Abstract
Olfactory impairment has been reported in patients with schizophrenia and individuals with a high risk of psychosis, but its neural basis is largely unknown. We used magnetic resonance imaging to investigate the morphology of the olfactory sulcus (an indicator of olfactory system development) and its relation to olfactory function in 38 persons with an at-risk mental state (ARMS), 62 patients with schizophrenia, and 61 healthy controls. Odor detection and identification were examined with a T & T olfactometer. Compared with the controls, the olfactory sulcus was significantly shallower and odor identification was inferior among the ARMS and schizophrenia subjects. Across all subjects, but not within each group, the olfactory sulcus depth was significantly related to better identification of odors. Our results support the concept that olfactory sulcus morphology reflects the neurodevelopmental process of the olfactory system.
Keywords: Anatomy, Medical imaging, Neuroscience, Psychiatry, Schizophrenia, Olfactory sulcus, At-risk mental state, Neurodevelopment, Olfaction
Anatomy; Medical imaging; Neuroscience; Psychiatry; Schizophrenia; Olfactory sulcus; At-risk mental state; Neurodevelopment; Olfaction
1. Introduction
Neuroimaging has generally demonstrated an abnormally shallow olfactory sulcus, possibly reflecting abnormal embryonic development of the olfactory system [8], in various stages of psychosis [23, 24, 30]. This suggests that olfactory sulcus morphology could be a marker of vulnerability to psychosis. It was reported that olfactory sulcus depth may be related to prodromal symptomatology [24], but the pathophysiological role of the morphology of this sulcus in psychosis remains unclear.
The potential influence of olfactory system underdevelopment on vulnerability to psychosis is supported by impairment of odor identification among schizophrenia patients, their unaffected family members, and persons with a high risk of psychosis [at-risk mental state (ARMS)] [10, 20, 28]. Olfactory deficits may be related to negative symptoms and cognitive/social impairment of schizophrenia patients [6, 16], which could be partly due to the same neural region [e.g., the orbitofrontal cortex (OFC)] being involved in both olfaction and processing of social/affective information [2, 21]. However, it remains unclear whether olfactory sulcus morphology is associated with olfactory deficits and other clinical characteristics in persons with schizophrenia or ARMS.
The present study was performed to confirm previous magnetic resonance imaging (MRI) findings of an association between an abnormally shallow olfactory sulcus and ARMS or schizophrenia [23, 24] in a different cohort, and to further elucidate the relation of olfactory sulcus morphology to olfactory function and other clinical characteristics.
2. Materials and methods
2.1. Subjects
Thirty-eight persons with ARMS, 62 patients with schizophrenia, and 61 healthy controls participated in the study (Table 1). These subjects did not overlap with those in our previous studies of the olfactory sulcus [23, 24], while olfactory function of 30/38 ARMS subjects, 50/62 schizophrenia patients, and 40/61 healthy subjects has been reported previously [28]. The demographic and clinical characteristics of the study participants, as well as the inclusion criteria, have been described elsewhere [27].
Table 1.
Demographic/clinical data, olfactory function, and brain measures in the ARMS, schizophrenia, and control groups.
| Controls (n = 61) | ARMS (n = 38) | Sz (n = 62) | Group differencea | |
|---|---|---|---|---|
| Age | 25.6 ± 3.2 | 18.4 ± 3.9 | 28.2 ± 9.3 | F (2, 158) = 28.40, p < 0.001; ARMS < Contols, Sz |
| Male/female | 32/29 | 24/14 | 29/33 | Chi-square = 2.54, p = 0.281 |
| Height (cm) | 166.0 ± 8.3 | 165.3 ± 9.0 | 163.3 ± 8.5 | F (2, 158) = 1.63, p = 0.199 |
| JART-IQ | 110.2 ± 5.9 | 98.0 ± 10.2 | 99.9 ± 9.4 | F (2, 158) = 32.75, p < 0.001; ARMS, Sz < Controls |
| Handedness (right/mixed/left) | 40/15/6 | 22/12/4 | 51/9/2 | Fisher's exact test, p = 0.074 |
| Age of onset (years) | - | - | 22.5 ± 7.4 | - |
| Duration of illness (years) | - | - | 5.6 ± 6.0 | - |
| Medication dose (HPD equiv., mg/day) | - | 2.0 ± 1.6 (n = 11) | 11.1 ± 7.8 (n = 50) | F (1, 58) = 13.76, p < 0.001; ARMS < Sz |
| Medication type (atypical/typical/mixed) | - | 9/1/1 | 44/1/5 | Fisher's exact test, p = 0.379 |
| Duration of medication (years) | - | 0.7 ± 1.3 (n = 14)b | 5.2 ± 6.2 (n = 52)b | F (1, 63) = 0.05, p = 0.832 |
| PANSS positive | - | 11.4 ± 3.6 | 14.0 ± 5.6 | F (1, 97) = 6.23, p = 0.014; ARMS < Sz |
| PANSS negative | - | 15.4 ± 6.7 | 16.2 ± 6.2 | F (1, 97) = 3.92, p = 0.051 |
| PANSS general | - | 30.4 ± 8.1 | 31.1 ± 9.8 | F (1, 97) = 1.71, p = 0.195 |
| SOFASc,d | - | 52.2 ± 10.8 | 48.1 ± 14.0 | F (1, 96) = 4.75, p = 0.032; not significant (post-hoc test) |
| SCoRS global rating scorec | - | 5.4 ± 2.4 | 5.2 ± 2.5 | F (1, 96) = 0.31, p = 0.580 |
| BACS subdomain z-scorese | Group x domain interaction, F (5, 490) = 5.45, p < 0.001 | |||
| Verbal memory | - | -0.9 ± 1.6 | -1.4 ± 1.4 | p = 0.930 |
| Working memory | - | -0.8 ± 1.4 | -1.0 ± 1.3 | p = 1.000 |
| Motor function | - | -0.8 ± 1.4 | -1.9 ± 1.5 | p = 0.007; Sz < ARMS |
| Verbal fluency | - | -1.0 ± 1.6 | -0.8 ± 1.1 | p = 1.000 |
| Attention and processing speed | - | -0.3 ± 1.3 | -1.4 ± 1.5 | p = 0.023; Sz < ARMS |
| Executive function | - | -0.5 ± 1.3 | -0.8 ± 1.6 | p = 1.000 |
| BACS mean z-score | - | -0.7 ± 1.1 | -1.2 ± 1.0 | F (1, 97) = 3.54, p = 0.063 |
| Odor detection thresholdf | ||||
| Mean | 0.05 ± 0.42 | 0.17 ± 0.83 | 0.10 ± 0.84 | F (2,154) = 0.26, p = 0.775 |
| Odor A (rose) | -0.13 ± 0.85 | 0.00 ± 1.16 | 0.23 ± 1.23 | F (2,154) = 0.71, p = 0.494 |
| Odor B (caramel) | 0.07 ± 0.54 | 0.05 ± 0.93 | -0.06 ± 0.94 | F (2,154) = 1.41, p = 0.247 |
| Odor C (rotten food or sweaty clothes) | 0.30 ± 0.74 | 0.53 ± 1.11 | 0.27 ± 1.07 | F (2,154) = 0.48, p = 0.619 |
| Odor D (sweet fruit) | 0.33 ± 0.79 | 0.34 ± 0.97 | 0.35 ± 1.04 | F (2,154) = 0.09, p = 0.913 |
| Odor E (fecal material) | -0.30 ± 0.64 | -0.08 ± 1.02 | -0.27 ± 1.03 | F (2,154) = 1.58, p = 0.210 |
| Odor identification thresholdf | ||||
| Mean | 0.75 ± 0.45 | 1.29 ± 0.88 | 1.18 ± 0.90 | F (2,154) = 6.24, p = 0.002; C < ARMS, Sz |
| Odor A (rose) | 0.89 ± 0.82 | 1.71 ± 1.64 | 1.69 ± 1.65 | F (2,154) = 5.36, p = 0.006; C < ARMS, Sz |
| Odor B (caramel) | 0.59 ± 0.59 | 0.87 ± 1.04 | 0.76 ± 0.78 | F (2,154) = 0.82, p = 0.444 |
| Odor C (rotten food or sweaty clothes) | 0.66 ± 0.54 | 1.37 ± 1.08 | 1.00 ± 0.83 | F (2,154) = 5.16, p = 0.007; C < ARMS |
| Odor D (sweet fruit) | 1.00 ± 0.63 | 1.29 ± 0.93 | 1.42 ± 1.42 | F (2,154) = 1.50, p = 0.227 |
| Odor E (fecal material) | 0.64 ± 1.46 | 1.24 ± 1.26 | 1.03 ± 1.46 | F (2,154) = 3.04, p = 0.051 |
| Subjects with olfactory deficitsg [n (%)] | 12 (19.7) | 23 (60.5) | 28 (45.2) | Chi-square = 17.95, p < 0.01; Controls < ARMS, Sz |
| Olfactory sulcus length (mm) | F (2,153) = 3.40, p = 0.036; not significant (post-hoc test) | |||
| left | 43.3 ± 3.2 | 42.0 ± 3.8 | 42.1 ± 3.4 | |
| right | 44.4 ± 3.4 | 42.7 ± 4.5 | 42.6 ± 3.5 | |
| Olfactory sulcus depth (mm) | F (2,153) = 108.70, p < 0.001; ARMS, Sz < C | |||
| left | 11.1 ± 0.8 | 9.6 ± 0.8 | 9.5 ± 0.9 | |
| right | 11.9 ± 0.9 | 9.9 ± 0.9 | 9.8 ± 0.9 | |
| Intermediate orbital sulcus depth (mm) | F (2,153) = 0.22, p = 0.799 | |||
| left | 8.7 ± 1.4 | 8.7 ± 1.4 | 8.8 ± 1.3 | |
| right | 8.9 ± 1.4 | 9.0 ± 1.4 | 8.8 ± 1.6 | |
| Heschl's sulcus depth (mm) | F (2,153) = 0.48, p = 0.618 | |||
| left | 7.7 ± 1.4 | 7.6 ± 1.4 | 7.9 ± 1.7 | |
| right | 6.4 ± 1.0 | 6.3 ± 0.8 | 6.5 ± 1.4 | |
| Intracranial volume (ml)h | 1459 ± 126 | 1408±127 | 1436±145 | F (2,157) = 1.21, p = 0.300 |
Values represent the mean ± SD unless otherwise stated.
ARMS, at-risk mental state; BACS, Brief Assessment of Cognition In Schizophrenia; HPD, haloperidol; JART, Japanese version of National Adult Reading Test; PANSS, Positive and Negative Syndrome Scale; SCoRS, Schizophrenia Cognition Rating Scale; SOFAS, Social and Occupational Functioning Assessment Scale; Sz, schizophrenia.
Demographic differences between groups were examined with one-way analysis of variance (ANOVA) or the chi-square test. Intergroup differences of social and cognitive function, symptom severity, and intracranial volume were tested using analysis of covariance (ANCOVA) with age as a covariate because the groups showed a significant difference of age. Intergroup differences of olfactory function (detection and identification thresholds) were tested by ANCOVA with age as a covariate and diagnosis and gender as between-subject factors. See the text for details of the statistical analysis of sulcus morphology.
Some participants were medication-free at the time of scanning but had a history of antipsychotic medication.
Data were missing for one schizophrenia patient.
Healthy subjects generally have a score ranging from 90-100 [15].
The primary measure from each test of the BACS was standardized by creating z-scores, with the mean score of Japanese healthy controls set to zero and the standard deviation set to one [11].
Analyzed using log-transformed values because of their skewed distributions (p < 0.001, Kolmogorov-Smirnov test). Note that lower threshold scores indicate better olfactory performance (range, -2 to 5).
Subjects with a mean identification threshold > 1.0.
Estimated using SPM 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/).
Briefly, the ARMS group was recruited from the Consultation and Support Service in Toyama (CAST), which is a service specifically for young persons at risk of developing psychosis [17]. ARMS was diagnosed according to the Comprehensive Assessment of At-Risk Mental States (CAARMS) criteria [33]. Most participants also fulfilled criteria for Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I disorders [1], including anxiety disorders (n = 9), depressive disorders (n = 6), and schizotypal personality disorder (n = 6). During follow up for a mean of 896.1 ± 841.6 days, 4/38 ARMS subjects (10.5%) transitioned to overt schizophrenia, but the others did not develop psychosis.
The patients with schizophrenia were recruited from Toyama University Hospital. Schizophrenia was diagnosed by using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Patient Edition [4].
Healthy volunteers were hospital staff and local community members. They were screened by using the SCID-I Non-patient Edition [4], and none of them had a past or family history of neuropsychiatric disease.
Subjects were excluded if they had a history of a serious medical (e.g., thyroid diseases, diabetes, or hypertension) or surgical condition, head trauma, neurological illness, steroid use, substance abuse, or nasal trauma. Subjects with nasal congestion (e.g., rhinitis) at the time of the study were also excluded. This study was approved by the Ethics Committee of Toyama University. Written informed consent was obtained from the subjects in agreement with the Declaration of Helsinki. If a subject was under 20 years old, written consent was also obtained from a parent or legal guardian.
2.2. Clinical assessment
Clinical characteristics (i.e., symptoms, social function, and cognitive function) were assessed at the time of MRI by the same methods as previously reported [26, 27, 28]. Briefly, the Brief Assessment of Cognition in Schizophrenia (BACS) [13], the Social and Occupational Functioning Assessment Scale (SOFAS) [5], and the Schizophrenia Cognition Rating Scale (SCoRS) [7, 14] were used to assess the cognitive and social function of subjects with ARMS or schizophrenia, while the Positive and Negative Syndrome Scale (PANSS) [12] was employed for symptoms.
2.3. Olfactory function testing
As described elsewhere in detail [25, 28], olfactory function was tested at the time of MRI with a T & T olfactometer (Daiichi Yakuhin Sangyo, Tokyo, Japan), which quantitatively assesses both odor detection sensitivity and odor identification based on the average threshold scores of five different odors (Table 1).
2.4. Magnetic resonance imaging
Subjects underwent brain MRI with a 3-T Magnetom Verio (Siemens, Erlangen, Germany) at Toyama University Hospital. A three-dimensional MPRAGE sequence was used to acquire 176 contiguous T1-weighted sagittal slices with a thickness of 1.2 mm. Imaging parameters were as follows: repetition time = 2300 ms, echo time = 2.9 ms, flip angle = 9°, field of view = 256 mm, matrix = 256 × 256, and voxel size = 1.0 × 1.0 × 1.2 mm.
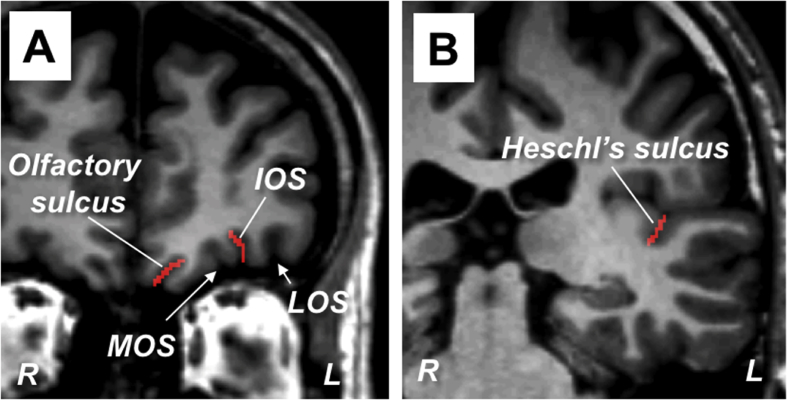
Images were reconstructed by using Dr. View software (Infocom, Tokyo, Japan), to obtain contiguous coronal slices with a thickness of 1.0 mm perpendicular to a line connecting the anterior and posterior commissures. As detailed elsewhere [23, 24], one rater (T Takahashi) who was blinded to the identity of the subjects, assessed the mean depth of the olfactory sulcus (i.e., total depth in all coronal slices containing the sulcus divided by the number of slices) and its anteroposterior length (mm). Two control sulci [i.e., the intermediate orbital sulcus (IOS) on the OFC surface and Heschl's sulcus (HS) in the superior temporal plane (Fig. 1)] were identified as described previously [18, 22], and were also evaluated to test the specificity of our findings regarding the olfactory sulcus. Due to their complexity and variability, the average depth was estimated for a portion of each control sulcus; the IOS was assessed in 10 contiguous slices anterior to the plane showing its connection to the transverse orbital sulcus, and the HS was assessed in the posterior-most 10 slices in which it could be identified. When there were two or three IO sulci, we assessed the depth of the most prominent one. The intra-rater and inter-rater (T Takahashi and YN/DS) reliability (intraclass correlation coefficient) of sulcus measurements in 10 randomly selected brains was >0.9.
Fig. 1.
Representative coronal slices showing the orbitofrontal (A) and superior temporal (B) regions. The olfactory sulcus, intermediate orbital sulcus (IOS), and Heschl's sulcus on the left hemisphere are colored red. Because we traced the surface of the intrasulcal gray matter using a line 1 mm in width, the number of pixels (1.0 × 1.0 mm) in each coronal slice was equal to the sulcus depth (mm). LOS, lateral orbital sulcus; MOS, medial orbital sulcus.
2.5. Statistical analysis
Analysis of covariance (ANCOVA) was performed to assess the sulcus data, with group and gender as between-subject factors, age and intracranial volume (ICV) as covariates, and side as a within-subject variable. A post hoc Scheffé's test was performed.
Pearson's partial correlation analysis adjusted for ICV was done to evaluate the relationship between sulcus measurements and the odor threshold, after log-transformation because of the skewed distribution (p < 0.001, Kolmogorov-Smirnov test), as well as between sulcus measurements and clinical factors [age of onset and duration of schizophrenia, dose and duration of medication, and PANSS subscores] or social/cognitive factors (SOFAS, SCoRS, and BACS scores). A p value <0.05 was regarded as indicating significance.
3. Results
3.1. Characteristics of the subjects
Demographic and clinical characteristics of the subjects, including olfactory function, are summarized in Table 1. The groups were matched for gender, but there were differences of age and IQ. Patients with schizophrenia had more severe positive symptoms, higher doses of antipsychotics, and lower BACS measures compared to the subjects with ARMS [27]. Both the ARMS group and the schizophrenia group had a higher mean odor identification threshold (i.e., impaired identification) than the controls [28].
3.2. Sulcus morphology
With regard to olfactory sulcus depth, ANCOVA showed main effects of group (Table 1) and side [F (1, 155) = 56.73, p < 0.001], as well as their interaction [F (2, 155) = 7.13, p = 0.001]. Compared with healthy controls, the olfactory sulcus was significantly shallower bilaterally in the ARMS group and the schizophrenia groups (p < 0.001), while it was deeper in the right hemisphere of the schizophrenia group (p = 0.004) and the control group (p < 0.001). However, olfactory sulcus length did not differ among the groups (Table 1).
The HS was significantly deeper in the left hemisphere [F (1, 155) = 98.04, p < 0.001], but there were no intergroup differences in the depth of the IOS and HS (Table 1).
3.3. Correlation analyses
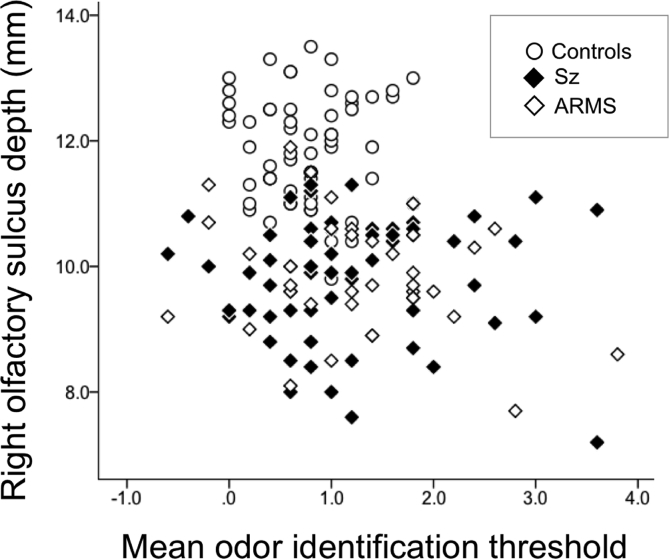
Among all patients, but not within each group, the depth of the left (r = -0.202, p = 0.010) and right (r = -0.265, p < 0.001) olfactory sulcus was significantly correlated with the odor identification threshold (Fig. 2). The correlations did not differ among the groups (left, χ2 = 0.48, p = 0.787; right, χ2 = 0.84, p = 0.658), and were specific to odors A and E when we separately assessed each odor (Table 2). The depths of the two control sulci (IOS and HS) were not correlated with the odor identification threshold. Sulcus morphology [olfactory sulcus (length, depth), IOS depth, and HS depth] was not correlated with the odor detection threshold across all patients or in each group.
Fig. 2.
Correlation between the right olfactory sulcus depth and the mean odor identification threshold in all subjects.
Table 2.
Correlation between the olfactory sulcus depth and identification threshold for each odor.
| Entire sample (n = 161) |
||||
|---|---|---|---|---|
| Left depth |
Right depth |
|||
| r | p | r | p | |
| Odor identification threshold | ||||
| Odor A (rose) | -0.2130 | 0.007 | -0.274 | >0.001a |
| Odor B (caramel) | -0.1200 | 0.130 | -0.067 | 0.400 |
| Odor C (rotten food or sweaty clothes) | -0.0570 | 0.472 | -0.153 | 0.054 |
| Odor D (sweet fruit) | -0.1010 | 0.203 | -0.127 | 0.110 |
| Odor E (fecal material) | -0.1630 | 0.040 | -0.231 | 0.003a |
Significant even after Bonferroni's correction for multiple comparisons.
In the schizophrenia and ARMS groups, sulcus morphology was not correlated with clinical or social/cognitive measures after Bonferroni's correction.
4. Discussion
The present study replicated our previous finding that the olfactory sulcus is shallow in ARMS and in schizophrenia [23, 24]. In addition, the depth of the olfactory sulcus was significantly associated with olfactory function, but this relation was not specific to any of the groups, suggesting that olfactory sulcus depth may be a general marker of olfactory system development. Furthermore, the control sulci on the OFC surface and in another brain region showed no relation to olfactory function.
The olfactory sulcus can be detected in the forebrain of the fetus around 16 weeks of gestation [3] and its integrity is thought to be associated with development of other olfactory structures [19]. Olfactory sulcus depth was reported to show a correlation with olfactory function in healthy persons and in patients with olfactory impairment [9, 10], suggesting that it may reflect both healthy and pathological embryonic development of olfaction. In agreement with previous reports of no significant correlation, or only a weak correlation, between olfactory sulcus depth and olfaction in schizophrenia [19] and ARMS [30] groups, our study suggested that olfactory sulcus depth reflects olfactory function in general, but not specifically in schizophrenia and related conditions. Our results also suggest a significant influence of the test odors on this relationship, which requires further clarification under various conditions.
In the ARMS and schizophrenia groups, our study showed that olfactory sulcus morphology was not related to clinical characteristics (symptom severity, duration of illness, medications, and/or social and cognitive function), indicating that it represents a stable trait marker of psychosis. Despite progressive brain atrophy in the early stages of psychosis [29], our longitudinal assessment found no changes of olfactory sulcus morphology during the first episode of schizophrenia [23]. However, as suggested in our previous study [24], it is still possible that olfactory sulcus morphology may be affected by the chronicity of illness and that its association with clinical characteristics (e.g., prodromal symptoms, social and cognitive functions) is only evident in ARMS subjects with later onset of psychosis.
This study had several limitations. First, there was no assessment of other olfactory structures like the olfactory bulb, which also reflects olfactory system underdevelopment in psychosis [31], but could not be reliably measured on T1-weighted images. Second, while the olfactory deficit in our ARMS and schizophrenia subjects may imply orbitofrontal dysfunction [2, 21], neurocognitive (e.g., decision-making [32]) and neuroimaging data on OFC function were not available. Third, this preliminary study could not assess the outcomes of ARMS subjects (i.e., later psychosis onset) because of a small sample size and this point requires further investigation in a larger high-risk cohort.
In conclusion, individuals with a high risk of developing psychosis shared an abnormally shallow olfactory sulcus and impairment of odor identification with schizophrenia patients, potentially reflecting a common psychopathologic vulnerability. Our finding of a significant correlation between olfactory sulcus morphology and olfactory function suggested that the depth of this sulcus may reflect both normal and pathological olfactory system development.
Declarations
Author contribution statement
T. Takahashi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Nakamura, Y. Nishikawa, D. Sasabayashi, H. Itoh: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Y. Takayanagi, Yuko Higuchi: Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Furuichi, S. Nishiyama, K. Noguchi: Contributed reagents, materials, analysis tools or data.
M. Kido, Y. Komori, T. Tateno: Performed the experiments.
Y. Masaoka, M. Suzuki: Conceived and designed the experiments.
Funding statement
This work was supported in part by JSPS KAKENHI Grant Number No. JP18K07550, JP18K15509, and JP18K07549, and by the Health and Labour Sciences Research Grants for Comprehensive Research on Persons with Disabilities from the Japan Agency for Medical Research and Development (AMED) Grant Number 16dk0307029h0003.
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study has been deposited at COCORO (Cognitive Genetics Collaborative Research Organization) as Toyama B cohort.
References
- 1.American Psychiatric Association . fourth ed. Text Revised, American Psychiatric Association; Washington, D.C.: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Atanasova B., Graux J., EI Hage W., Hommet C., Camus V., Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci. Biobehav. Rev. 2008;32:1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Chi J.G., Dooling E.C., Gilles F.H. Gyral development of the human brain. Ann. Neurol. 1997;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 4.First M.B., Gibbon M., Spitzer R.L., Williams J.B.W. American Psychiatric Publishing; Washington, D.C.: 1997. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- 5.Goldman H.H., Skodol A.E., Lave T.R. Revising axis V for DSM-IV: a review of measures of social functioning. Am. J. Psychiatry. 1992;149:1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- 6.Good K.P., Tibbo P., Milliken H., Whitehorn D., Alexiadis M., Robertson N., Kopala L.C. An investigation of a possible relationship between olfactory identification deficits at first episode and four-year outcomes in patients with psychosis. Schizophr. Res. 2010;124:60–65. doi: 10.1016/j.schres.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi Y., Sumiyoshi T., Seo T., Suga M., Takahashi T., Nishiyama S., Komori Y., Kasai K., Suzuki M. Associations between daily living skills, cognition, and real-world functioning across stages of schizophrenia; a study with the Schizophrenia Cognition Rating Scale Japanese version. Schizophr. Res. Cogn. 2017;7:13–18. doi: 10.1016/j.scog.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummel T., Damm M., Vent J., Schmidt M., Theissen P., Larsson M., Klussmann J.P. Depth of olfactory sulcus and olfactory function. Brain Res. 2003;975:85–89. doi: 10.1016/s0006-8993(03)02589-7. [DOI] [PubMed] [Google Scholar]
- 9.Hummel T., Urbig A., Huart C., Duprez T., Rombaux P. Volume of olfactory bulb and depth of olfactory sulcus in 378 consecutive patients with olfactory loss. J. Neurol. 2015;262:1046–1051. doi: 10.1007/s00415-015-7691-x. [DOI] [PubMed] [Google Scholar]
- 10.Kamath V., Turetsky B.I., Calkins M.E., Kohler C.G., Conroy C.G., Borgmann-Winter K., Gatto D.E., Gur R.E., Moberg P.J. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World J. Biol. Psychiatry. 2014;15:209–218. doi: 10.3109/15622975.2011.615862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneda Y., Ohmori T., Okahisa Y., Sumiyoshi T., Pu S., Ueoka Y., Takaki M., Nakagome K., Sora I. Measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery: validation of the Japanese version, Psychiatry Clin. Neuroscience. 2013;67:182–188. doi: 10.1111/pcn.12029. [DOI] [PubMed] [Google Scholar]
- 12.Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 13.Keefe R.S., Goldberg T.E., Harvey P.D., Gold J.M., Poe M.P., Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Keefe R.S., Poe M., Walker T.M., Kang J.W., Harvey P.D. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am. J. Psychiatry. 2006;163:426–432. doi: 10.1176/appi.ajp.163.3.426. [DOI] [PubMed] [Google Scholar]
- 15.Madeira L., Bonoldi I., Rocchetti M., Samson C., Azis M., Queen B., Bossong M., Perez J., Stone J., Allen P., Howes O.D., McGuire P., Raballo A., Fusar-Poli P., Ballerini M., Stanghellini G. An initial investigation of abnormal bodily phenomena in subjects at ultra high risk for psychosis: their prevalence and clinical implications. Compr. Psychiatr. 2016;66:39–45. doi: 10.1016/j.comppsych.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Malaspina D., Keller A., Antonius D., Messinger J.W., Goetz D.M., Harkavy-Friedman J., Goetz P.R., Harlap S. Olfaction and cognition in schizophrenia: sex matters. J. Neuropsychiatry Clin. Neurosci. 2012;24:165–175. doi: 10.1176/appi.neuropsych.11070154. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno M., Suzuki M., Matsumoto K., Murakami M., Takeshi K., Miyakoshi T., Ito F., Yamazawa R., Kobayashi H., Nemoto T., Kurachi M. Clinical practice and research activities for early psychiatric intervention at Japanese leading centres. Early Interv. Psychiatr. 2009;3:5–9. doi: 10.1111/j.1751-7893.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M., Takahashi T., Takayanagi Y., Sasabayashi D., Katagiri N., Sakuma A., Obara C., Koike S., Yamasue H., Furuichi A., Kido M., Nishikawa Y., Noguchi K., Matsumoto K., Mizuno M., Kasai K., Suzuki M. Surface morphology of the orbitofrontal cortex in individuals at risk of psychosis: a multicenter study. Eur. Arch. Psychiatry Clin. Neurosci. 2019;269:397–406. doi: 10.1007/s00406-018-0890-6. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen A.D., Pelavin P.E., Shenton M.E., Chilakamarri P., McCarley R.W., Nestor P.G., Levitt J.J. Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: an MRI study. Brain Imaging Behav. 2011;5:252–261. doi: 10.1007/s11682-011-9129-0. [DOI] [PubMed] [Google Scholar]
- 20.Rupp C.I. Olfactory function and schizophrenia: an update. Curr. Opin. Psychiatr. 2010;23:97–102. doi: 10.1097/YCO.0b013e328336643f. [DOI] [PubMed] [Google Scholar]
- 21.Soudry Y., Lemogne C., Malinvaud D., Consoli S.M., Bonfils P. Olfactory system and emotion: common substrates. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011;128:18–23. doi: 10.1016/j.anorl.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T., Suzuki M., Zhou S.Y., Tanino R., Hagino H., Kawasaki Y., Matsui M., Seto H., Kurachi M. Morphologic alterations of the parcellated superior temporal gyrus in schizophrenia spectrum. Schizophr. Res. 2006;83:131–143. doi: 10.1016/j.schres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T., Nakamura Y., Nakamura K., Ikeda E., Furuichi A., Kido M., Kawasaki Y., Noguchi K., Seto H., Suzuki M. Altered depth of the olfactory sulcus in first-episode schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:167–172. doi: 10.1016/j.pnpbp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T., Wood S.J., Yung A.R., Nelson B., Lin A., Yücel M., Phillips L.J., Nakamura Y., Suzuki M., Brewer W.J., Proffitt T.M., McGorry P.D., Velakoulis D., Pantelis C. Altered depth of the olfactory sulcus in ultra high-risk individuals and patients with psychotic disorders. Schizophr. Res. 2014;153:18–24. doi: 10.1016/j.schres.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T., Itoh H., Nishikawa Y., Higuchi Y., Nakamura M., Sasabayashi D., Nishiyama S., Mizukami Y., Masaoka Y., Suzuki M. Possible relation between olfaction and anxiety in healthy subjects. Psychiatry Clin. Neurosci. 2015;69:431–438. doi: 10.1111/pcn.12277. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T., Higuchi, Komori Y., Nishiyama S., Nakamura M., Sasabayashi D., Nishikawa Y., Sumiyoshi T. Suzuki, Quality of life in individuals with attenuated psychotic symptoms: possible role of anxiety, depressive symptoms, and socio-cognitive impairments. Psychiatry Res. 2017;257:431–437. doi: 10.1016/j.psychres.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T., Higuchi Y., Komori Y., Nishiyama S., Takayanagi Y., Sasabayashi D., Kido M., Furuichi A., Nishikawa Y., Nakamura M., Noguchi K., Suzuki M. Pituitary volume and socio-cognitive functions in individuals at risk of psychosis and patients with schizophrenia. Front. Psychiatry. 2018;9:574. doi: 10.3389/fpsyt.2018.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi T., Nakamura M., Sasabayashi D., Komori Y., Higuchi Y., Nishikawa Y., Nishiyama S., Itoh H., Masaoka Y., Suzuki M. Olfactory deficits in individuals at risk for psychosis and patients with schizophrenia: relationship with socio-cognitive functions and symptom severity. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268:689–698. doi: 10.1007/s00406-017-0845-3. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T., Suzuki M. Brain morphologic changes in early stages of psychosis: implications for clinical application and early intervention. Psychiatry Clin. Neurosci. 2018;72:556–571. doi: 10.1111/pcn.12670. [DOI] [PubMed] [Google Scholar]
- 30.Turetsky B.I., Crutchley P., Walker J., Gur R.E., Moberg P.J. Depth of the olfactory sulcus: a marker of early embryonic disruption in schizophrenia? Schizophr. Res. 2009;115:8–11. doi: 10.1016/j.schres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turetsky B.I., Moberg P.J., Quarmley M., Dress E., Calkins M.E., Ruparel K. Structural anomalies of the peripheral olfactory system in psychosis high-risk subjects. Schizophr. Res. 2018;195:197–205. doi: 10.1016/j.schres.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallis J.D. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 33.Yung A.R., Yuen H.P., McGorry P.D., Phillips L.J., Kelly D., Dell'Olio M., Francey S.M., Cosgrave E.M., Killackey E., Stanford C., Godfrey K., Buckby J. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust. N. Z. J. Psychiatr. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]