Abstract
Background
Human multipotent adult progenitor cells (MAPC®) are an emerging therapy for traumatic brain injury (TBI); however, clinically translating a therapy involves overcoming many factors in vivo which are not present in pre-clinical testing. In this study we examined clinical parameters in vitro that may impact cell therapy efficacy.
Methods
MAPC were infused through varying gauged needles and catheters with and without chlorhexidine, and their viability tested with trypan blue exclusion. MAPC were co-cultured with phenytoin and celecoxib at relevant clinical concentrations for 1 h and 24 h. Anti-inflammatory potency was tested using a stimulated rat splenocyte co-culture and ELISA for TNF-α production. MAPC were cultured under different osmolar concentrations and stained with propidium iodide for viability. Anti-inflammatory potency was tested by co-culture of MAPC with naïve lymphocytes activated by CD3/CD28 beads, and Click-iT® Plus EdU was used to quantify proliferation by flow cytometry.
Results
The mean viability of the MAPC infused via needles was 95 ± 1%; no difference was seen with varying flow rate, but viability was notably reduced by chlorhexidine. MAPC function was not impaired by co-culture with phenytoin, celecoxib, or combination with both. Co-culture with phenytoin showed a decrease in TNF-α production as compared to the MAPC control. MAPC cultured at varying osmolar concentrations all had viabilities greater than 90% with no statistical difference between them. Co-culture of MAPC with CD3/CD28 activated PBMCs showed a significant reduction in proliferation as measured by EdU uptake.
Discussion
Needle diameter, phenytoin, celecoxib, and a relevant range of osmolarities do not impair MAPC viability or anti-inflammatory potency in vitro.
Keywords: Stem cell research, Cell biology, Immunology, Medicine, Stem cells research, Inflammation, Regenerative medicine, Cell therapy, Traumatic brain injury, Translational medicine
1. Introduction
The Center for Disease Control (CDC) estimates that traumatic brain injury (TBI) accounted for 2.87 million emergency room visits, hospitalizations, and deaths in the United States in 2014 [1]. Those who survive face functional limitation or disability as well as lasting cognitive and behavioral disorders [2]. Currently, there is no effective treatment for primary injury associated with TBI, however many are focusing on interventions for damaging secondary injury sequelae. Though multiple therapies for TBI have emerged with strong pre-clinical evidence for therapeutic benefit, none have successfully translated from the bench to the clinic [3]. Over the past decade cell therapies derived from multiple sources, such as adipose tissue, bone marrow, amniotic fluid, umbilical cord blood, and other tissues, have emerged as a potential treatment. Although their exact mechanisms of action in the brain and other organs is pleiotropic, cell therapies improve the integrity of the blood-brain-barrier, decreasing edema in the brain after TBI [4], and may promote an anti-inflammatory and pro-regenerative phenotype in the brain [5]. Phase I studies in pediatric populations have shown clinical safety [6], and retrospective cohort data has shown decreased therapeutic intensity for increased intracranial pressures over the first seven days of hospitalization [7].
Currently, this cell therapy treatment paradigm requires a bone marrow harvest and a team of specialists, as well as a laboratory compliant with Current Good Manufacturing Practices (cGMP), to make the mononuclear product. This model may not be generalizable outside large medical centers without modification. As an alternative to this model, we have performed pre-clinical experiments with multipotent adult progenitor cells (MAPC®, or MultiStem®) which can be manufactured and stored for use at the appropriate time without the acute need for autologous bone marrow harvest and cell processing [8]. Like mesenchymal stromal cells, MAPC are post-natal nonhematopoeitic adherent stem cells, however they have the ability to be expanded for more than 30 passages (in contrast to mesenchymal stem cells, which can only be maintained for 12–18 passages) [9]. MAPC have also been shown to stimulate superior angiogenesis, decrease left ventricular injury, and have a more favorable cytokine profile than other cellular therapies in studies of myocardial infarction [10]. MAPC have also demonstrated promise in pre-clinical studies of TBI [11, 12], as well as in clinical trials of ischemic stroke [13]. Cellular therapies continue to gain momentum in both preclinical and clinical studies. Here we detail some common components of clinical cell infusion that may have an effect on cell viability and immunobiology.
We examine the effects of common clinical conditions, such as infusion through needles and catheters (chlorhexidine impregnated and non-impregnated), co-culture with phenytoin and celecoxib, and in diluents with different osmolarities, on the viability of MAPC. Antiseptic-impregnated catheters decrease the prevalence of catheter-associated bloodstream infections, as well as number of ICU days [14, 15, 16], and are used extensively for central vascular access in critically ill patients. Phenytoin is used as a prophylactic anticonvulsant in TBI, and celecoxib is increasingly used as part of a multi-modal pain regimen designed to minimize narcotic use. We examined the effects of variation in osmolar environment on MAPC because hyperosmolar therapy is a mainstay of TBI treatment and recommended by the American Association of Neurological Surgeons [17] for decreasing intracranial hypertension. In addition, MAPC were co-cultured with stimulated human peripheral blood mononuclear cells (PBMC) and examined for their proliferation with flow cytometry. These parameters were selected because they are key components of current neurocritical care for TBI. As one of the potential mechanisms of action for MAPC relates to immunomodulatory properties of pro-inflammatory cytokine suppression and T-cell inhibition, we defined “potency” as anti-inflammatory TNF-α suppression and inhibition of T-cell proliferation.
2. Methods
2.1. Infusion via needles
Briefly, vials of frozen MAPC obtained from Athersys, Inc. [Cleveland, OH, USA, http://athersys.com] [8, 9, 18] were rapidly thawed, washed in phosphate buffered solution (PBS). Next, cell were re-suspended in an assay media consisting of RPMI media supplemented with 10% heat-inactivated fetal bovine serum (FBS) [Atlanta Biological, Atlanta, Ga, USA] and 1% PenStrep (100 U/mL penicillin and 100 μg/mL streptomycin) [Sigma-Aldrich, St. Louis, MO, USA] at 1 × 106 cells/mL. They were tested for baseline viability via trypan blue exclusion and then loaded into a BD 10mL syringe, which was secured to a Harvard Apparatus PHD 2000 Syringe Pump Series infusion pump, and the respective 30, 25, and 20 gauge 1-inch needles were attached. Each needle was primed with 100μL of cell solution, then 0.25mL was infused through each needle at rates of 60, 120, 240, and 500 mL/h, collected in a 15mL conical vial, and viability tested again with trypan blue exclusion. Needle infusion tests were run in duplicate.
2.2. Infusion via central venous catheters
Freshly thawed MAPC, as previously described [8, 9], at 1 × 106 cells/mL were loaded into a 10 mL BD syringe and secured to a Harvard Apparatus PHD 2000 Syringe Pump Series infusion pump. The chlorhexidine-impregnated ARROWgard Blue PLUS Pressure Injectable Quad-Lumen (ASK-45703-AKP1A) [Arrow International, Reading, PA, USA] central venous catheter was secured to the syringe and 9 mL of MAPC solution was infused through it. Each successive milliliter was collected in a separate 15mL conical vial. Viability of each sample was obtained using trypan blue exclusion every 10 min for 40 min. The syringe was then removed and agitated, and the ARROW Multi-Lumen Central Venous Catheter (AK-15703) [Arrow International, Reading, PA, USA] was attached, and the syringe replaced in the pump. The experiment was then repeated as for the first catheter. N for each catheter = 4.
2.3. MAPC co-culture with splenocytes in the presence of phenytoin and celecoxib
Freshly thawed and washed MAPC, as previously described [8, 9], were suspended at 1 × 105 cells/mL in previously prepared media containing either clinical target concentrations of phenytoin and celecoxib, 15 μg/mL of phenytoin [Sigma-Aldrich, St. Louis, MO, USA], 2 μg/mL of celecoxib [LC Laboratories, Woburn, MA, USA], or a combination with 15 μg/mL of phenytoin and 2 μg/mL of celecoxib. In addition, we ran experiments a log-fold above and below these concentrations. These concentrations were chosen based on pharmacokinetics of these drugs [16, 19]. One hundred μL of each MAPC solution was plated in triplicate in a 96-well plate. After 24h of incubation at 37 °C the plate was washed with fresh media, and all wells replenished with media containing the same total concentrations of the drugs described above and allowed to incubate for 5 min. Next, 100 μL of freshly harvested rat splenocytes were added to the wells at a concentration of 1 × 106 cells/mL and then activated with 0.1μl of lipopolysaccharide (LPS) [Invivogen, San Diego, CA, USA] at 1 μg/mL. The conditioned media was then collected 1 h and 24 h post-LPS activation and assayed by ELISA [Biolegend, San Diego, CA, USA] for TNF-α levels following manufacturer's protocol.
2.4. Osmolarity studies
Freshly thawed and washed MAPC, as previously described [8, 9], were cultured under different osmolar concentrations (250, 270, 290, 310, 330, 350, 370, and 390 mOsm). Osmolarities were measured by a Wescor 5500 vapor pressure osometer [Wescor, Inc., Logan, UT, USA]. On day 3 the cells were evaluated for viability. Cells were harvested and stained for propidium iodide exclusion [BD Biosciences, San Jose, California, USA] at manufacturer recommended concentrations and analyzed by flow cytometry on a Beckman Coulter LSRII [Beckman Coulter, Inc., Miami, FL, USA], data analysis was performed using DIVA software.
2.5. PBMC isolation
Fresh human blood was collected under university approved IRB protocol MSC-MS-10-0190. Healthy volunteers donated whole blood which was then layered over Ficoll-Paque [GE Healthcare Bio-Sciences, Uppsala, Sweden] and separated by centrifugation according to manufacturer directions. The mononuclear layer was then washed in PBS and counted in a hemocytometer.
2.6. PBMC proliferation assay
To evaluate MAPC ability to inhibit PBMC proliferation, freshly thawed and washed MAPC, as previously described [8, 9], were plated into a 6-well plate [Greiner Bio-One North America, Inc., Monroe, NC, USA] at 3 different treatment concentrations (3 × 105, 1.5 × 105, and 7.5 × 104 cells/well). Peripheral blood mononuclear cells were activated to proliferate via manufacturer's protocol using CD3/CD28 biotinylated beads [Miltenyi Biotec, San Diego, CA, USA], and then 2.4 × 106 activated cells were added to each well to make treatment groups of 1:32, 1:16, and 1:8 (MAPC:PBMC). PBMC cultured alone (without MAPC) with or without activation beads served as positive and negative controls, respectively. The plates incubated for 3 days, at which time EdU [ThermoFisher Click-iT® Plus EdU Alexa Fluor® 488 Flow Cytometry Assay Kit, Life Technologies, Carlsbad, CA, USA] was added to each well at a final concentration of 10μM and allowed to incorporate for 24 h according to manufacturer suggested protocol. MAPC which had undergone needle infusion through a 30 gauge 1-inch needle were then plated in media that contained a combination of 15 μg/mL phenytoin and 2 μg/mL of celecoxib. These cells were incubated for 24 h and then the media was removed, fresh media plated, and freshly collected PBMC plated in co-culture to achieve the doses stated above. On day 4, PBMC were harvested with TrypLE™ [Gibco by Life Technologies, Grand Island, NY, USA] and counted by hemocytometer. The cells were then stained for T-cell surface markers, fixed and permeabilized, and Click-iT® EdU was detected. The cells were then re-suspended in stain buffer (3% goat serum in PBS) and analyzed by flow cytometry. Suspended PBMC were washed with buffer and stained for V450-conjugated mouse anti-human CD8 and APC-conjugated mouse anti-human CD3 [BD Biosciences, San Jose, CA, USA] on ice in the dark. After washing with PBS they were fixed and permeabilized, and Click-iT® EdU was detected. The cells were then re-suspended in saponin-based permeabilization and wash reagent (from Click-iT® kit) and analyzed by flow cytometry on a Beckman Coulter Gallios flow cytometer using Kaluza Acquisition Software [Beckman Coulter, Inc., Miami, FL, USA] for acquisition and analysis.
2.7. Statistical analysis
Statistical analysis was performed using GraphPad Prism, version 5.04 [GraphPad Software, Inc, La Jolla, CA, USA]. Data are expressed as the mean ± SEM. Two-tailed t-test is used to analyze the chlorhexidine versus non-chlorhexidine catheter studies, and chlorhexidine-catheter versus needle studies. One-way analysis of variance (ANOVA) was used to analyze all other data, with the Tukey post-hoc test. Significance was set at p < 0.05.
3. Results
3.1. The effect of needle gauge, infusion rate, catheters, and osmolarity on the infusion of MAPC
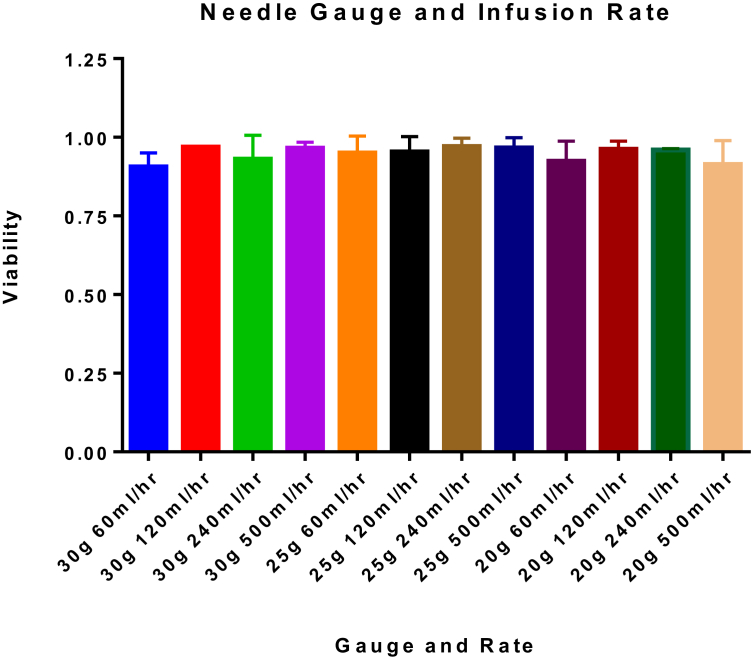
To study the potential effects of needle-associated shear stress on MAPC viability, we compared 20, 25, and 30 gauge, 1 inch needles with infusion rates at 60, 120, 240, or 500 ml/h then measured viability with trypan blue exclusion. We observed a significant variation in needle gauge (p = 0.0368), according to Two-Way ANOVA. However, there was no significant variation in viability based upon infusion rate (p value = 0.2071). Our results show that the mean viability of MAPC infused at all rates, via all needles was 95 ± 0.1%. Based on these results flow rate did not significantly affect cell viability, maintaining high cell viability across all tested factors. Full results are shown in Table 1 and Fig. 1. Values represent mean ± SD. Statistical analysis performed by Two-Way ANOVA.
Table 1.
Percent viabilities of MAPC after infusion via needles. MAPC were infused via needles of different size and at different rates. Neither needle gauge nor infusion rate significantly altered viability of MAPC. The data presented in this table represents the mean percent viability ±SD. N = 2.
| Gauge | Rate (mL/hr) |
|||
|---|---|---|---|---|
| 60 | 120 | 240 | 500 | |
| 30 | 0.91 ± 0.03 | 0.97 ± 0.00 | 0.93 ± 0.05 | 0.97 ± 0.01 |
| 25 | 0.95 ± 0.04 | 0.95 ± 0.03 | 0.97 ± 0.02 | 0.97 ± 0.02 |
| 20 | 0.92 ± 0.05 | 0.96 ± 0.02 | 0.96 ± 0.00 | 0.91 ± 0.05 |
Fig. 1.
MAPC viability after infusion through needles. This data complements Table 1. MAPC were infused via needles of different size and at different rates. Neither needle gauge nor infusion rate significantly altered viability of MAPC. The data presented in this table represents the mean percent viability ±SD. Statistical analysis was performed by Two-Way ANOVA. N = 2.
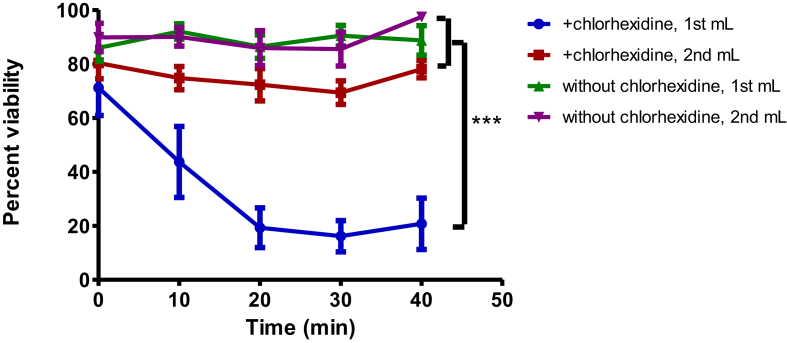
We also examined cell viability after infusion through catheters with or without chlorhexidine coating. Mean viability of MAPC infused via non-chlorhexidine treated catheters significantly decreased by 5.75% (94.96% ± 3.97 vs. 89.21 ± 8.79, p < 0.01). The first 1mL of MAPC infused through a chlorhexidine-treated catheter had 54% reduced viability as compared to the first 1mL infused via a non-chlorhexidine catheter (32.26% ± 25.98 vs. 88.71% ± 8.15, p < 0.001). The viability of the second mL of MAPC solution infused through a chlorhexidine-treated catheter showed no statistical difference in viability compared to the catheter that had not been treated with chlorhexidine. When the catheter was flushed with 10mL of saline before infusion, as is clinically required when central lines are placed, the viability increased to greater than 80%. In addition, the first mL of MAPC solution that went through the chlorhexidine-impregnated catheter continued to decrease in viability in the collection vial over the 40 min after infusion (Fig. 2). Values represent mean ± SD. Statistical analysis performed by Two-Way ANOVA.
Fig. 2.
Chlorhexidine effect on MAPC viability. Percent viability versus time of the first and second mL of a 1 × 106 MAPC/mL solution when infused through a chlorhexidine-impregnated central venous catheter versus the same type of catheter which had not been treated with chlorhexidine. Data show a significant decrease in cell viability in the first mL infused by a chlorhexidine-impregnated catheter as compared to the second mL infused through a chlorhexidine-impregnated catheter, and a catheter that had not been treated with chlorhexidine (p < 0.001). There was no statistical difference between the viability of the first and second mL of a catheter that had not been treated with chlorhexidine and the second mL of solution infused through a chlorhexidine-impregnated catheter. Values represent mean ± SD. Statistical analysis performed by Two-Way ANOVA. N = 4 per group.
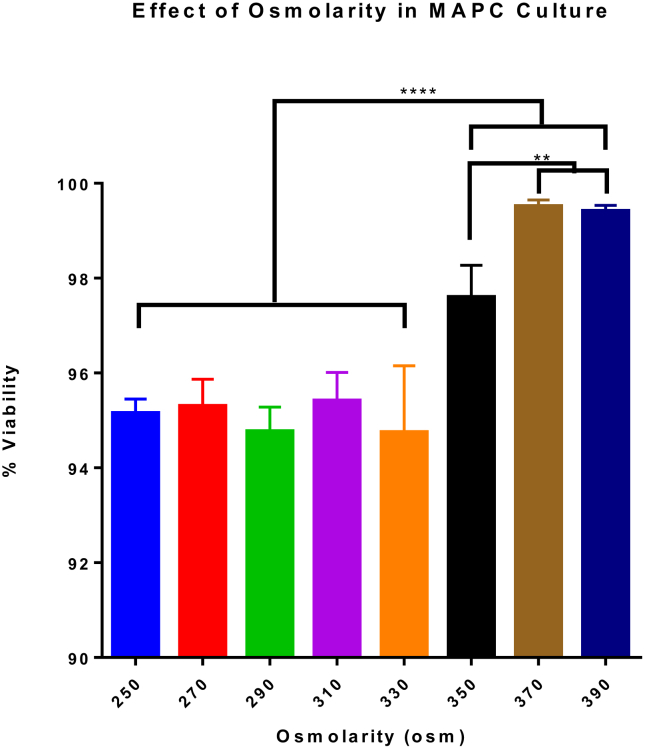
MAPC were cultured under different osmolar concentrations to determine potential effects on viability. When MAPC were cultured at osmolar concentrations ranging from 250 osm to 390 osm, all viabilities were greater than 94% when stained with propidium iodide. Specifically, MAPC cultured at higher osmolar concentrations (350, 370, 390 osm) yielded the highest viability and were significantly higher (p value <0.0001) than those cultured at lower concentrations. Highest viability was achieved at 370 and 390 osm, with 99.42% ± 0.12 and 99.52% ± 0.13 viability, respectively (Fig. 3). These results indicate that increasing osmolar concentrations in culture can enhance viability in MAPC. Values represent mean ± SD. Statistical analysis performed by One-Way ANOVA.
Fig. 3.
Effect of osmolarity on MAPC infusion. MAPC were cultured at osmolar concentrations ranging from 250 osm to 390 osm and measured for percent viability. Specifically, MAPC cultured at higher osmolar concentrations (350, 370, 390 osm) yielded the highest viability and were significantly higher (p value <0.0001) than those cultured at lower concentrations. These results indicate that increasing osmolar concentrations in culture can enhance viability in MAPC. Values represent mean ± SD. Statistical analysis performed by One-Way ANOVA. N = 6.
3.2. MAPC effect on T cell proliferation
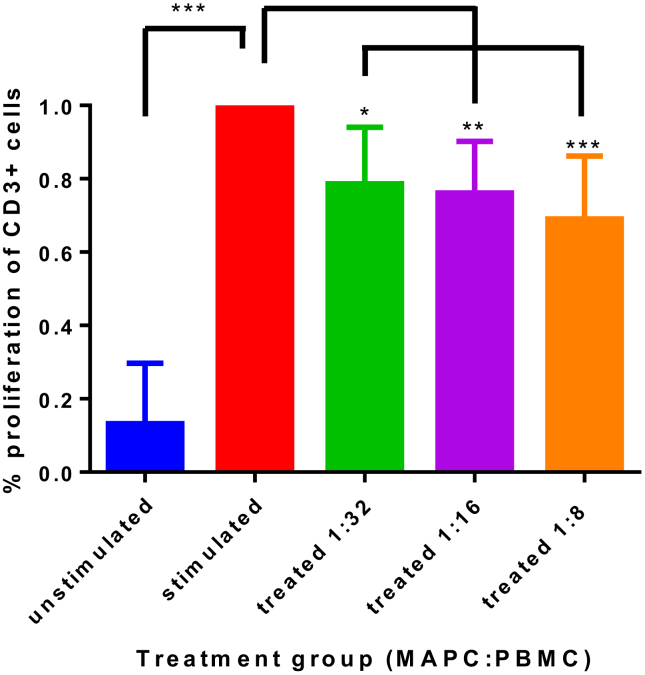
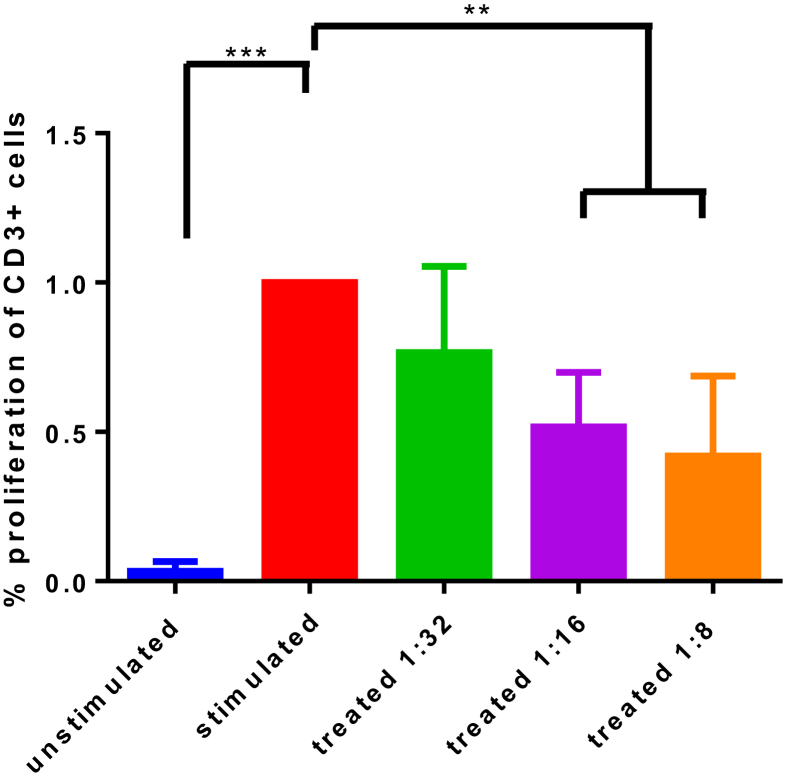
To evaluate their biological function, we studied the effect of MAPC on activated PBMC ability to proliferate. A co-culture of MAPC with CD3/CD28-activated PBMC showed a statistically significant reduction in proliferation (Fig. 4) compared to the activated control. Overall, higher ratios of MAPC:PBMC resulted in stronger suppression of PBMC proliferation. Specifically, suppression was measured at 79% ± 0.15 with 1:32 MAPC:PBMC treatment group (p < 0.05), 76% ± 0.14 in the 1:16 treatment group (p < 0.01), and 69% ± 0.17 reduction in the 1:8 treatment group (p < 0.001) compared to an activated PBMC control. Values represent mean ± SD. Statistical analysis performed by One-Way ANOVA.
Fig. 4.
MAPC effect on T cell proliferation. The percent of T-cells proliferating as normalized to the CD3/CD28 stimulated control are presented. The basal proliferation is indicated by the unstimulated control. CD3/CD28 stimulated PBMC proliferate less robustly at all MAPC activation groups (1:32, p < 0.05; 1:16, p < 0.01; 1:8, p < 0.001), with a dose-dependent trend ranging from 21% to 31% reduction in proliferation as MAPC concentration increases. Application of MAPC to activated PBMC results in a suppression of PBMC proliferation ability. Values represent mean ± SD. Statistical analysis performed by One-Way ANOVA. N = 5 per group.
3.3. Effect of infused MAPC co-cultured with phenytoin and celecoxib on T cell proliferation
MAPC that had been infused via needles and then co-cultured with phenytoin and celecoxib were also co-cultured with PBMC. Flow cytometry analysis of T-cell proliferation showed statistically significant 48% reduction of proliferation in the 1:16 treatment dose (51.74% ± 0.18, p < 0.01) and a 58% reduction in the 1:8 treatment dose (41.98% ± 0.27, p < 0.01). Treatment with a ratio of 1:32 MAPC to PBMC showed an average reduction in proliferation which was not statistically significant (76.6% ± 0.29, p = 0.21) (Fig. 5). Values represent mean ± SD. Statistical analysis performed by One-Way ANOVA.
Fig. 5.
MAPC effect on T cell proliferation, under the influence of clinical parameters. Results of co-culture proliferation assay as normalized to positive control. MAPC were first infused via a 30 gauge needle, then co-cultured with phenytoin and celecoxib for 24 h, after which the media was changed and MAPC were plated with activated healthy donor PBMC. There was a statistically significant decrease in proliferation in the higher doses (1:16 and 1:8 treatment groups, p < 0.001). Anti-inflammatory potency of the needle-infused and drug-treated MAPC to reduce proliferation was not statistically different from the MAPC which were not treated with needle-infusion or drug co-culture. Values represent mean ± SD. Statistical analysis performed by One-Way ANOVA. N = 5 per group.
3.4. TNF-α suppression by MAPC co-cultured with phenytoin, celecoxib, or both
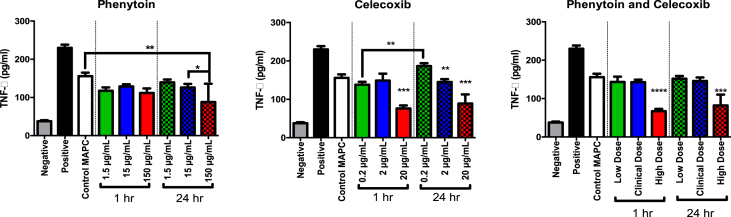
MAPC co-cultured with phenytoin, celecoxib, or both, were co-cultured with stimulated PBMC to determine the effect of the treatment on MAPC ability to inhibit TNF-α. ELISA was used to measure these effects. Our results showed that MAPC suppress PBMC TNF-α production in the presence of clinical antiseptics. At clinical target concentrations, MAPC ability to suppress PBMC TNF-α secretion showed no statistical impairment after co-culture with phenytoin, celecoxib, or combination with both, for 1 or 24 h (Fig. 6).
Fig. 6.
TNF-α suppression by MAPC co-cultured with phenytoin, celecoxib or combination, at low, clinical, or high doses. MAPC Splenocyte TNF-α Inhibition Assay Data showing TNF- α production as measured by ELISA. Rat splenocytes were activated with lipopolysaccharide and then treated with MAPCs in culture. They were also treated with either celecoxib, phenytoin, or both as shown. There was a statistically significant difference between the activated control and all groups treated with MAPCs. In addition, there was a synergistic decrease in TNF- α production in the groups that were treated with phenytoin as compared to MAPCs alone. Values represent mean ± SD. Statistical analysis performed by One-Way ANOVA. N = 3 per group.
Co-cultured MAPC with a clinically relevant dose of 15 μg/ml phenytoin, as well as 1.5 μg/ml (low) and 150 μg/ml (high) concentrations. All MAPC treated with phenytoin significantly suppressed TNF-α compared to positive controls at 1hr and 24hr, including the clinical dose (15 μg/ml) (1hr: 128.8 pg/ml ± 5.67, 24hr: 126.1 pg/ml ± 9.26, vs 230.0 pg/ml ± 8.36, p value < 0.0001). When compared to MAPC alone, phenytoin co-cultured cells were only significantly different at the high dose at 24hr (87.97 pg/ml ± 47.97 vs 155.8 pg/ml ± 9.009, p value < 0.01).
MAPC co-cultured with a clinically relevant dose of 2 μg/ml celecoxib also significantly suppressed TNF-α compared to positive controls at 1hr and 24hr (148.9 pg/ml ± 18.04, 144.9 pg/ml ± 7.31, respectively, vs. 230.0 pg/ml ± 8.36, p value < 0.0001), with no significant difference when compared to control MAPC. As above celecoxib was also tested at 0.2 μg/ml (low) and 20 μg/ml (high) doses, finding that all doses of celecoxib significantly suppressed TNF-α compared to positive controls. Compared to MAPC alone, celecoxib co-cultured cells were significantly different at the high dose (20 μg/ml) at both 1hr and 24hr (76.09 pg/ml ± 7.74 and 88.99 pg/ml ± 23.55, respectively, vs. 230.0 ± 8.34, p value < 0.0001).
Finally, MAPC were co-cultured with phenytoin and celecoxib together, where all doses of celecoxib significantly suppressed TNF-α compared to positive controls (p value <0.0001). As with the individual treatments, high doses significantly suppressed TNF-α at both 1hr and 24hr. compared to untreated MAPC controls (67.03 pg/ml ± 6.0, 82.1 pg/ml ± 28.32, respectively, vs. 155.8 pg/ml ± 9.01, p value < 0.01).
4. Discussion
As promising as cell based therapies are, by principle, they differ from the conventional usage of synthetic drugs. Re-injecting live, potent cells as is done when treating TBI patients with progenitor stem cells, requires thorough investigation of their potency and deep understanding of their actual biological function. By mimicking the process of their use in in-vitro settings, we can better understand/predict the consequences that may present when they are applied in the clinic. Though this is, by no means, an exhaustive list of physiologic factors influencing patients, we have attempted to re-create in vitro clinical scenarios that are mainstays of treatment. With these, we investigated the effect of the clinical parameters on MAPC, and consequently, their target cells the PBMC.
Shear stress on mesenchymal stromal cells has been shown to affect micro-RNA expression as well as cell phenotype [20, 21]. However, our experiments show no change in the anti-inflammatory potency of MAPC to suppress T-cell proliferation after being infused through 30 gauge catheters. It is not likely that a smaller bore than would ever be in clinical use, exposing them to more shear stress than would be expected with clinical administration. Most US institutions use antibiotic or antiseptic-impregnated central venous access lines as a matter of policy for patient safety. Though there is copious literature documenting the impact of these catheters on catheter-associated blood stream infections, ICU-days, and hospital cost [14, 15, 16], as well as evidence that exposure to chlorhexidine decreases cell viability [22], no studies have been published which investigate the effects of antiseptic agents on cellular therapies or blood transfusions. Our work shows that chlorhexidine decreases cell viability as measured only by trypan blue exclusion which does not indicate whether there may be additional apoptosis or cellular stress as a result of exposure to chlorhexidine, however this can be easily prevented by routine flushing of the catheters. Though the current standard of care at most hospitals is to use chlorhexidine-impregnated catheters for central venous access, routine flushing of the lumen on placement will prevent the chlorhexidine from affecting MAPC viability.
We know that MAPC are resistant to hyperosmolar conditions, which are a clinical standard of severe TBI treatment and is recommended as an adjunct to decrease intracranial hypertension, a contributor to secondary injury after TBI [17]. Although our studies with propidium iodide do not shed light on the mechanics of cell death [22], they do show preservation of MAPC viability in a hyperosmolar environment. While there is a significantly higher percent viability at the highest osmolar concentrations, these differences are likely negligible with viability over 94% at all tested concentrations. Extreme osmolar conditions may result in an impactful decrease in viability, however the concentrations selected for our tests were chosen due to physiologic relevance. Overall, the physical conditions of shear stress, presence of antiseptic, and osmolar variation tested here revealed MAPC to be quite resilient.
According to the Brain Trauma Foundation Guidelines for the Management of Severe Traumatic Brain Injury, anti-epileptic drugs are recommended for the first week after injury to prevent early PTS immediately after TBI [17]. Phenytoin has been shown to be effective at preventing early PTS, as well as cost-effective [23, 24], and for that reason we chose it for our investigations. In addition, celecoxib is increasingly being used as part of a multi-modal pain regimen, and as a COX-2 inhibitor there is rationale to believe it might facilitate an anti-inflammatory response. MAPC that were treated with these drugs in media at therapeutic concentrations had no statistical difference in performance in our anti-inflammatory potency assay when compared to MAPC that had been freshly defrosted and placed in co-culture. Furthermore, MAPC treated with phenytoin for 1 h and for 24 h had better suppression of TNF-α, suggesting a synergistic anti-inflammatory response with phenytoin. Additionally, at higher and lower concentrations of these drugs, no decrease in the ability of MAPC to ameliorate the TNF-α response was seen. At higher concentrations celecoxib was shown to further potentiate MAPC TNF-α suppression, although it is unclear whether this is due to residual celecoxib or a change in MAPC biology.
Our anti-inflammatory potency assays determined the ability of MAPC to suppress T-cell proliferation and showed a replicable, dose-dependent suppression of T-cell proliferation, even after MAPC have been infused via needles and exposed to celecoxib and phenytoin. As critical care and clinical practice evolves to include new therapeutics, pharmaceuticals, and techniques, this assay can be revisited in future studies to rapidly assess any subsequent effects on MAPC and other immunomodulatory and anti-inflammatory cell therapies.
Limitations of this study include the use of rat splenocytes as the source of a mixed immune cell population, and since we used LPS to stimulate monocytes/macrophages and B-cells, these results are limited to only a portion of the reactive leukocyte population. Future studies on other lymphoid cells may show different results or utilize an independent mechanism. In addition we only looked at the effect of co-culture on the suppression of TNF-α. Though TNF-α has been shown to be a robust indicator of acute inflammatory-modulation effects associated with trauma, going forward studies may wish to move to human immune cells and examine a more diverse set of assays including other cytokines such as IFN-γ, IL-1β, IL-2, and IL-6, among others [25]. In addition, celecoxib and phenytoin are by no means the only pharmaceuticals that will be encountered in the bloodstream. Other common therapies include propofol, benzodiazepines, and opiates. These should be targets of future studies in MAPC physiology.
MAPC are currently being evaluated in six clinical trials dealing with diverse conditions such as stroke, acute respiratory distress syndrome, myocardial infarction, liver transplantation, and hematologic malignancies. Though more work needs to be done to illuminate the putative mechanism of action of cellular therapies, we have demonstrated that the modulation of the inflammatory response and preservation of microvascular permeability are reproducible effects [7]. Thus, other potential factors that may influence clinical cell performance are translationally relevant. MAPC may be an avenue to reduce cost and increase efficacy of TBI treatment; this study paves the way for their use in randomized clinical trials in TBI.
5. Conclusions
Though the parameters that surround cells in vitro may be different than those parameters seen in vivo, multipotent adult progenitor cells show preserved viability when undergoing shear stress from needle and catheter infusion as well as hyperosmolar treatment. They also show preserved anti-inflammatory potency as measured by dose-dependent reduction in T-cell proliferation when infused via needles and co-cultured with celecoxib and phenytoin.
Declarations
Author contribution statement
Margaret L. Jackson: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; wrote the paper.
Benjamin M. Aertker, Supinder Bedi, Katherine A. Ruppert: analyzed and interpreted the data; wrote the paper.
Daniel J. Kota, Karthik S. Prabhakara, Robert A. Hetz: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data.
Robert W. Mays: contributed reagents, materials, analysis tools or data; wrote the paper.
Scott D. Olson: conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Charles S. Cox: conceived and designed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper.
Funding statement
This work was supported by an SBIR grant from the NIH, NINDS (#U44-NS077511-01) awarded to Dr. Cox and Athersys, Inc as well as support from NIGMS T32 GM 0879201–11.
Competing interest statement
The authors declare the following conflict of interests: Charles Cox, Scott Olson; conduct additional sponsored research with Athersys, Inc. Robert Mays; is employed by Athersys, Inc. and receives compensation from Athersys, Inc.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge Amy Hazen, Ph.D. and John Norman, B.M.E. for their valuable technical support.
Contributor Information
Scott D. Olson, Email: Scott.D.Olson@uth.tmc.edu.
Charles S. Cox, Jr., Email: Charles.S.Cox@uth.tmc.edu.
References
- 1.Prevention, C. F. D. C. a. Surveillance Report of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014. 2019. https://stacks.cdc.gov/view/cdc/78062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention, C. F. D. C. a. Traumatic Brain Injury & Concussion: Get the Facts. 2019. [Google Scholar]
- 3.Cox C.J., Juranek J., Bedi S. Clinical trials in traumatic brain injury: cellular therapy and outcome measures. Transfusion. 2019;59:858–868. doi: 10.1111/trf.14834. [DOI] [PubMed] [Google Scholar]
- 4.Walker P.A. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp. Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker P.A. Bone marrow–derived stromal cell therapy for traumatic brain injury is neuroprotective via stimulation of non-neurologic organ systems. Surgery. 2012;152:790–793. doi: 10.1016/j.surg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Cox C.J. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68:588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 7.Liao G. Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatr. Crit. Care Med. 2015;16:245–255. doi: 10.1097/PCC.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boozer S. Global characterization and genomic stability of human MultiStem, a multipotent adult progenitor cell. J. Stem Cells. 2009;4:17–28. [PubMed] [Google Scholar]
- 9.Roobrouck V. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29:871–882. doi: 10.1002/stem.633. [DOI] [PubMed] [Google Scholar]
- 10.Pelacho B. Plasticity and cardiovascular applications of multipotent adult progenitor cells. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:S15–20. doi: 10.1038/ncpcardio0735. [DOI] [PubMed] [Google Scholar]
- 11.Bedi S.S. Therapeutic time window of multipotent adult progenitor therapy after traumatic brain injury. J. Neuroinflammation. 2018;15:84. doi: 10.1186/s12974-018-1122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker P.A. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp. Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess D.C. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 14.Lai N. Catheter impregnation, coating or bonding for reduced central venous catheter-related infections in adults. Cochrane Database Syst. Rev. 2016;3:CD007878. doi: 10.1002/14651858.CD007878.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittet D., Tarara D., Wenzel R. Nosocomial bloodstream infection in critically ill patients. J. Am. Med. Assoc. 1994;271:1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 16.Maki D., Stolz S., Wheeler S., Mermel L. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. Ann. Intern. Med. 1997;127:257–266. doi: 10.7326/0003-4819-127-4-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, A. C. Bratton S.L., Chestnut R.M., Ghajar J., McConnell Hammond F.F., Harris O.A., Hartl R., Manley G.T., Nemecek A., Newell D.W., Rosenthal G., Schouten J., Shutter L., Timmons S.D., Ullman J.S., Videtta W., Wilberger J.E., Wright D.W. Guidelines for the management of severe traumatic brain injury. II. Hyperosmolar therapy. J. Neurotrauma. 2007;24:S14–20. doi: 10.1089/neu.2007.9994. [DOI] [PubMed] [Google Scholar]
- 18.Bedi S. Therapeutic time window of multipotent adult progenitor therapy after traumatic brain injury. J. Neuroinflammation. 2018;15:84. doi: 10.1186/s12974-018-1122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane S., Bress A., Tesoro E. Characterization of unbound phenytoin concentrations in neurointensive care unit patients using a revised Winter-Tozer equation. Ann. Pharmacother. 2013;47:628–636. doi: 10.1345/aph.1R651. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci. Rep. 2015;5:16522. doi: 10.1038/srep16522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondadori dos Santos A. mi-R-126 is involved in vascular remodeling under laminar shear stress. BioMed Res. Int. 2015;2015:497280. doi: 10.1155/2015/497280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroemer G G.L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R., Hengartner M., Knight R.A., Kumar S., Lipton S.A., Malorni W., Nuñez G., Peter M.E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G. Nomenclature committee on cell death 2009. Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotton B., Kao L., Kozar R., Holcomb J. Cost-utility analysis of levetiracetam and phenytoin for posttraumatic seizure prophylaxis. J. Trauma. 2011;71:375–379. doi: 10.1097/TA.0b013e318224d307. [DOI] [PubMed] [Google Scholar]
- 24.Temkin N. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N. Engl. J. Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 25.Lozano D. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatric Dis. Treat. 2015;11:97–106. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]