Abstract
In this study, the dominant lactic acid bacteria present in whole wheat sourdough was isolated and identified as Lactobacillus plantarum by molecular methods, before being utilized as the starter culture in the processing of sourdough cup breads. Subsequently, the effects of sourdough fermentation time, ash content, and sugar content on bread quality attributes were investigated by response surface methodology. In terms of the independent variables, the best polynomial models were fitted for bread hardness, chewiness, specific volume, total color difference (TCD), porosity and overall acceptability (OAA). Based on statistical analysis (P < 0.05), the effect of fermentation time on hardness and TCD was significant; ash content influenced specific volume and TCD significantly. In comparison with other functions, the interaction between fermentation time and sugar content had a significant (P < 0.05) influence on bread OAA. Furthermore, a strong positive correlation was ascertained between sourdough total titratable acidity and most of the quality attributes of cup bread (especially hardness, specific volume and OAA). Accordingly, the control of sourdough fermentation conditions is influential on microbial activity and metabolites, which affect bread quality characteristics.
Keywords: Food science, Iranian cup breads, Sourdough, Response surface methodology, Quality characteristics
Food Science; Iranian cup breads, Sourdough, Response surface methodology, Quality characteristics.
1. Introduction
Breads produced from wheat flours with 67.5–95% extraction rates play an important role in human nutrition in Iran. The production of common flat breads in this country is mainly based on a rapid, semi-automatic process, with sodium bicarbonate being used as the chemical leavening agent. Such bread processing methods and additives reduce both product quality and shelf life, leading to a huge waste of wheat per year (Fazeli et al., 2004; Gargari et al., 2007). Cup breads are not common in Iran. However, in the past decade, several studies such as Sadeghi et al., (2008) and Pontonio et al., (2015) have been conducted on the use of sourdough fermentation under controlled conditions as an alternative to common additives in the processing of cup breads in the country.
The results of a recent study demonstrated that the use of certain starters isolated from Iranian flours in sourdough fermentation can increase the concentrations of total free amino acids and organic acids, enhance textural characteristics and antioxidant capacity, and decrease the phytate content of produced cup breads in comparison with spontaneous sourdough fermentation. Based on these results, sourdough utilization in the processing of Iranian cup breads has technological, nutritional, and economical significance (Pontonio et al., 2015).
Sourdough is essentially water mixed with cereal flour, though it comprises a very complex, non-aseptic fermentation ecosystem that can be utilized as a bread-making additive due to its generally recognized as safe status. Sourdough also has crucial effects on final product quality, including antimicrobial, nutritional, functional and anti-staling effects (Gobbetti et al., 2014). The most important factors that influence sourdough fermentation include the microbial starter, fermentation conditions (time and temperature; number of back-sloppings) and flour composition (especially protein, carbohydrate and ash contents).
Among the mentioned parameters, fermentation time as well as flour ash and sugar contents have the largest effects on sourdough acidification. Usually, by increasing the flour extraction rate and ash content, the growth of microbial starters and the sourdough acidification power both increase given the buffering capacity, minerals and micronutrients of bran. Furthermore, there is an obvious relationship between acidification and the quality, shelf life, and sensory attributes of sourdough bread (Aplevicz et al., 2013).
Due to the fact that sourdough fermentation is a multivariable process that features various simultaneous interactions, the response surface methodology (RSM) is a suitable technique for its optimization, which facilitates the selection of ideal fermentation conditions (Katina et al., 2006). RSM, as a statistical and mathematical approach, can be used for solving similar equations with several parameters and optimizing a set of experimental factors by reducing the number of trials required to investigate combined effects on a desired response (Khare et al., 2015).
Considering the huge waste of bread as well as the importance of bread in Iranian diet, the key objectives of the present study were to control sourdough fermentation conditions (specific starter culture; fermentation time; flour ash and sugar contents) in the processing of cup breads prepared using the main industrial wheat flours utilized in Iran, and to subsequently investigate the effect of fermentation conditions on final product quality via RSM.
2. Materials and methods
2.1. Raw materials
Wheat flours (Null, Star and Whole) were purchased from a local milling factory (Zahedi, Gorgan, Iran) and assessed in terms of chemical properties using AACC standard methods (44-19 moisture content, 46-10 protein (N×5.70), 38-12 wet gluten and 08-01 ash content) (AACC, 2010). Based on these assessments, Null, Star and Whole flours had in order: 67.5, 76, and 92% extraction rate; 14.2, 13.80, and 8.10% moisture content; 8.5, 10.90, and 12.25% protein; 23.4, 25.85, and 26.40% wet gluten; 0.45, 0.75, and 1.55% ash content; and 2.4, 3.5, and 4.1% acidity. Active dry yeast extract containing Saccharomyces cerevisiae was acquired from the Iran-Mellas company (Iran). All chemical reagents and microbial media (MRS broth, MRS agar and Nutrient broth) were purchased from Merck (Germany).
2.2. Methods
2.2.1. Spontaneous fermentation of whole wheat sourdough
Spontaneous fermentation was facilitated in whole weat sourdough using the following method: whole wheat flour was mixed with table water to give a dough yield (DY: dough weight×100/flour weight) of 160. The mixture was incubated at 28 °C for the first 24 h. On the second day, 25% of the fermented sourdough from the first day was used for back-slopping, meaning that it was mixed with freshly prepared sourdough; back-slopping was repeated daily for ten consecutive days (Venturi et al., 2013).
2.2.2. Isolation and molecular identification of dominant lactic acid bacteria
The dominant lactic acid bacteria (LAB) of whole wheat sourdough were determined by a surface plate count of the final back-slopping on MRS agar medium (Merck, Germany). Pure single colonies of LAB isolates were obtained from the streak plates and then subjected to Gram staining, catalase assay, and species-specific PCR. Subsequently, the genomic DNA of the isolates was extracted with a DNA Extraction Kit (Bioneer's AccuPrep®, South Korea) and subjected to specific PCR according to Leite et al. (2015). PCR led to the amplification of 1500 base pairs (bp), with the target sequence being from variable regions of 16S rDNA in LAB. The sequences of the forward and reverse specific primers were 27F: 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R: 5′-GGTTACCTTGTTACGACTT-3′, respectively. After agarose gel electrophoresis, the PCR products were sequenced by MWG Biotech (Germany). Sequences were edited and aligned by ClustalW in BioEdit. Results were compared against genomic data available on NCBI using the BLASTn procedure (http://www.ncbi.nlm.nih.gov/genbank/). For estimating the phylogenetic relationships, phylogenetic trees were constructed using the neighbor-joining (NJ) method with 500 bootstrap replicates. All phylogenetic analyses were performed in MEGA 7 using the Tamura-Nei model (Tamura et al., 2013).
2.2.3. Controlled fermentation of wheat sourdough
To control the fermentation of wheat sourdough, the LAB isolate was first cultured in MRS broth at 30 °C for 48 h to 108 colony forming units per gram. Then, the produced biomass was collected and centrifuged (Hanil, Combi 514R, South Korea) for 15 min at 5000 g and 4 °C, before being resuspended in sterile water. In the next stage and in order to form sourdough (DY 160), the active dry yeast extract that contained S. cerevisiae (equivalent to 0.25% of the flour) and the cell suspension of the LAB isolate (equivalent to 1.5% of the flour) were rapidly mixed with wheat flour (Dal Bello et al., 2007). Finally, the mixture obtained from each of the three wheat flours (0.45, 0.75, and 1.55% ash content) was fermented under different fermentation times (8, 16, and 24 h) and sugar contents (0.5, 1, 1.5%) at 30 °C (Meignen et al., 2001).
2.2.4. Sourdough pH and total titratable acidity (TTA)
A pH meter (Knick 766, Germany) equipped with a food penetration probe was used to measure the pH values related to the sourdough samples. To measure TTA, sterile distilled water (90 mL) was used to produce homogenous solutions containing 10 g of the sourdough samples. These solutions were titrated with 0.1 N NaOH to a final pH of 8.5. The TTA was expressed in mL of consumed 0.1 N NaOH (Katina et al., 2006).
2.2.5. Bread making
The control cup bread formulation per 100 g of flour included 2 g of active dry yeast extract containing S. cerevisiae, 1.5 g of salt (NaCl) and 1.5 g of sugar. With the help of a Brabender Farinograph (FE022N, Germany), the suitable amount of water to prepare dough with 60% absorption was ascertained. Then, the listed ingredients were mixed for 20 min at 60 rpm in a laboratory dough mixer (EB124101, Germany). Next, the dough was left for initial proofing at 30 ± 1 °C and 75% relative humidity for 30 min and subsequently divided into pieces of 150 g before being moulded by hand. Final proofing was then carried out on the rounded dough pieces over 90 min under a relative humidity of 85% and a temperature of 45 ± 1 °C. An electrical baking oven (Leisure, Italy) was then used to bake the dough for 15 min at 220 ± 5 °C. The baked bread samples were then left to cool down for 2 h under sanitary conditions at room temperature (25 °C), before being packed within polyethylene bags until analysis. For preparation of sourdough breads, before the final proofing step, 25% (w/w) of each sourdough treatment was added to dough prepared as the control sample, which was then processed under identical conditions (Meignen et al., 2001).
2.2.6. Crumb texture analysis and specific volume
A texture analyzer device (TAXT, Pluse Stable Micro Systems, England) was used to perform texture profile analysis (TPA) and assess the crumb texture (hardness and chewiness) with a cylindrical aluminum probe (25 mm diameter). In brief, a 30 mm/min crosshead speed was utilized for crumb compression to 50% of the initial height. Measurements were made on three slices (20 ± 2 mm thickness) obtained from the middle of the loaf 2 h after baking (Gomez et al., 2008). To determine the bread loaf specific volume, the rapeseed displacement method was used according to A-A-20126E Metric (Hallen et al., 2004).
2.2.7. Crust total color difference (TCD), crumb porosity and bread sensory evaluation
For determination of the color values of the samples, a cross sectional picture was scanned (HP Scanjet G2710, China) from one-fourth into each loaf 2 h post-baking. The obtained images were then transformed into L∗a∗b∗ units because the colors were detected as RGB signals by the computer vision system, which is device-dependent. The crust color values included a∗ (–a∗ = greenness and +a∗ = redness), b∗ (–b∗ = blueness, +b∗ = yellowness) and L∗ (L∗ = darkness/lightness; 0 = black; 100 = white). Based on the mentioned values of color, Eq. (1) was used to measure the TCD index (Mohd Jusoh et al., 2009):
| (1) |
where and are the initial values of L*, a* and b*, respectively.
For determining the crumb porosity of the samples, pictures were converted to a binary form. Finally, image analysis was performed using Image J (Image J Portable Software, 2013) to calculate average pore size and percent area of pores (Carson et al., 2000). The hedonic test was used for the evaluation of the breads’ overall acceptability (OAA) at 2 h after baking. Ten trained consumers rated the intensity of the samples for sensory attributes using a 5-point hedonic scale (1 = dislike extremely, 3 = neither like nor dislike, 5 = like extremely). Samples were identified with 3-digit random codes and panelists during the test had access to the control sample as well as drinking water to minimize errors. Bread sensory properties included form and shape, chewability, color, flavor and taste. OAA was attained on the basis of the mean panelist scores, which took into account all organoleptic attributes (Pourfarzad et al., 2013).
2.2.8. Experimental design and statistical analysis
RSM was used to estimate the simultaneous impacts of the variables involved (fermentation time, X1; sugar contents, X2 and ash contents, X3) on response functions (Y) including sourdough TTA and pH, bread hardness (N), chewiness (g), specific volume (cm3/g), color values (L*a*b* and TCD index), porosity (%) and OAA.
The maximum and minimum values of the independent variables were determined after pretreatments. A central composite design was used and the actual values of fermentation time, sugar and ash contents were chosen by Design-Expert version 9.01 (Statease Inc., Minneapolis, USA) as 8–24 h, 0.5–1.5% and 0.45–1.55%, respectively (Table 1 A & B). Randomized experiments were prepared from three central points to evaluate the repeatability of the method. For each function, model selection was based on the lack-of-fit test, sum of squares (SS), correlation coefficient (R2) and significant effect of F-test (P < 0.05). The coefficients of all independent variables in the equation were also determined by analysis of variance (ANOVA) for each function; finally, the independent and response variables were fitted to a suitable model (Koocheki et al., 2009).
Table 1.
Composition of various runs of central composite design (A) and experimental data (B).
| (A) | |||
|---|---|---|---|
| Run | Fermentation time (h) | Sugar contents (%) | Ash contents (A, g/100 g, d.w) |
| 1 | 24.00 | 0.50 | 1.55 |
| 2 | 8.00 | 1.50 | 0.45 |
| 3 | 16.00 | 1.00 | 1.00 |
| 4 | 16.00 | 1.00 | 0.45 |
| 5 | 16.00 | 1.00 | 1.00 |
| 6 | 24.00 | 1.50 | 0.45 |
| 7 | 16.00 | 0.50 | 1.00 |
| 8 | 8.00 | 0.50 | 0.45 |
| 9 | 24.00 | 0.50 | 0.45 |
| 10 | 16.00 | 1.00 | 1.55 |
| 11 | 8.00 | 0.50 | 1.55 |
| 12 | 24.00 | 1.50 | 1.55 |
| 13 | 24.00 | 1.00 | 1.00 |
| 14 | 16.00 | 1.00 | 1.00 |
| 15 | 8.00 | 1.50 | 1.55 |
| 16 | 8.00 | 1.00 | 1.00 |
| 17 | 16.00 | 1.50 | 1.00 |
| (B) | |||||||
|---|---|---|---|---|---|---|---|
| Run | TTA | Hardness (N) | Chewiness (g) | Specific volume (cm3/g) | TCD index | Porosity (%) | OAA |
| 1 | 2.3 | 13.33 | 983.29 | 1.81 | 11.97 | 19.4 | 11.7 |
| 2 | 1.13 | 14.01 | 985.54 | 1.49 | 18.54 | 18.4 | 12.1 |
| 3 | 2.56 | 23.97 | 2444.54 | 2.10 | 39.32 | 20.6 | 11.9 |
| 4 | 1.43 | 17.85 | 1113.04 | 1.58 | 21.28 | 18.9 | 14.9 |
| 5 | 2.43 | 20.976 | 2134.23 | 1.89 | 39.30 | 21.3 | 12.1 |
| 6 | 1.73 | 9.71 | 717.90 | 1.69 | 14.00 | 27.6 | 12.5 |
| 7 | 2.63 | 22.67 | 1600.09 | 2.22 | 40.19 | 27.5 | 11.8 |
| 8 | 0.66 | 22.06 | 1409.92 | 1.51 | 11.38 | 22.1 | 11.7 |
| 9 | 1.3 | 13.08 | 963.92 | 1.81 | 21.80 | 20.01 | 11.8 |
| 10 | 1.86 | 22.92 | 1615.99 | 1.58 | 23.62 | 29.8 | 9.01 |
| 11 | 1.3 | 8.81 | 3142.24 | 1.59 | 11.38 | 13.8 | 8.8 |
| 12 | 2.93 | 6.71 | 1117.14 | 1.59 | 14.00 | 22.7 | 12 |
| 13 | 2.61 | 20.1 | 1164.40 | 2.26 | 11.85 | 14.02 | 12.3 |
| 14 | 2.56 | 21.2 | 2224.54 | 2.10 | 39.32 | 20.6 | 12 |
| 15 | 1.03 | 15.1 | 2333.34 | 1.59 | 17.64 | 14.8 | 7.2 |
| 16 | 1.53 | 11.87 | 849.96 | 2.35 | 32.63 | 14.7 | 11.8 |
| 17 | 2.76 | 27.19 | 2740.93 | 2.01 | 37.54 | 12.76 | 12.6 |
3. Results and discussion
3.1. Adequacy of the model
The results of ANOVA related to the combined effects of sourdough fermentation time, sugar content and ash content on sourdough TTA, cup bread hardness, chewiness, specific volume, TCD, porosity and OAA (as linear, quadratic and interaction effects) are presented in Table 2. In this study relatively high R2 values were obtained in the quadratic models for all functions. Moreover, polynomial models of hardness, chewiness and porosity were not significant (ns). The adequacy of the polynomial models was also confirmed based on the statistical analysis, and the following coefficients were used: β0 (constant term); β1, β2 and β3 (linear); β11, β22 and β33 (quadratic); β12, β13 and β23 (interaction effects); and ε (error).
| Y = β0 + β1 X1 + β2 X2 + β3 X3 + β11 X12 + β22 X22 + β33 X32 + β12 X1X2 + β13 X1X3 + β23 X2X3+ε | (2) |
Table 2.
ANOVA and regression coefficients of the linear and secondorder polynomial models for the response variables (actual values).
| Source | DF | TTA |
Hardness (N) |
Chewiness |
Specific volume (cm3/g) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Sum of squares | p-Value | Coefficient | Sum of squares | p-Value | Coefficient | Sum of squares | p-Value | Coefficient | Sum of squares | p-Value | ||
| Model | 9 | 2.48 | 7.97 | <0.0001 | 23.03 | 415.76 | 0.1968 | 1941.01 | 632.2 | 0.1892 | 2.10 | 1.22 | 0.0039 |
| Linear | |||||||||||||
| X1 | 1 | 0.52 | 2.72 | 0.0126 | -0.89 | 7.96 | 0.5810 | -377.43 | 142.5 | 0.0848 | 0.062 | 0.039 | 0.1486 |
| X2 | 1 | 0.14 | 0.20 | 0.4507 | -0.72 | 5.25 | 0.6529 | -20.46 | 4186.42 | 0.9164 | -0.057 | 0.032 | 0.1811 |
| X3 | 1 | 0.32 | 1.00 | 0.1023 | -0.98 | 9.66 | 0.5443 | 400.17 | 160.1 | 0.0710 | 930.01 | 864.9 | 0.8155 |
| Quadratic | |||||||||||||
| X11 | 1 | -0.38 | 0.39 | 0.0052 | -7.78 | 162.13 | 0.0349 | -688.17 | 127.1 | 0.0999 | 0.15 | 0.062 | 0.0788 |
| X22 | 1 | 0.25 | 0.16 | 0.0369 | 1.17 | 3.64 | 0.707 | 474.17 | 603.4 | 0.2329 | -0.034 | 310.5 | 0.6601 |
| X33 | 1 | -0.80 | 1.73 | <0.0001 | -3.38 | 30.59 | 0.2941 | -331.43 | 294.3 | 0.3921 | -0.57 | 0.87 | 0.0001 |
| Interaction | |||||||||||||
| X12 | 1 | 0.11 | 0.094 | 0.0904 | -1.03 | 8.48 | 0.5693 | 140.14 | 157.1 | 0.5266 | -0.040 | 0.013 | 0.3837 |
| X13 | 1 | 0.21 | 0.35 | 0.0069 | 1.18 | 11.08 | 0.5169 | -332.62 | 885.5 | 0.1577 | -0.037 | 0.011 | 0.4216 |
| X23 | 1 | -0.067 | 0.036 | 0.2654 | 1.38 | 15.32 | 0.4487 | -0.58 | 2.72 | 0.9979 | -937.5 | 703.1 | 0.8333 |
| Residual | 7 | 0.17 | 166.51 | 247.7 | 0.10 | ||||||||
| Lack of fit | 5 | 0.16 | 0.1713 | 160.93 | 0.0818 | 242.6 | 0.0506 | 0.072 | 0.5883 | ||||
| Pure error | 2 | 0.012 | 5.59 | 50949.40 | 0.031 | ||||||||
| Total | 16 | 8.14 | 582.27 | 879.9 | 1.33 | ||||||||
| R2 | 0.979 | 0.7140 | 0.7187 | 0.9223 | |||||||||
| Adj- R2 | 0.952 | 0.3464 | 0.3565 | 0.8225 | |||||||||
| CV | 8.08 | 28.44 | 36.72 | 6.60 | |||||||||
| Source | DF | TCD index |
Porosity (%) |
OAA |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Sum of squares | p-Value | Coefficient | Sum of squares | p-Value | Coefficient | Sum of squares | p-Value | ||
| Model | 9 | 37.03 | 1863.85 | 0.0156 | 20.23 | 177.73 | 0.7702 | 12.28 | 40.24 | 0.0409 |
| Linear | ||||||||||
| X1 | 1 | -1.80 | 32.24 | 0.3759 | 1.99 | 39.72 | 0.3101 | 0.87 | 7.57 | 0.0355 |
| X2 | 1 | 0.50 | 2.49 | 0.8005 | -0.65 | 4.29 | 0.7298 | 0.060 | 0.036 | 0.8629 |
| X3 | 1 | -0.84 | 7.04 | 0.6720 | -0.65 | 4.24 | 0.7313 | -1.43 | 20.42 | 0.0037 |
| Quadratic | ||||||||||
| X11 | 1 | -13.06 | 457.27 | 0.0092 | -5.42 | 78.63 | 0.1676 | -0.44 | 0.51 | 0.5228 |
| X22 | 1 | 3.56 | 33.95 | 0.3642 | 0.35 | 0.33 | 0.9230 | -0.29 | 0.22 | 0.6727 |
| X33 | 1 | -12.56 | 442.88 | 0.0099 | 4.57 | 56.02 | 0.2349 | -0.53 | 0.75 | 0.4395 |
| Interaction | ||||||||||
| X12 | 1 | -2.40 | 45.99 | 0.2960 | 1.70 | 23.09 | 0.424 | 0.27 | 0.60 | 0.4984 |
| X13 | 1 | -1.12 | 9.96 | 0.6155 | 0.80 | 5.10 | 0.7039 | 0.90 | 6.48 | 0.0443 |
| X23 | 1 | 1.12 | 9.96 | 0.6155 | 0.051 | 0.021 | 0.9805 | -0.30 | 0.72 | 0.4612 |
| Residual | 7 | 252.45 | 223.24 | 7.85 | ||||||
| Lack of fit | 5 | 252.45 | 0.0601 | 231.92 | 0.0035 | 7.83 | 0.0604 | |||
| Pure error | 2 | 377.4 | 0.33 | 0.020 | ||||||
| Total | 16 | 2116.30 | 409.98 | 48.09 | ||||||
| R2 | 0.8807 | 0.4335 | 0.8363 | |||||||
| Adj- R2 | 0.7273 | 0.2948 | 0.6270 | |||||||
| CV | 25.16 | 28.89 | 9.17 | |||||||
3.2. Molecular identification of LAB isolate
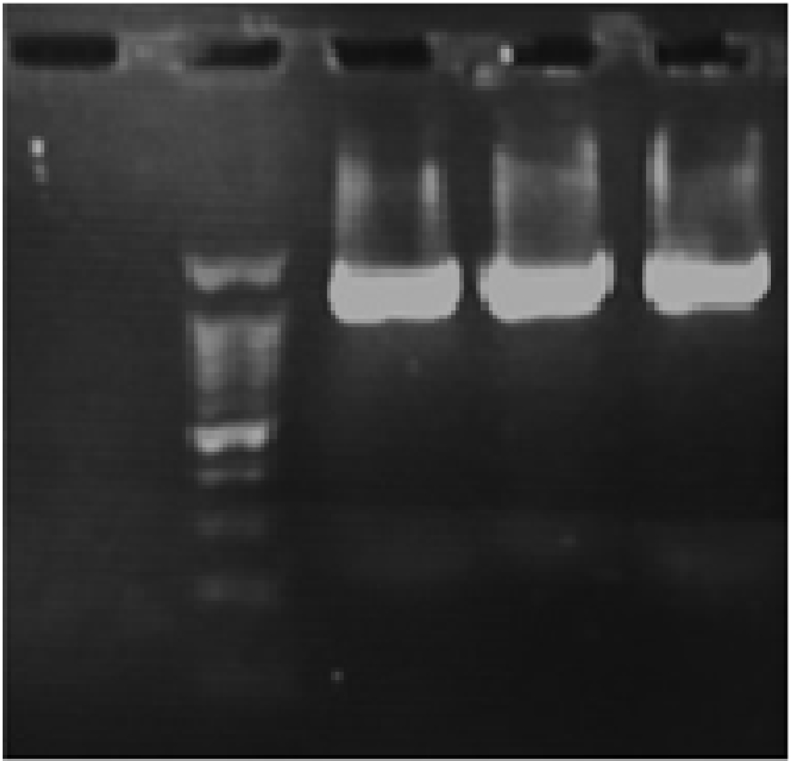
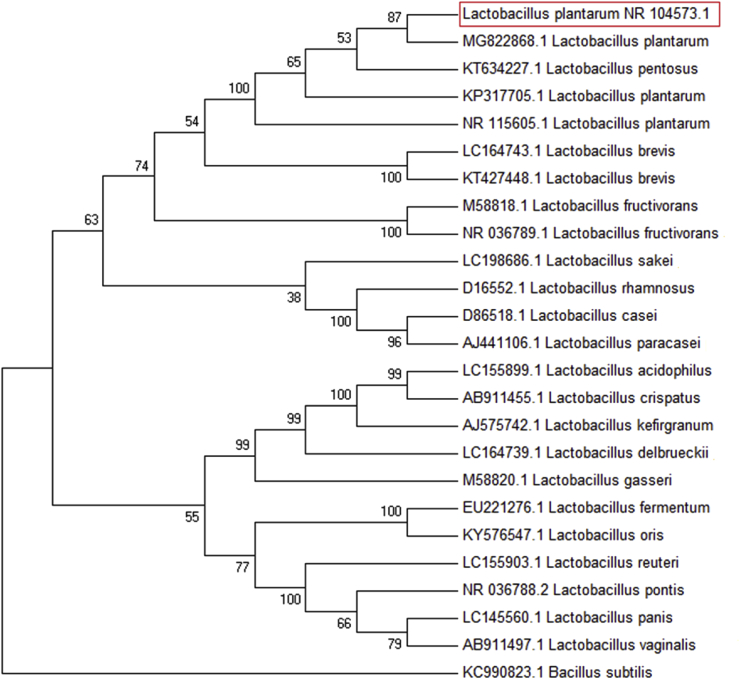
The products of PCR were subjected to gel electrophoresis in order to specifically detect the dominant LAB isolate, as shown in Fig. 1. After electrophoresis, no primer dimers or nonspecific products appeared, with only the 1500 bp amplicon that was expected being observed. The BLASTn search was used to confirm the identity of the amplicon, and L. plantarum was identified as the dominant LAB isolated from whole wheat sourdough after sequencing the PCR products. Fig. 2 represents the MEGA7 cluster alignments of the 16S rRNA genes of one distinct strain in sourdough (L. plantarum), thereby demonstrating its phylogenetic relationships. The higher frequency of Lactobacillus strains in sourdough compared with species such as Pediococcus, Weissella, and Leuconostoc has also been confirmed by microbiological studies; such dominance may be due to their adaptative and competitive abilities within this ecocystem (De Vuyst and Neysens, 2005).
Fig. 1.
Agarose gel electrophoresis of PCR products obtained under optimized conditions for identification of dominant LAB isolates (1500 bp). 100 bp DNA ladder (lane 2), extracted DNA from cultured cells of Lactobacillus spp. in MRS broth as positive control (lane 3), DNA extracted from single colonies of sourdough cultures (lane 4 and 5) and no DNA as negative control (lane 1).
Fig. 2.
Dendogram showing multiple sequence alignment of 16S rRNA gene sequences of the wheat bran sourdough of the strains.
3.3. Sourdough pH and TTA
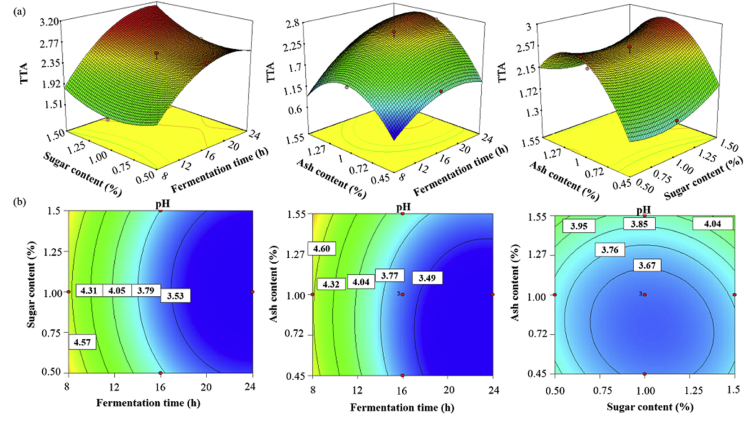
All of the independent variables had significant (P < 0.05) quadratic effects on sourdough TTA (Table 2). Furthermore, as expected, when fermentation time and sugar content were increased across all sourdough treatments, TTA also increased and pH decreased. The sourdoughs obtained from 24 h of fermentation, 1.5% sugar, and 1.55 or 0.45% ash had the highest TTA and lowest pH values, respectively. The sourdough TTA values increased with ash content in the range of 0.45–1%, but further addition of ash had a negative effect on TTA. Conversely, a low level of ash (up to 1%) decreased the pH, but higher levels partly induced a rise in pH (Fig. 3).
Fig. 3.
Response surface plot of interactive effects of independent variables on sourdough TTA (a) and pH (b).
The polynomial models generated for the relationsip between the three independent variables (fermentation time; sugar and ash contents) and sourdough TTA and pH are shown in Eqs. (3) and (4), respectively.
| TTA = –1.66 + 1.18 X1 – 1.87 X2 + 5.37 X3 + 0.02 X1X2 + 0.04 X1X3 – 0.24 X2X3 – 0.09 X12 + 0.98 X22 – 2.65 X32 (R2 = 0.979) | (3) |
| pH = 7.80–0.26 X1 – 1.73 X2 – 1.25 X3 – 0.01 X1X2 + 0.01 X1X3 + 0.12 X2X3 + 0.05 X12 + 0.80 X22 + 0.58 X32 (R2 = 0.915) | (4) |
The biochemical features of sourdough, including pH and TTA, are impacted by various factors including fermentation time and temperature, flour type, dough yield, number of back-sloppings and, especially, the complexity of the microbial ecology. In general, by utilization of whole flour and by increasing the temperature or water content of wheat sourdoughs, TTA is increased (Clarke et al., 2004). Moreover, Simonson et al. (2003) reported that with sucrose addition (0–6%), the TTA of wheat sourdough increased mainly due to the accumulation of acetic acid. In the present study, the highest amount of TTA and lowest value of pH were observed at the same values of fermentation time (24 h) and sugar content (1.5%), but at the maximum (1.55%) and minimum (0.45%) amounts of ash content, respectively. It may be assumed that microbial metabolism is influenced by fiber's buffering capacity and the role of bran as a nutritional supplement, which leads to changes in the TTA and pH profiles under the same fermentation conditions (Simonson et al., 2003). It should be noted that TTA correlates with most of the positive features of sourdough, including its direct effect on dough properties and its indirect effects on enzymatic activity (both cereal and microbial) (Clarke and Arendt, 2005).
3.4. Hardness, chewiness and specific volume
The quadratic models fitted for the effects of three independent variables on hardness, chewiness and specific volume of the cup breads are presented in Eqs. (5), (6), and (7), respectively.
| Hardness = –4.38 + 3.76 X1 – 11.68 X2 + 11.24 X3 – 0.25 X1X2 + 0.26 X1X3 + 5.03 X2X3 – 0.12 X12 + 4.66 X22 – 11.17 X32 (R2 = 0.714 ns) | (5) |
| Chewiness = –594.50 + 337.77 X1 – 4395.94 X2 + 4130.73 X3 + 35.03 X1X2 – 75.61 X1X3 - 2.11 X2X3 – 10.76 X12 + 1898.29 X22 – 1095.62 X32 (R2 = 0.718 ns) | (6) |
| Specific volume = + 0.34–0.05 X1 + 0.35 X2 + 3.94 X3 – 0.09 X1X2 – 8.32 X1X3 – 0.03 X2X3 + 2.38 X12 – 0.13 X22 – 1.88 X32 (R2 = 0.922) | (7) |
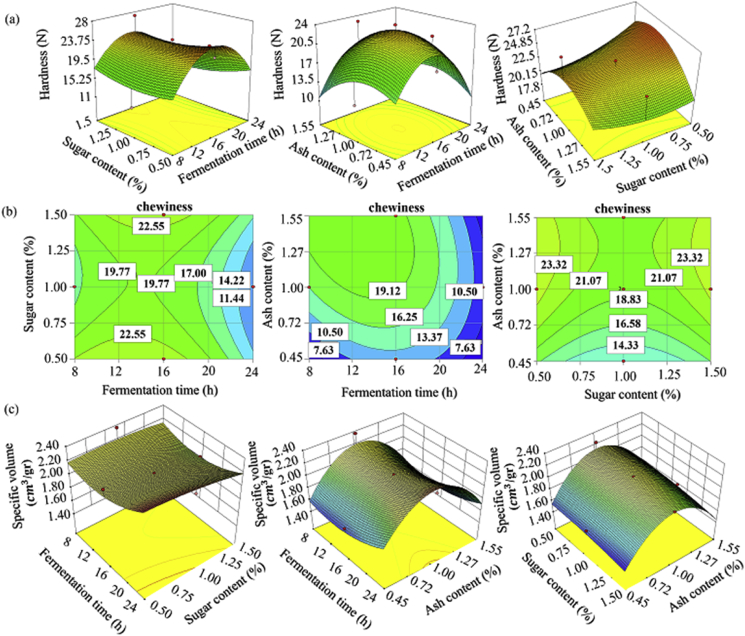
As shown in Table 2, among the independent variables, only fermentation time had a positive second order effect on bread hardness, while none of the variables affected chewiness (P > 0.05). Furthermore, ash content had a significant (P < 0.05) effect on the specific volume, while the effects of fermentation time and sugar content on the specific volume were insignificant. The second order effect of fermentation time on bread hardness indicates that there is an optimum fermentation time window in which the maximum/minimum value of hardness is reached. Cup bread prepared from sourdough obtained after 16 h of flour fermentation with 1% ash content and 1.5% sugar content had the highest amount of hardness, while cup bread prepared from sourdough obtained after 24 h of flour fermentation with 1.55% ash and 1.5% sugar had the least hardness (Fig. 4a). By increasing fermentation time, the chewiness decreased, though ash and sugar contents had different effects on bread chewiness (Fig. 4b). With increasing fermentation time and sugar content, crumb specific volume decreased moderately, and the maximum amount of specific volume was reached at 1% ash content (Fig. 4c).
Fig. 4.
Response surface plot of interactive effects of independent variables on bread hardness (a), chewiness (b) and specific volume (c).
From the consumer's point of view, the key textural attribute related to bread quality is crumb hardness as it is a major indicator of bread freshness. Bread freshness is maintained by biological acidification, which affects moisture redistribution throughout the crumb (Corsetti et al., 2000). This is due to the fact that these compounds strengthen the gluten network, which prevents moisture loss during baking by absorbing water, and have the ability to react with starch molecules, which retards starch retrogradation in the final product and thereby delays bread staling (Sudha et al., 2007). The decrease in bread hardness via sourdough acidification is also due to the stimulation of flour proteolytic enzymes, which improves gluten solubility, increases the activity of alpha-amylase thereby changing the moisture absorption of polysaccharides, and eventually degrades the cross-linked gluten proteins that are responsible for bread firmness. Loaf specific volume is mainly affected by the magnitude of gas that is produced over fermentation and the gas-retaining capacity of the dough, which in turn are dependent on the dough's physicochemical structure and macromolecular degradation. Improvement in bread specific volume and softness are related to a reduction of staling and extended bread shelf life (Clarke et al., 2002). In this study, the effect of fermentation time on crumb hardness and the influence of ash content on loaf specific volume were clarified. Accordingly, the control of sourdough fermentation conditions influences the microbial activity and the types of microbial metabolites produced, thereby affecting bread texture.
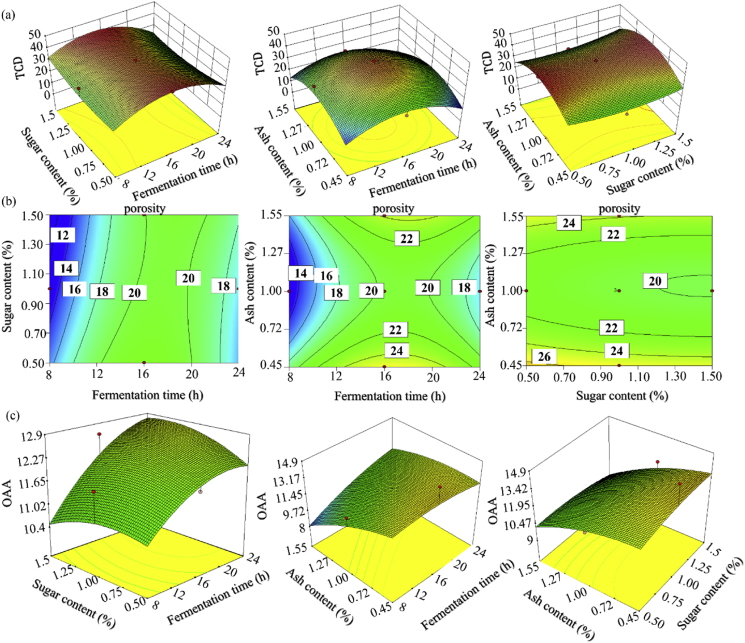
3.5. TCD, porosity and OAA
As exemplified in Table 2, positive second order effects of fermentation time and ash content on TCD index (P < 0.05) were observed. After 16 h of fermentation in the presence of 1% ash, TCD reached its maximum amount. By increasing the sugar content up to 1.5%, the TCD index also increased (Fig. 5a). As it can be seen in Table 1, none of the independent variables significantly influenced crumb porosity (P < 0.05). Furthermore, as fermentation time and ash content increased in the range of 8–16 h and 1–1.55%, respectively, the porosity increased. Moreover, a slight decrease in porosity was observed when the sugar content was increased (Fig. 5b).
Fig. 5.
Response surface plot of interactive effects of independent variables on TCD (a), porosity (b) and OAA (c).
On the other hand, none of the independent variables had second order influences on bread OAA, though significant impacts were induced by fermentation time and ash content according to the linear model (Table 2). In comparison with the other functions, the interaction between fermentation time and sugar content had a significant (P < 0.05) influence on bread OAA. Thus, it appears that the interaction between the mentioned parameters improved the organoleptic characteristics of bread under controlled sourdough fermentation. When the ash content was augmented, the bread OAA diminished. However, the OAA increased when fermentation time and sugar content were increased (Fig. 5c). The bread with the highest OAA was that prepared after 16 h of flour fermentation with 0.45% ash and 1% sugar. On the other hand, 8 h of fermentation, 1.55% ash and 1.5% sugar led to the lowest OAA. The TCD index, porosity and OAA were estimated using the following response surface polynomial models (Eqs. (8), (9), and (10)):
| TCD index = – 48.96 + 7.16 X1 – 21.94 X2 + 83.47 X3 – 0.59 X1X2 – 0.25 X1X3 + 4.05 X2X3 – 0.20 X12 + 14.23 X22 – 42.50 X32 (R2 = 0.880) | (8) |
| Porosity = + 23.48 + 2.35 X1 – 11.11 X2 – 34.50 X3 + 0.42 X1X2 + 0.18 X1X3 + 0.18 X2X3 – 0.08 X12 + 1.41 X22 + 15.11 X32 (R2 = 0.433 ns) | (9) |
| OAA = 11.66 + 0.05 X1 + 2.39 X2 – 1.27 X3 + 0.06 X1X2 + 0.20 X1X3 – 1.09 X2X3 – 0.06 X12 – 1.14 X22 – 1.75 X32 (R2 = 0.836) | (10) |
The key use of the TCD index is for comparing the overall difference in color between reference and processed samples. This index is a valuable measurement of crust color and is positively correlated with bread appearance. In general, crust color values are mostly affected by Maillard and caramelization reactions, which are in turn dependent on the flour components, the other ingredients of the bread formulation, the ratio between amino acids (amassed due to augmented proteolytic activity during sourdough fermentation) and reducing carbohydrates, pH, and, especially, the two parameters of baking time and temperature (Martins et al., 2000; Mohd Jusoh et al., 2009). In this study, it can be assumed that due to a boost in the types and amounts of metabolites produced during sourdough fermentation, the TCD index increased with increasing sugar content.
There is also evidence from the literature indicating that hetero-fermentative lactic acid bacteria and yeasts in sourdough may influence gas production. Furthermore, proteolytic and amylolytic activity cause a rise in the acidity of sourdough, which is responsible for the better gas holding capacity, expanded gluten network, and increased porosity (Thiele et al., 2002; Clarke et al., 2003). Usually, longer fermentation times and higher protein contents lead to increased acidity and expanded gluten networks.
In flour, fiber has a crucial role as it stabilizes the air cells and improves the water binding ability, thereby preventing the coalescence of CO2 bubbles and reinforcing the gluten network (Arendt et al., 2007). This point is in agreement with our findings and can be used to explain the influence of fermentation time, sugar content and ash content on crumb porosity.
It has been reported that the effects of elevated ash content and fermentation time on OAA are mostly due to enhanced acidification, proteolysis, and formation of flavor-active volatile compounds (Katina et al., 2004). In contrast to the mentioned studies, we found that an increasing ash content led to the reduction of OAA. Hence, it can be assumed that the interaction between sugar content and ash content affects the acidic flavor intensity and undesired odor formation. Our findings are not in agreement with those of Katina et al. (2006), who indicated that ash content had the greatest influence on bread OAA. However, in the present study, increased ash content led to increased chewability but decreased crumb softness and decreased OAA in sourdough breads.
3.6. Correlation between sourdough TTA and pH with bread quality properties
As shown in Table 3, sourdough TTA had a strong, positive correlation with most of the quality attributes of sourdough bread (especially with OAA (0.89), hardness (0.84) and specific volume (0.81)) fermented with L. plantarum. Furthermore, the following strong, positive correlations were found: specific volume with hardness; porosity and TCD index with specific volume; and OAA with chewiness (Table 3). Based on these results, the regulation of sourdough TTA may be utilized as an important parameter for the optimization of bread quality characteristics. Moreover, changes in bread texture or sensory parameters vary depending on the different sourdough fermentation conditions (especially time and substrates) due to the metabolic activity of sourdough, and optimum conditions are necessary for enhancing the quality attributes of prepared breads (Katina et al., 2006).
Table 3.
Correlation coefficients matrix between sourdough bread quality attributes fermented with L. plantarum.
| Parameter | TTA | pH | Hardness | Chewiness | Specific volume | TCD | Porosity | OAA |
|---|---|---|---|---|---|---|---|---|
| TTA | 1 | 0.921 | 0.845 | 0.732 | 0.813 | 0.762 | 0.534 | 0.896 |
| pH | 1 | 0.711 | 0.522 | 0.781 | 0.628 | 0.499 | 0.820 | |
| Hardness | 1 | 0.322 | 0.621 | 0.784 | 0.421 | 0.616 | ||
| Chewiness | 1 | 0.443 | 0.623 | 0.531 | 0.789 | |||
| Specific volume | 1 | 0.799 | 0.654 | 0.661 | ||||
| TCD | 1 | 0.510 | 0.541 | |||||
| Porosity | 1 | 0.626 | ||||||
| OAA | 1 |
4. Conclusions
RSM is a suitable technique for sourdough optimization through the selection of appropriate fermentation conditions. In this study, significant influences of sourdough fermentation time, ash content and sugar content on bread quality responses were demonstrated. Specifically, sourdough fermentation time significantly influenced bread hardness and TCD, while ash content significantly affected specific volume and TCD, and a strong correlation was found between sourdough TTA and the parameters of bread hardness, specific volume and OAA. Finally, model equations were developed, which can be used for predicting the attributes of sourdough cup breads and for designing sourdough baking processes suitable for achieving improved bread quality.
Declaration
Author contribution statement
Abbas Abedfar: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Alireza Sadeghi: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- AACC international . Moisture 44-19, protein 46-10, wet gluten 38-12, and ash 08-01 methods. In: Paul St., editor. American Association of Cereal Chemists 357 (AACC) International. 2010. MN, USA. [Google Scholar]
- Aplevicz K.S., Ogliari P.J., Sant'Anna E.S. Influence of fermentation time on characteristics of sourdough bread. Braz. J. Pharm. Sci. 2013;49(2):233–239. [Google Scholar]
- Arendt E.K., Ryan L.A., Dal Bello F. Impact of sourdough on the texture of bread. Food Microbiol. 2007;24(2):165–174. doi: 10.1016/j.fm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Carson L., Setser C., Sun X.S. Sensory characteristics of sorghum composite bread. Int. J. Food Sci. Technol. 2000;35(5):465–471. [Google Scholar]
- Clarke C.I., Arendt E.K. A review of the application of sourdough technology to wheat breads. Adv. Food Nutr. Res. 2005;49(1):137–161. doi: 10.1016/S1043-4526(05)49004-X. [DOI] [PubMed] [Google Scholar]
- Clarke C.I., Schober T.J., Arendt E.K. Effect of single strain and traditional mixed strain starter cultures on rheological properties of wheat dough and on bread quality. Cereal Chem. 2002;79(5):640–647. [Google Scholar]
- Clarke C.I., Schober T.J., Angst E., Arendt E.K. Use of response surface methodology to investigate the effects of processing conditions on sourdough wheat bread quality. Eur. Food Res. Technol. 2003;217(1):23–33. [Google Scholar]
- Clarke C.I., Schober T.J., Dockery P., O'Sullivan K., Arendt E.K. Wheat sourdough fermentation: effects of time and acidification on fundamental rheological properties. Cereal Chem. 2004;81(3):409–417. [Google Scholar]
- Corsetti A., Gobbetti M., De Marco B., Balestrieri F., Paoletti F., Russi L., Rossi J. Combined effect of sourdough lactic acid bacteria and additives on bread firmness and staling. J. Agric. Food Chem. 2000;48(7):3044–3051. doi: 10.1021/jf990853e. [DOI] [PubMed] [Google Scholar]
- Dal Bello F., Clarke C.I., Ryan L.A.M., Ulmer H., Schober T.J., Ström K., Sjögren J., Van Sinderen D., Schnürer J., Arendt E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007;45(3):309–318. [Google Scholar]
- De Vuyst L., Neysens P. The sourdough microflora: biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005;16(1-3):43–56. [Google Scholar]
- Fazeli M.R., Shahverdi A.R., Sedaghat B., Jamalifar H., Samadi N. Sourdough-isolated Lactobacillus fermentum as a potent anti-mould preservative of a traditional Iranian bread. Eur. Food Res. Technol. 2004;218(6):554–556. [Google Scholar]
- Gargari B.P., Mahboob S., Razavieh S.V. Content of phytic acid and its mole ratio to zinc in flour and breads consumed in Tabriz, Iran. Food Chem. 2007;100(3):1115–1119. [Google Scholar]
- Gobbetti M., Rizzello C.G., Di Cagno R., De Angelis M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014;37:30–40. doi: 10.1016/j.fm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Gomez M., Ronda F., Caballero P.A., Blanco C.A., Rosell C.M. Functionality of different hydrocolloids on the quality and shelf-life of yellow layer cakes. Food Hydrocoll. 2008;21(2):167–173. [Google Scholar]
- Hallen E., Ibanoglu S., Ainsworth P. Effect of fermented/germinated cowpea flour addition on the rheological and baking properties of wheat flour. J. Food Eng. 2004;63(2):177–184. [Google Scholar]
- Jusoh Y.M., Chin N.L., Yusof Y.A., Rahman R.A. Bread crust thickness measurement using digital imaging and Lab colour system. J. Food Eng. 2009;94(3-4):366–371. [Google Scholar]
- Katina K., Kaisa P., Karin A. Influence and interactions of processing conditions and starter culture on formation of acids, volatile compounds, and amino acids in wheat sourdoughs. Cereal Chem. 2004;81(5):598–610. [Google Scholar]
- Katina K., Heiniö R.L., Autio K., Poutanen K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2006;39(10):1189–1202. [Google Scholar]
- Khare A.K., Biswas A.K., Balasubramanium S., Chatli M.K., Sahoo J. Optimization of meat level and processing conditions for development of chicken meat noodles using response surface methodology. J. Food Sci. Technol. 2015;52(6):3719–3729. doi: 10.1007/s13197-014-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koocheki A., Taherian A.R., Razavi S.M., Bostan A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocolloids. 2009;23(8):2369–2379. [Google Scholar]
- Leite A.M.O., Miguel M.A.L., Peixoto R.S., Ruas-Madiedo P., Paschoalin V.M.F., Mayo B., Delgado S. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 2015;98:3622–3632. doi: 10.3168/jds.2014-9265. [DOI] [PubMed] [Google Scholar]
- Martins S.I., Jongen W.M., Van Boekel M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000;11(9-10):364–373. [Google Scholar]
- Meignen B., Onno B., Gélinas P., Infantes M., Guilois S., Cahagnier B. Optimization of sourdough fermentation with Lactobacillus brevis and baker's yeast. Food Microbiol. 2001;18(3):239–245. [Google Scholar]
- Pontonio E., Nionelli L., Curiel J.A., Sadeghi A., Di Cagno R., Gobbetti M., Rizzello C.G. Iranian wheat flours from rural and industrial mills: exploitation of the chemical and technology features, and selection of autochthonous sourdough starters for making breads. Food Microbiol. 2015;47:99–110. doi: 10.1016/j.fm.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Pourfarzad A., Mahdavian-Mehr H., Sedaghat N. Coffee silverskin as a source of dietary fiber in bread-making: optimization of chemical treatment using response surface methodology. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2013;50(2):599–606. [Google Scholar]
- Sadeghi A., Shahidi F., Mortazavi A., Mahallati M.N. Evaluation of Lactobacillus sanfransicencis (ATCC 14917) and Lactobacillus plantarum (ATCC 43332) effects on Iranian Barbari bread shelf life. Afr. J. Biotechnol. 2008;7(18):3346–3351. [Google Scholar]
- Simonson L., Salovaara H., Korhola M. Response of wheat sourdough parameters to temperature, NaCl and sucrose variations. Food Microbiol. 2003;20(2):193–199. [Google Scholar]
- Sudha M.L., Vetrimani R., Leelavathi K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007;100(4):1365–1370. [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C., Gänzle M.G., Vogel R.F. Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chem. 2002;79(1):45–51. [Google Scholar]
- Venturi F., Andrich G., Sanmartin C., Zinnai A. The kinetics of fermentations in sourdough bread stored at different temperature and influence on bread quality. J Bioprocess. 2013;3(2):134–138. [Google Scholar]