Abstract
Background
From fetal life until cardiac surgery, complex congenital heart diseases (CHD) exhibit different hemodynamic and oxygenation patterns that can lead to alteration of the metabolic profile. We used a metabolomic approach to identify urine metabolic markers before cardiac surgery, aiming to define the physiology of patients with complex CHD and to contribute to predict their neurodevelopmental outcome.
Methods
In a prospective, observational, single-center study we enrolled 28 patients with complex biventricular and univentricular CHD aged less than 5 years, on stable hemodynamic conditions, and with no genetic anomalies. We analyzed urine samples, collected at the induction of anesthesia, by 1H NMR spectroscopy. Profiles of 1H NMR spectra were submitted to unsupervised (principal component) and supervised (partial least squares-discriminant) multivariate analysis. Neurodevelopment was assessed by neuropsychological and adaptive functioning testing.
Results
Principal components analysis divided CHD patients metabolic profiles in two distinct clusters (RED and BLACK). Metabolic profiles belonging to the RED cluster showed higher levels of accumulation of citric acid cycle intermediates and glucose compared to the profiles in the BLACK cluster, indicating a possible switching to anaerobic metabolism. Patients belonging to the RED cluster were significantly more prone to show an adverse neurodevelopment pattern (p = 0.01).
Conclusions
The application of metabolomic analysis to CHD children permitted a deeper insight on their metabolic status that could help to obtain a better understanding of the physiological implications and to predict long-term neurodevelopmental outcome.
Keywords: Cardiology, Medicine, Neuroscience, Pediatrics, Physiology, Biological psychiatry, Metabolomics, Congenital heart diseases
1. Introduction
Congenital heart diseases (CHD) are the most common congenital defects, affecting nearly 1% of all newborns [1]. There are different types of CHD involving the abnormal development of the right or left ventricular outflow tract of the heart septation. This implies an altered blood flow and oxygen delivery to the tissues during fetal and neonatal life, leading to organ dysfunctions, including the brain. About 25% of children with a CHD are critical and life-saving surgical correction is employed in the first months of life [2]. These children are at risk of suboptimal developmental outcome as consequence of cardiac surgery [3], although there is an increasing awareness that CHD patients may exhibit anatomic and metabolic cerebral abnormalities [4, 5], suggesting alterations in early stages of brain development independently from surgical procedures [6].
Despite medical advancements have increased the survival rate of CHD patients, the incidence of neurodevelopmental sequelae remains high [7, 8], since patients without neuro-motor impairments may exhibit subtle cognitive difficulties in several domains, such as visuospatial, information processing speed, language and communication, attention, and social skills [8, 9].
Overall, several studies demonstrated that children with CHD are at high risk to develop a wide spectrum of neurodevelopmental sequelae but early prognostic indicators are lacking. The assessment of the untargeted metabolic profile (metabolomics) could be used as biomarker of impaired neurological outcome and specific metabolites can be identified after an initial untargeted screening. This approach has been used in a wide range of diseases, including surgical oncology, sepsis, obesity, and malnutrition [10, 11, 12]. Types and amounts of metabolites are influenced by various physiological and pathological stimuli including age, gender, drugs, toxins, and diseases. New developments in metabolic profiling techniques such as nuclear magnetic resonance (1H NMR) spectroscopy and mass spectrometry have opened up the possibility to build a picture of the metabolic environment in cells, tissues, or biofluids. Typical analytical samples for metabolomic studies are easily accessible fluids (i.e. plasma/serum or urine) and their metabolome represents the end-product of genetic fingerprinting, thus providing a more complete characterization of cell phenotype than genome, transcriptome, and proteome [13].
In this study, we correlate the metabolic profile of urine samples collected before cardiac surgery in patients with CHD with their long-term neuropsychological outcome. We selected various CHD types, in order to increase the generalizability of results across heart defects. We hypothesized that metabolic profiles could reflect the physiological status before surgery and could contribute predicting clinical and neurodevelopmental outcome.
2. Materials and methods
2.1. Patients
This is a prospective, observational, single-center study in children with CHD. The study was approved by the Institutional Review Board and Ethics Committee, Padova University Hospital. Inclusion criteria were: children with complex CHD that would require a cardiopulmonary-bypass (CPB) during surgery; elective cardiac surgery (patient on spontaneous breathing before surgery), stable hemodynamic conditions (constant inotropic support if needed, no volume load at admission or during the hospital stay prior to surgery), and written informed consent obtained from parents/legal guardians. Exclusion criteria were: age >5 years, liver damage defined as coagulation factor V <20%, kidney failure with creatinine clearance <30%, and preoperative diagnosis of chromosomal abnormalities.
We prospectively collected the following demographic data and clinical outcome parameters: birth weight, gestational age, gender, weight and age at the time of surgery, presence of hypoxia (defined as arterial oxygen saturation <90%, recorded before surgery), pre-surgery oxygen saturation, cerebral regional saturation, plasma arterial lactate, intensive care unit and hospital length of stay, and survival.
2.2. Sample collection and analysis
A urinary catheter was placed after induction of anesthesia in the operating room before cardiac surgery. Samples were collected from the urinary reservoir, aliquoted, and immediately stored at −80 °C until analysis.
2.3. 1H NMR spectroscopic analysis
NMR measurements were carried out on a Bruker DRX 600 MHz Avance Spectrometer equipped with a Selective Inverse Probe (SEI) equipped with Z gradient coil Spectra were acquired at the constant temperature of 298.0 ± 0.1 K by using a 90° pulse. A 10 s delay was included in the pulse sequence to allow T1 relaxation. In fact, T1 values (in the range 1.5–2.8 s) of the considered metabolites are such that a 10 s delay allows full recovery of longitudinal magnetization after a 90° pulse, as verified by constant integral values for D1 ≥5 s. A 0.3 Hz line broadening function was applied before Fourier transformation. Suppression of the water signal was achieved by applying a saturation pulse of 2 s duration at the water resonance. 32k data points per scan were used and 128 transients were accumulated. Samples were prepared as described by Groenen et al. [14]. For deproteinization, 2 ml of the sample was centrifuged over a 10 KDa filter for ½ h at 3000 x g (10 kDa Sartorius AG, Goettingen, Germany), pre-washed with 6 ml of NaOH 0.01 N and with 8 ml of water to avoid contamination with glycerol, always present in commercial membranes. 550 μl of filtrate plus 50 μl of a TSP-d4 20 mM solution were measured into a 0.5 mm (outer diameter) NMR tube. All spectra were run at 2.50 ± 0.02 pH measured with a microelectrode. The chemical shift of ionizable substances is highly dependent on pH. At pH 2.50 all chemical shift values are reproducible within ±0.01 ppm 10. Moreover, under these conditions, the methyl signals of creatine and creatinine are clearly separated (3.05 ppm for the methyl signal of creatine and 3.13 ppm for creatinine) and the methyl signal of lactic acid (1.41 ppm) is not overlapped by the methyl resonance of threonine (1.33 ppm). The pH was adjusted with a minimal volume of HCl, starting from a 3 M and ending with a 0.05 M.
2.4. Outcome assessment
At least 18 months after surgery, a brief neuropsychological examinations and an evaluation of the adaptive functioning were conducted by a psychologist trained in test administration and scoring.
2.4.1. Neuropsychological testing
The following neuropsychological tests were used: the Raven Colored Matrices [15]., which evaluates abstract reasoning and allow an estimate of general cognitive functioning; the visual attention test of the NEPSY-II [16, 17]; the Coding test of the WISC-IV [18], which evaluates working memory, manual dexterity and attention; the visual-motor integration test [19], which evaluates visual-motor integration abilities. The entire test battery required nearly 1 h to be completed.
2.4.2. Adaptive functioning
Adaptive functioning was assessed by the administration to parents of the Vineland Adaptive Behavior Scales (VABS-I) [20], which is a psychometrically validated interview that assesses adaptive behaviors at developmental levels from birth through adulthood. Several domains are evaluated, yielding index scores for socialization, communication, daily living, and motor skills (for children up to age 5). Each domain includes several subdomains with developmentally sequenced items, starting with skills typically observed in infancy.
2.5. Data reduction and multivariate analysis
Pareto scaling has the advantage, over the classical autoscaling preprocessing procedure, of boosting the importance of smaller peaks, without increasing instrumental noise in a significant way. In the pre-processing step, NMR spectra are phase and baseline corrected, using a weighted least square second order polynomial regression, and referenced to TSP (δ 0.0 ppm) position. Then data have been aligned by means of an open source algorithm, iCOSHIFT [21], which is based on rigid shift of intervals and which uses Fast Fourier Transform computation to boost the simultaneous alignment of all spectra in a dataset. At the end of the pre-processing, useless spectrum parts, like the high field and the very low field, plus the central part (about 4.80–5.20 ppm), where the water resonates, are removed.
In order to analyze the differences in metabolic profile that are correlated to the different patients' status, we used a two-step analytical procedure. First we used a Principal Components Analysis (PCA) [22, 23], an unsupervised technique that it is generally used to find trajectories and clustering, then Partial Least Square Discriminant Analysis (PLSDA) was used for data modelling. PLSDA [24] was carried out with the R package ‘pls’ [25]. The “leaving one out” cross validation determined the optimal number of components. PLSDA models are reported in term of the coefficients of determination calculated both from the fitted values on the overall dataset (R2cal) and on the cross-validated one. Feature selection has been accomplished using variable importance values in the projection (VIP), computed according to Chong Il-Gyo and Jun Chi-Hyuck's definition [26].
2.6. Neuropsychological data
Scores for the neuropsychological and adaptive results were age-corrected and converted into z, scaled or standard scores, as appropriate, using published normative data. The z scores indicate the deviation from the mean population score, which is set to 0, standard deviation 1. A z score of -2 (or less) comprises 2.5 % of the normal distribution and is considered to be significantly lower than average. Scaled scores indicate the deviation from the mean population score, which is set to 10, standard deviation 3. A scaled score of 4 (or less) is considered to be significantly lower than average. Standard scores have an age-referenced mean (intelligence quotient, IQ) of 100 and a standard deviation of 15. A standard score of 70 (or less) is considered to be significantly lower than average.
Regarding to neuropsychological testing, Clinical Index (CI) of dysfunction was calculated for each patient considering impairments of single functions: we classified as dysfunction if a patient obtained an impaired score (<2 standard deviations) on at least two cognitive tasks or VABS-I subscales (i.e. of socialization, communication, daily living, and motor skills). This methodology provided dichotomous values and it is useful in order to quantify a wide range of impairments in a unitary measure (a single, dichotomous score from different tests). Fisher's Exact test was used for dichotomous variables. Positive predictive value (PPV), negative predictive value (NPV), sensitivity, specificity, and accuracy were calculated in order to evaluate the prognostic value of metabolomics.
3. Results
3.1. Patients
Twenty-eight children with CHD were studied in the Pediatric Cardiovascular Surgery Unit, “V. Gallucci” center, Padova University Hospital, Italy. Eleven patients presented with a main diagnosis of tetralogy of Fallot (TOF), 3 had septal defects (ventricular or atrial, VSD/ASD), 6 had transposition of the great arteries (TGA), and 8 had diagnosis of univentricular heart. Four children died, 3 during or immediately after surgery and 1 later for non-surgical related cause.
Median age at cardiac surgery was 3.0 (0.4–6.5) months, and median weight was 4.8 (3.4–6.6) kg. They had impaired growth, with a median weight-to-age zscore of -1.4 (-2.5, -0.5). Clinical details are shown in Table 1.
Table 1.
Clinical details on the patient clinical variables.
| Clinical variable | All (n = 28) | TGA (n = 6) | TOF (n = 11) | Septal defects (n = 3) | Univentricular (n = 8) |
|---|---|---|---|---|---|
| Age (months) | 3.0 (0.4–6.5) | 0.3 (0.2–1.2) | 3.3 (2.8–5.2) | 0.4 (2.3–4.4) | 8.0 (0.4–39.0) |
| Gender (M/F) | 14/14 | 0/6 | 7/4 | 3/0 | 4/4 |
| SaO2 (%) | 88 (85–95) | 87 (82–92) | 94 (89–94) | 88 (95–97) | 85 (72–88) |
| Cerebral saturation (rO2) | 55 (45–63) | 52 (45–60) | 57 (52–63) | 43 (45–48) | 58 (41–66) |
| Blood pH before surgery | 7.42 (7.34–7.49) | 7.45 (7.37–7.55) | 7.41 (7.34–7.48) | 7.42 (7.43–7.80) | 7.40 (7.31–7.47) |
| Serum Lactate before surgery (mmol/L) | 1.10 (0.80–2.06) | 2.18 (1.66–2.84) | 0.84 (0.71–1.22) | 0.88 (1.00–2.97) | 1.07 (0.91–1.65) |
Data are expressed as median (interquartile range) or median (min - max) for septum defects. TGA: transposition of the great arteries; TOF: tetralogy of Fallot.
3.2. PCA loading scores
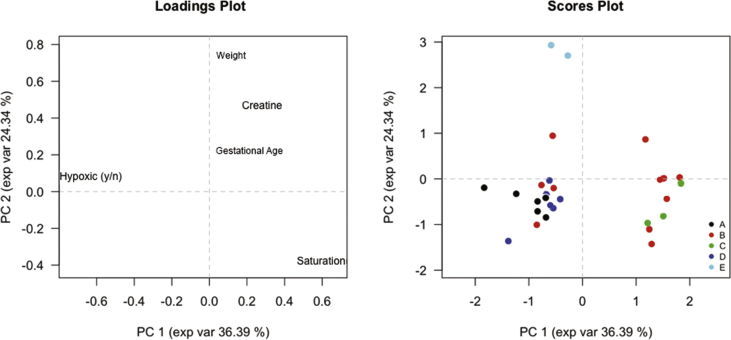
In Fig. 1, we reported the grouping obtained using only pre-surgery clinical characteristics. Oxygen-related variables are the main factors affecting clustering, revealed mainly along the first principal component (PC1) axis. Hypoxia, defined as preoperative arterial saturation <90 %, was first selected for a rough classification and was then refined through the actual oxygen saturation (second principal component, PC2 axis). All CHD, except TOF, tended to form a compact cluster and outliers could be explained by definite clinical characteristics (e.g. particularly complex heart anatomy or comorbidity).
Fig. 1.
Principal component analysis (PCA) loadings and scores. A: Transposition of the great arteries; B: Tetralogy of Fallot; C: Septum defects; D: Hypoplastic left-heart syndrome - Norwood first stage surgery; E: Hypoplastic left-heart syndrome - Fontan surgery. Left panel represents variables (clinical characteristics) used for PCA scoring, while right panel represents patients collocation based on their clinical characteristics. PC1 and PC2: principal component 1 and 2.
TOF patients (red) are distributed along the PC1 axis reflecting the heterogeneity of the disease. These patients are distinguished as normo-saturated (on the right side) vs. hypoxic TOF (left side of PC1 axis) according to their oxygen saturation recorded before surgery. VSD/ASD (green) are clustered on the right side of the graph and they are therefore not hypoxic (normoxic) subjects. Instead, on the left of the graph are grouped all hypoxic CHD infants. Within this group, there are three types of CHD: TGA and Norwood patients that have a similar pattern, and Fontan patients that are in the highest part of the Fig. 1 because of their higher weight.
3.3. NMR spectra
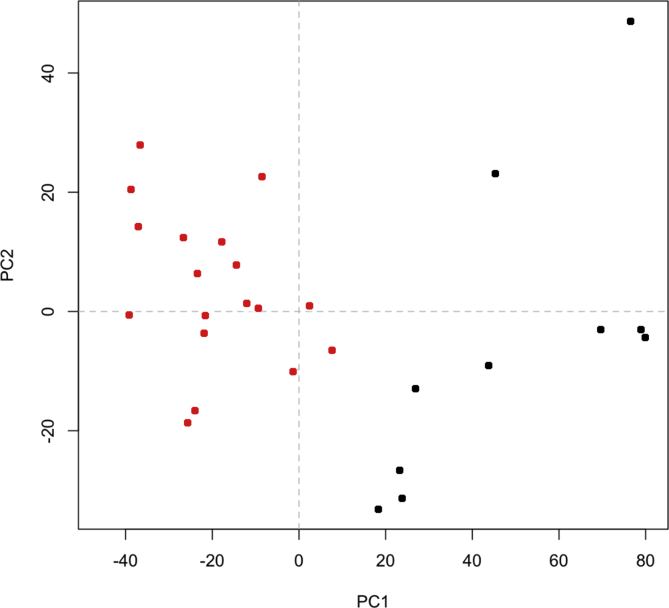
PCA clustering of urinary NMR spectra resulted in two distinct metabolic profiles, represented as RED and BLACK profiles in Fig. 2, that identifies two groups of infants. Glucose, sucrose, acetate, formate, lactate, succinate, alpha-ketoglutarate, creatine, and urea were the compounds over-represented in the RED cluster. Metabolic profiles belonging to the RED cluster showed higher levels of accumulation of citric acid cycle intermediates and glucose compared to the profiles in the BLACK cluster, indicating a possible switching to anaerobic metabolism.
Fig. 2.
Principal component analysis (PCA) of NMR spectra. NMR spectra reflecting the metabolites composition of urines in each patient were analyzed with an unsupervised method (PCA) resulting in a classification of CHD patients in two distinct clusters (RED and BLACK). PC1 and PC2: principal component 1 and 2.
These infants’ clusters did not correspond to the pre-surgery clinical clusters depicted in Fig. 1. Moreover, metabolic clustering was not associated with the type of CHD, oxygen saturation before surgery, or age (data not shown).
3.4. Neurodevelopmental outcome
We were able to administer the brief neuropsychological battery and VABS-I interview to 19 out of 24 patients alive at the 18 months follow-up after cardiac surgery: five parents refused the interview or were unreachable.
By considering individual impairments, 52% of the children obtained an impaired score to at least one subscale of the VABS-I, 9% to the Raven Colored Matrices, 17% to the visual attention test, 33% to the Coding test, 9% to the visual-motor integration test.
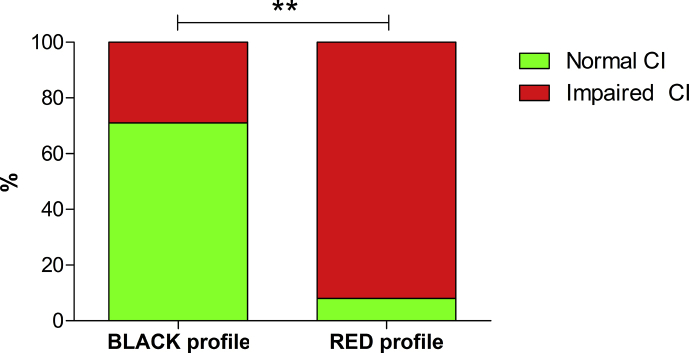
Considering the CI, 11/12 children in the RED metabolic cluster vs 2/7 in the BLACK cluster meet the criteria of neuropsychological dysfunctions (Table 2, Fig. 3). The two patients meeting criteria of neuropsychological dysfunctions in the BLACK cluster showed both an impairment in daily abilities and in social functioning.
Table 2.
Cross tabulation of children with impaired and normal Clinical Index with respect to metabolic clustering.
| Metabolic cluster | Clinical index |
Total | |
|---|---|---|---|
| Normal | Impaired | ||
| BLACK | 5 | 2 | 7 |
| RED | 1 | 11 | 12 |
| Total | 6 | 13 | 19 |
RED and BLACK refer to the dichotomous classification of patients obtained by the analysis of urinary NMR spectra.
Fig. 3.
Clinical Index of neurodevelopment. Clinical Index (CI) of dysfunction was calculated for each patient considering impairments of single functions in neurodevelopmental testing. RED and BLACK refer to the clustered classification of patients obtained by the principal component analysis of metabolomic urinary NMR spectra. p = .01 (Fisher's Exact test).
The diagnostic performance of metabolic clustering on impairments of neuropsychology were: PPV 92%, NPV 71%, specificity 83%, sensitivity 85% and overall accuracy 84%, p = .01 (Fisher's Exact test).
4. Discussion
In this study, we enrolled patients with the major CHD diseases and we cluster them according to urine NMR metabolomic analysis. The metabolic clustering showed a good prognostic performance on neurodevelopmental outcome, with both high sensitivity and specificity.
CHD children represent a unique model for biomarker testing and validation since most of them undergo elective, mostly standardized, surgery after prenatal diagnosis [27]. CHD are a heterogeneous group of diseases that, in our case, were not particularly linked to a single genetic mutation or common group of mutations. Metabolomics was previously successfully employed to detect foetuses with CHD using only a single sample of maternal serum. This method seemed to be more effective than ultrasound screening (the current standard for diagnosis) in detecting CHD [28].
Here we applied a metabolomic approach in an exploratory pilot study designed to test a classification that could predict the neurodevelopmental outcome of CHD patients, enhancing the anatomical-clinical and morphological classification.
Our data suggest that NMR spectra predict the infants’ neurodevelopment in a better fashion than their anatomical and/or clinical classification. In fact, we did not identify a single or group of clinical variables, such as pre-operative oxygen saturation or CHD type, able to reflect the classification obtained from the NMR metabolic profile. This is in line with the assumption that metabolome is the most “up-to-date” insight on the status of the system, compared to proteomics and genomics that are thought to be less actual [29]. The metabolites we identified, involving an accumulation of citric acid cycle intermediates and glucose, indicated a shift toward the anaerobic metabolism typical of cyanotic patients. Since key cluster-defining metabolites were from the energy metabolism, we firstly speculated that oxygen saturation could explain the majority of the clustering obtained by NMR spectra analysis but it was not true. This is probably due to the fact that the overall chronic oxygenation status is not precisely described by the oxygen saturation near surgery, and thus metabolomics could help to have a more precise look into energy metabolism modification rather than a mere oxygen saturation measurement.
An accurate determination of the risk of neurodevelopmental abnormalities in this population is challenging, but our data support the hypothesis of a prognostic role of metabolomics on neurodevelopment. Metabolite clustering demonstrated to correctly identify the majority of children with neurodevelopmental sequelae (sensitivity 85%), while maintaining a low rate of children incorrectly defined as not impaired (NPV 71%). Since sensitivity increases as the number of false negatives reduces, it represents a useful index especially in those mild-moderate cases in which other reliable clinical or instrumental prognostic indicators are lacking.
In children who underwent surgery for complex CHD, a wide spectrum of neuro-cognitive deficits have been reported at the beginning of school age (about 50%) [30], with differential effects of the severity of CHD emerging gradually over time [7, 31]. The etiology of neurodevelopmental sequelae is not well understood, but neuroimaging and neurodevelopmental studies have identified an altered brain maturation with a disruption of cognitive network as a probable cause [8]. The cerebral damage could originate in utero and before surgery from disorders of cerebral blood flow due to the heart condition [8], in a period of crucial importance for the development of the nervous system. In severely hypoxic children the lack of ketone production could act in two ways toward brain injury: depriving the brain of its principal anaerobic source of energy, and taking away a neuroprotective class of molecules possibly exposing the brain to further insults during surgery (i.e. cardiopulmonary bypass) [9]. Given the immaturity of the nervous system at this stage, the real effects of these processes on cortical circuitries are not evident since several years after the cerebral damage, as more complex functions emerge, revealing the underlying neurobiological vulnerability, as shown in other pathological conditions [32].
Moreover, late identification of ongoing abnormal neurodevelopment, often at school age, means that an important opportunity for therapeutic interventions during the early formative developmental window may be lost. Early identification of children at risk of developing neurodevelopmental sequelae is crucial for interventions and rehabilitative programs: in early infancy changes on brain circuitry and on cortical refinement can still occur; at this time the milestones at the bottom of subsequent maturation of more complex cognitive abilities were posed. In the absence of interventions, disrupted cerebral circuitries may undergo a cumulative effect on ongoing development, showing a worsening of cognitive functioning during childhood [33].
No different clinical outcome (i.e. ICU stay, total length of stay, and duration of mechanical ventilation) was observed among the clusters. Moreover, all patients that died in the post-surgery period (n = 3) are grouped in the BLACK metabolic cluster suggesting that surgical challenges and complex physiological interactions may still be important factors that influence the overall short-term outcome of surgery.
Major limitations of this study were the small number of patients that precluded a deeper analysis of CHD children metabolism and neurodevelopmental outcome in different diseases and through the same disease. Moreover, an external validation in an independent cohort is lacking.
In conclusion, we found that metabolic profile before CHD surgery could be useful for providing a more precise classification of CHD patients based on their neurodevelopmental outcome, possibly enhancing the anatomical-clinical and morphological classification. This classification, once validate in a larger cohort, could be useful to stratify CHD children for their neurodevelopmental risk in order to plan timely interventions aimed to minimize their burden.
Declarations
Author contribution statement
Luca Vedovelli: conceived and designed the experiments; wrote the paper.
Paola Cogo, Virgilio Carnielli: conceived and designed the experiments; contributed reagents, materials, analysis tools or data.
Elisa Cainelli, Agnese Suppiej, Massimo Padalino, Giuseppe Buonocore: performed the experiments; analyzed and interpreted the data.
Maria Tassini, Giovanni Stellin: performed the experiments; contributed reagents, materials, analysis tools or data.
Manuel Simonato: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data.
Mariangela Longini: conceived and designed the experiments; analyzed and interpreted the data.
Funding statement
This work was supported by the Grant Program for Young Investigators on Pediatric Research, CARIPARO Foundation 2013 (LV), Fondazione Umberto Veronesi Fellowships 2017 (LV) and from the Just Foundation grant 2016 (PC). Funding sources were not involved in any part of the study.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would thank Dr. Marco Calderisi from Kode Solutions s.r.l. for the statistical analysis of NMR spectra.
References
- 1.Hoffman J.I., Kaplan S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Oster M.E., Lee K.A., Honein M.A., Riehle-Colarusso T., Shin M., Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131:e1502–e1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger D.C., Wypij D., Du Plessis A.J., Rappaport L.A., Riviello J., Jonas R.A., Newburger J.W. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J. Thorac. Cardiovasc. Surg. 2001;121:374–383. doi: 10.1067/mtc.2001.111206. [DOI] [PubMed] [Google Scholar]
- 4.Licht D.J., Wang J., Silvestre D.W., Nicolson S.C., Montenegro L.M., Wernovsky G., Tabbutt S., Durning S.M., Shera D.M., Gaynor J.W., Spray T.L., Clancy R.R., Zimmerman R.A., Detre J.A. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J. Thorac. Cardiovasc. Surg. 2004;128:841–849. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Dent C.L., Spaeth J.P., Jones B.V., Schwartz S.M., Glauser T.A., Hallinan B., Pearl J.M., Khoury P.R., Kurth C.D. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J. Thorac. Cardiovasc. Surg. 2005;130:1523–1530. doi: 10.1016/j.jtcvs.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 6.Limperopoulos C., Tworetzky W., McElhinney D.B., Newburger J.W., Brown D.W., Robertson R.L., Guizard N., McGrath E., Geva J., Annese D., Dunbar-Masterson C., Trainor B., Laussen P.C., Du Plessis A.J. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandlistuen R.E., Stene-Larsen K., Holmstrom H., Landolt M.A., Eskedal L.T., Vollrath M.E. Motor and social development in 6-month-old children with congenital heart defects. J. Pediatr. 2010;156:265–269.e1. doi: 10.1016/j.jpeds.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Morton P.D., Ishibashi N., Jonas R.A. Neurodevelopmental abnormalities and congenital heart disease. Circ. Res. 2017;120:960–977. doi: 10.1161/CIRCRESAHA.116.309048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puchowicz M.A., Zechel J.L., Valerio J., Emancipator D.S., Xu K., Pundik S., Lamanna J.C., Lust W.D. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J. Cereb. Blood Flow Metab. 2008:2879. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riekeberg E., Powers R. New frontiers in metabolomics: from measurement to insight. F1000Research. 2017;6:1148. doi: 10.12688/f1000research.11495.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klupczyńska A., Dereziński P., Kokot Z.J. Metabolomics in medical sciences--trends, challenges and perspectives. Acta Pol. Pharm. 2015;72:629–641. http://www.ncbi.nlm.nih.gov/pubmed/26647618 [PubMed] [Google Scholar]
- 12.Davis V.W., Bathe O.F., Schiller D.E., Slupsky C.M., Sawyer M.B. Metabolomics and surgical oncology: potential role for small molecule biomarkers. J. Surg. Oncol. 2011;103:451–459. doi: 10.1002/jso.21831. [DOI] [PubMed] [Google Scholar]
- 13.Wishart D.S. Advances in metabolite identification. Bioanalysis. 2011;3:1769–1782. doi: 10.4155/bio.11.155. [DOI] [PubMed] [Google Scholar]
- 14.Groenen P.M.W., Engelke U.F., Wevers R.A., Hendriks J.C.M., Eskes T.K.A.B., Merkus H.M.W.M., Steegers-Theunissen R.P.M. High-resolution 1H NMR spectroscopy of amniotic fluids from spina bifida fetuses and controls. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004;112:16–23. doi: 10.1016/s0301-2115(03)00279-3. http://www.ncbi.nlm.nih.gov/pubmed/14687733 [DOI] [PubMed] [Google Scholar]
- 15.Raven J., Raven J. Raven progressive Matrices. Handb. Nonverbal Assess. 2003:223–237. [Google Scholar]
- 16.Korkman M., Kirk U., Kemp S. second ed. Psychological Corporation; San Antonio, TX: 2007. NEPSY-II: a Developmental Neuropsychological Assessment. [Google Scholar]
- 17.Urgesi C., Campanella F., Fabbro F. second ed. Contributo alla taratura italiana, Giunti Organizzazioni Speciali; Firenze: 2011. NEPSY-II. [Google Scholar]
- 18.Orsini A., Pezzuti L., Picone L. Giunti Organizzazioni Speciali; Firenze: 2012. WISC-IV. Contributo Alla Taratura Italiana. [Google Scholar]
- 19.Beery K., Beery N. fifth ed. Modern Curriculum Press; Cleveland, OH: 2004. The Beery-Buktenica Developmental Test of Visual Motor Integration: Administration, Scoring, and Teaching Manual. [Google Scholar]
- 20.Sparrow S.S., Balla D.A., Cichetti D.V. National Council on Measurement in Education; Circle Pines, MN: 1984. Vineland Adaptive Behavior Scales. [Google Scholar]
- 21.Savorani F., Tomasi G., Engelsen S.B., icoshift A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010;202:190–202. doi: 10.1016/j.jmr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Jackson J.E. 1991. A User’s Guide to Principal Components. [Google Scholar]
- 23.Jolliffe I.T. Principal component analysis. J. Am. Stat. Assoc. 2002;98:487. [Google Scholar]
- 24.Wold S., Sjöström M., Eriksson L. Chemom. Intell. Lab. Syst.; 2001. PLS-regression: a basic tool of chemometrics; pp. 109–130. [Google Scholar]
- 25.BjørnHelge L.K., Wehrens R.M. PLS Partial Least Squared Princ. Compon. Regression. R Packag. Version 2.43. 2013. PLS: partial least squared and principal component regression.http://cran.rproject.org/package=pls [Google Scholar]
- 26.Chong I.G., Jun C.H. Performance of some variable selection methods when multicollinearity is present. Chemometr. Intell. Lab. Syst. 2005;78:103–112. [Google Scholar]
- 27.Vedovelli L., Padalino M., Simonato M., D’Aronco S., Bertini D., Stellin G., Ori C., Carnielli V.P., Cogo P.E. Cardiopulmonary bypass increases plasma glial fibrillary acidic protein only in first stage palliation of hypoplastic left heart syndrome. Can. J. Cardiol. 2016;32:355–361. doi: 10.1016/j.cjca.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Bahado-Singh R.O., Ertl R., Mandal R., Bjorndahl T.C., Syngelaki A., Han B., Dong E., Liu P.B., Alpay-Savasan Z., Wishart D.S., Nicolaides K.H. Metabolomic prediction of fetal congenital heart defect in the first trimester. Am. J. Obstet. Gynecol. 2014;211:240.e1–240.e14. doi: 10.1016/j.ajog.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 29.Kordalewska M., Markuszewski M.J. Metabolomics in cardiovascular diseases. J. Pharm. Biomed. Anal. 2015;113:121–136. doi: 10.1016/j.jpba.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Majnemer A., Limperopoulos C., Shevell M., Rohlicek C., Rosenblatt B., Tchervenkov C. Developmental and functional outcomes at school entry in children with congenital heart defects. J. Pediatr. 2008;153:55–60. doi: 10.1016/j.jpeds.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Sarrechia I., Miatton M., Francois K., Gewillig M., Meyns B., Vingerhoets G., De Wolf D. Neurodevelopmental outcome after surgery for acyanotic congenital heart disease. Res. Dev. Disabil. 2015;45–46:58–68. doi: 10.1016/j.ridd.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Cainelli E., Trevisanuto D., Cavallin F., Manara R., Suppiej A. Evoked potentials predict psychomotor development in neonates with normal MRI after hypothermia for hypoxic-ischemic encephalopathy. Clin. Neurophysiol. 2018;129:1300–1306. doi: 10.1016/j.clinph.2018.03.043. [DOI] [PubMed] [Google Scholar]
- 33.Aarsen F.K., Paquier P.F., Reddingius R.E., Streng I.C., Arts W.-F.M., Evera-Preesman M., Catsman-Berrevoets C.E. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106:396–402. doi: 10.1002/cncr.21612. [DOI] [PubMed] [Google Scholar]