Abstract
Total phenolic content (TPC) and antioxidant properties of xanthone extract from mangosteen pericarp via microwave-assisted extraction (MAE) method was optimized by response surface methodology (RSM). The MAE extraction conditions to obtain optimum antioxidant-rich xanthone extract were at 2.24 min of irradiation time, 25 mL/g of solvent-to-solid ratio and 71% of ethanol concentration. The predicted results for four responses were as follows; 320.31 mg gallic acid equivalent/g extract, 83.63% and 93.77% inhibition (DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulfonic acid) assays), and 144.56 mg Trolox equivalent/g extract (FRAP, Ferric reducing antioxidant power). The predicted and actual values were statistically insignificant (P > 0.05). Therefore, these results confirmed that the examined model was acceptable and relevant. MAE led to a slightly similar antioxidant capacity and a higher extraction of α-mangostin, a major xanthone of mangosteen pericarp as compared to water bath-maceration technique.
Keywords: Biochemical engineering, Food engineering, Food technology, Food processing, Bioactive compound, Antioxidant, Phenolic compound, Biochemical characterization of food, Antioxidant, Mangosteen pericarp, Microwave-assisted extraction, Total phenolic content, Xanthone, α-mangostin
1. Introduction
Mangosteen (Garcinia mangostana L.) is a popular exotic “superfruit” and can be found widely in Southeast Asia. Recently, the interest in in-vitro and in-vivo investigation of mangosteen fruit has increased rapidly. The fruit exhibits several pharmacological properties, including antioxidant (Chhouk et al., 2016), antiproliferative (Yoo et al., 2011), anti-inflammatory (Nakatani et al., 2002) and anticarcinogenic (Shan et al., 2011). Xanthones are polyphenol compound that are found abundantly in mangosteen pericarp which contain α-mangostin (69.01%) as the major xanthones compound followed by γ-mangostin (17.86%), while the minor xanthones compounds (13.13%) include gartanin, 8-deoxygartanin, garcinon E, 1,7-dihydroxy-3-methoxy-2-(3-methylbut-2-enyl)xanthone, and 1,3,7-tri-hydroxy-2,8-di (3-methylbut-2-enyl)-xanthone (Wittenauer et al., 2012). α-mangostin is a hydrophobic polyphenol compound that accounted for the superior antioxidant potential (Jung et al., 2006; Suvarnakuta et al., 2011; Sukatta et al., 2013).
There are many extraction techniques to recover bioactive xanthones from mangosteen pericarp. Most of the conventional extraction methods such as maceration in water bath method consume a high quantity of solvent and extended extraction time. Therefore, due to the drawbacks of the conventional extraction methods, various advanced extraction techniques have been examined to extract bioactive and phenolic compounds. For instance, supercritical carbon dioxide (SC–CO2) method (Chhouk et al., 2016; Zarena et al., 2012), ultrasonic bath (Suvarnakuta et al., 2011) and microwave-assisted extraction (MAE) (Ghasemzadeh, Jaafar, Baghdadi, & Tayebi-meigooni, 2018) have shown high capability to extract α-mangostin compound from mangosteen pericarp.
Previous studies have reported the advantages of MAE method such as low solvent consumption, less time consuming, and fast energy transfer through the irradiation that permit well diffusion of solvent within the extraction medium (Nayak et al., 2015; Hayat et al., 2010) especially for the preparation of antioxidant-rich plant extracts. M'hiri et al. (2015) has evaluated that MAE and ultrasound-assisted extraction (UAE) could extract the highest value of total phenolic content (TPC) and antioxidant activity from orange peel which led to the most prevailing extraction techniques compared to other extraction methods; conventional solvent extraction (CSE), SC-CO2, and high-pressure extraction (HPE).
The yield extract and the amount of total xanthone of mangosteen pericarp are strongly affected by the extraction solvent (Kusmayadi et al., 2018; Suttirak and Manurakchinakorn, 2014). Aisha, Abu-Salah, Ismail and Abdul-Majid (2013) has reported that toluene is the most efficient extraction solvent to extract mangosteen pericarp when comparing to 75% ethanol and methanol. Ethanol which is an amphipathic solvent could be a potential solvent to extract the α-mangostin compound and other xanthones derivatives from mangosteen pericarp (Yoshimura et al., 2015). For MAE method, several primary factors could affect the extraction, such as solvent types, solvent volume, power, temperature, irradiation time, and size of raw material. These factors affect the extracted yield and the amount of total phenolic content (TPC) (Desai et al., 2010). More evaluations on the extraction factors using various extraction techniques are needed to produce the antioxidant-rich xanthones extract from mangosteen pericarp. Even though many studies have attempted to extract optimum yield extraction via advanced methods, the condition at which the optimum antioxidant-rich extract obtained has not been fully reported. Thus, this study is aimed to investigate the optimum MAE extraction conditions by using response surface methodology (RSM) for the high recovery of antioxidant-rich xanthones from mangosteen pericarp. MAE and water bath-maceration (WBE) extract were analyzed to compare the TPC, α-mangostin content and antioxidant potential. This study hypothesizes that the different extraction methods could affect the amount of α-mangostin content, TPC and antioxidant properties of the xanthone extract.
2. Materials and methods
2.1. Materials
Mangosteen (Garcinia mangostana L.) pericarp was purchased from a wholesaler and supplier of herbal raw materials (Delima Jelita Herbs Pvt. Ltd., Kedah, Malaysia). Mangosteen pericarp powder (MPP) in 120 mesh sieve size stored in the dark airtight bottles until use for analyses.
Trolox standard, TPTZ (2,4,6-Tris (2-pyridyl)-s-triazine), DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulfonic acid) were supplied by Sigma-Aldrich (M) Sdn. Bhd, Selangor, Malaysia. Potassium persulphate was obtained from R & M Chemicals, United Kingdom. Ferric chloride hexahydrate was bought from Merck KGaA, Darmstadt, Germany. α-mangostin standard was purchased from Tokyo Chemicals Industry, Co., Ltd. Tokyo, Japan. Gallic acid was obtained from Acros Organics, Fisher Scientific, Fair Lawn, New Jersey. Analytical-grade or HPLC-grade chemicals were chosen for this study and were used without further treatment unless otherwise mentioned.
2.2. Microwave-assisted extraction (MAE) of mangosteen pericarp powder
MAS-Ⅱ plus microwave synthesis workstation (2450 MHz, Sineo Microwave Chemistry Technology Co. Ltd, Shanghai, China) with 1000 W of maximum power supply (Jusoh et al., 2018) was employed for the extraction process. It was digitally controlled to regulate irradiation time, temperature, microwave power, stirring system and also fitted with a reflux condenser. The reaction process was operated by placing mangosteen pericarp powder (MPP) in a 4-neck round bottom flask containing 20 mL ethanol. The ranges for extraction variables were set based on the generated experimental value from Design Expert software (Version 7.1.5, Stat-Ease Inc., Minneapolis, USA) as referred to Table 1. After MAE extraction, the crude extract was left at room temperature to enable cooling and centrifuged at the conditions of 5000 rpm, 5 °C and 15 min. Thus, the supernatant was dried to evaporate the ethanol solvent by using rotary evaporator and then dried to evaporate the water by using freeze drier (Alpha 1-2-LDplus, Germany). The recovered dried mangosteen pericarp extracts (DMPE) were kept at 5 °C. DMPE was analyzed for TPC, antioxidant activities and α-mangostin content by HPLC.
Table 1.
TPC and antioxidant properties of dried mangosteen pericarp extract from MAE experimental sets designed by BBD.
| Run | Irradiation time (min), X1 | Solvent-to-solid ratio (mL/g), X2 | Ethanol concentration (%), X3 | TPC (mg GAE/g extract) | Antioxidant properties |
||
|---|---|---|---|---|---|---|---|
| DPPH (%) | ABTS (%) | FRAP (mg TE/g extract) | |||||
| 1 | 2.50 | 25 | 60 | 296.2 | 78.45 | 92.08 | 137.9 |
| 2 | 1.75 | 25 | 80 | 309.2 | 75.99 | 89.58 | 143.6 |
| 3 | 1.75 | 35 | 60 | 243.4 | 71.09 | 75.95 | 105.5 |
| 4 | 1.75 | 45 | 40 | 54.63 | 46.16 | 44.86 | 73.97 |
| 5 | 1.75 | 35 | 60 | 235.1 | 70.79 | 79.31 | 107.7 |
| 6 | 1.75 | 25 | 40 | 44.16 | 48.72 | 64.77 | 73.86 |
| 7 | 1.75 | 45 | 80 | 246.6 | 69.28 | 80.69 | 103.9 |
| 8 | 2.50 | 35 | 80 | 270.6 | 74.29 | 89.80 | 121.6 |
| 9 | 1.75 | 35 | 60 | 247.9 | 73.62 | 75.92 | 109.1 |
| 10 | 1.00 | 25 | 60 | 252.9 | 69.37 | 89.61 | 110.9 |
| 11 | 1.00 | 35 | 80 | 255.1 | 63.44 | 83.88 | 117.2 |
| 12 | 1.75 | 35 | 60 | 254.8 | 71.42 | 79.31 | 112.8 |
| 13 | 2.50 | 45 | 60 | 264.8 | 68.47 | 84.77 | 96.35 |
| 14 | 1.75 | 35 | 60 | 244.1 | 74.64 | 75.95 | 114.1 |
| 15 | 2.50 | 35 | 40 | 68.59 | 33.04 | 60.19 | 75.29 |
| 16 | 1.00 | 35 | 40 | 49.89 | 42.30 | 41.59 | 60.96 |
| 17 | 1.00 | 45 | 60 | 225.2 | 71.05 | 74.40 | 94.38 |
2.3. Water bath-maceration extraction (WBE) of mangosteen pericarp powder
The maceration process was operated according to M'hiri et al. (2015) method with some modifications. MPP was dissolved in 95% ethanol. The MPP was extracted by using stirring-water bath (SWB-20L-3 Cleaver Scientific Ltd., USA) for about 4 h and at 65 °C. The irradiation time, solvent-to-solid (S/S) ratio and ethanol concentration were fixed by following the optimum MAE extraction conditions. Dried mangosteen pericarp extract from WBE method (denoted as WDMPE) was obtained by following the procedure mentioned in method 2.2. The extract was collected and evaluated for TPC, antioxidant activities and α-mangostin content by HPLC.
2.4. Determination of total phenolic content of dried mangosteen pericarp extract
TPC of DMPE was evaluated by implementing the Folin-Ciocalteu method (Folin and Ciocalteau, 1927) but was done with some adjustments. DMPE at 400 ppm or Gallic acid at a series of concentration was dissolved in 95% ethanol. DMPE (0.5 mL) or Gallic acid was mixed with 0.5 mL of Folin-Ciocalteu reagent for about 2–3 min, and softly shaken with 7% sodium carbonate solution (10 mL). After the solution being incubated at 28 ± 1 °C (room temperature), the absorbance was analyzed using a microplate reader (Epoch Microplate Spectrophotometer, USA) at 750 nm. The results were reported as milligram of gallic acid equivalent per gram extract (mg GAE/g extract).
2.5. Determination of antioxidant activity of dried mangosteen pericarp extract
2.5.1. DPPH radical scavenging activity
DMPE was examined for its DPPH radical scavenging activity by following Parry et al. (2005) method but was done with some adjustments. DMPE was dissolved in 95% ethanol and was prepared for a series of concentrations. About 40 μL of DMPE sample was mixed with 220 μL freshly prepared DPPH reagent (0.1 mM) and was incubated for 1 h without direct light exposure. The absorbance was observed using a microplate reader at 517 nm. The DPPH scavenging activity of the DMPE was measured as DPPH inhibition (%) as displayed in Eq. (1) (A0 is blank; A1 is absorbance of sample) and estimated according to milligram of Trolox equivalent per gram dry extract (mg TE/g extract). IC50 value (μg/mL) was also determined by plotting a linear regression analysis of dose response curve to observe the effective concentration that could reach 50% of a maximum scavenging capacity by DPPH radical.
| (1) |
2.5.2. ABTS radical scavenging activity
Trolox Equivalent Antioxidant Capacity (TEAC) of DMPE was determined according to ABTS assay established by Re et al. (1999) but with some alterations. A stable stock solution of 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulfonic acid) (ABTS•+) was generated by blending 7.0 mM ABTS solution and 2.45 mM potassium persulfate solution at a ratio of 1:1. After undergoing 12 h incubation time at room temperature with no light exposure, the mixture was regulated to attain an absorbance of 0.70 ± 0.05 at 734 nm by adding an appropriate amount of 95% ethanol. The radical scavenging activity was initiated by mixing 40 μL of the DMPE in 95% ethanol and 220 μL ABTS•+ reagent. After undertaken 1 h incubation time at room temperature, the absorbance was observed at 734 nm through microplate reader. ABTS scavenging activity of the DMPE was reported as the inhibition percentage of ABTS radical and calculated by following Eq. (2) and also was estimated according to milligram of Trolox equivalent per gram extract (mg TE/g extract) and milligram of α-mangostin per gram extract (mg α-mangostin/g extract). IC50 value (μg/mL) was also determined by plotting a linear regression analysis of dose-response curve to observe the effective concentration that could reach half capacity (50%) of a maximum ABTS scavenging activity.
| (2) |
2.5.3. Ferric reducing antioxidant power (FRAP)
FRAP was examined for DMPE by referring to Zarena et al. (2012) method with minor changes. After incubated for 60 min, the absorbance of the reaction mixture was observed at 593 nm. The FRAP of the DMPE were exhibited as mg of TE (Trolox equivalent) per g extract which referring to Trolox standard curve.
2.6. Quantification of α-mangostin content using High Performance Liquid Chromatography
α-mangostin content was investigated by High Performance Liquid Chromatography (HPLC) with referring to Jujun et al. (2009) method and subjected to minor modifications. The HPLC was fitted with an autosampler (Waters 2690) and operated using a C18 column (100 mm × 4.6 mm, 5 μm particle size). An isocratic solvent system, 95% of acetonitrile and 5% of 0.1% ortho-phosphoric acid in deionized water were prepared for mobile phase solution. The mobile phase was run at a flow rate of 0.8 ml min−1. DMPE (400 μg) was solubilized in 1 mL 95% ethanol and filtered via 0.45 μm nylon membrane filter. DMPE sample (20 μL) solution was injected to the system and the eluate was monitored by UV-vis detector at wavelength 319 nm. The α-mangostin content was measured according to the calibrated standard α-mangostin curve plotted by peak area versus α-mangostin concentration. The results were expressed as milligram of α-mangostin per gram extract (mg α-mangostin/g extract).
2.7. Experimental design by response surface methodology (RSM)
The experimental design was performed using Design Expert software (Version 7.1.5, Stat-Ease Inc., Minneapolis, US). The four responses (Y); total phenolic content (YTPC), DPPH radical scavenging activity (YDPPH), ABTS radical scavenging activity (YABTS) and ferric reducing antioxidant power (YFRAP) of DMPE were optimized by employing a Box Behnken Design (BBD) with five center points. As referring to the findings of the single-factor experiments (Fig. 1) which evaluated the influences of ethanol concentration and solvent-to-solid ratio (S/S, mL/g ratio) while the other factors were referred to literature (Alara et al., 2018; M'hiri et al., 2014; Dahmoune et al., 2013). The optimization process was explored by response surface methodology (RSM) design with selected three independent variables (X); irradiation time (X1, min), solvent-to-solid ratio (X2, mL/g) and ethanol concentration (X3, %) to obtain optimized dried mangosteen pericarp extract (ODMPE). The independent factors, including the ranges, were tabulated in Table 1. The significance of regression coefficients and the suitability of the established model were statistically examined by analysis of variance (ANOVA). A quadratic (second-order) polynomial model was fitted to the factors and response value of 1 set experimental (Table 1) with the equation as expressed in Eq. (3):
| (3) |
Fig. 1.
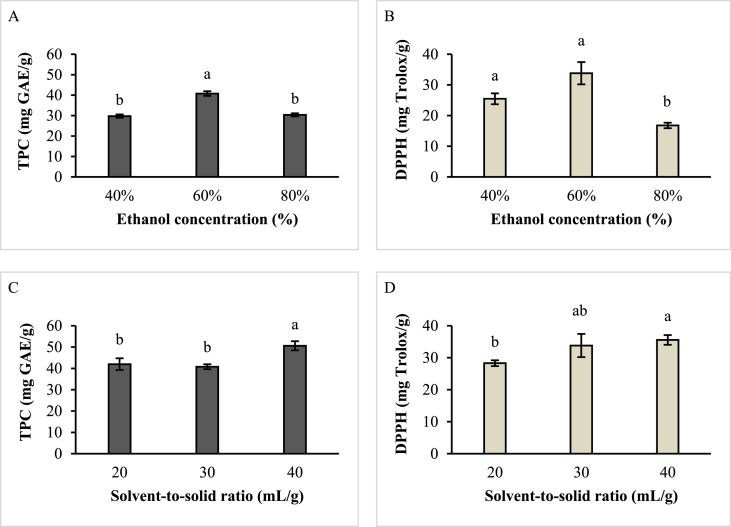
The effect of A & B: ethanol concentration (at fixed variables of solvent-to-solid ratio,S/S: 30 mL/g, power: 300W, time:2 min, and temperature: 65 °C) and C & D: S/S ratio (at fixed variables of ethanol concentration: 60%, power: 300W, time:2 min, and temperature: 65 °C) on TPC and DPPH radical scavenging activity of ME. Values are mean ± SD of triplicate analysis; Means with different letters denote significant differences of TPC and DPPH values within ranges of ethanol concentrations and within ranges of S/S ratio.
Three-dimensional (3D) response surface plots and two dimensions (2D) contour plots were generated from the regression equations to observe the influence of the factors and their correlative relations on all four responses (Kang et al., 2016).
2.8. Statistical analysis
The experimental data for the optimization process in finding the effect of the factors and the interaction between them was analyzed by response surface methodology (Design Expert software) using analysis of variance (ANOVA). Other quantitative data was investigated in three replications and was denoted as mean ± standard deviation. The data was evaluated by paired-samples t-test using IBM SPSS Statistic software (Version 25.0.0.0, IBM, New York, US).
3. Results and discussion
3.1. Antioxidant recovery by MAE and selection of parameters for MAE optimization
This study attempted to find the most effective conditions of MAE for high TPC recovery with high antioxidant properties by considering green extracting solvent, irradiation time, solvent-to-solid ratio, power and temperature. Preliminary single-factor experiments were applied to evaluate the impacts of two factors including ethanol concentration (%) and solvent-to-solid, S/S ratio (mL/g) on TPC and DPPH radical scavenging activity of the DMPE (Fig. 1). The TPC yield of the DMPE was significantly higher when extracted using 60% ethanol (40.77 ± 1.14a mg GAE/g) compared to 40% ethanol (29.74 ± 0.81b mg GAE/g) and 80% ethanol (30.34 ± 0.79b mg GAE/g) (Fig. 1A). The most intense antioxidant level of DMPE was shown by 60% ethanol (33.81 ± 3.63a mg TE/g) and 40% ethanol (25.48 ± 1.77a mg TE/g) while 80% ethanol (16.78 ± 0.91a mg TE/g) significantly exhibited the lowest antioxidant potential (Fig. 1B). A prenylated xanthone is a nonpolar compound, which could be extracted from mangosteen pericarp by ethanol solvent (Jung et al., 2006). The polarity of ethanol increased with the addition of water. Thus, the decreasing of water proportion in the mixture of water and ethanol enhanced TPC yield. Solvent selection is the most significant factor for microwave-assisted extraction (MAE) to recover the targeted bioactive compound from plant tissues (Desai et al., 2010). Microwave irradiation will rapidly trigger the heat and pressure and then continue to modify the physical characteristics of the plant cells and enhances the porosity of the biological matrix (M'hiri et al., 2014). The mixtures of organic solvent and water possessed a higher efficiency for antioxidant compounds recovering rather than the use of single pure organic solvent (Boeing et al., 2014). The proper solvent with higher tan δ value (dissipation factor) for MAE could assist a better solvent diffusion across the extraction medium. This phenomenon enhances the phenolic compound yield of the sample. Previously, Dahmoune et al. (2013) has reported that the optimum ethanol concentration (%) to obtain lemon peels extract with the highest antioxidant potential was at 48% for MAE and 63.93% for UAE. Therefore, the range of ethanol concentration between 40%-80% was chosen in this study for further investigation in the optimization process by RSM based on the influence of ethanol concentration (%) on the TPC yield (mg GAE/g dried MPP) and DPPH radical scavenging activity (mg TE/g dried MPP).
The ethanol concentration was fixed at 60% to investigate the effect of S/S ratio due to the greatest potential in achieving the highest TPC value and the highest antioxidant activity of the DMPE. The recovery of TPC of DMPE was significantly highest at 40 mL/g (50.60 ± 2.12a mg GAE/g) of S/S ratio rather than 20 mL/g (41.99 ± 2.74b mg GAE/g) and 30 mL/g (40.77 ± 1.14b mg GAE/g) (Fig. 1C). The highest antioxidant potential was expressed significantly at 40 mL/g (35.55 ± 1.52a mg TE/g) of S/S ratio than 20 mL/g (28.30 ± 0.91 b mg TE/g) but not significantly different with 30 mL/g (33.81 ± 3.63 ab mg TE/g) (Fig. 1D). According to literature, Prakash Maran et al. (2017) has determined an ideal S/S ratio of 18.6 mL/g in achieving TPC yield at 5.526 ± 1.57 mg GAE/g from rambutan peel. Dahmoune et al. (2014) have found that 28 mL/g was the optimum S/S ratio for the extraction of the total polyphenol from Pistacia lenticus leaves. Dahmoune et al. (2015) has reported the MAE optimal conditions to extract polyphenols from Myrtus communis L. leaves at S/S ratio of 32 mL/g, 42% of ethanol concentration, 500 W of microwave power, and 62 s of irradiation time. The authors also reported that MAE extracts exhibited the strongest antioxidant potential, consumed the least volume of extraction solvent and the shortest time among other extracts such as UAE and CSE. MAE and HPE demonstrated the highest antioxidant potential of orange peel by the extraction condition at constant S/S ratio (10 mL/g) (M'hiri et al., 2015). The S/S ratio significantly influenced the extraction equilibrium constant (Dahmoune et al., 2013). The higher level of S/S ratio could enhance the extraction yield as the mass transfer of the immersed solutes into the solution allows a steeper concentration gradient (Qu et al., 2010). Considering all these factors and results from previous studies, 25–45 mL/g was the selected S/S ratio range used in this study for RSM experimental design.
It has been reported that the optimal MAE extraction parameters to recover phenolic compounds from citrus peel were using methanol or ethanol, at 135 °C–140 °C of temperature, 49 s to 8 min of irradiation time and up to 400 W of microwave power (M'hiri et al., 2014). Antioxidant-enrich Citrus limon residues were successfully extracted by MAE at optimum condition of 123 s, 400 W of power, 48% ethanol of solvent and 28 mL/g of S/S ratio (Dahmoune et al., 2013). Dahmoune et al. (2015) has also reported that 500 W was the optimal microwave power for MAE to obtain the polyphenols from the Myrtle leaves. Alara, Abdurahman and Olalere (2018) determined the condition of the optimum variables for maximum extraction of total flavonoid content (TFC) and antioxidant were at 416 W, 7 min, 100 °C, and 0.10 g/mL S/S ratio. These results from previous studies indicated that high microwave power promotes the solvent's motion, cell break and dispersion of the extracted compound into the extraction medium which leads to enhance the antioxidant capacity. However, a very high level of microwave power could deteriorate antioxidant capacity of bioactive compound. Study by Li et al. (2017) has reported a decrease of Trolox equivalent antioxidant capacity (TEAC) value when the microwave power reached 800 W for Gordonia axillaris fruit. With these considerations, the MAE extraction condition was selected from 60 s to 150 s of irradiation time, 400 W of microwave power and 65 °C of temperature.
3.2. Optimization of antioxidant recovery by MAE
3.2.1. Model fitting
RSM experimental design with response data is shown in Table 1 which includes three parameters; irradiation time (X1), S/S ratio (X2), and ethanol concentration (X3) as well as four responses including TPC, DPPH and ABTS radical scavenging, and FRAP assay. Table 2 represents the analysis of variance (ANOVA) which shows the models of the second-order polynomial regression were significantly (P < 0.0001) fitted for the models and the regression coefficients of the intercept, linear, quadratic and interaction parameters of all models. From the ANOVA results, the value of lack of fit for the models was as follows: 0.1072, 0.2091, 0.1188, and 0.3502, respectively, expressing no significant differences (P < 0.05) which suited the model. The correlation between the predicted and observed data was relevant due to R2 (determination coefficients) values, adjusted R2, and predicted R2 (0.9938, 0.9859, and 0.9236; 0.9887, 0.9742, and 0.8777; 0.9854, 0.9667, and 0.8225; 0.9867, 0.9696, and 0.8788) for each response, respectively. Moreover, the values of coefficient of variation (C.V. %) were 5.17, 3.34, 3.59, and 3.79 whereas adequate precision ratios were 32.06, 25.76, 24.10, and 28.68, respectively, recommended that the models were dependable and repeatable agreeing previous literature investigated by Alara et al. (2018) and Dahmoune et al. (2015). A lower level of C.V., generally below 10% represents low inconsistency of the mean value indicating an adequate response model has been satisfactorily developed (Karazhiyan et al., 2011).
Table 2.
Analysis of variance (ANOVA) for the experimental results obtained using microwave-assisted extraction (MAE) a) TPC (mg GAE/g extract), b) DPPH inhibition (%), c) ABTS inhibition (%), d) FRAP (mg TE/g extract).
| Source |
Sum of Squares |
df |
Mean Square |
F-Value |
p-value Prob > F |
| a) | |||||
| Model | 1.322E+005 | 9 | 14687.80 | 125.23 | <0.0001 |
| X1-time | 1716.53 | 1 | 1716.53 | 14.64 | 0.0065 |
| X2-S/S ratio | 1545.70 | 1 | 1545.70 | 13.18 | 0.0084 |
| X3-ethanol % | 93349.88 | 1 | 93349.88 | 795.92 | < 0.0001 |
| X1X2 | 3.50 | 1 | 3.50 | 0.030 | 0.8678 |
| X1X3 | 2.63 | 1 | 2.63 | 0.022 | 0.8853 |
| X2X3 | 1334.20 | 1 | 1334.20 | 11.38 | 0.0119 |
| 153.61 | 1 | 153.61 | 1.31 | 0.2901 | |
| 315.62 | 1 | 315.62 | 2.69 | 0.1449 | |
| 34163.22 | 1 | 34163.22 | 291.28 | < 0.0001 | |
| Model | 821.00 | 7 | 117.29 | ||
| Lack of Fit | 615.43 | 3 | 205.14 | 3.99 | 0.1072 |
| Pure Error | 205.57 | 4 | 51.39 | ||
| Cor Total | 1.330E+005 | 16 | |||
| C.V. % | 5.17 | ||||
| PRESS | 10168.15 | ||||
| Adeq precision | 32.059 | ||||
| R2 | 0.9938 | ||||
| Adj R2 | 0.9859 | ||||
| Pred R2 |
0.9236 |
||||
| b) | |||||
| Model | 2878.39 | 9 | 319.82 | 68.12 | <0.0001 |
| X1-time | 8.17 | 1 | 8.17 | 1.74 | 0.2286 |
| X2-S/S ratio | 38.58 | 1 | 38.58 | 8.22 | 0.0241 |
| X3-ethanol % | 1589.78 | 1 | 1589.78 | 338.62 | < 0.0001 |
| X1X2 | 34.02 | 1 | 34.02 | 7.25 | 0.0310 |
| X1X3 | 101.15 | 1 | 101.15 | 21.54 | 0.0024 |
| X2X3 | 4.30 | 1 | 4.30 | 0.92 | 0.3702 |
| 55.23 | 1 | 55.23 | 11.76 | 0.0110 | |
| 41.67 | 1 | 41.67 | 8.88 | 0.0205 | |
| 1000.94 | 1 | 1000.94 | 213.19 | < 0.0001 | |
| Residual | 32.86 | 7 | 4.69 | ||
| Lack of Fit | 21.11 | 3 | 7.04 | 2.39 | 0.2091 |
| Pure Error | 11.76 | 4 | 2.94 | ||
| Cor Total | 2911.26 | 16 | |||
| C.V. % | 3.34 | ||||
| PRESS | 356.10 | ||||
| Adeq precision | 25.756 | ||||
| R2 | 0.9887 | ||||
| Adj R2 | 0.9742 | ||||
| Pred R2 | 0.8777 | ||||
| c) | |||||
| Model | 3477.55 | 9 | 386.39 | 52.61 | <0.0001 |
| X1-time | 174.43 | 1 | 174.43 | 23.75 | 0.0018 |
| X2-S/S ratio | 329.21 | 1 | 329.21 | 44.82 | 0.0003 |
| X3-ethanol % | 2195.99 | 1 | 2195.99 | 298.99 | < 0.0001 |
| X1X2 | 15.62 | 1 | 15.62 | 2.13 | 0.1881 |
| X1X3 | 40.16 | 1 | 40.16 | 5.47 | 0.0520 |
| X2X3 | 30.37 | 1 | 30.37 | 4.14 | 0.0815 |
| 48.93 | 1 | 48.93 | 6.66 | 0.0364 | |
| 86.06 | 1 | 86.06 | 11.72 | 0.0111 | |
| 589.42 | 1 | 589.42 | 80.25 | < 0.0001 | |
| Residual | 51.41 | 7 | 7.34 | ||
| Lack of Fit | 37.83 | 3 | 12.61 | 3.71 | 0.1188 |
| Pure Error | 13.59 | 4 | 3.40 | ||
| Cor Total | 3528.96 | 16 | |||
| C.V. % | 3.59 | ||||
| PRESS | 626.43 | ||||
| Adeq precision | 24.097 | ||||
| R2 | 0.9854 | ||||
| Adj R2 | 0.9667 | ||||
| Pred R2 |
0.8225 |
||||
| d) | |||||
| Model | 7996.09 | 9 | 888.45 | 57.76 | <0.0001 |
| X1-time | 283.80 | 1 | 283.80 | 18.45 | 0.0036 |
| X2-S/S ratio | 1192.87 | 1 | 1192.87 | 77.55 | < 0.0001 |
| X3-ethanol % | 5113.45 | 1 | 5113.45 | 332.43 | < 0.0001 |
| X1X2 | 155.63 | 1 | 155.63 | 10.12 | 0.0155 |
| X1X3 | 24.53 | 1 | 24.53 | 1.59 | 0.2471 |
| X2X3 | 396.93 | 1 | 396.93 | 25.81 | 0.0014 |
| 26.61 | 1 | 26.61 | 1.73 | 0.2298 | |
| 27.83 | 1 | 27.83 | 1.81 | 0.2206 | |
| 772.98 | 1 | 772.98 | 50.25 | 0.0002 | |
| Residual | 107.67 | 7 | 15.38 | ||
| Lack of Fit | 56.39 | 3 | 18.80 | 1.47 | 0.3502 |
| Pure Error | 51.28 | 4 | 12.82 | ||
| Cor Total | 8103.76 | 16 | |||
| C.V. % | 3.79 | ||||
| PRESS | 982.40 | ||||
| Adeq precision | 28.675 | ||||
| R2 | 0.9867 | ||||
| Adj R2 | 0.9696 | ||||
| Pred R2 | 0.8788 | ||||
The regression coefficients for TPC response indicated that all linear (X1, X2, and X3) and quadratic () parameters were greatly significant (P < 0.05), while the interaction of S/S ratio and ethanol concentration (X2X3) was also significant (P < 0.05) (Table 2A). Table 2B has shown that DPPH radical scavenging activity was significantly affected by two linear parameters, X2 and X3, two interactions X1X2 and X1X3, and also all quadratic parameters (P < 0.05). ABTS radical scavenging activity was significantly influenced by all the linear parameters and their quadratic parameters while all the interaction parameters did not demonstrate significant differences (P > 0.05) (Table 2C). All the linear and quadratic parameters (), as well as two interactions, X1X2 and X2X3 (Table 2D) significantly (P < 0.05) influenced the FRAP activity. The optimal values for TPC, antioxidant activities including DPPH, ABTS radical scavenging and FRAP assay of optimized dried mangosteen pericarp extract (ODMPE) can be obtained from the final predictive quadratic equations through the multiple regression analysis as shown in Eqs. (4), (5), (6), and (7), respectively.
| (4) |
| (5) |
| (6) |
| (7) |
3.2.2. Response surface plots
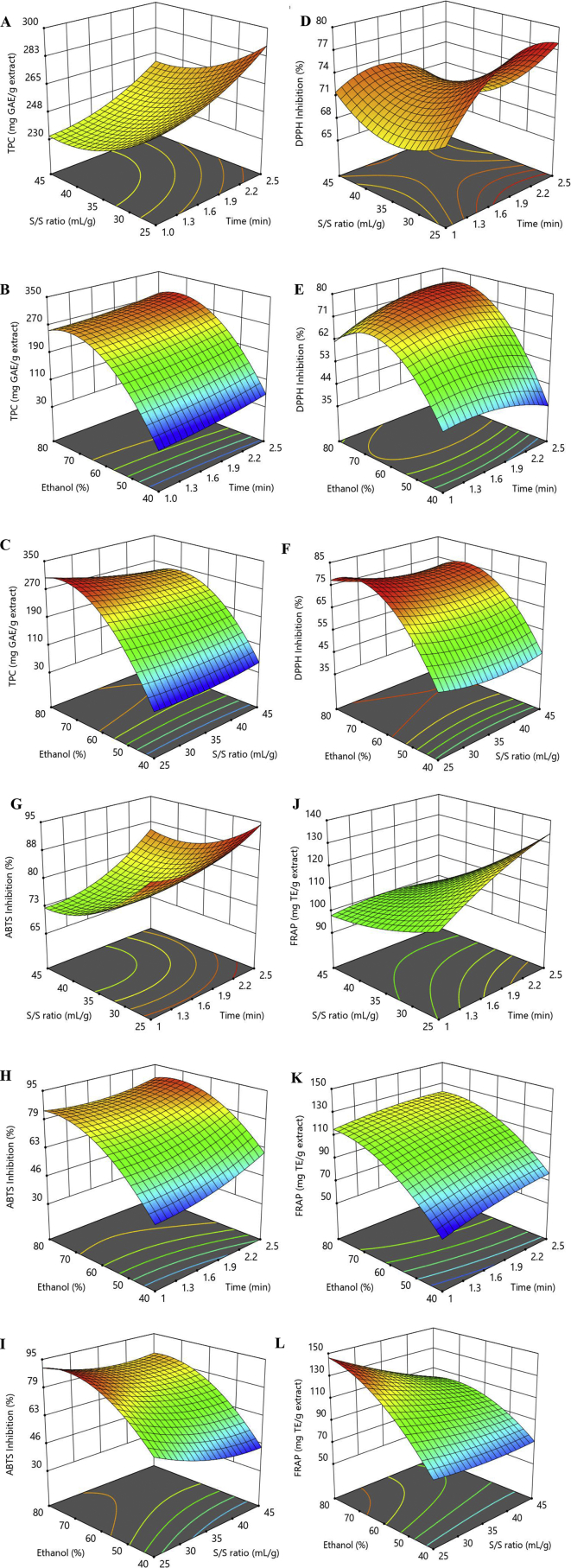
Interaction between the S/S ratio and ethanol concentration on TPC is presented in Fig. 2C. The significance of correlative interactions can be predicted from the distinct shapes of contour plots (Fig. 2); the insignificant interactions between the corresponding factors were indicated by circular contour plots, whereas the significant interactions between the corresponding factors were estimated by elliptical contours (Liu, 2013). The TPC of ME was significantly increased (P < 0.05) from 44.16 to 309.193 mg GAE/g extract when the S/S ratio decreased from 45 mL/g to 25 mL/g and the ethanol concentration raised from to 40%–80%. The recovery of TPC was significantly affected by ethanol concentration through its linear, quadratic and interaction with S/S ratio (Table 2A). Fig. 2D-E expressed the interactions between the irradiation time and each of the two factors; S/S ratio and ethanol concentration on the DPPH. The DPPH capacity of DMPE was significantly increased from 68.47 to 78.45% with the decrease of S/S ratio from 45 mL/g to 25 mL/g and the increase of irradiation time from 1 min to 2.5 min. The highest DPPH activity was obtained with the extraction conditions at 60% of ethanol concentration, 25 mL/g of S/S ratio and 2.5 min of irradiation time. The highest DPPH activity was significantly affected by ethanol concentration, linearly (X3), quadratically () as well as by the interaction with irradiation time (X1) and S/S ratio (X2) (Table 2). Fig. 2G illustrates the interaction between the irradiation time and the S/S ratio on the ABTS activity. ABTS capacity of the DMPE increased from 74.40 to 92.08% with the decrease of S/S ratio from 45 mL/g to 25 mL/g and the increase of irradiation time from to 1 min to 2.5 min. The maximum ABTS activity could be reached by similar extraction conditions to achieve the highest DPPH activity. ABTS activity was not significantly affected by the interaction effects between the independent variables but was significantly affected by the linear (X1, X2, and X3) and quadratic (X12, X22 and X32) effects. Fig. 2L exhibits the interactions between the S/S ratio and ethanol concentration on the FRAP assay. The FRAP activity of the DMPE was significantly increased from 73.86 to 143.64 mg TE/g extract with the decrease of S/S ratio from 45 mL/g to 25 mL/g and the increase of ethanol concentration from to 40%–80%. The maximum FRAP activity could be attained by similar extraction conditions to obtain the highest TPC which were 25 mL/g (S/S ratio), 80% (ethanol concentration), and 1.75 min (irradiation time). Ethanol concentration variable showed a significantly strong effect on FRAP activity through its linear (X3), quadratic () and interaction with S/S ratio (X2X3). From this finding, ethanol concentration showed a significant effect in obtaining the highest yield of TPC and the highest antioxidant activity when referring to DPPH, ABTS and FRAP assay of the DMPE. This finding is in concurrence with Yoshimura et al. (2015) which also reported a high amount of xanthones extracted using ethanol. The amphiphilic properties of ethanol solvent make it miscible in both polar (hydrophilic) and non-polar (hydrophobic) molecule. Xanthone is not miscible in a high polarity solvent especially water (Chhouk et al., 2016). Ethanol is the solvent of medium polarity index that could extract the highest total of xanthone (Aisha et al., 2013) with the highest antioxidant activity (Kusmayadi et al., 2018).
Fig. 2.
Respose surface plots for TPC (A, B & C), DPPH (D, E & F) and ABTS (G, H & I) radical scavening activity, and FRAP (J, K & L) versus S/S ratio and irradiation time, ethanol (%) and irradiation time, and ethanol (%) and S/S ratio, respectively.
3.2.3. Model validation
Table 3 exhibited the optimal MAE extraction conditions that were generated by response surface methodology (RSM). From the RSM results, the optimal values of TPC, DPPH, ABTS and FRAP activities were obtained at the following extraction condition; 2.24 min of irradiation time, 25 mL/g of solvent-to-solid ratio and 71% of ethanol concentration. This optimal condition was repeated to obtain an actual response. Thus it was used to evaluate the predicted response and validate the relevance of the model equations. The predicted and the actual values for TPC, DPPH, ABTS, and FRAP, were as follows; 320 and 316.92 ± 3.72 mg GAE/g extract, 83.63 and 70.33 ± 5.40%, 93.77 and 88.26 ± 4.97%, 144.56 and 143.70 ± 1.84 mg TE/g extract, respectively. From these results it can be concluded that the model was acceptable and relevant for this investigation.
Table 3.
Comparison of total phenolic content, α-mangostin content and antioxidant properties from dried mangosteen pericarp extract by microwave-assisted extraction (MAE) and water bath extraction (WBE) techniques.
| Sample | TPC | FRAP | Antioxidant properties |
α-M content (HPLC) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH |
ABTS |
|||||||||
| % | IC50 | TE | % | IC50 | TE | α-M | ||||
| ODMPE (Predicted) | 320.31 | 144.56 | 83.63 | - | - | 93.77 | - | - | - | - |
| ODMPE (Actual) | 316.92 ± 3.72a | 143.70 ± 1.84a | 70.33 ± 5.40a | 176.90 ± 6.36b | 245.22 ± 8.77a | 88.26 ± 4.97a | 94.21 ± 8.57b | 288.07 ± 29.15a | 1921.34 ± 267.41a | 100.48 ± 2.12a |
| WDMPE | 192.29 ± 11.51b | 125.36 ± 7.41b | 63.22 ± 2.28a | 186.17 ± 4.43c | 234.75 ± 9.00a | 99.85 ± 0.07a | 78.90 ± 2.66b | 327.77 ± 12.52a | 2285.49 ± 114.84a | 93.56 ± 1.63b |
| Trolox | - | - | - | 31.68 ± 0.03a | - | - | 23.59 ± 0.00a | - | - | - |
| α-M | - | - | - | - | - | - | 144.25 ± 0.03c | - | - | - |
Results are expressed as means ± standard deviation. Different letters within the same column denote significant differences among extraction methods (p < 0.05). Expression of units are as follows: TPC: mg GAE/g extract; FRAP: mg TE/g extract; IC50: μg/mL; TE (Trolox equivalent): mg TE/g extract; α-M (α-mangostin): mg α-M/g extract. ODMPE is optimized dried mangosteen pericarp extract from MAE; WDMPE is dried mangosteen pericarp extract from WBE.
3.3. Comparison of optimized dried mangosteen pericarp extract using MAE with WBE method
3.3.1. Total phenolic content
Table 3 presented the results of TPC and antioxidant capacity of optimized dried mangosteen pericarp extract (ODMPE) from MAE and dried mangosteen pericarp extract from WBE method (WMDPE). MAE expressed a significantly higher extraction efficiency than WBE (P < 0.05). Higher TPC values of ODMPE indicates that MAE provides strong penetration forces to the mangosteen pericarp powder (MPP) to efficiently extract the phenolic compound. MAE derived the microwave heat from the electromagnetic energy where the ionic conduction and dipole rotation permit microwaves to hit the inner glandular, trichomes, and vascular systems of MPP while for WBE, conduction and convection allowed the conventional heating of water bath to transfer the thermal energy from the source to directly hit the surface of MPP (Desai et al., 2010). The degradation of the phenolic compound may occur along the extraction process as WBE required a longer extraction time than MAE. Chaovanalikit et al. (2012) has reported that 29.30 ± 3.18 mg GAE/g extract was obtained from mangosteen outer pericarp and 34.04 ± 3.22 mg GAE/g extract from inner pericarp, while lower TPC values were extracted using acetone solvent and an ultrasonic bath.
3.3.2. Antioxidant properties
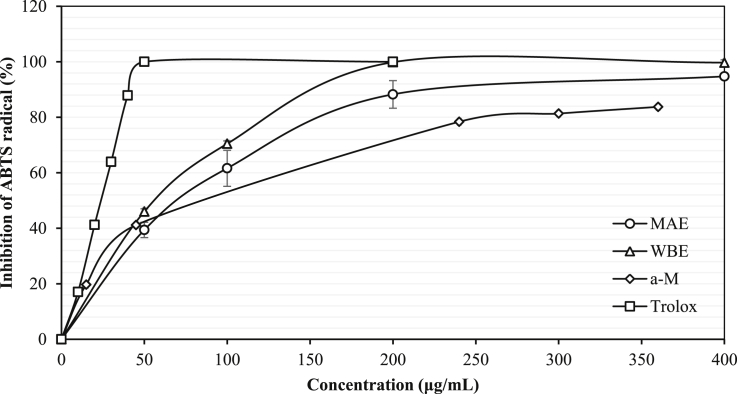
The inhibition percentage of DPPH and ABTS radical scavenging assay as well as FRAP activity (mg TE/g dried extract) was done to evaluate the antioxidant properties of the ODMPE. Based on DPPH assay, results from both ODMPE and WDMPE showed a slightly similar capacity (P > 0.05) of the percentage of DPPH inhibition (P = 0.09) and Trolox equivalent (P = 0.19) whereas ODMPE exhibited significantly lower IC50 value than WDMPE. Moreover, the inhibition percentage of ABTS radical of ODMPE and WDMPE had a statistically significant result (P < 0.05). While referring to ABTS assay, the inhibition percentage of ABTS radical, IC50 value, Trolox equivalent and α-mangostin (α-M) equivalent of ODMPE and WDMPE had no significant difference. Additionally, Ferric reducing antioxidant potential (FRAP) exhibited that ODMPE had a significantly more proficient antioxidant capacity than WBE (P < 0.05). OME and WBE had expressed about 1.7 and 1.5 times higher FRAP capacity than total FRAP of mangosteen pericarp and its yellow gum (24.6 ± 0.7 mg TE/g and 59.6 ± 2.5 mg TE/g, respectively) that were reported by Sukatta et al. (2013). The ODMPE and WDMPE had shown about 2.0 and 1.9 times, respectively higher Trolox equivalent (TE) value than total TE from ethanolic extracts of mangosteen pericarp and its yellow gum which reported by Sukatta et al. (2013) (25.2 ± 2.5 mg TE/g and 99.4 ± 3.7 mg TE/g, respectively) as referring to DPPH assay. IC50 value indicates the concentration of ODMPE that could scavenge the DPPH radical by 50%. ODMPE expressed at about 5.6 and 8.6 times less efficient whereas WDMPE exhibited 6.0 and 9.0 times less efficient in comparison with standard Trolox (Table 3) and result from previous study by Ghasemzadeh et al. (2018), respectively. The ODMPE and WDMPE had IC50 values of ABTS assay as represented in Fig. 3, which expressed at about 1.5 and 1.8 times, respectively more efficient than α-mangostin standard. These IC50 values (Table 3) demonstrated a lower capacity than the finding by Ghasemzadeh et al. (2018). This occurrence might be associated with the particle size of MPP (120 mesh size versus 80 mesh size) as well as the solvent type (71% ethanol versus 72% ethyl acetate). Therefore, a too small and/or too large particle size could diminish the extraction efficiency. Particle size and solvent type are among the influential factors that affect MAE extraction efficiency (Desai et al., 2010). This finding is supported by Dahmoune et al. (2013) who proposed MAE to be a potential technique for antioxidant compound extraction from lemon peels as MAE attained the lowest IC50 value of DPPH assay (203.59 ± 5.59 μg GAE/mL) rather than UAE (286.24 ± 10.62 μg GAE/mL) and conventional solvent extraction, CSE (298.82 ± 8.6 μg GAE/mL). The authors suggested that the lower antioxidant capacity of UAE and CSE extracts might be linked to a greater extraction time consumed as well as more detrimental exposure such as light and oxygen.
Fig. 3.
ABTS radical scavenging activities of MAE and WBE extract, trolox and α-mangostin standard.
3.3.3. α-mangostin content of optimized mangosteen pericarp extract (ODMPE) by HPLC
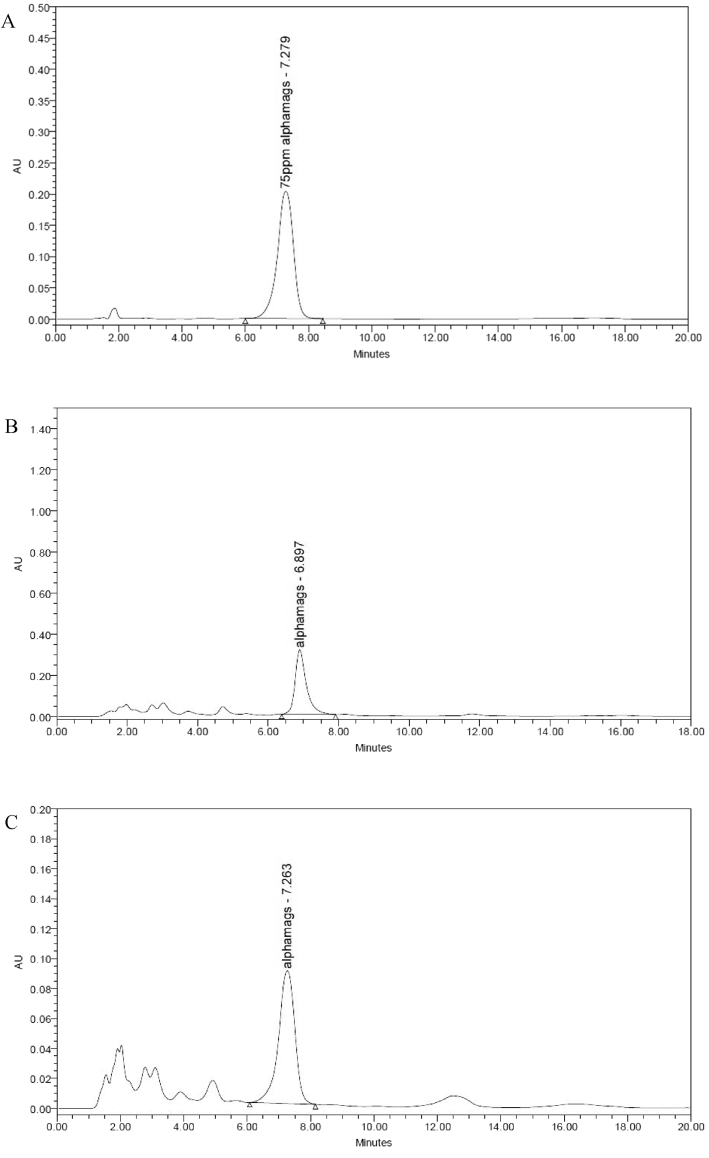
The quantification of α-mangostin (α-M) was measured based on a calibration standard curve of α-mangostin which plotted by peak area versus a series of α-mangostin concentration (Fig. 4). According to quantification of α-mangostin content using a reverse-phase HPLC column, ODMPE (100.48 ± 2.12a mg α-M/g extract) demonstrated a significantly higher α-mangostin content as compared to WDMPE (93.56 ± 1.63b mg α-M/g extract) (P < 0.05). Thus, α-mangostin might be correlated to TPC value as ODMPE also exhibited a significantly higher TPC value than WDMPE. This finding is in agreement with previous study by Ghasemzadeh et al. (2018) (121.01 mg α-M/g dry matter). Mangosteen pericarp extract had a much lower xanthone content when extracted using UAE (0.1760 mg/g dried weight), soxhlet (0.1221 mg/g dried weight) and maceration techniques (0.0565 mg/g dried weight) (Yoswathana, 2013) which might occur due to the different solvent concentration (95% ethanol versus 71% ethanol). Water is an excellent microwave absorptive solvent due to higher tan δ value (dissipation factor) (Desai et al., 2010). Therefore, water could enhance the dissipation factor of other solvents to fit the microwave responsive heating.
Fig. 4.
HPLC analysis of (A) α-mangostin standard; (B) MAE extract; and (C) WBE extract.
4. Conclusion
This study attempted to find the most effective MAE conditions for high TPC recovery with the optimum antioxidant-rich properties of xanthone extract from mangosteen pericarp. The results from response surface methodology has shown that all the independent variables affected the values of TPC, DPPH, ABTS and FRAP significantly. In addition, the ANOVA of each models displayed that the models were significantly (P < 0.0001) fitted and the lack of fit value of the models were insignificant (P > 0.05) expressing that the models are reliable and repeatable. The optimal TPC, DPPH, ABTS, FRAP values were obtained by following MAE optimum conditions; 2.24 min of irradiation time, 25 mL/g of solvent-to-solid ratio and 71% of ethanol concentration. The predicted values for TPC, DPPH, ABTS, and FRAP assay were as followed; 320 mg GAE/g extract, 83.63%, 93.77%, and 144.56 mg TE/g extract, respectively. The optimized dried mangosteen pericarp extract (ODMPE) expressed a significantly higher TPC and FRAP values than dried mangoesteen pericarp extract from WBE (WDMPE). However, DPPH and ABTS radical scavenging assays showed that ODMPE and WDMPE had a slightly similar antioxidant capacity. ODMPE (100.48 ± 2.12a mg α-M/g extract) also demonstrated significantly more prominent α-mangostin content when compared to WDMPE. α-mangostin represents the major constituent of the xanthone compound as well as contributing to the highest antioxidant potential. For industrial application, the obtained models can be the basis for pilot-scale in operating MAE as a green and safer extraction technology for the extraction of antioxidant compounds from bio-waste, mangosteen pericarp. For future research, MAE is a sustainable technology that can also be applied in the enhancement of stability of high-value antioxidant compound to produce and develop the potent natural antioxidant for food and pharmaceutical applications.
Declarations
Author contribution statement
Nor Azizah: Mohammad Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Dayang Norulfairuz Abang Zaidel: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ida Idayu Muhamad: Contributed reagents, materials, analysis tools or data.
Mariani Abdul Hamid, Harisun Yaakob: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yanti Maslina Mohd Jusoh: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Ministry of Education Malaysia and Universiti Teknologi Malaysia for the financial support from Research Grant Q.J130000.3551.05G90 and R.J130000.7851.4F993, as well as the Zamalah UTM Scholarship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aisha A.F.A., Abu-Salah K.M., Ismail Z., Abdul-Majid A.M.S. Determination of total xanthones in Garcinia mangostana fruit rind extracts by ultraviolet (UV) spectrophotometry. J. Med. Plants Res. 2013;7(1):29–35. [Google Scholar]
- Alara O.R., Abdurahman N.H., Olalere O.A. Optimization of microwave-assisted extraction of flavonoids and antioxidants from Vernonia amygdalina leaf using response surface methodology. Food Bioprod. Process. 2018;107:36–48. [Google Scholar]
- Boeing J.S., Barizão É.O., e Silva B.C., Montanher P.F., de Cinque Almeida V., Visentainer J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem. Cent. J. 2014;8(1):48. doi: 10.1186/s13065-014-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaovanalikit A., Mingmuang A., Kitbunluewit T., Choldumrongkool N., Sondee J., Chupratum S. Anthocyanin and total phenolics content of mangosteen and effect of processing on the quality of Mangosteen products. Int. Food Res. J. 2012;19(3):1047–1053. [Google Scholar]
- Chhouk K., Quitain A.T., Gaspillo P.D., Maridable J.B., Sasaki M., Shimoyama Y., Goto M. Supercritical carbon dioxide-mediated hydrothermal extraction of bioactive compounds from Garcinia Mangostana pericarp. J. Supercrit. Fluids. 2016;110:167–175. [Google Scholar]
- Dahmoune F., Boulekbache L., Moussi K., Aoun O., Spigno G., Madani K. Valorization of Citrus limon residues for the recovery of antioxidants: evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crops Prod. 2013;50:77–87. [Google Scholar]
- Dahmoune F., Spigno G., Moussi K., Remini H., Cherbal A., Madani K. Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crops Prod. 2014;61:31–40. [Google Scholar]
- Dahmoune F., Nayak B., Moussi K., Remini H., Madani K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015;166:585–595. doi: 10.1016/j.foodchem.2014.06.066. [DOI] [PubMed] [Google Scholar]
- Desai M., Parikh J., Parikh P.A. Extraction of natural products using microwaves as a heat source. Separ. Purif. Rev. 2010;39(1–2):1–32. [Google Scholar]
- Folin O., Ciocalteau V. Tyrosine and tryptophane in proteins. J. Biol. Chem. 1927;73(2):627–648. [Google Scholar]
- Ghasemzadeh A., Jaafar H.Z.E., Baghdadi A., Tayebi-meigooni A. Alpha-mangostin-rich extracts from mangosteen pericarp: optimization of green extraction protocol and evaluation of biological activity. Molecules. 2018;23(1852):1–16. doi: 10.3390/molecules23081852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat K., Zhang X., Chen H., Xia S., Jia C., Zhong F. Liberation and separation of phenolic compounds from citrus Mandarin peels by microwave heating and its effect on antioxidant activity. Separ. Purif. Technol. 2010;73(3):371–376. [Google Scholar]
- Jujun P., Pootakham K., Pongpaibul Y., Tharavichitkul P., Ampasavate C. HPLC determination of mangostin and its application to storage stability study. Chiang Mai Univ. J. Nat. Sci. 2009;8(1):43–53. [Google Scholar]
- Jung H.-A., Su B.-N., Keller W.J., Mehta R.G., Kinghorn A.D. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen) J. Agric. Food Chem. 2006;54(6):2077–2082. doi: 10.1021/jf052649z. [DOI] [PubMed] [Google Scholar]
- Jusoh Y.M.M., Idris A.A., Khairuddin N., Zaidel D.N.A., Hashim Z., Mahmood N.A.N. Effect of solvent pH, microwave power and extraction time on microwave-assisted extraction of Hibiscus rosa-sinensis. Chem. Eng. Trans. 2018;63:541–546. [Google Scholar]
- Kang J., Kim S., Moon B. Optimization by response surface methodology of lutein recovery from paprika leaves using accelerated solvent extraction. Food Chem. 2016;205:140–145. doi: 10.1016/j.foodchem.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Karazhiyan H., Razavi S.M.A., Phillips G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocolloids. 2011;25(5):915–920. [Google Scholar]
- Kusmayadi A., Adriani L., Abun A., Muchtaridi M., Tanuwiria U.H. The effect of solvents and extraction time on total xanthone and antioxidant yields of mangosteen peel (Garcinia mangostana L.) extract. Drug Invent. Today. 2018;10(12):2572–2576. [Google Scholar]
- Li Y., Li S., Lin S.-J., Zhang J.-J., Zhao C.-N., Li H.-B. Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: optimization and identification of phenolic compounds. Molecules. 2017;22(1481):1–16. doi: 10.3390/molecules22091481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wei S., Liao M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crops Prod. 2013;49:837–843. [Google Scholar]
- Prakash Maran J., Manikandan S., Nivetha C.V., Dinesh R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arabian J. Chem. 2017;10:S1145–S1157. [Google Scholar]
- M’hiri N., Ioannou I., Ghoul M., Boudhrioua N.M. Extraction methods of citrus peel phenolic compounds. Food Rev. Int. 2014;30(4):265–290. [Google Scholar]
- M’hiri N., Ioannou I., Boudhrioua N.M., Ghoul M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Process. 2015;96:161–170. [Google Scholar]
- Nakatani K., Nakahata N., Arakawa T., Yasuda H., Ohizumi Y. Inhibition of cyclooxygenase and prostaglandin E2 synthesis by γ-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem. Pharmacol. 2002;63(1):73–79. doi: 10.1016/s0006-2952(01)00810-3. [DOI] [PubMed] [Google Scholar]
- Nayak B., Dahmoune F., Moussi K., Remini H., Dairi S., Aoun O., Khodir M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015;187:507–516. doi: 10.1016/j.foodchem.2015.04.081. [DOI] [PubMed] [Google Scholar]
- Parry J., Su L., Luther M., Zhou K., Peter Yurawecz M., Whittaker P., Yu L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005;53(3):566–573. doi: 10.1021/jf048615t. [DOI] [PubMed] [Google Scholar]
- Qu W., Pan Z., Ma H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010;99(1):16–23. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Shan T., Ma Q., Guo K., Liu J., Li W., Wang F., Wu E. Xanthones from mangosteen extracts as natural chemopreventive agents: potential anticancer drugs. Curr. Mol. Med. 2011;11(8):666–677. doi: 10.2174/156652411797536679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukatta U., Takenaka M., Ono H., Okadome H., Sotome I., Nanayama K. Distribution of major xanthones in the pericarp, aril, and yellow gum of mangosteen (Garcinia mangostana linn.) fruit and their contribution to antioxidative activity. Biosci. Biotech. Biochem. 2013;77(5):984–987. doi: 10.1271/bbb.120931. [DOI] [PubMed] [Google Scholar]
- Suttirak W., Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014;51(12):3546–3558. doi: 10.1007/s13197-012-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarnakuta P., Chaweerungrat C., Devahastin S. Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem. 2011;125(1):240–247. [Google Scholar]
- Wittenauer J., Falk S., Schweiggert-Weisz U., Carle R. Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC-DAD-MS n. Food Chem. 2012;134(1):445–452. [Google Scholar]
- Yoo J.H., Kang K., Jho E.H., Chin Y.W., Kim J., Nho C.W. α- and γ-Mangostin inhibit the proliferation of colon cancer cells via β-catenin gene regulation in Wnt/cGMP signalling. Food Chem. 2011;129(4):1559–1566. [Google Scholar]
- Yoshimura M., Ninomiya K., Tagashira Y., Maejima K., Yoshida T., Amakura Y. Polyphenolic constituents of the pericarp of mangosteen (Garcinia mangostana L.) J. Agric. Food Chem. 2015;63(35):7670–7674. doi: 10.1021/acs.jafc.5b01771. [DOI] [PubMed] [Google Scholar]
- Yoswathana N. Accelerated extraction of Xanthone from Mangosteen pericarp using ultrasonic technique. Afr. J. Pharm. Pharmacol. 2013;7(6):302–330. [Google Scholar]
- Zarena A.S., Manohar B., Udaya Sankar K. Optimization of supercritical carbon dioxide extraction of xanthones from mangosteen pericarp by response surface methodology. Food Bioprocess Technol. 2012;5(4):1181–1188. [Google Scholar]