in recent decades. The majority of reports came from fMRI and PET confirming spatially structured brain activation during rest [1, 2, 3, 4]. It has been suggested that the function of this neural activity is to maintain both physiological functions [5] and consciousness [6, 7]. The content of human consciousness consists of a sequence of internally experienced states that form a single stream of consciousness or mind wandering [8, 9].
Quite a few questionnaires have been designed to qualitatively assess the cognitive state during mind wandering based on retrospective self-reports. One of them is the New York Cognition Questionnaire (NYC-Q) [10], which is an improved and revised version of the Dundee Stress State Questionnaire [11]. NYC-Q organizes mind wandering into categories of content (future, past, positive, negative, social) and form (words, images, specificity). The other is the Amsterdam Resting-State Questionnaire (ARS Q), which constitutes both a qualitative and quantitative assessment of specific dimensions of subjective experience during rest [12, 13]. The first version of the ARSQ contained 50 question-statements estimating seven dimensions of thoughts and feelings during a 5-min eyes-closed rest: (1) Discontinuity of Mind, (2) Theory of Mind, (3) Self, (4) Planning, (5) Sleepiness, (6) Comfort, and (7) Somatic Awareness [12]. In the second version of the questionnaire (ARSQ 2.0), the number of questions was increased to 54 and, following confirmatory factor analysis; the number of factors was expanded to 10 with the addition of (8) Health Concerns, (9) Visual Thought, and (10) Verbal Thought [13].
Unlike other resting-state questionnaires, the ARSQ has been mentioned more frequently in subsequent studies. The ARSQ scores of Sleepiness, Imagery, and Discontinuity of Mind have been shown to correlate with resting-state functional connectivity in an fMRI study of 106 participants [7]. An EEG study revealed a correlation between alpha and theta oscillations and ARSQ-factors of the Theory of Mind, Planning and Sleepiness in that linear patterns of EEG such as power spectral density (PSD) of alpha and theta bands were significant predictors of the Theory of Mind, Self, Sleepiness, and Comfort, while alpha/theta ratio was correlated positively with just Planning at an individual level [14]. Interestingly, stochastic or dynamic patterns of resting-state EEG measured by long-range temporal correlations of alpha/theta oscillations were associated with Discontinuity of Mind [14]. Later on, Pipinis et al. [15] also explored an association of EEG dynamic with ARSQ ratings and showed a significant correlation of specific microstates in the resting-state EEG with Somatic awareness [15].
There are also data confirming that even deviation in Hilbert Envelope frequency and standard deviation may serve as a powerful predictor of minor changes in human consciousness, reflect emotional or mood associated states [16, 17], and be an index of speech recognition or enhancement [18, 19]. Stochastic features of EEG, Hilbert envelope frequency and its deviation reflecting the different emotional states could be detected using nonlinear methods rather than the traditional methods of EEG analysis.
Additionally, k-mean clustering of frequency and topography in EEG oscillations build on Hilbert envelope may reflect neural underpins of resting-state experience as it was shown early [20] and may indicate about abnormalities of the consciousness content associated with cognitive deficit [21].
In this study, we aimed to check the hypothesis that patterns in EEG recordings both linear (PSD of specific frequency bands) and dynamic (Hilbert envelope frequency and its standard deviation along with presence of rhythmic clusters in EEG) could predict the ARSQ ratings. By applying a combination of linear and nonlinear approaches in EEG analysis, we aimed to detect previously hidden connections between EEG features and cognitive and mental activity of the resting state assessed by ARSQ.
2. Materials and methods
2.1. Participants
The study sample consisted of 61 healthy volunteers recruited for the project of memory reactivation during daytime sleep (RFBR # 16-04-01403). Participants were recruited according to preliminary screening with the following eligibility criteria: right-handed; aged 18–35 years; non-smokers; not using medication; absence of excessive daytime sleepiness; and no history of hearing impairments, sleep disorders, brain injury, neurological diseases and/or hormonal diseases (hypothyreosys, thyrotoxicosis). We offered our female participants to choose a date for the lab visit in the follicular phase (4–12 day of their menstrual cycle). All participants were instructed to refrain from alcohol for 24 h before the study as well as from the intake of tea and coffee for 6 h before the laboratory visit.
The research protocol was compiled following the requirements of the Helsinki Declaration and approved by the local ethical committee in the Institute of Higher Nervous Activity and Neurophysiology of the Russian Academy of Sciences. All participants provided written informed consent and received instructions regarding all the experimental details, as well as their right to withdraw at any time. Participants received a monetary reward (750 RUB) for their participation.
2.2. Procedure
All participants arrived at the laboratory approximately at the same time of the day - around 13h00. After acquaintance with the study design and signing the informed consent, participants filled in paper-based questionnaires about their sleepiness at the moment of the study—Visual Analogue Sleepiness Scale (VASS) and Stanford Sleepiness Scale (SSS). Participants also completed a questionnaire about their regular daytime sleepiness: Epworth Sleepiness Scale (ESS). Volunteers with excessive daytime sleepiness (ESS> 10) were excluded from the study. After that, we attached electrodes for registration of EEG, electrocardiogram (ECG), electromyogram (EMG) and electrooculogram (EOG), and by 14h00 participants lay supine in the bed in an acoustically and electrically shielded chamber. We instructed participants to lay relaxed with eyes closed for 10 min, not to think of anything particularly, not to move and to try not to fall asleep. After recording the resting-state session, we asked participants to sit up and complete a paper-based version of the ARSQ.
2.3. Amsterdam Resting-State Questionnaire
The resting-state subjective experience was assessed using the Russian version of ARSQ 2.0 [13]. The questionnaire includes 54 statements representing thoughts and feelings during the resting-state condition. Participants completed the ARSQ on paper immediately after a 10 min eyes-closed rest. All items were scored from 'Completely Disagree' to 'Completely Agree' on a 5-point scale.
2.3.1. ARSQ data analysis
The answers of the participants were summed up according to the model of factors comprising 30 statements of ARSQ 2.0 for 10 dimensions: (1) Discontinuity of Mind, (2) Theory of Mind, (3) Self, (4) Planning, (5) Sleepiness, (6) Comfort, (7) Somatic Awareness, (8) Health Concern, (9) Visual Thought, and (10) Verbal Thought. Answers to the statements (Table 1), like in the version of Diaz et al. [13] corresponded to the following scores: completely disagree (--) – 1 point, disagree (-) – 2 points, do not know (+-) – 3 points, agree (+) - 4 points, completely agree (++) - 5 points.
Table 1.
ARSQ dimension and corresponding items (Diaz et al. 2014).
| Number of factor | Factor | Statements |
|---|---|---|
| 1 | Discontinuity of Mind | “I had busy thoughts”, “I had rapidly switching thoughts”, “I had difficulty holding on to my thoughts” |
| 2 | Theory of Mind | “I thought about others”, “I thought about people I like”, “I placed myself in other peoples' shoes” |
| 3 | Self | “I thought about my feelings”, “I thought about my behavior”, “I thought about myself” |
| 4 | Planning | “I thought about things I need to do”, “I thought about solving problems”, “I thought about the future” |
| 5 | Sleepiness | “I felt tired”, “I felt sleepy”, “I had difficulty staying awake” |
| 6 | Comfort | “I felt comfortable”, “I felt relaxed”, “I felt happy” |
| 7 | Somatic Awareness | “I was conscious of my body”, “I thought about my heartbeat”, “I thought about my breathing” |
| 8 | Health Concern | “I felt ill”, “I thought about my health”, “I felt pain”. |
| 9 | Visual Thoughts | “I thought in images”, “I pictured events”, “I pictured places” |
| 10 | Verbal Thoughts | “I thought in words”, “I had silent conversations”, “I imagined talking to myself” |
2.4. EEG registration
During the 5 min EEG recording, participants lay supine in a bed in an acoustically and electrically shielded chamber. The participants were instructed to close their eyes, remain calm, avoid falling asleep and avoid thinking about anything specific. EEG was recorded using a 19-channel EEG amplifier Encephalan with the recording of polygraphic channels: EOG, ECG and EMG (Poly4, Medikom MTD, Taganrog, Russian Federation). ECG was recorded by 0.5 cm surface electrode placed at the left wrist. Facial EMG was acquired by two surface electrodes placed at the chin. The amplifier bandpass filter was nominally set to 0.05–70 Hz. Continuous EEG was acquired with AgCl electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2) according to the International 10–20 system with a sampling rate of 250 Hz. The electrodes placed on the left and right mastoids served as joint references under unipolar montage. The vertical EOG was measured with AgCl cup electrodes placed 1 cm above and below the left eye, and the horizontal EOG was measured with electrodes placed 1 cm lateral from the outer canthi of both eyes. The electrode impedances were kept below 10 kΩ.
2.5. EEG preprocessing
Before the preprocessing of EEG, two experts inspected continuous EEG for signs of drowsiness or transition to sleep. Visual scoring of each 30-s epoch of EEG recording was performed according to standard criteria [22]. Inter-score reliability was greater than 92%. Continuous EEG of each subject was filtered with bandpass filter 0.5–30 Hz and cleaned artifacts from EMG, ECG and eye movements by an ICA-based algorithm in the EEGLAB plugin for Matlab 7.11.0 (Mathwork Inc.). Muscle artifacts were cut out through manual data inspection. The EEG data from 12 of the 61 participants were rejected after analysis due to the insufficient duration of artifact-free EEG epoch corresponding to the resting-state session (less than 240 s) or due to periods of drowsiness detected with off-line polysomnography analysis. Finally, 49 participants (the average age 23.3 ± 3 y. o., 29 females and 20 males) were included in the EEG and behavioral analysis.
2.6. EEG analysis
2.6.1. Power spectral density and Hilbert Envelope
For each EEG channel, except Fp1 and Fp2, we calculated absolute PSD using the Fast Fourier Transform (FFT). The EEG spectrum was estimated for every 300 ± 7.8 s intervals. The resulting normalized spectra were integrated over intervals of unit width in the range of interest (2–3 Hz, 3–4 Hz, … 19–20 Hz). Besides, we estimated the alpha peak frequency (8–13 Hz) and calculated Hilbert envelope standard deviation and frequency (HESD and HEF, respectively) for wideband (1.6–30 Hz) and alpha band separately (8–13 Hz). Only wideband (1.6–30 Hz) HESD showed significant Spearman correlations supported by Permutation test.
2.6.2. K-mean clustering
Using k-mean clustering, we analyzed the spectral and spatial parameters of EEG data after FFT in series of 10-seconds EEG epochs to find stable EEG states during the resting-state condition. Artifact-free continuous resting-state EEG lasting at least 240 s was segmented into 10 ms epochs. Localization and PSD of each component from 2–20 Hz were concatenated into one feature vector and then transformed using PCA to reduce dimensionality. In order to isolate different functional states of resting-state EEG, we conducted k-mean clustering 45 of feature vectors of all components for the entire set of individual data from all participants.
Further, k-mean clustering was performed on PSD data. The cluster attribute for each dipole component was determined by the minimal distance to the cluster center in EEG data vector space. Duration of a specific cluster in EEG was calculated by summing up durations of epochs, which contained at least one dipole component belonging to the specific cluster (C1-5). Duration of the cluster (or percentage of epochs with a dominant cluster) was calculated for each subject separately. Thus, each cluster duration reflected how frequently and how long a particular electrical activity with a specific location and rhythmic pattern appeared in the resting-state EEG. We also analyzed the association of contribution of each cluster with factors of ARSQ, VASS and SSS values by calculating Spearman's rank correlation. The same procedure was completed for wideband (1.6–30 Hz) HESD and HEF and alpha-rhythm (8–13 Hz) HESD and HEF. However, significant correlations with ARSQ were not found.
2.7. Correlation analysis of EEG data
We checked the normality of distribution for the studied parameters using the Shapiro-Wilk test. As the ARSQ ratings were not normally distributed, we applied nonparametric correlation analysis. We analyzed a possible association of resting-state EEG patterns (PSD for each 1-Hz band, HESD and HEF, duration of EEG clusters) with ARSQ ratings using Spearman correlation coefficients corrected for multiple comparisons by cluster-based permutation test using clustering method (Matlab toolbox for BCI) with 500 permutations at each node (the Bonferroni corrected p-value of 0.05). Only significant results were discussed further.
3. Results
3.1. Psychological assessment results
According to ESS, the general level of daytime sleepiness in all participants was in the healthy range (7.7 ± 2.3). Results of sleepiness assessment just before the resting-state session showed that participants did not fill relaxed or sleepier than usual: SSS 3.2 ± 1.1 and VASS 4.9 ± 2.0.
The participants had the following mean ratings: Discontinuity of Mind – 9.1 ± 2.8, Theory of Mind – 7.9 ± 2.5, Self – 6.5 ± 1.9, Planning – 7.4 ± 3.8, Sleepiness – 9.5 ± 2.7, Comfort – 12.6 ± 1.4, Somatic Awareness – 7.9 ± 3.0, Health Concern – 3.7 ± 1.0, Visual Thought – 9.9 ± 2.9, and Verbal Thought – 6.7 ± 2.8. The Mean scores of the evaluated ARSQ domains and intra-class correlation coefficients of ARSQ dimensions are presented in Tables 2 and 3, respectively. There was no significant correlation of ARSQ ratings with age and the effect of gender.
Table 2.
Mean scores of the evaluated ARSQ domains.
| ARSQ domains |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DoM | ToM | Self | Planning | Sleepiness | Comfort | SA | Health | Visual | Verbal | |
| Mean | 3.03 | 2.65 | 2.16 | 2.46 | 3.16 | 4.20 | 2.63 | 1.22 | 3.30 | 2.22 |
| SD | 0.97 | 0.86 | 0.66 | 1.29 | 0.92 | 0.46 | 1.04 | 0.34 | 1.01 | 0.96 |
Table 3.
Intra-class correlation coefficients and corresponding p values of ARSQ dimensions. Bold and underlined - Correlation is significant at the 0.01 level (2-tailed). Bold - Correlation is significant at the level p < 0.05 (2-tailed). Underlined numbers denote significant correlations after Bonferroni-Holm correction.
| PCC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | DoM | ToM | Self | Planning | Sleepiness | Comfort | SA | health | visual | verbal |
| DoM | 0.28 | 0.09 | 0.15 | 0.22 | -0.05 | -0.14 | -0.09 | 0.37 | 0.19 | |
| ToM | 0.06 | 0.24 | 0.26 | 0.08 | 0.18 | -0.18 | -0.16 | 0.29 | 0.18 | |
| Self | 0.55 | 0.10 | 0.14 | -0.32 | -0.19 | 0.09 | -0.17 | 0.06 | 0.04 | |
| Planning | 0.31 | 0.07 | 0.32 | -0.03 | 0.26 | -0.06 | 0.06 | 0.16 | 0.37 | |
| Sleepiness | 0.12 | 0.58 | 0.02 | 0.83 | -0.07 | -0.18 | 0.01 | 0.04 | -0.06 | |
| Comfort | 0.73 | 0.22 | 0.19 | 0.08 | 0.62 | -0.06 | -0.16 | 0.11 | -0.04 | |
| SA | 0.35 | 0.21 | 0.55 | 0.67 | 0.22 | 0.69 | 0.22 | 0.11 | 0.28 | |
| Health | 0.54 | 0.27 | 0.24 | 0.69 | 0.97 | 0.28 | 0.13 | -0.04 | 0.11 | |
| Visual | 0.01 | 0.04 | 0.66 | 0.27 | 0.78 | 0.44 | 0.47 | 0.78 | -0.07 | |
| Verbal | 0.19 | 0.22 | 0.77 | 0.01 | 0.68 | 0.78 | 0.05 | 0.43 | 0.66 | |
3.2. ARSQ & resting-state EEG
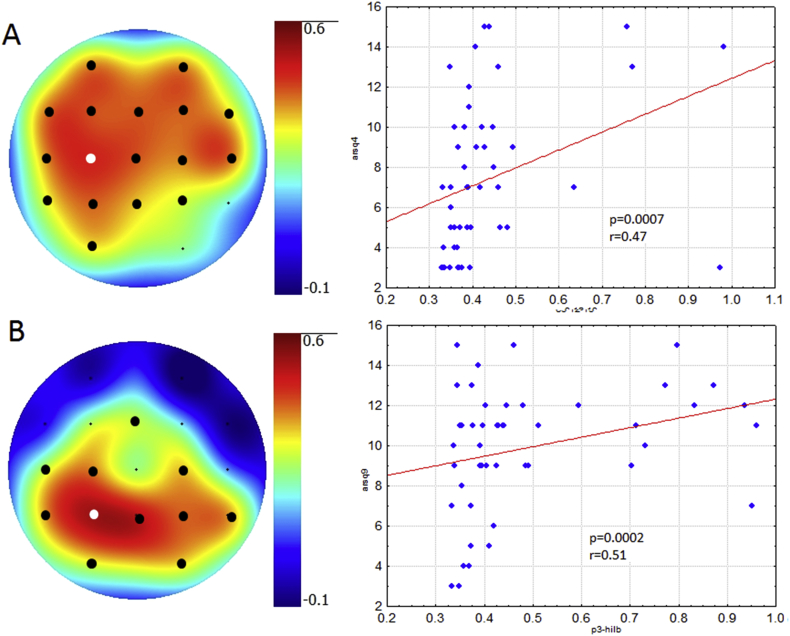
The cluster-based permutation test of possible correlations between ARSQ and studied EEG patterns revealed two positive electrode clusters. The positive correlation between 12-13 Hz PSD and Planning (r = 0.47, p = 0.0007) dominated in the frontal and central areas (Fig. 1A). The second positive correlation (r = 0.51, p = 0.0002) was found between HESD for wideband (1.6–30 Hz) and experienced Visual Thoughts in parietal and occipital areas (Fig. 1B).
Fig. 1.
Topographical plots showing the strength of Spearman correlation for all channels: A - between Planning of ARSQ and PSD of 12–13 Hz, B –between Visual Thought of ARSQ and HESD of 1.6–30 Hz. Black dots indicate channels with significant correlation (p < 0.05). Scatterplots of EEG patterns and ARSQ scores correspond to the channels marked as white dots.
3.3. Association of ARSQ with K-mean clustering of EEG power spectral density and localization
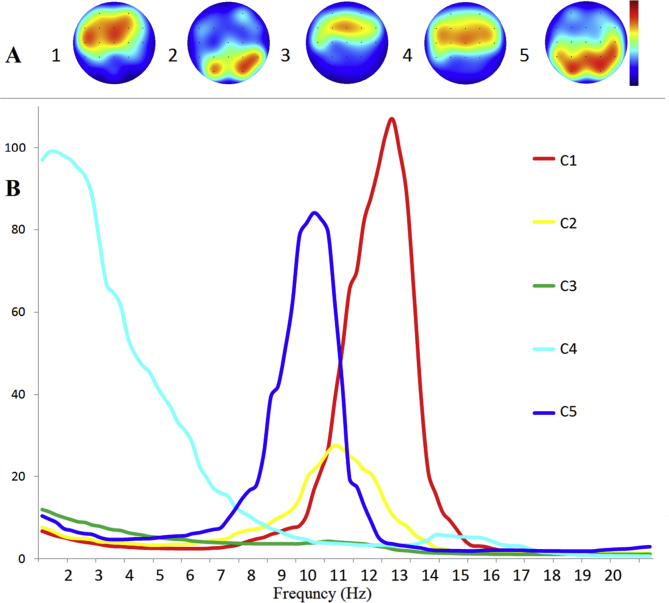
K-mean clustering revealed five clusters presented in the resting-state EEG over two-thirds of individuals (Fig. 2AB). Among them, 2 clusters showed significant association with ARSQ ratings.
Fig. 2.
Resting-state EEG clusters, measured using localization and frequency parameters of power spectral density (PSD). A: C (1–5) topographic maps of peak PSD for five clusters on the head view, B: frequency peak of PSD for each cluster. Colored lines represent five clusters.
Cluster 1 (C1) consisted of high PSD of high frequency (12–13 Hz) alpha-rhythm (mean peak frequency 12.7 Hz and mean PSD 109 μV2), localized in frontal and central areas and more pronounced in the left hemisphere (Fig. 2_A1). This cluster had an average contribution of 17.2 ± 27.0% in the resting-state EEG in 87.8% of the participants. The C1 contribution (Fig. 2A1, 2B) positively correlated with Planning (Spearman's rank correlation: rs = 0.44, p = 0.0016).
Cluster 3 (C3) was characterized by low PSD (10 μV2) with peak density in delta rhythm (2–3 Hz) in bilateral frontal areas and the contribution of 55.5 ± 37.0% in EEG of 100% of participants. The C3 contribution (Fig. 2_A3) negatively correlated with Planning (Spearman's rank correlation: rs = -0.52, p 0.00012).
4. Discussion
We used linear and stochastic EEG patterns to investigate a possible association between resting-state brain activity and subjective evaluation of mind-wandering experience assessed by ARSQ. To our knowledge, this is the first study demonstrating a reliable correlation of retrospective rating of thoughts related to Planning with both a contribution and power of EEG oscillations in upper alpha band of 12–13 Hz. Moreover, our results show that dynamic changes in EEG, reflected in the higher standard deviation of envelope frequency in the wide-band oscillations (1.6–30 Hz), were associated with higher ratings of Visual Thoughts.
The contribution of EEG-cluster with high PSD of 12–13 Hz positively correlated with ARSQ rating of Planning. The upper alpha band, 12–13 Hz in frontal areas, was previously associated with specific attention [23], planning of motor tasks [24, 25] and social interactions [26]. All these processes including mental attention, motor imaginary, and social cognition could contribute to the planning thoughts, as an estimation of Planning based on the summarized rating of three ARSQ statements: "I thought about things I need to do", "I thought about solving problems", "I thought about the future". Our findings are partially consistent with data of Diaz et al. [14] reported that higher alpha/theta ratio was correlated positively with Planning. Importantly, our results indicate that not wideband alpha but the upper alpha band of 12–13 Hz, its power and contribution in the resting-state EEG with specific localization in the frontal and central areas, predicts higher ratings of Planning in ARSQ. On the contrary, a contribution of the cluster with low PSD EEG, which could be associated with alpha-rhythm desynchronization in the frontal areas, showed a negative association with Planning. Previous studies reported that less frontal desynchronization was associated with the experiencing novelty during the action's execution and perception [27] as well as evaluative cognitive activity [28].
In this work, we also analyzed the dynamic patterns of resting-state EEG using frequency and deviation in frequency of amplitude envelope for alpha (8–13 Hz) and wideband EEG (1.6–30 Hz). Dynamic on nonlinear features of EEG may prove an additional tool to study neural correlates of consciousness. For example, in nonlinear dynamical analysis, the EEG signal can be embedded into a so-called “state space” to derive values for the chaotic complexity [29] or synchronicity [30] of particular states. Several methods to define the entropy as a state characteristic of the EEG signal have been proposed to recognize ictal patterns [31]. Microstate analysis, partially adapted in this study as K-mean clustering of the EEG PSD, is another such method where states are defined by topographies of electric potentials over a multichannel electrode array [32].
We found a positive correlation between the standard deviation of wideband frequency in parietal and occipital areas band and subjective ratings on Visual thoughts. The higher standard deviation of envelope frequency indicates higher variability in EEG dynamic. Previous studies showed that the variability of EEG increases with the degree of cognitive activity [33], visual information processing [34]. Moreover, it was found that more complex visual stimulus produced the highest increase in the complexity of brain activity [35]. Using MRI researchers succeeded in isolating a typical activation for visual tasks, which included the memory-related brain areas [36]. The last data evidenced that the processes of the visual thoughts were inherently associated with memory processes, which could also be reflected in the nonlinear dynamic [37]. Moreover, memory tasks required visualization components were accompanied by the rise in complexity of the EEG signals, which increased proportionally to the task difficulty [37, 38].
However, we failed to replicate previous findings on significant association PSD of EEG with ARSQ resting on Theory of Mind, Self, Sleepiness, and Comfort [14]. This may be accounted by the different PSD analysis. While we measured absolute power of 1 Hz -wideband, Diaz and colleagues did study the ratio between them [14]. Another possible factor that had an impact in the results could be related with the prolongation of the resting state session up to 10 min. We may only hypothetically suggest that extension of the resting-state session led to the restructuring of the consciousness content, which was reflected on the EEG. First of all, we found the elimination of experimental-dependent factors. Novelty induced by the experimental setting ("what can the researcher think about me?" or "could researchers found any pathologic symptoms in my EEG") might contribute to Somatic Awareness or Theory of Mind [14]. However, during 10 min the way of thinking could switch to solving daily problems: Planning of own individual activities, the actualization of memories or imagination.
Unexpectedly, we did not observe a clear association of EEG patterns with Sleepiness, while the downside of the resting extension could result in drowsiness. Moreover, Diaz reported that the ARSQ dimension of Sleepiness was associated with the long-range temporal correlations of parietal theta oscillations [14]. The factor Sleepiness also exhibited significant associations with functional connectivity within Visual and Default Mode networks [7]. In our study, we minimized the influence of drowsiness by the inclusion of participants only with low daytime sleepiness according to preliminary scoring by ESS. We also performed a polysomnographic analysis of EEG to exclude participants having transitioned from wakefulness to light sleep.
One of the limitations of this current study could be a possible influence of hormonal fluctuations at bot EEG dynamic and mind-wandering in female participants. Further research with control of hormonal level might help to validate an effect of neurohormonal status at resting-state brain dynamic and retrospective self-reports about mind-wandering in women. Nevertheless, our findings might serve as a baseline for comparison in the future studies of neural underprints of altered mind-wandering in psychopathology.
5. Conclusion
Our data suggest that the dynamic properties of EEG reflect cognitive states assessed by subjective ARSQ ratings, which had not been previously reported. In particular, we were the first to show that the ratings of Planning were higher in participants with higher power and duration of 12–13 Hz oscillations in the frontal areas. The subjective ratings of Visual thoughts positively correlated with a standard deviation of wideband Hilbert envelope frequency of EEG in the parietal areas.
Declarations
Author contribution statement
Galina V. Portnova: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yulia V. Ukraintseva: Conceived and designed the experiments; Performed the experiments.
Krystsina M. Liaukovich: Performed the experiments.
Olga V. Martynova: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Russian Scientific Foundation (grant No. 16-15-00300).
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study has been deposited at https://data.mendeley.com/datasets/wnp3jnymvn/draft?a=7ce76728-c0ff-42fa-b551-7ae8396df675.
References
- 1.Raichle M.E., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 2.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sojoudi A., Goodyear B.G. Statistical inference of dynamic resting-state functional connectivity using hierarchical observation modeling. Hum. Brain Mapp. 2016;37:4566–4580. doi: 10.1002/hbm.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krug K., Daniel Salzman C., Waddell S. Understanding the brain by controlling neural activity. Philos. Trans. R. Soc. Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine L., Soddu A., Gómez F., Vanhaudenhuyse A., Tshibanda L., Thonnard M., Charland-Verville V., Kirsch M., Laureys S., Demertzi A. Resting state networks and consciousness Alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness states. Front. Psychol. 2012;3:295. doi: 10.3389/fpsyg.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoffers D., Diaz B.A., Chen G., Den Braber A., Van’t Ent D., Boomsma D.I., Mansvelder H.D., De Geus E., Van Someren E.J.W., Linkenkaer-Hansen K. Resting-state fMRI functional connectivity is associated with sleepiness, imagery, and discontinuity of mind. PLoS One. 2015;10:e0142014. doi: 10.1371/journal.pone.0142014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James W. Ch 21: the perception of reality. Princ. Psychol. 1890;2:281–302. [Google Scholar]
- 9.Killingsworth M.A., Gilbert D.T. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 10.Gorgolewski K.J., Lurie D., Urchs S., Kipping J.A., Craddock R.C., Milham M.P., Margulies D.S., Smallwood J. A correspondence between individual differences in the brain’s intrinsic functional architecture and the content and form of self-generated thoughts. PLoS One. 2014;9:e97176. doi: 10.1371/journal.pone.0097176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews G., Campbell S.E., Falconer S., Joyner L.A., Huggins J., Gilliland K., Grier R., Warm J.S. Fundamental dimensions of subjective state in performance Settings : task engagement, distress, and worry. Emotion. 2002;2:315–340. doi: 10.1037/1528-3542.2.4.315. [DOI] [PubMed] [Google Scholar]
- 12.Diaz B.A., Van Der Sluis S., Moens S., Benjamins J.S., Migliorati F., Stoffers D., Den Braber A., Poil S.-S., Hardstone R., Van't Ent D., Boomsma D.I., De Geus E., Mansvelder H.D., Van Someren E.J.W., Linkenkaer-Hansen K. The Amsterdam Resting-State Questionnaire reveals multiple phenotypes of resting-state cognition. Front. Hum. Neurosci. 2013;7:446. doi: 10.3389/fnhum.2013.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz B.A., Van Der Sluis S., Benjamins J.S., Stoffers D., Hardstone R., Mansvelder H.D., Van Someren E.J.W., Linkenkaer-Hansen K. The ARSQ 2.0 reveals age and personality effects on mind-wandering experiences. Front. Psychol. 2014;5:271. doi: 10.3389/fpsyg.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz B.A., Hardstone R., Mansvelder H.D., Van Someren E.J.W., Linkenkaer-Hansen K. Resting-state subjective experience and EEG biomarkers are associated with sleep-onset latency. Front. Psychol. 2016;7:492. doi: 10.3389/fpsyg.2016.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pipinis E., Melynyte S., Koenig T., Jarutyte L., Linkenkaer-Hansen K., Ruksenas O., Griskova-Bulanova I. Association between resting-state microstates and ratings on the Amsterdam resting-state questionnaire. Brain Topogr. 2017;30:245–248. doi: 10.1007/s10548-016-0522-2. [DOI] [PubMed] [Google Scholar]
- 16.Portnova G.V., Atanov M.S. Nonlinear EEG parameters of emotional perception in patients with moderate traumatic brain injury, coma, stroke and schizophrenia. AIMS Neurosci. 2018;5:221–235. doi: 10.3934/Neuroscience.2018.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portnova G.V., Tetereva A., Balaev V., Atanov M., Skiteva L., Ushakov V., Ivanitsky A., Martynova O. Correlation of BOLD signal with linear and nonlinear patterns of EEG in resting state EEG-informed fMRI. Front. Hum. Neurosci. 2018;11:654. doi: 10.3389/fnhum.2017.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atal B.S. Proc. ICC. 1984. Stochastic coding of speech at very low bit rates. [Google Scholar]
- 19.Ganapathy S., Thomas S., Motlicek P., Hermansky H. IEEE Work. Appl. Signal Process. To Audio Acoust. 2009. Applications of signal analysis using autoregressive models for amplitude modulation. [Google Scholar]
- 20.Martynova O., Portnova G., Sushinskaia-Tetereva A., Balaev V., Atanov M., Ivanitsky A. Changes in EEG complexity of brain dynamics associated with bold fluctuations during resting state. Clin. Neurophysiol. 2017;128:e279. [Google Scholar]
- 21.Portnova G.V. Moderate and severe TBI patients distinguish emotional stimuli unlikely to healthy adults: EEG and behavioural research. Brain Inj. 2016;30:508. [Google Scholar]
- 22.Iber C., Ancoli-Israel S., Chesson A.L., Jr., Quan S.F. 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 23.Klimesch W., Pfurtscheller G., Schimke H. Pre- and post-stimulus processes in category judgement tasks as measured by event-related desynchronization (ERD) J. Psychophysiol. 1992;6:185–203. [Google Scholar]
- 24.Deiber M.-P., Sallard E., Ludwig C., Ghezzi C., Barral J., Ibañez V. EEG alpha activity reflects motor preparation rather than the mode of action selection. Front. Integr. Neurosci. 2012;6:59. doi: 10.3389/fnint.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaepffel M., Trachel R., Kilavik B.E., Brochier T. Modulations of EEG beta power during planning and execution of grasping movements. PLoS One. 2013;8:e60060. doi: 10.1371/journal.pone.0060060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naeem M., Prasad G., Watson D.R., Kelso J.A.S. Electrophysiological signatures of intentional social coordination in the 10-12Hz range. Neuroimage. 2012;59:1795–1803. doi: 10.1016/j.neuroimage.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Quandt L.C., Marshall P.J., Bouquet C.A., Young T., Shipley T.F. Experience with novel actions modulates frontal alpha EEG desynchronization. Neurosci. Lett. 2011;499:37–41. doi: 10.1016/j.neulet.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Parvaz M.A., MacNamara A., Goldstein R.Z., Hajcak G. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognit. Affect Behav. Neurosci. 2012;12:730–740. doi: 10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wackermann J., Lehmann D., Michel C.M., Strik W.K. Adaptive segmentation of spontaneous EEG map series into spatially defined microstates. Int. J. Psychophysiol. 1993;14:269–283. doi: 10.1016/0167-8760(93)90041-m. [DOI] [PubMed] [Google Scholar]
- 30.Carmeli C., Knyazeva M.G., Innocenti G.M., De Feo O. Assessment of EEG synchronization based on state-space analysis. Neuroimage. 2005;25:339–354. doi: 10.1016/j.neuroimage.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 31.Kannathal N., Choo M.L., Acharya U.R., Sadasivan P.K. Entropies for detection of epilepsy in EEG. Comput. Methods Progr. Biomed. 2005;80:187–194. doi: 10.1016/j.cmpb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Koenig T., Lehmann D., Merlo M.C.G., Kochi K., Hell D., Koukkou M. A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. Eur. Arch. Psychiatry Clin. Neurosci. 1999;249:205–211. doi: 10.1007/s004060050088. [DOI] [PubMed] [Google Scholar]
- 33.Natarajan K., Acharya U.R., Alias F., Tiboleng T., Puthusserypady S.K. Nonlinear analysis of EEG signals at different mental states. Biomed. Eng. Online. 2004;3 doi: 10.1186/1475-925X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stam C.J., Van Woerkom T.C.A.M., Pritchard W.S. Use of nonlinear EEF measures to characterize EEG changes during mental activity. Electroencephalogr. Clin. Neurophysiol. 1996;99:214–224. doi: 10.1016/0013-4694(96)95638-2. [DOI] [PubMed] [Google Scholar]
- 35.Müller V., Lutzenberger W., Preißl H., Pulvermüller F., Birbaumer N. Complexity of visual stimuli and nonlinear EEG dynamics in humans. Cogn. Brain Res. 2003;16:104–110. doi: 10.1016/s0926-6410(02)00225-2. [DOI] [PubMed] [Google Scholar]
- 36.Guidotti R., Del Gratta C., Baldassarre A., Romani G.L., Corbetta M. Visual learning induces changes in resting-state fMRI multivariate pattern of information. J. Neurosci. 2015;35:9786–9798. doi: 10.1523/JNEUROSCI.3920-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarjam P., Epps J., Lovell N.H., Chen F. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS. 2012. Characterization of memory load in an arithmetic task using nonlinear analysis of EEG signals. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Bermúdez G., García-Laencina P.J. Analysis of EEG signals using nonlinear dynamics and chaos: a review. Appl. Math. Inf. Sci. 2015;9:2309. [Google Scholar]