Abstract
Background
Early postoperative intraperitoneal chemotherapy (EPIC) can be used in combination with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) to treat patients with peritoneal carcinomatosis (PC) of multiple origins. The present study is a systematic review to evaluate the role of EPIC after CRS + HIPEC for appendiceal and colorectal cancers with PC.
Content
We conducted a systematic search in PubMed according to the PRISMA guidelines and included all studies published before June 27 of 2019 comparing EPIC to HIPEC or the combination of both. Our search found 79 articles. After excluding non-relevant articles, a total of 13 retrospective clinical studies reporting on the efficacy and safety of EPIC compared to HIPEC or as a combination therapy for lower gastrointestinal neoplasms were analyzed. Initial EPIC reports led to its declined usage because of concerns with increased postoperative morbidity and uncertain added benefit on survival. Recent retrospective studies have been promising, showing significant improvements in OS and fewer issues with complications when adding EPIC to CRS + HIPEC.
Conclusions
Current evidence is entirely retrospective and is conflicting. It is hoped that ongoing clinical trials and additional studies will clarify EPIC’s role in the treatment of patients with PC.
Keywords: appendiceal cancer, colon cancer, EPIC, HIPEC, peritoneal carcinomatosis, peritonectomy
Introduction
Peritoneal spread of advanced neoplasms arising from the gastrointestinal and gynecological systems are some of the most challenging cases to manage in surgical oncology. Because of the poor blood supply of the peritoneum and the abundance of viscera in the abdomen, this disease responds poorly to conventional chemotherapy and radiotherapy [1]. When added to other classical treatment modalities, CRS and intraperitoneal chemotherapy dramatically changed the regional management of peritoneal disease of multiple origins [2]. CRS consists of removing all visible tumor nodules, including diseased organs from the abdominal cavity and is usually followed by intraperitoneal chemotherapy to target microscopic residual tumor cells. This strategy allows the local administration of higher concentrations of chemotherapy agents while decreasing their multiple systemic toxicities [3].
There are many ways of effectively delivering chemotherapy in the peritoneum perioperatively and several techniques have been refined over time [4]. Since the 1990s, CRS with either HIPEC or normothermic EPIC have been developed to treat PC mainly for advanced colorectal and appendiceal tumors [5, 6]. Heating the intraperitoneal solution to 41–43 °C can have a direct cytotoxic effects on tumor [7] while enhancing the effectiveness of chemotherapeutic agents [8].
In the recent years, EPIC has fallen out of favor because of concerns about increased postoperative morbidity [9, 10, 11, 12, 13], superiority of HIPEC over EPIC in terms of survival [14, 15, 16, 17] and because of the increased resources needed to manage EPIC in the ICU or on the ward. Undoubtedly, HIPEC is the current standard for the delivery of intraperitoneal therapy. This article aims to review the whole body of literature surrounding the use of EPIC for lower gastrointestinal neoplasms with PC. Most recent publications from our group and others, suggest that EPIC may have a survival advantage when added to HIPEC, without increasing postoperative complications [18, 19, 20, 21, 22, 23]
Methods
A systematic PubMed database search was conducted on the 27th of June 2019 using the following keywords: ((((((early[All Fields] AND (“postoperative period”[MeSH Terms] OR (“postoperative”[All Fields] AND “period”[All Fields]) OR “postoperative period”[All Fields] OR “postoperative”[All Fields]) AND intraperitoneal[All Fields] AND (“drug therapy” [Subheading] OR (“drug”[All Fields] AND “therapy”[All Fields]) OR “drug therapy”[All Fields] OR “chemotherapy” [All Fields] OR “drug therapy”[MeSH Terms] OR (“drug”[All Fields] AND “therapy”[All Fields]) OR “chemotherapy” [All Fields])) AND (“peritoneal neoplasms” [MeSH Terms] OR (“peritoneal”[All Fields] AND “neoplasms”[All Fields]) OR “peritoneal neoplasms”[All Fields] OR (“peritoneal” [All Fields] AND “carcinomatosis”[All Fields]) OR “peritoneal carcinomatosis” [All Fields])) AND English[Language]) NOT ovarian[All Fields]) NOT (“stomach”[MeSH Terms] OR “stomach”[All Fields] OR “gastric”[All Fields])) NOT (“mesothelioma”[MeSH Terms] OR “mesothelioma” [All Fields])) NOT review[Publication Type] AND “humans” [MeSH Terms].
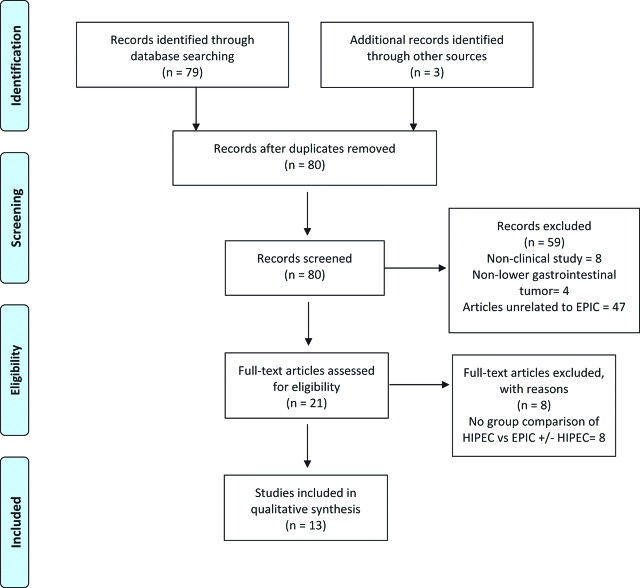
The search yielded 79 studies and 3 more were identified by screening previous systematic reviews on the use of intraperitoneal chemotherapy for lower gastrointestinal tumors with peritoneal metastases. The PRIMA Flow diagram is presented in Figure 1. Clinical studies containing patients who received EPIC after CRS for PC of appendiceal or colorectal origin were included in our review. We reviewed in detail a total of 21 clinical studies and excluded 8 additional studies because they were lacking group comparisons of HIPEC vs. EPIC ± HIPEC.
Figure 1:
PRISMA flow diagram for this systematic review.
Results
Based on inclusion and exclusions criteria, a total of 13 studies were analyzed and are detailed in Tables 1 and 2. All studies were retrospective cohort or case control studies with high risk of selection bias. There are currently no prospective randomized published studies looking at EPIC for PC of appendiceal and colorectal origin. Most of the studies were small and non-powered but there were three large international cohort studies [9, 22, 25] less likely to be underpowered. One must be cautious when interpreting Tables 1 and 2 as individual patients are likely to be represented in multiple studies, contributing to both national and international series. Reporting of morbidity and survival differed significantly between studies, making comparisons tenuous. Nevertheless, 9 out of 13 studies found that EPIC was associated with increased morbidity compared to HIPEC alone or as a dual therapy. Only 4 of those were statistically significant differences. Out of the 7 studies comparing HIPEC to HIPEC + EPIC that reported on survival, 5 showed a survival advantage with the combination therapy. Only the two most recent studies from our group were statistically significant [19, 20].
Table 1:
Studies comparing HIPEC to EPIC for lower gastrointestinal tumors with peritoneal metastasis following cytoreductive surgery.
| Author, year, country | Origin | n= | Treatment regimen | Grade 3 + morbidity | Survival analysis |
|---|---|---|---|---|---|
| Elias [14] France | Colorectal | 23 23 | HIPEC EPIC | 0 fistula p=0.0216 6 fistulas | 54% 5Y OS. NS 28% 5Y OS |
| Sideris [24] | Appendix | 11 13 | HIPEC EPIC | Not reported | 60% 5Y OS. NS 58% 5Y OS |
| Elias [25]International | Colorectal | 439 84 | HIPEC EPIC | No difference | 26% 5Y OS. NS 30% 5Y OS |
| Sorensen [16]Norway | PMP | 45 48 | HIPEC EPIC | 17% NS 29% | 79% 7Y OS. NS 75% 7Y OS |
HIPEC, Hyperthermic intraperitoneal chemotherapy; EPIC, Early postoperative intraperitoneal chemotherapy; NS, Non statistically significant; PMP, pseudomyxoma peritonii; OS Overall survival.
Table 2:
Studies comparing HIPEC to the combination of HIPEC + EPIC for lower gastrointestinal tumors with peritoneal metastasis following cytoreductive surgery.
| Author, year, country | Origin | n= | Treatment regimen | Grade 3 + morbidity | Survival analysis |
|---|---|---|---|---|---|
| Glehen [9]International | Colorectal | 271 112 | HIPEC HIPEC + EPIC | EPIC=more fistulasRR 1.7. p=0.032 | HIPEC + EPIC Better than HIPEC and EPIC alone but NS p=0.61 |
| Saxena [26]Australia | Colorectal | 12 34 | HIPEC HIPEC + EPIC | 50% NS 30% | Not reported |
| Chua [22]international | PMP | 1382 668 | HIPEC HIPEC + EPIC | No difference | HIPEC found to be an independent factor of better OS but not EPIC |
| Chua [21]Australia | Colorectal Subgroupa | 30 45 | HIPEC HIPEC + EPIC |
13% 16% | 19 months RFS. 19 months OS 33 months RFS. 38 months OS p=0.046. p=0.38 |
| Lam [15] Canada | Colorectal + High grade appendix | 37 56 | HIPEC HIPEC + EPIC | 19.6% p=0.01 43.2% | 6% 3Y RFS 46% 3Y OS. 21% 3Y RFS 50% 3Y OS. NS. NS |
| Sparks [13]Australia | Appendix | 13 17 | HIPEC HIPEC + EPIC | Trend toward more complications with EPIC group NS | No difference |
| Tan [12]Singapore | multiple | 69 42 | HIPEC HIPEC + EPIC | 25% p=0.048 58% | Not reported |
| Huang [19]Australia | LAMN | 74 176 | HIPEC HIPEC + EPIC | 44.6% 48.3% | 64.5% 5Y OS. p=0.001 93.0% 5Y OS |
| Huang [20]Australia | PMCA | 118 67 | HIPEC + EPIC | 47.9% 53.7% | 30.5% 5Y OS. p=0.003 62.3% 5Y OS |
HIPEC, Hyperthermic intraperitoneal chemotherapy; EPIC, Early postoperative intraperitoneal chemotherapy; NS, Non statistically significant; PMP, pseudomyxoma peritonii; OS Overall survival; RFS, Recurrence free survival; LAMN, Low-grade appendiceal mucinous neoplasm; PMCA, Peritoneal mucinous carcinomatosis of the Appendix.
Summary of evidence – foundation of EPIC
EPIC was first introduced by Sugarbaker in the 1990s in an effort to reduce disease recurrence and to prolong long-term survival of patients with PC [27]. Given the high risk of peritoneal recurrence, even after optimal cytoreduction, EPIC was a simple way of delivering high doses of cytotoxic agents targeted at the peritoneal surfaces without systemic compromise [28]. Sugarbaker’s design consisted of administering dilute 5-FU through a Tenckhoff catheter on postoperative days 1 to 5 in order to eliminate any residual microscopic tumor deposits before the formation of fibrinous adhesions [29]. The most commonly used protocol for appendiceal and colorectal neoplasms is 650 mg/m2 of 5-FU infused in hypertonic, high molecular weight solution to reduce its clearance speed from the abdominal cavity [30]. This solution is infused for 23 h with the surgical drains clamped, followed by 1 h of free drainage. This protocol overcomes the disadvantage of 5-FU’s short half-life because of its high intraperitoneal/intravenous area under the curve ratio, which allows the administration of higher doses with a resultant 250-fold increased tissue exposure [31]. Even with very high doses of 5-FU, systemic toxicities are much lower than systemic infusion because of the first pass metabolism through the liver. 5-Fu toxicity can be markedly increased in dihidropyrimidine dehydrogenase (DPD) deficient subjects, which represents between 3% and 15% of the population. Most cancer associations now recommend systematic screening for DPD deficiency before initiating any 5-FU based therapy [32]. In a murine experimental study by Klaver et al., comparing CRS alone vs. CRS + HIPEC vs. CRS + EPIC vs. CRS + HIPEC + EPIC, both EPIC and HIPEC were shown to prolong the rat’s survival [33]. A 1:2 matched case-control study comparing EPIC (n=30) vs. CRS only (n=15) for colorectal cancers identified EPIC as an independent prognostic factor for both OS and DFS [34]. This is one of the few clinical studies to have studied EPIC’s standalone efficacy when added to an optimal CRS for lower gastrointestinal tumors. Armstrong et al. reported in 2006 the results of an RCT which demonstrated significantly better OS with EPIC vs. IV chemotherapy after optimal CRS for ovarian PC (23.8 vs. 18.3 months respectively) [35]. Another RCT published in 2001 demonstrated similar results in favor of adding EPIC after radical surgery for locally advanced gastric cancers [36]. Multiple studies have compared HIPEC and EPIC head to head after CRS and all have found HIPEC to be either equivalent or superior in terms of survival as shown in Table 1 [14, 16, 24, 25]. A retrospective matched case control study by Elias et al. of 46 patients found that HIPEC was associated with a better 5-year OS compared to EPIC (54% vs. 28%), although not statistically significant [14]. Concerningly, patients in the EPIC group had more postoperative fistulas, more peritoneal recurrences and worse long-term survival compared to patients who received HIPEC. In 2003, Verwaal et al. published the first randomized controlled study using HIPEC for PC of colorectal origin [37]. This trial demonstrated a significant increase in OS with the use of CRS + HIPEC compared to systemic therapy alone. Being the only published RCT for PC at that time, this trial set CRS + HIPEC as the new standard for the treatment of selected appendiceal and colorectal neoplasm with PC.
Summary of evidence HIPEC + EPIC combination therapy
After 2003, many units around the world continued to use Sugarbaker’s initial protocol consisting of Mitomycin C (MMC) HIPEC and 5-FU EPIC. In 2012, Chua et al. conducted a large multicenter retrospective study of 2298 patients with PMP of appendiceal origin treated with CRS + HIPEC + EPIC [22]. In this study, although EPIC was significantly associated with longer OS in the univariate analysis, this effect was no longer present in the multivariate analysis, which could mean that the use of EPIC is more of a surrogate for another factor which positively influences OS rather than an independent factor.
Our group reported on a retrospective cohort study comparing different intraperitoneal chemotherapy regimens for PC of colorectal and appendiceal origin and found no significant impact of adding EPIC to HIPEC on OS [21]. In this cohort, the addition of EPIC to HIPEC was associated with longer recurrence free survival (33 vs. 19 months p=0.046) only in patients with PC of colorectal origin. These results have to be interpreted with caution as there was a significant risk of selection bias in the study design. The standard intended treatment in our unit at that time was HIPEC + EPIC and the control group consisted of patients who were not eligible to receive EPIC. In circumstances where high risk surgical procedures were performed and with reasonable risk of complications, leakage of intraperitoneal chemotherapy, major organ failure, intra-abdominal hypertension and hemodynamic instability, EPIC was withheld.
Lam et al. published the Canadian’s experience with EPIC in 2015 for the treatment of PC of appendiceal and colorectal origin. After reports of increased complications with the use of EPIC [9, 14], this unit changed their intraperitoneal chemotherapy protocol in 2008. Initially, their protocol was HIPEC with MMC (12–15 mg for 60 min) followed by 5-FU EPIC (1000 mg daily from post-operative day 1 to 5). After 2008, the protocol was changed to HIPEC with 400 mg of oxaliplatin for 60 min with a simultaneous IV dose of 5-FU, but no further EPIC was given. This particular historical context provided the opportunity to examine differences in complications and survival without significant selection bias. The authors first reported the impact of the different regimen on major complications and found that the combination of HIPEC + EPIC and PCI>26 were the only two independent factors associated with grade 3 + morbidity [10]. The concern for a learning curve effect with this study design was addressed by analysing overall major complications according to the year of surgery without finding any trend. Two years later, they reported their survival data of the same historical cohort and found no significant difference in the 3-year OS [15]. The two groups were very similar but more chemotherapy was given to the HIPEC group (76.8% vs. 54% p=0.05). The 60-minute fixed 12–15 mg dose of MMC used in the HIPEC + EPIC group is one of the lowest doses reported. Doses between 20 and 40 mg of MMC for 90 min are commonly seen in large cohort studies. These two factors should be considered when analysing the survival of the two groups, because they both favor the HIPEC only group. The authors reported no difference in RFS but when analysing the data, the 3-year RFS was 21% in the HIPEC + EPIC group compared to 6% in HIPEC group. Although not statistically significant, there is still an observable difference in RFS between the two groups in favor of HIPEC + EPIC.
In 2016, the Singapore group also reported their data which were consistent with previously published studies [12]. In this retrospective study of 111 subjects with tumors of different origins, patients all received HIPEC but did not get EPIC if they underwent a very extensive surgery. In fact, the no EPIC group had significantly more blood losses, longer ICU stay and more transfusions than the EPIC group. Despite this, patients with less extensive surgery who receive EPIC still presented more grade 3 and above complications (58% vs. 25%) and a longer hospital stay (16 vs. 13 days). Survival analysis pointed toward better OS in favor of the combined therapy with an HR of 0.62 (0.28–1.37, p=0.231).
In the past two years, new evidence arising mainly from our center in Sydney Australia has emerged and differs from previous findings, reopening a debate that many thought was closed. EPIC or no EPIC?
In 2017, Huang et al. published two papers on our experience with EPIC for Low grade appendiceal mucinous neoplasms (LAMN) [20] and appendiceal adenocarcinomas (PMCA) [19].
In the first study, 250 consecutive surgeries for LAMNs were retrospectively analyzed to compare HIPEC vs. HIPEC + EPIC. The two groups were generally comparable but the HIPEC group was on average 4 years older, putting them more at risk than the combined therapy group. No difference in postoperative mortality or morbidity was found and patients who received EPIC had a significantly better 5-year OS (93.0% vs. 64.5% p=0.001). When comparing the five year OS of both groups to a large retrospective multicenter cohort study [22], LAMNS who received HIPEC + EPIC seem to have comparable 5-year OS (93% vs. 91%) whereas patients receiving HIPEC only had a worse prognosis than what is quoted in the literature (64% vs. 76%), despite excluding CCR-2 patients, which were included in the large cohort study. With the two cohorts having similar mean PCI (22 and 21) and median age (53 and 53), it seems that the 5-year OS difference identified in this study is related to the HIPEC only group having a worse prognosis, raising the suspicion for selection bias, although we cannot draw conclusions from comparing the two study cohorts. In fact, as we noted in the text, the default treatment in our unit for soft or LAMN appearing tumors is HIPEC + EPIC and the decision to withhold EPIC is either subjective or because of contraindications to EPIC. The second study [19] with a similar retrospective case-control design (HIPEC + EPIC vs. HIPEC only), focused on patients with PMCAs. The groups differed significantly in terms of chemotherapy agent used for the HIPEC, MMC was used 95.5% of the time in the HIPEC + EPIC group whereas Oxaliplatin was used in 76% of the time in the HIPEC only group. There was no significant difference in terms of hospital mortality (p=0.632), major morbidity rate (i.e. Grade III/IV) (p=0.444), ICU stay (p=0.638) and total hospital stay (p=0.078). Patients who received HIPEC and EPIC had a significantly better 5-year OS than those who received HIPEC alone (62.3% for HIPEC + EPIC, 30.5% for HIPEC alone, p=0.002) which reflected on better PFS as well (18.0 vs. 12.3 months, p=0.002). Although there was a significant difference in the type of chemotherapy agent used in the two groups, Levine et al. published an RCT in 2018 demonstrating no significant survival advantage between MMC and Oxaliplatin for appendiceal tumors with PC treated by CRS + HIPEC [38].
We later published interesting findings on the correlation between subjective tumor consistency of PMCAs and long-term survival [23]. In this retrospective study of 192 patients, subjects with softer tumors tended to have received EPIC more commonly on top of HIPEC but when adjusting for EPIC in the multivariate analysis, subjective tumor consistency was found as an independent survival prognostic factor.
Analyzing the only two studies to have found such a positive impact of adding EPIC over HIPEC [19, 20], nearly doubling the survival of patients with LAMNs and PMCAs, one must suspect significant selective bias and it is now clear that choosing which patient receives EPIC according to tumor’s consistency is potentially one of them. After realizing the impact of tumor consistency on survival and the potential for bias, we did a post hoc analysis to adjust for tumor consistency in the multivariate analysis. After adjustments, EPIC remained an independent factor for better OS of soft (5Y OS 76.4% vs. 44.0%, p=0.005) and intermediate (p=0.06) tumors [23].
Interestingly, most recent published data do not seem to show increased morbidity with the use of EPIC compared to older series. Because of the retrospective nature of these studies, this could be due to selection bias but could also be due to better postoperative care in general.
Discussion
Current available data on the efficacy of EPIC when combined with HIPEC for the treatment of PC of appendiceal and colorectal origin is conflicting and difficult to interpret due to the retrospective nature of all the studies and the possibility of bias. To date, there are no published prospective data regarding the use of EPIC for PC of appendiceal and colorectal origin which, combined with concerns of increased postoperative morbidity and inconsistent effects on survival, explains why EPIC has fallen out of favor over time. Although decreasing postoperative complications is something every surgeon seeks, survival and quality of life is really what matters most to cancer patients [39]. Specifically in the CRS and HIPEC literature, it is not clear if postoperative complications correlate with long term OS or not [22, 40, 41, 42].
All the studies to have compared HIPEC vs. HIPEC + EPIC are presented in Tables 1 and 2. Most studies reported increased morbidity with the combined therapy. Although most initial papers comparing HIPEC + EPIC vs. HIPEC did not find statistically significant survival differences, all pointed toward better survival with the combined therapy. Concluding there is no difference in survival because the p-value is not below 0.05 is a common mistake and is a false statement [43]. In such small samples, it is merely impossible to know if there is a Beta error or if no association exists. Although not exempt from bias, the fact that two recent studies have found clinically and statistically significant better survival with HIPEC + EPIC warrants further investigations.
ICARuS is an actively recruiting multicenter RCT in the US, evaluating the effectiveness of EPIC after optimal CRS + MMC HIPEC for patients with isolated peritoneal metastasis of appendiceal and colorectal origin (NCT01815359). This trial will certainly be very informative on the effectiveness of EPIC, but survival results won’t be available for many years. Additional propensity matched case control studies from other high-volume centers could potentially enlighten us in the meantime. Making a parallel with malignant peritoneal mesothelioma, Sugarbaker et al. recently published their long experience, comparing three different chemotherapy regimens (HIPEC vs. HIPEC + EPIC vs. HIPEC + EPIC + normothermic intraperitoneal chemotherapy long term (NIPEC)) [18]. No significant survival benefit was observed when adding EPIC over HIPEC, but interestingly, a long-term administration of paclitaxel (NIPEC) significantly increased the 5-year OS from 44 to 75%. As local recurrence and complications such as malignant bowel obstruction is still an important problem for patients with PC, further investigations on the use of EPIC and other postoperative intraperitoneal regimens are needed and should not be hampered by the fear of possibly increasing postoperative complications, if the resultant effect is better survival and quality of life for our patients.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Mikael L. Soucisse, Email: mikael.soucisse@gmail.com.
Winston Liauw, Email: Winston.Liauw@health.nsw.gov.au.
Gabrielle Hicks, Email: Gabrielle.Hicks@health.nsw.gov.au.
David L. Morris, Email: David.Morris@unsw.edu.au.
References
- 1.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426–32. [DOI] [PubMed]
- 2.Stephens FO, Storey DW, Thompson JF, Marsden FW. Surgical oncology and the role of regional chemotherapy. Aust N Z J Surg 1992;62:691–6. [DOI] [PubMed]
- 3.Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacokinetics and pharmacodynamics of perioperative cancer chemotherapy in peritoneal surface malignancy. Cancer J 2009;15:216–24. [DOI] [PubMed]
- 4.Ortega-Deballon P, Facy O, Jambet S, Magnin G, Cotte E, Beltramo JL, et al. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann Surg Oncol 2010;17:1957–63. [DOI] [PubMed]
- 5.Avital I, Brucher BL, Nissan A, Stojadinovic A. Randomized clinical trials for colorectal cancer peritoneal surface malignancy. Surg Oncol Clin N Am 2012;21:665–88. [DOI] [PubMed]
- 6.Goere D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 2013;257:1065–71. [DOI] [PubMed]
- 7.Overgaard J. Effect of hyperthermia on malignant cells in vivo. Rev Hypothesis Cancer 1977;39:2637–46. [DOI] [PubMed]
- 8.Buell JF, Reed E, Lee KB, Parker RJ, Venzon DJ, Amikura K, et al. Synergistic effect and possible mechanisms of tumor necrosis factor and cisplatin cytotoxicity under moderate hyperthermia against gastric cancer cells. Ann Surg Oncol 1997;4:141–8. [DOI] [PubMed]
- 9.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284–92. [DOI] [PubMed]
- 10.McConnell YJ, Mack LA, Francis WP, Ho T, Temple WJ. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol 2013;107:591–6. [DOI] [PubMed]
- 11.Quenet F, Goere D, Mehta SS, Roca L, Dumont F, Hessissen M, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg 2011;254:294–301. [DOI] [PubMed]
- 12.Tan GH, Ong WS, Chia CS, Tham CK, Soo KC, Teo MC. Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Int J Hyperthermia 2016;32:281–8. [DOI] [PubMed]
- 13.Sparks DS, Morris B, Xu W, Fulton J, Atkinson V, Meade B, et al. Cytoreductive surgery and heated intraperitoneal chemotherapy for peritoneal carcinomatosis secondary to mucinous adenocarcinoma of the appendix. Int Surg 2015;100:21–8. [DOI] [PMC free article] [PubMed]
- 14.Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol 2007;14:509–14. [DOI] [PubMed]
- 15.Lam JY, McConnell YJ, Rivard JD, Temple WJ, Mack LA. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg 2015;210:424–30. [DOI] [PubMed]
- 16.Sorensen O, Flatmark K, Reed W, Wiig JN, Dueland S, Giercksky KE, et al. Evaluation of complete cytoreductive surgery and two intraperitoneal chemotherapy techniques in pseudomyxoma peritonei. Eur J Surg Oncol 2012;38:969–76. [DOI] [PubMed]
- 17.Cashin PH, Graf W, Nygren P, Mahteme H. Intraoperative hyperthermic versus postoperative normothermic intraperitoneal chemotherapy for colonic peritoneal carcinomatosis: a case-control study. Ann Oncol 2012;23:647–52. [DOI] [PubMed]
- 18.Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur J Surg Oncol 2017;43:1228–35. [DOI] [PubMed]
- 19.Huang Y, Alzahrani NA, Liauw W, Soudy H, Alzahrani AM, Morris DL. Early postoperative intraperitoneal chemotherapy is associated with survival benefit for appendiceal adenocarcinoma with peritoneal dissemination. Eur J Surg Oncol 2017;43:2292–8. [DOI] [PubMed]
- 20.Huang Y, Alzahrani NA, Liauw W, Traiki TB, Morris DL. Early postoperative intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasms with pseudomyxoma peritonei: is it beneficial? Ann Surg Oncol 2017;24:176–83. [DOI] [PubMed]
- 21.Chua TC, Liauw W, Zhao J, Morris DL. Comparative analysis of perioperative intraperitoneal chemotherapy regimen in appendiceal and colorectal peritoneal carcinomatosis. Int J Clin Oncol 2013;18:439–46. [DOI] [PubMed]
- 22.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449–56. [DOI] [PubMed]
- 23.Huang Y, Alzahrani NA, Fisher OM, Chua TC, Kozman MA, Liauw W, et al. Intraoperative macroscopic tumour consistency is associated with overall survival after cytoreductive surgery and intraperitoneal chemotherapy for appendiceal adenocarcinoma with peritoneal metastases: a retrospective observational study. Am J Surg 2019;217:704–12. [DOI] [PubMed]
- 24.Sideris L, Mitchell A, Drolet P, Leblanc G, Leclerc YE, Dube P. Surgical cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis arising from the appendix. Can J Surg 2009;52:135–41. [PMC free article] [PubMed]
- 25.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63–8. [DOI] [PubMed]
- 26.Saxena A, Yan TD, Morris DL. A critical evaluation of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. World J Surg 2010;34:70–8. [DOI] [PubMed]
- 27.Sugarbaker PH, Cunliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol 1989;16:83–97. [PubMed]
- 28.Cunliffe WJ, Sugarbaker PH. Gastrointestinal malignancy: rationale for adjuvant therapy using early postoperative intraperitoneal chemotherapy. Br J Surg 1989;76:1082–90. [DOI] [PubMed]
- 29.Sugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE, Hull WE, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res 1990;50:5790–4. [PubMed]
- 30.Pestieau SR, Schnake KJ, Stuart OA, Sugarbaker PH. Impact of carrier solutions on pharmacokinetics of intraperitoneal chemotherapy. Cancer Chemother Pharmacol 2001;47:269–76. [DOI] [PubMed]
- 31.Van der Speeten K, Govaerts K, Stuart OA, Sugarbaker PH. Pharmacokinetics of the perioperative use of cancer chemotherapy in peritoneal surface malignancy patients. Gastroenterol Res Pract 2012;2012:378064. [DOI] [PMC free article] [PubMed]
- 32.Loriot MA, Ciccolini J, Thomas F, Barin-Le-Guellec C, Royer B, Milano G, et al. [Dihydropyrimidine dehydrogenase (DPD) deficiency screening and securing of fluoropyrimidine-based chemotherapies: update and recommendations of the French GPCO-Unicancer and RNPGx networks]. Bull Cancer 2018;105:397–407. [DOI] [PubMed]
- 33.Klaver YL, Hendriks T, Lomme RM, Rutten HJ, Bleichrodt RP, de Hingh IH. Intraoperative versus early postoperative intraperitoneal chemotherapy after cytoreduction for colorectal peritoneal carcinomatosis: an experimental study. Ann Surg Oncol 2012;19:S475–82. [DOI] [PubMed]
- 34.Park SY, Choi GS, Park JS, Kim HJ, Yang CS, Kim JG, et al. Efficacy of early postoperative intraperitoneal chemotherapy after complete surgical resection of peritoneal metastasis from colorectal cancer: a case-control study from a single center. Ann Surg Oncol 2016;23:2266–73. [DOI] [PubMed]
- 35.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34–43. [DOI] [PubMed]
- 36.Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg 2001;25:985–90. [DOI] [PubMed]
- 37.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43. [DOI] [PubMed]
- 38.Levine EA, Votanopoulos KI, Shen P, Russell G, Fenstermaker J, Mansfield P, et al. A multicenter randomized trial to evaluate hematologic toxicities after hyperthermic intraperitoneal chemotherapy with oxaliplatin or mitomycin in patients with appendiceal tumors. J Am Coll Surg 2018;226:434–43. [DOI] [PMC free article] [PubMed]
- 39.Hansen F, Berntsen GKR, Salamonsen A. “What matters to you?” A longitudinal qualitative study of Norwegian patients’ perspectives on their pathways with colorectal cancer. Int J Qual Stud Health Well-being 2018;13:1548240. [DOI] [PMC free article] [PubMed]
- 40.Ihemelandu C, Mavros MN, Sugarbaker P. Adverse events postoperatively had no impact on long-term survival of patients treated with cytoreductive surgery with heated intraperitoneal chemotherapy for appendiceal cancer with peritoneal metastases. Ann Surg Oncol 2016;23:4231–7. [DOI] [PubMed]
- 41.Lee L, Alie-Cusson F, Dube P, Sideris L. Postoperative complications affect long-term outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. J Surg Oncol 2017;116:236–43. [DOI] [PubMed]
- 42.Baratti D, Kusamura S, Iusco D, Bonomi S, Grassi A, Virzi S, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 2014;57:858–68. [DOI] [PubMed]
- 43.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019;567:305–7. [DOI] [PubMed]