Abstract
Prophylactic use of antibiotics in poultry diets has been identified as a problematic practice because of its potential to exacerbate the spread of antibiotic resistance to human pathogens. A range of countries have opted to completely ban the use of antibiotics in animal feed. The animal production industries are looking for alternative ways to effectively control pathogens while providing the performance benefits previously secured by antibiotics in feed. Here, we present evidence that oregano (Origanum vulgare) could be a potential alternative for pathogen control in the poultry industry. Broiler diets were supplemented with oregano powder (0%, 0.5%, 1%, and 2%) for six weeks. The capacity for pathogen control was estimated by microbiota profiling of the jejunum, ileum, and caecum content, and in the faeces, by 16S rRNA gene amplicon sequencing. The concentrations of short-chain fatty acids in the caecal content were also measured, as were villus/crypt parameters in the ileum. There were no differences among treatments in weight gain, feed intake, or the concentration of short-chain fatty acids. The height, width, and the surface area of villi in the ileum were not influenced by oregano addition. However, 1% and 2% of oregano produced a significant increase in the villus height to crypt depth ratio. There were no visible histopathological changes in the liver in control and treated groups. Although oregano had no significant effect on overall microbial diversity and gross composition, some specific genera, like Proteus, Klebsiella and Staphylococcus, which include known pathogens, were reduced in relative abundance by oregano treatment. Bifidobacterium, recognized as a beneficial and probiotic genus, was also suppressed by the oregano treatment.
Keywords: Food microbiology, Animal science, Microbiology, Public health, Gastrointestinal system, Oregano, Chicken, Microbiota, Antibiotic alternative
Food microbiology; Animal science; Microbiology; Public health; Gastrointestinal system; Oregano, Chicken, Microbiota, Antibiotic alternative
1. Introduction
The poultry industry has to deal with high pathogen load environments, influenced by high bird stocking density and faecal loaded surroundings, requiring control measures to reduce the incidence and risk of disease outbreaks. For many years the industry has used in-feed supplementation of antibiotic growth promoters (AGPs) to control poultry pathogens. The beneficial effects of sub-therapeutic use of AGPs on bird health have been particularly evident on farms where biosecurity and bird living conditions were the poorest. This suggests that improving the living conditions and animal husbandry techniques could be an effective way to reduce the requirement for AGPs. However, it is prudent to maintain multiple lines of defence against pathogens to prevent disease outbreaks. A major challenge for pathogen control in broiler sheds is the high excreta pathogen load that can be found in the litter, particularly as coprophagous activity is normal amongst broilers. Interactions with the litter have been implicated in the bird to bird transmission of pathogens such as Campylobacter jejuni [1]. The use of AGPs and their ability to control pathogens and improve the overall health and performance of birds has contributed to the immense growth of the poultry industry. However, the use of AGPs in production has been gradually overshadowed by the public concern of antibiotics in the human food chain as well as the potential for farms to become breeding grounds for antibiotic-resistant bacteria.
Phytobiotics, such as oregano's volatile compounds - carvacrol, thymol, and their pre-cursors p-cymene and γ-terpinene, have been demonstrated to have synergistic/additive effects, such as antifungal, antiparasitic, antioxidant and antimicrobial activities. This suggests that oregano could be a viable alternative to AGPs provided there are no negative effects on the birds' health and that the efficiency of controlling the pathogen load in the gastrointestinal tract community is confirmed in vivo. Carvacrol and other oregano compounds can be cytotoxic [2, 3] at high doses indicating the need for optimising the concentration of oregano in feed to achieve maximum positive effects without cytotoxic effects. Additionally, there is a valid concern that phytobiotics may induce microbial resistance [4]. Use of phytobiotics in the form of essential oil is complicated by the volatility of compounds, which leads to rapid absorption in the upper gastrointestinal tract and difficulty in delivering sufficient dosages to the lower regions of the gut.
In the current study, chicken feed was supplemented with a range of oregano powder concentrations (0%, 0.5%. 1% and 2%) to investigate the effects on the pathogen load in the gastrointestinal tract and on short-chain fatty acid (SCFA) production in the caecum of broiler chickens. We evaluated liver cytotoxicity and ileal digestive capacity using histological assessment of these sections. Our results demonstrate the ability of oregano to control some poultry and human pathogens without visible effect on ileal and liver morphology.
2. Materials and methods
2.1. Oregano powder preparation
The dried aerial parts of oregano (Turkish, Saucy Spice Company, NSW) were used to make the powder. We have previously evaluated the effect of the oregano spice particle size on the growth of poultry pathogens in-vitro and concluded that the oregano powder with particle size lower than 80μm are the most efficient in pathogen control. Oregano was processed by blending (100g, 1.5min/max, 1500W, Nutri Ninja Auto iQ Duo, SharkNinja, USA) to reduce particle size. That powder was then processed in a Planetary Ball Mill Machine (speed no. 5, 2 hrs, 40g*each run; Changsha Yonglekang Equipment, China). The oregano was then placed in an electric sieve machine (Changsha Yonglekang Equipment, China); the powder used in the trial was the material that passed through a 75μm sieve. The particle size of the final oregano powder product was determined by laser diffraction (Mastersizer 2000, Malvern, ATA scientific, Australia) to have an average diameter of 10μm.
2.2. Feed preparation
Chicken starter diet (Red Hen, Laucke Mills, Australia) with no antimicrobials or coccidiostats was used throughout the trial. The feed was formulated to meet or exceed the National Research Council guidelines for broiler chickens [5]. The oregano was mixed into the feed to make 3 treatment groups 2% (0.02 kg/kg w/w), 1% (0.01 kg/kg w/w) and 0.5% (0.005 kg/kg w/w) in an electric mixer (125L cement mixer CMX-125, Ozito, China).
2.3. Birds and management
The study was approved by the Animal Ethics Committee of Central Queensland University under the approval number 0000020312. Forty eight one-day-old chicks (Ross Broiler 308, Bond Enterprises, Toowoomba) were randomly distributed into four pens, with 12 birds per pen. Each pen received feed supplemented with a different amount of oregano: the control 0%, 0.5%, 1% and 2%. The purpose of this experiment was not to evaluate bird performance but rather to evaluate the effects of oregano on gastrointestinal sections and on pathogen load reduction. All birds were fed ad libitum and had unrestricted access to drinking water. Birds were individually tagged using leg bands and weighed every week. The trial ran for a total of 42 days. Faecal material was collected for each bird by placing a transparent loose chicken wire enclosure around individual birds and waiting until fresh faeces were deposited and collected. Birds were euthanised at day 42 post hatch (CO2, BOC, Australia) and dissected. Jejunum, ileum and caecum contents were taken for microbiota analysis, and liver and ileum sections were collected for histology.
2.4. Short chain fatty acids measurement
The standards and samples were analysed on a GC-MS (GC-MS-QP2010 Ultra) fitted with a AOC-20s Shimadzu autosampler and a Shimadzu AOC-20i auto-injector with a polar column (Agilent J&W GC, 30m, 0.250 diameters (mm), film 0.25 (μm) temperature limits form 40 °C–260 °C).
SCFAs were determined by injecting a 1 μl sample at 250 °C with helium (1.97 ml/min, 5.0, Coregas, Australia) as the carrier gas. The injection mode had a 5.0 split. The pressure was maintained at 143.3 kPa and a helium flow of 103.4 ml/min. The mass spectrometer operated in the electron ionization mode at 0.2kV, source temperature was 220 °C, and the scan mode was between 33 to 150m/z.
2.5. Histology
The tissue samples of liver and the midpoint of the ileum were collected and fixed in 10% buffered formalin solution. Fixation, paraffin embedding, deparaffinization, rehydration and staining with hematoxylin and eosin (H&E), were done by routine laboratory procedures. Glass slides were scanned at the TRI Microscopy Core Facility (Brisbane, Australia) using a Nikon Brightfield, Olympus VS120 slide scanner and analysed using Olympus microscopy software Olyvia. Morphometric analysis of the ileum was performed using Olympus software SensEntry 1.13. For each tissue sample, 20 well-oriented villi and crypts were examined. The measured parameters were: villus height (distance from the tip to the bottom of the villi), villus width (mean value between basal and apical villi width) and the crypt depth (distance between the crypt neck and its base). These morphometric measures were also utilised for the calculation of the villus surface area and the villus height to crypt depth ratio. The villus surface area was calculated using the equation [6]: Villus surface area [μm2] = π × Villus height [μm] × Villus width [μm].
As datasets were homogenous (cv<30%), the groups were compared using one-way ANOVA followed by Tukey's multiple comparison test. A significant difference was estimated at P < 0.05 and P < 0.01 significance levels. Statistical analysis of the results obtained in the experiment was carried out using statistical software GraphPad Prism version 6 (GraphPad, San Diego, CA, USA).
2.6. DNA extraction
DNA was extracted from jejunal, ileal, caecal and faecal samples. Approximately 0.2 g of sample was transferred into tubes containing 0.2 g of glass beads (0.1 mm diameter) and 0.7 ml of lysis buffer (500 mM NaCl, 50 mM EDTA, 50 mM TrisHCl (pH 8), 4% SDS). Samples were homogenised at maximum speed for 5 min (Mini-Beadbeater, Biospec products) and incubated at 75 °C for 15 min with vortexing at 5 min intervals. Samples were then centrifuged (16,000 rcf, 5 min) and 0.4 ml of the supernatant was combined with 500 μl of binding buffer (5 M Gu-HCl, 30% isopropanol) and transferred into a DNA spin column with a collection tube (Enzymax LLC, Cat# EZC101, Kentucky, US). The spin column was centrifuged (8,000 rcf, 1 min) and the contents of the collection tube discarded. The spin column was then washed twice with 800 μl of wash buffer (10 mM Tris-HCl, 80% ethanol (pH7.5) centrifuging at 8,000 rcf for 1 min. The spin columns were dried by centrifugation (8,000 rcf, 1 min) and placed in new collection tubes and eluted with 50 μl of elution buffer (10 mM Tris-HCl). The DNA quality and quantity was estimated using a NanoDrop spectrophotometer.
2.7. 16S rRNA gene sequencing
Primers used for amplification of the V3–V4 region of 16S rRNA genes were: forward ACTCCTACGGGAGGCAGCAG, reverse GGACTACHVGGGTWTCTAAT. The primers contained barcodes, spacers and Illumina sequencing linkers that have been previously described [7]. The 16S rRNA gene sequencing library preparation and amplification followed the manufacturer's protocol (Illumina Inc., San Diego, CA, USA). Sequencing was conducted on the Illumina MiSeq platform using 2 × 300 bp paired-end sequencing.
The microbial communities of each sample were initially analysed using Quantitative Insights Into Microbial Ecology (QIIME v.1.9.1) [8]. Paired-end sequences were combined using the Fastq-Join algorithm, allowing no mismatches within the region of overlap. Phred quality threshold had a minimum of 20. OTUs were picked at 97% similarity using Uclust [9] and inspected for chimeric sequences using Pintail [10]. All taxonomic assignments were performed in QIIME against the GreenGenes database and QIIME default parameters [11]. A UniFrac matrix was calculated in QIIME using a rarefied table of OTU abundance. Calypso was used to further explore and present the data [12]. After quality filtering, 16S rRNA gene amplicon data for 36 faecal, 39 caecum, 41 ileum and 41 jejunum, samples were included in the analysis. The sequence data is publicly available at the MG-RAST database under library accession number mgl745316 and a project ID mgp89580.
All OTUs with less than 0.01% abundance were removed. Statistical analysis including Spearman correlations, alpha and beta diversity were done on Hellinger transformed [13] OTU table. Significantly differentially abundant taxa were analysed using ANOVA. Bodyweight data were analysed using a one-way ANOVA in IBM SPSS Statistics. Significance was considered at P < 0.05.
3. Results
3.1. Bird performance
The experiment had a relatively low mortality rate (4%) and there were no significant differences in bird weights (P = 0.514) or feed intake between treatment groups over the 42 day grow-out period.
3.2. Gut histology and SCFA
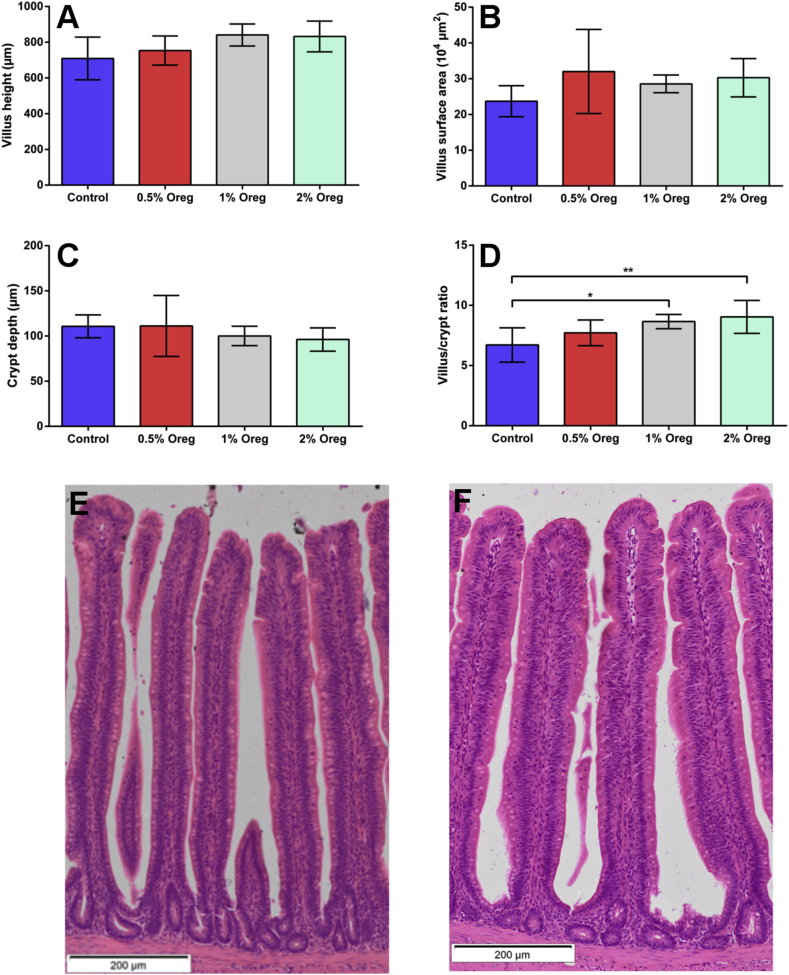
Light microscopic observation revealed there were no significant differences in height, width and the villus surface area in the ileum in oregano supplemented groups (Fig. 1). The morphology of mucosal epithelium was well preserved in all groups. Depth of the crypts was not significantly different; however, the villus height to crypt depth ratio was significantly increased in groups treated with 1% P < 0,05) and 2% oregano (P < 0,01) (Fig. 1). Histological analysis of liver tissue showed no histological appearance of pathological changes and no visible difference between the groups. There were no significant differences in either acetic, butyric, isobutyric nor in valeric acid concentrations in caecal content.
Fig. 1.
Effect of oregano supplementation on the ileal morphology. Statistical analysis of villus height (A); villus surface area (B); crypt depth (C); villus/crypt ratio (D). Results shown as mean ± SD and P < 0.05 was considered statistically significant (*P < 0.05 and **P < 0.01). Microphotography of ileum of the control (E) and 2% oregano supplemented broiler (F); H&E, bar = 200μm.
3.3. Microbiota data summary
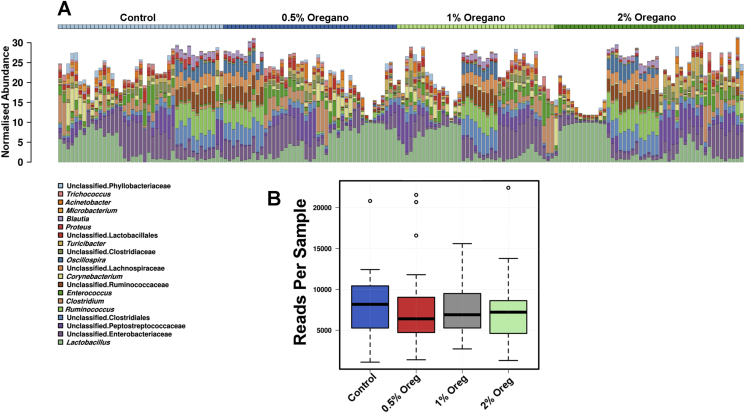
A total of 157 samples from different oregano concentrations (0, 0.5, 1 and 2%) and the gut origins (cecum, ileum, jejunum and faeces) were sequenced (Fig. 2).
Fig. 2.
Hierarchical sample clustering bar-chart showing 20 most abundant genera (A) and the sequencing reads per sample barchart (B).
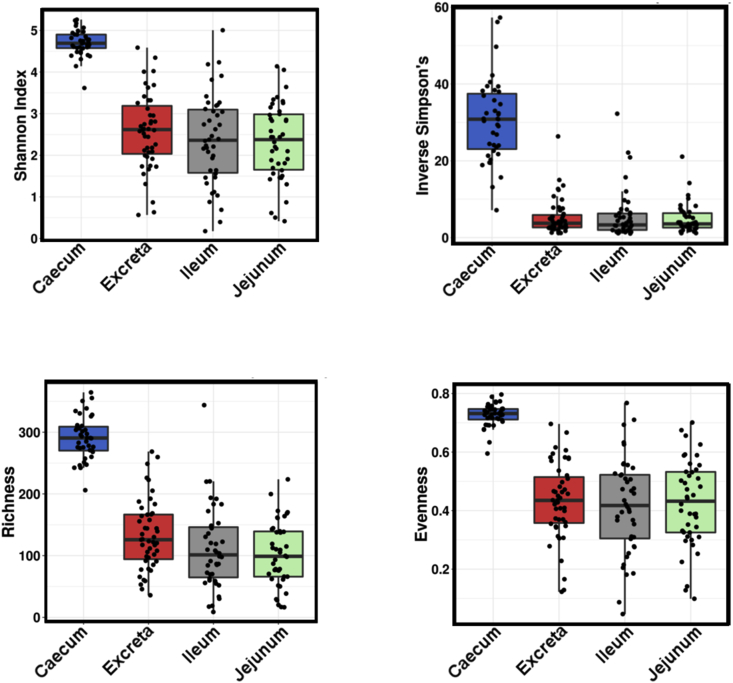
3.4. Alpha diversity
There were no statistically significant differences in alpha diversity indices due to the oregano supplementation, as assessed by Shannon (P = 0.66), Inverse Simpson (P = 0.70), Richness (P = 0.61) or Evenness indices (P = 0.64). However, as expected, the gut sections had very different diversity structure, with caecum showing higher richness and taxa evenness using all indexes analysed: Shannon (P = 3.1e−29), Inverse Simpson (P = 9.1e−45), Richness (P = 7.2e−39) and Evenness index (P = 2.7e−24) as shown in Fig. 3.
Fig. 3.
Influence of oregano on alpha diversity.
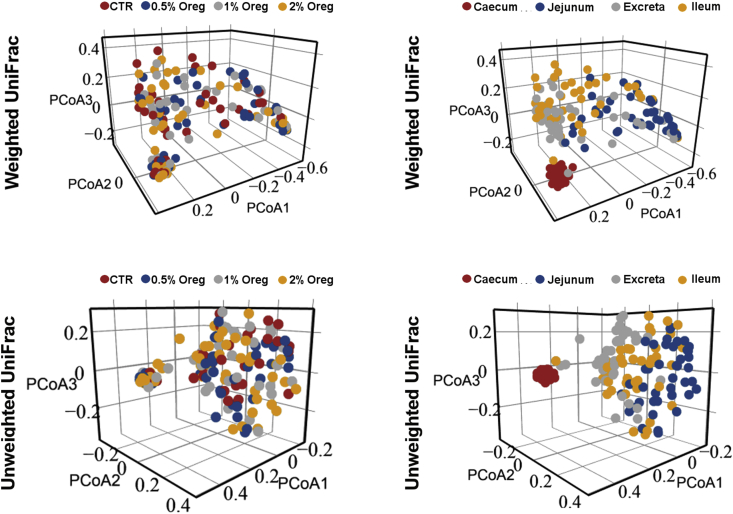
Weighted and unweighted UniFrac at an OTU level and a Bray Curtis matrix at genus and phylum levels were used inspect the differences in beta diversity. Based on weighted UniFrac there was no significant difference between the microbiotas from the treatment groups fed different oregano concentrations (Adonis P = 0.359) nor based on bird sex (P = 0.396) or weight (P = 0.554). However, the gut origin had a very distinct microbiota structure (Adonis P = 3.3e−4). Similarly, based on unweighted UniFrac oregano concentration was not a major influencer (P = 0.456) and neither were the bird sex (P = 0.234) or their weight (P = 0.178), but the gut origin had a significant role in microbiota structure (Adonis P = 3.3e−4) (Fig. 4). Additionally, using Primer-e based PERMANOVA and Bray Curtis distance, at the genus level oregano has no significant influence (P = 0.064), the gut origin was very distinct (P = 1e−4) and there was no significant interaction between the gut origins and oregano concentration (P = 0.194). Based on the above, oregano had a marginal influence on overall gut microbiota independent from the gut sections inspected, thus targeting specific taxa rather than a total community.
Fig. 4.
Oregano and beta diversity expressed as weighted and unweighted UniFrac. Samples are coloured by concentrations of oregano (left) and sampling origin (right).
3.5. Oregano and microbiota
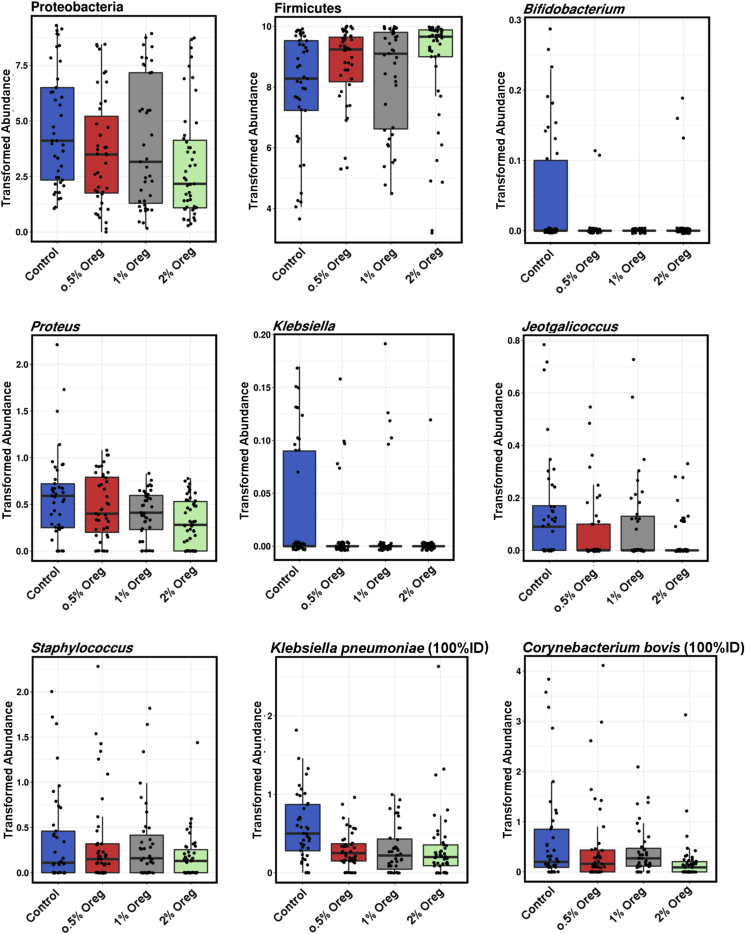
Several taxa at all taxonomic levels were differentially abundant between the groups treated with different oregano concentrations. The phyla that significantly (P < 0.05) differed between the treatments included Tenericutes (higher in oregano treatments), Chloroflexi (lower in oregano groups) and Proteobacteria, reduced in oregano treatments (Table 1). Both Firmicutes and Proteobacteria were significantly correlated (Pearson) with oregano concentration, with Proteobacteria significantly (P = 0.009, R = -0.19) reduced in the higher concentrations of oregano and Firmicutes significantly (P = 0.045, R = 0.15) increased in higher oregano concentrations (Fig. 5). At the genus level (detailed in Table 2), the genera that were differentially abundant between the treatments (Fig. 5) included Bifidobacterium (P = 4.4e−5), Proteus (P = 6.7e−4), Klebsiella (P = 0.003) and Jeotgalicoccus (P = 0.015); all reduced in oregano groups. Proteus (P = 6.3e−5 R = -0.3) and Staphylococcus (P = 0.027, R = -0.17; Fig. 5) were significantly negatively correlated with oregano concentrations.
Table 1.
Phylum level: ANOVA significant and Pearson significantly correlated with oregano concentration phyla.
| Taxa (ANOVA) |
P- value |
FDR corrected |
| Tenericutes | 0.00019 | 0.0011 |
| Chloroflexi | 0.026 | 0.062 |
| Proteobacteria | 0.031 | 0.062 |
| Firmicutes | 0.068 | 0.1 |
| Bacteroidetes | 0.58 | 0.7 |
| Actinobacteria |
0.77 |
0.77 |
| Taxa (Pearson correlated) |
P-value |
R |
| Proteobacteria | 0.0096 | -0.1981 |
| Firmicutes | 0.045 | 0.1541 |
Fig. 5.
Taxa at different phylogenetic levels significantly (P < 0.05) responding to different oregano concentrations.
Table 2.
Genus level: ANOVA significant and Pearson significantly correlated with oregano concentration genera.
| Taxa (ANOVA) |
P- value |
FDR corrected |
| Oligella | 0.0000059 | 0.00067 |
| Bifidobacterium | 0.000044 | 0.0025 |
| Unclassified.RF39 | 0.00019 | 0.0072 |
| Proteus | 0.00067 | 0.019 |
| Klebsiella | 0.0035 | 0.068 |
| Solibacillus | 0.0036 | 0.068 |
| Jeotgalicoccus | 0.015 | 0.24 |
| Unclassified.Alcaligenaceae | 0.017 | 0.24 |
| Unclassified.Bacillales | 0.021 | 0.27 |
| Unclassified.JG30KFCM45 | 0.026 | 0.27 |
| Salinicoccus | 0.026 | 0.27 |
| Granulicatella | 0.041 | 0.36 |
| Unclassified.Carnobacteriaceae | 0.043 | 0.36 |
|
Rhodococcus |
0.049 |
0.36 |
| Taxa (Pearson correlated) |
P- value |
R |
| Proteus | 0.000063 | -0.3018 |
| Unclassified Planococcaceae | 0.018 | -0.1811 |
| Unclassified Enterobacteriaceae | 0.025 | -0.1713 |
| Staphylococcus | 0.027 | -0.1693 |
| Unclassified Gemellales | 0.027 | -0.1701 |
| Unclassified Lactobacillales | 0.044 | -0.155 |
At an OTU level, there were several OTUs that varied in relative abundance between treatments (Fig. 5), with Enterobacteriaceae OTU 291838 (100% identical to Klebsiella pneumoniae subsp. ozaenae strain ATCC 11296 using Blastn across the amplified region), and Corynebacterium OTU 173995 (100% identical to Corynebacterium bovis DSM 20582), negatively correlated with oregano concentration (P = 8.7e−3, R = -0.2 and P = 3.9e−3, R = -0.22, respectively, Fig. 5) and a Clostridiales OTU 284045 (most similar to Ruminococcus lactaris ATCC 29176 (99%)) that was slightly positively correlated (P = 0.012, R = 0.19) with oregano concentration.
3.6. High concentration of oregano
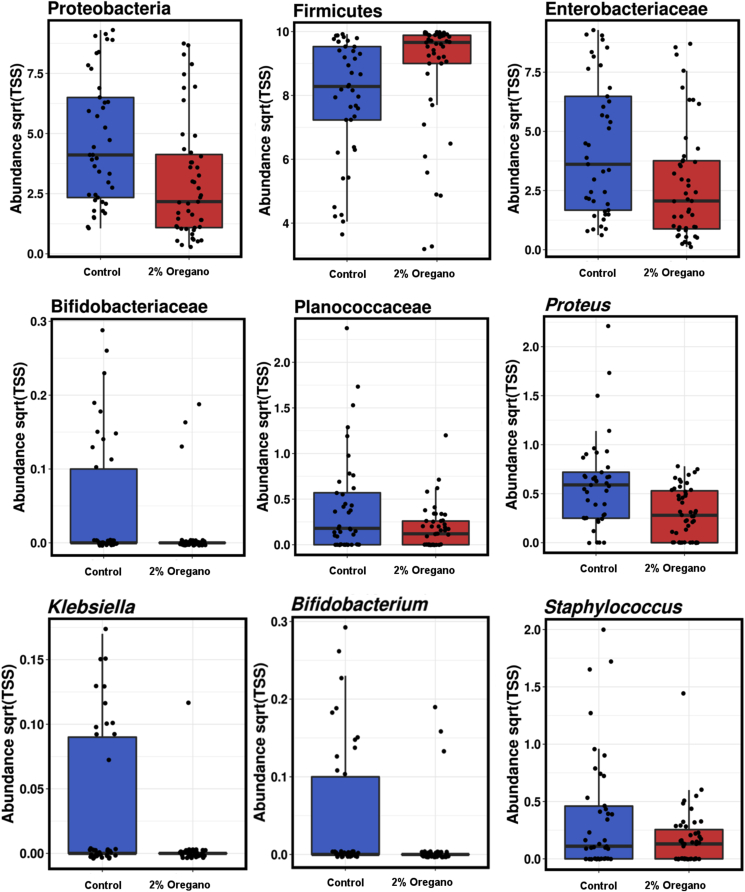
The comparison between the microbiota of the birds at 0% and 2% oregano shows the effects of relatively high concentrations of oregano. Proteobacteria and Firmicutes phyla significantly changed in relative abundance, Enterobacteriaceae and Planococcaceae families were reduced while Bifidobacteriaceae were dramatically inhibited by 2% oregano. At the genus level Proteus, Klebsiella, Bifidobacterium and Staphylococcus were all significantly reduced by 2% oregano in the feed (Fig. 6).
Fig. 6.
Taxonomic levels significantly affected by high concentrations of oregano.
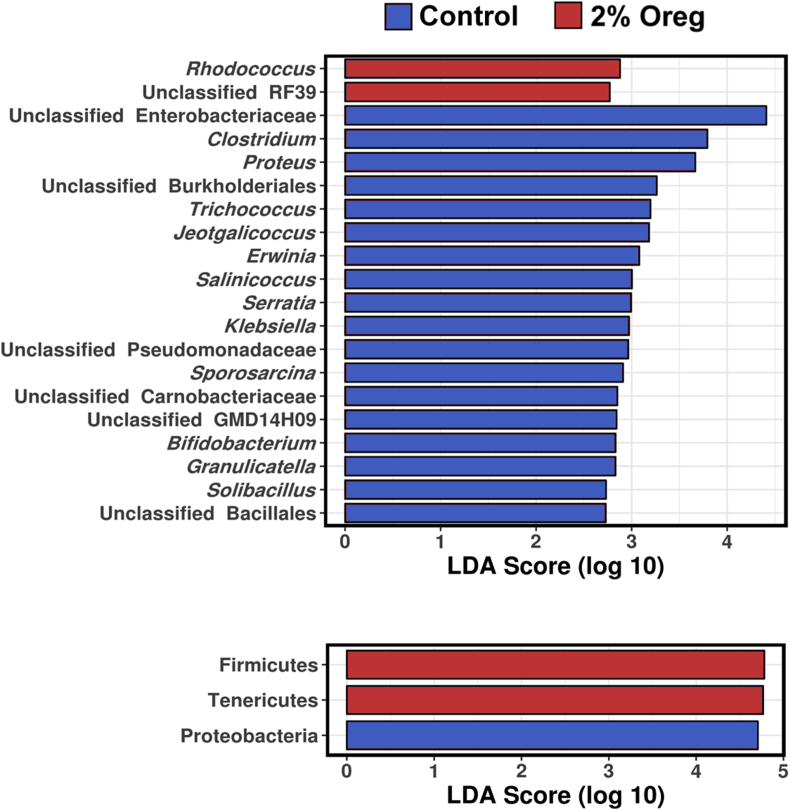
LefSe analysis (Fig. 7) confirmed that poultry pathogen-rich genera Clostridium, Proteus, Serratia, Klebsiella and Sporosarcina as well as beneficial Bifidobacterium, were the genera that most defined the microbiota differences between the control group and the 2% oregano treated group. Proteobacteria was the most defining phylum carried by the control group and higher levels of Firmicutes and Tenericutes were the most defining phyla of the microbiota within the 2% oregano group.
Fig. 7.
LefSe analysis representing taxa that define control and oregano microbial structures.
3.7. Influence of oregano in different gut sections
PERMANOVA analysis demonstrated no difference in response to oregano in different gut sections. Due to the high data volume presented in the manuscript, the detailed account of oregano influence in each intestinal compartment; caecum, ileum, jejunum and faeces, is given in Supplementary File 1. Each sheet within the file presents a data summary for a different sample origin, each containing genus level clustered bar chart, RDA multivariate plot and significance, DAPC group to group association plot, richness and evenness bar charts and a table with ANOVA significant genera with a bar chart figure for all ANOVA significant taxa. Based on RDA analysis, differences between oregano concentrations significantly affected microbial communities in caecum (P < 0.001) and in faeces (P = 0.037) while there were no significant differences in the ileum and jejunum microbiota compositions. There were significant differences in richness only in the jejunum and faecal microbiota, but not in caecum or ileum microbiota (Supplementary File 1).
4. Discussion
A desirable property of potential AGP replacements for pathogen control is that they should not induce major shifts in the gut microbiota, thus avoiding unforeseen consequences of microbial disruption [14].
Proteobacteria are a microbial signature of dysbiosis, and an increase in Proteobacteria has been associated with several GIT diseases including metabolic syndromes, diabetes, cancer, obesity, Chron's disease, inflammatory bowel disease and ulcerative colitis [15]. Additionally, their reduction has been shown to increase bird performance [16]. Enterobacteriaceae was the only family significantly reduced by oregano treatments. Four out of five Enterobacteriaceae genera detected in the study were significantly decreased in various oregano concentrations: Klebsiella, Proteus, Unclassified Enterobacteriaceae genus and Serratia. Klebsiella and Proteus members are associated with a wide range of diseases within human and animal health, as well as antimicrobial resistance. They are known to be related to diseases that result from biofilm production [17, 18], host cell invasion [19, 20], translocation-related [21, 22, 23] and foodborne illness [24, 25].
Enterobacteriaceae family members have been shown to rapidly produce biofilms in heterogeneous bacterial populations within the mucus layer of the intestinal epithelium [26]. Biofilm communities are capable of exclusion of antimicrobial agents and are associated with many persistent bacterial infections [27] and adherence to epithelial cells, which is essential for bacterial host invasion. However, the mechanisms are dynamic and still not well understood. For example, Klebsiella has been shown to use a transcellular pathway to translocate without the requirement for degradation of tight junction proteins, likely by hijacking eukaryotic signalling machinery to control downstream cytoskeleton dynamics [28]. In a study conducted in mice, Klebsiella increased inflammation by producing β-glucuronidase and endotoxin lipopolysaccharide, which resulted in reduced tight junction proteins [29]. Additionally, Klebsiella strains are becoming of increasing concern due to antimicrobial resistance particularly within the broiler industry where a study conducted in China has shown that 96.7% of Klebsiella were multidrug resistant [30, 31, 32]. Proteus has been correlated with 5.8% of deaths associated with bacterial related foodborne disease in China between 1994-2005 [33].
Carvacrol is the main antimicrobial compound found in oregano plants and is known to interact with cell membranes [34]. Carvacrol hydroxyl group has shown to facilitate the transport of cations across the cell membrane, which reduces membrane potential, eventually leading to cell death [35], subsequently, carvacrol's ability to interact with cell membranes means that there is potential for cytotoxicity [2, 36]. Our study revealed no significant changes in villi height, area or crypt depth or difference in weight gain, suggesting that birds fed 2% oregano did not compromise intestinal integrity or performance. While carvacrol has been shown to be a potent antimicrobial, it has also displayed the ability to disrupt the invasive ability of motile pathogens at sub-inhibitory levels [37]. Escherichia coli has been shown to produce increased amounts of heat shock protein 60 (HSP60) in the presence of 1mM of carvacrol and become aflagellate and therefore non-motile [38]. In a recent study, Inamuco [39] revealed that Salmonella typhimurium lost motility and invasive potential not as a result of malformed flagella, but potentially a loss of flagellum functionality. Carvacrol has been demonstrated to inhibit various strains of Klebsiella biofilm formation [40], although the precise mechanisms are not identified. Many other studies also show that carvacrol is able to disrupt biofilm formation at concentrations far below the minimum inhibitory levels [41, 42, 43]. A more comprehensive study conducted by Burt [44], demonstrated that carvacrol at sublethal levels (<0.5mM) could disrupt quorum sensing of Chromobacterium violaceum, inhibiting the formation of biofilms. The reduced load of Klebsiella and Proteus in faeces is likely to decrease the transfer of pathogens by coprophagous activities and other litter interactions.
Staphylococcus is another pathogen that is significantly affected by higher concentrations of oregano. Some Staphylococcus species are potential poultry pathogens with zoonotic significance. Staphylococcus aureus is a widely prevalent enterotoxin-producing pathogen in poultry, which is recognised to acquire resistance against methicillin and other common antibiotics such as ciprofloxacin, erythromycin, tetracyclin etc. [45, 46, 47, 48] and can transfer from poultry to humans and vice versa [49]. Staphylococcus can cause a wide variety of infections both in humans and in animals [50], with severe food poisoning with staphylococcal enterotoxins [51], often associated with poultry meat [52]. Moreover, translocation of Staphylococcus from intestine can infect the proximal epiphyseal plate of the femur, tibiotarsus and flexible thoracic vertebrae causing bacterial chondronecrosis with osteomyelitis (commonly called femoral head necrosis), which is one of the major causes of lameness in chickens [53]. Therefore, staphylococcal inhibitory effects of oregano are potentially of significant benefit to the poultry industry.
Microbiota analysis indicated that the relative abundance of Corynebacterium bovis in the intestine was negatively correlated to the oregano treatment. Although this bacterium is a normal inhabitant of mammary gland of bovine and opportunistically causes mastitis [54], the infection of chicken with this bacterium is not commonly reported.
Although oregano did not have any major influence on the total intestinal Lactobacillus, all oregano concentrations reduced Bifidobacterium carriage in the community. Intestinal Bifidobacterium are generally regarded as beneficial to the host due to digestion of oligo- and polysaccharides, producing beneficial short chain fatty acids and lactic acid [55, 56], inhibition of potential pathogens like Compylobacter jejuni [57] and reduction in carcass condemnation due to cellulitis [58]. However, different bifidobacterial strains have different abilities to utilise carbohydrates [56]. Therefore further studies are necessary to elucidate the specific impacts of bifidobacterial depletion on the host. Enrichment of the product with pre-biotics could be an option to nullify this bifidotoxic effect of oregano which needs further study to substantiate.
5. Conclusions
Oregano powder as a feed supplement suppressed the relative abundance of various classes of pathogens and did not cause any negative effects on liver and ileum histology. Oregano could have application in the poultry industry as one of a series of compounds that can be used to ameliorate some of the difficulties introduced with the removal of in-feed AGPs. The strong reduction of Bifidobacterium is concerning and requires further study to understand the breadth of Bifidobacterium strains affected. Finally, we wish to acknowledge that our conclusions are drawn from a single animal trial and that more work needs to be done to understand the role oregano supplementation has in the poultry intestine. We are currently running oregano trials in industry with multiple sheds and high bird number.
Declarations
Author contribution statement
Benjamin W Bauer: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nicky-Lee Willson, Thi Thu Hao Van: Performed the experiments.
Anita Radovanovic, Yadav Sharma Bajagai: Analyzed and interpreted the data.
Robert J Moore: Contributed reagents, materials, analysis tools or data.
Dragana Stanley: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work, including a scholarship for Benjamin W Bauer, was supported by the Poultry CRC established and supported under the Australian Government's Cooperative Research Centres Program.
Competing interest statement
The authors declare no conflict of interest.
Additional information
The sequence data associated with this study has been deposited at the MG-RAST database under the accession number mgl745316 and a project ID mgp89580.
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2019.e02625.
Acknowledgements
We wish to acknowledge Jason Bell and help he provided in all aspects of High-Performance Computing.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary File 1
References
- 1.Line J.E. Influence of relative humidity on transmission of Campylobacter jejuni in broiler chickens. Poult. Sci. 2006;85:1145–1150. doi: 10.1093/ps/85.7.1145. [DOI] [PubMed] [Google Scholar]
- 2.Llana-Ruiz-Cabello M., Gutierrez-Praena D., Pichardo S., Moreno F.J., Bermudez J.M. Cytotoxicity and morphological effects induced by carvacrol and thymol on the human cell line Caco-2. Food Chem. Toxicol. 2014;64:281–290. doi: 10.1016/j.fct.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 3.M'Barek L.A., Mouse H.A., Jaafari A., Aboufatima R., Benharref A. Cytotoxic effect of essential oil of thyme (Thymus broussonettii) on the IGR-OV1 tumor cells resistant to chemotherapy. Braz. J. Med. Biol. Res. 2007;40:1537–1544. doi: 10.1590/s0100-879x2007001100014. [DOI] [PubMed] [Google Scholar]
- 4.Ferket P.R. Alternatives to antibiotics in poultry production: responses, practical experience and recommendations. Nutr. Biotechnol. Feed Food Ind. 2004;20:13. [Google Scholar]
- 5.NRC . 9th Rev. ed. Natl Acad Press; Washington: 1994. Nutritional Requirements of Poultry. [Google Scholar]
- 6.Rubio L.A., Ruiz R., Peinado M.J., Echavarri A. Morphology and enzymatic activity of the small intestinal mucosa of Iberian pigs as compared with a lean pig strain. J. Anim. Sci. 2010;88:3590–3597. doi: 10.2527/jas.2010-3040. [DOI] [PubMed] [Google Scholar]
- 7.Fadrosh D.W., Ma B., Gajer P., Sengamalay N., Ott S. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2 doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 10.Ashelford K.E., Chuzhanova N.A., Fry J.C., Jones A.J., Weightman A.J. At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl. Environ. Microbiol. 2005;71:7724–7736. doi: 10.1128/AEM.71.12.7724-7736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zakrzewski M., Proietti C., Ellis J.J., Hasan S., Brion M.J. Calypso: a user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics. 2017;33:782–783. doi: 10.1093/bioinformatics/btw725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legendre P., Gallagher E.D. Ecologically meaningful transformations for ordination of species data. Oecologia. 2001;129:271–280. doi: 10.1007/s004420100716. [DOI] [PubMed] [Google Scholar]
- 14.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin N.R., Whon T.W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Prasai T.P., Walsh K.B., Bhattarai S.P., Midmore D.J., Van T.T. Biochar, bentonite and zeolite supplemented feeding of layer chickens alters intestinal microbiota and reduces Campylobacter load. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy C.N., Clegg S. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol. 2012;7:991–1002. doi: 10.2217/fmb.12.74. [DOI] [PubMed] [Google Scholar]
- 18.Rocha S.P., Elias W.P., Cianciarullo A.M., Menezes M.A., Nara J.M. Aggregative adherence of uropathogenic Proteus mirabilis to cultured epithelial cells. FEMS Immunol. Med. Microbiol. 2007;51:319–326. doi: 10.1111/j.1574-695X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 19.Sahly H.N.-V.S., Roesler L., Hay A., Carmeli Y., Podschun R., Hennequin C., Forestier C., Ofek I. Extended-spectrum β-lactamase production is associated with an increase in cell invasion and expression of fimbrial adhesins in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2008;52:3029–3034. doi: 10.1128/AAC.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latta R.K., Schur M.J., Tolson D.L., Altman E. The effect of growth conditions on in vitro adherence, invasion, and NAF expression by Proteus mirabilis 7570. Can. J. Microbiol. 1998;44:896–904. doi: 10.1139/cjm-44-9-896. [DOI] [PubMed] [Google Scholar]
- 21.Laffineur G., Lescut D., Vincent P., Quandalle P., Wurtz A. Bacterial translocation in Crohn disease. Gastroenterol. Clin. Biol. 1992;16:777–781. [PubMed] [Google Scholar]
- 22.Freid M.A., Vosti K.L. The importance of underlying disease in patients with gram-negative bacteremia. Arch. Intern. Med. 1968;121:418–423. [PubMed] [Google Scholar]
- 23.Ambrose N.S., Johnson M., Burdon D.W., Keighley M.R. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn's disease surgery. Br. J. Surg. 1984;71:623–625. doi: 10.1002/bjs.1800710821. [DOI] [PubMed] [Google Scholar]
- 24.Sabota J.M., Hoppes W.L., Ziegler J.R., DuPont H., Mathewson J. A new variant of food poisoning: enteroinvasive Klebsiella pneumoniae and Escherichia coli sepsis from a contaminated hamburger. Am. J. Gastroenterol. 1998;93:118–119. doi: 10.1111/j.1572-0241.1998.118_c.x. [DOI] [PubMed] [Google Scholar]
- 25.Xue J.H., Zhang W.J. Understanding China's food safety problem: an analysis of 2387 incidents of acute foodborne illness. Food Control. 2013;30:311–317. [Google Scholar]
- 26.Macfarlane S., Woodmansey E.J., Macfarlane G.T. Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl. Environ. Microbiol. 2005;71:7483–7492. doi: 10.1128/AEM.71.11.7483-7492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 28.Hsu C.R., Pan Y.J., Liu J.Y., Chen C.T., Lin T.L. Klebsiella pneumoniae translocates across the intestinal epithelium via rho GTPase- and phosphatidylinositol 3-kinase/akt-dependent cell invasion. Infect. Immun. 2015;83:769–779. doi: 10.1128/IAI.02345-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee I.A., Kim D.H. Klebsiella pneumoniae increases the risk of inflammation and colitis in a murine model of intestinal bowel disease. Scand. J. Gastroenterol. 2011;46:684–693. doi: 10.3109/00365521.2011.560678. [DOI] [PubMed] [Google Scholar]
- 30.Seth E.C., Taga M.E. Nutrient cross-feeding in the microbial world. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H., Wang M.Y., Liu Y.Q., Wang X.H., Wang Y.K. Characterization of antimicrobial resistance in Klebsiella species isolated from chicken broilers. Int. J. Food Microbiol. 2016;232:95–102. doi: 10.1016/j.ijfoodmicro.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Fielding B.C., Mnabisa A., Gouws P.A., Morris T. Antimicrobial-resistant Klebsiella species isolated from free-range chicken samples in an informal settlement. Arch. Med. Sci. 2012;8:39–42. doi: 10.5114/aoms.2012.27278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S.J., Duan H.L., Zhang W., Li J.W. Analysis of bacterial foodborne disease outbreaks in China between 1994 and 2005. FEMS Immunol. Med. Microbiol. 2007;51:8–13. doi: 10.1111/j.1574-695X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 34.Rao A.Z.Y., Muend S., Rao R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010;54:5062–5069. doi: 10.1128/AAC.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An H.S., Jee Y.J., Min K.S., Kim B.L., Han S.J. Phylogenetic analysis of six species of pacific abalone (Haliotidae) based on DNA sequences of 16s rRNA and cytochrome c oxidase subunit I mitochondrial genes. Mar. Biotechnol. 2005;7:373–380. doi: 10.1007/s10126-004-4405-2. [DOI] [PubMed] [Google Scholar]
- 36.Sivropoulou A., Papanikolaou E., Nikolaou C., Kokkini S., Lanaras T., Arsenakis M. Antimicrobial and cytotoxic activities of origanum essential oils. J. Agric. Food Chem. 1996;44:1202–1205. [Google Scholar]
- 37.van Alphen L.B., Burt S.A., Veenendaal A.K., Bleumink-Pluym N.M., van Putten J.P. The natural antimicrobial carvacrol inhibits Campylobacter jejuni motility and infection of epithelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burt S.A., van der Zee R., Koets A.P., de Graaff A.M., van Knapen F. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007;73:4484–4490. doi: 10.1128/AEM.00340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inamuco J., Veenendaal A.K., Burt S.A., Post J.A., Tjeerdsma-van Bokhoven J.L. Sub-lethal levels of carvacrol reduce Salmonella Typhimurium motility and invasion of porcine epithelial cells. Vet. Microbiol. 2012;157:200–207. doi: 10.1016/j.vetmic.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 40.Raei P., Pourlak T., Memar M.Y., Alizadeh N., Aghamali M. Thymol and carvacrol strongly inhibit biofilm formation and growth of carbapenemase-producing Gram negative bacilli. Cell. Mol. Biol. (Noisy-Le-Grand) 2017;63:108–112. doi: 10.14715/cmb/2017.63.5.20. [DOI] [PubMed] [Google Scholar]
- 41.Knowles J.R.R.S., Murray D.B., Naidu A.S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2005:797–803. doi: 10.1128/AEM.71.2.797-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nostro A., Sudano Roccaro A., Bisignano G., Marino A., Cannatelli M.A. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007;56:519–523. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- 43.Nostro A., Marino A., Blanco A.R., Cellini L., Di Giulio M. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol. 2009;58:791–797. doi: 10.1099/jmm.0.009274-0. [DOI] [PubMed] [Google Scholar]
- 44.Burt S.A., Ojo-Fakunle V.T., Woertman J., Veldhuizen E.J. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro C.M., Stefani L.M., Lucheis S.B., Okano W., Cruz J.C.M. Methicillin-resistant Staphylococcus aureus in poultry and poultry meat: a meta-analysis. J. Food Prot. 2018;81:1055–1062. doi: 10.4315/0362-028X.JFP-17-445. [DOI] [PubMed] [Google Scholar]
- 46.Sergelidis D., Angelidis A. Methicillin-resistant Staphylococcus aureus: a controversial food-borne pathogen. Lett. Appl. Microbiol. 2017;64:409–418. doi: 10.1111/lam.12735. [DOI] [PubMed] [Google Scholar]
- 47.Aarestrup F.M., Agersø Y., Ahrens P., Jørgensen J.C.Ø., Madsen M. Antimicrobial susceptibility and presence of resistance genes in staphylococci from poultry. Vet. Microbiol. 2000;74:353–364. doi: 10.1016/s0378-1135(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 48.Waters A.E., Contente-Cuomo T., Buchhagen J., Liu C.M., Watson L. Multidrug-resistant Staphylococcus aureus in US meat and poultry. Clin. Infect. Dis. 2011;52:1227–1230. doi: 10.1093/cid/cir181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowder B.V., Guinane C.M., Zakour N.L.B., Weinert L.A., Conway-Morris A. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. 2009;106:19545–19550. doi: 10.1073/pnas.0909285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong S.Y., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Argudín M.Á., Mendoza M.C., Rodicio M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins. 2010;2:1751–1773. doi: 10.3390/toxins2071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bortolaia V., Espinosa-Gongora C., Guardabassi L. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect. 2016;22:130–140. doi: 10.1016/j.cmi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Wideman R.F., Jr. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult. Sci. 2015;95:325–344. doi: 10.3382/ps/pev320. [DOI] [PubMed] [Google Scholar]
- 54.Watts J.L. Etiological agents of bovine mastitis. Vet. Microbiol. 1988;16:41–66. doi: 10.1016/0378-1135(88)90126-5. [DOI] [PubMed] [Google Scholar]
- 55.Simpson H.L., Campbell B.J. Dietary fibre–microbiota interactions. Aliment. Pharmacol. Ther. 2015;42:158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pokusaeva K., Fitzgerald G.F., van Sinderen D. Carbohydrate metabolism in bifidobacteria. Genes Nutr. 2011;6:285. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baffoni L., Gaggìa F., Di Gioia D., Santini C., Mogna L. A Bifidobacterium-based synbiotic product to reduce the transmission of C. jejuni along the poultry food chain. Int. J. Food Microbiol. 2012;157:156–161. doi: 10.1016/j.ijfoodmicro.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 58.Estrada A., Wilkie D., Drew M. Administration of Bifidobacterium bifidum to chicken broilers reduces the number of carcass condemnations for cellulitis at the abattoir. J. Appl. Poult. Res. 2001;10:329–334. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1