Abstract
Green route biosynthesis of silver nanoparticles (SNPs) using methanol extract of Oscillatoria sp. was investigated. The nanoparticles (OsSNPs) were characterized by UV-Vis spectrophotometry, FTIR, SEM, Thermogravimetry, EDX and DLS. The antibacterial, antibiofilm and in vitro cytotoxicity activity of the OsSNPs was determined. Surface Plasmon Resonance peak was at 500 nm. Functional groups such as hydroxyl; alcohol, phosphate, and amine among others were responsible for the capping and stabilization of proteins in the nanoparticles. The OsSNPs were spherical with size of 10 nm and are thermostable to an extent without totally losing its weight. EDX analysis revealed a strong signal of silver element. DLS shows the particle diameter average of 0.000 nm and 558.1 nm with a polydispersity index of 0.580. The OsSNPs had effective antibacterial activity against the test bacterial pathogens with zone of inhibition ranged from 1 - 21 mm. OsSNPs exhibited strong antibiofilm activity. However, the toxicity of the OsSNPs to Artemia salina (brine shrimp) was observed to be insignificant with the highest mortality at 4000 μg/mL and lethal dose (LC50) of 2630.3 μg/mL. Greenly synthesized OsSNPs had effective antibacterial potency and low cytotoxicity which suggests its use in biomedical and pharmacological applications.
Keywords: Chemical engineering, Chemistry, Biological sciences, Health sciences, Materials science, Oscillatoria sp, Nanoparticles, Characterization, Antibacterial, Antibiofilm, Cytotoxicity
1. Introduction
Oscillatoria sp. is blue - green algae which belong to Phylum Cyanobacteria, Class Cyanophyceae, Order Oscillatoriales and Family Oscillatoraceae. They are either straight or slightly irregular or cylindrical sometimes coiled at both ends and are usually between 4 – 50 μm long. They are mostly present in shady area and cells can be blue-green, olive or almost black in colour [1]. Chronic bacterial infection is of great concern in clinical sector and it is related to the emergence of persistent bacteria [2] which use survival mode that is different from that of resistance which is known to either occur through horizontal gene transfer or genetic mutation [3]. Persistent bacteria are usually stimulated by the exposure to low concentration of silver nanoparticles which leads to the oxidative stress in such bacteria [4]. Staphylococcus aureus is an example of earlier reported persistent bacterium [5]. The emergence of persistent bacteria in health sector in recent years has led to the wide application of nanotechnology which uses nanoscientific ideas to design materials whose structural component size is usually less than 100 nm [6, 7]. It also involves imaging, measuring, modeling and deformation of matter at nanoscale level [8]. Nanoparticles serve as fundamental building blocks of nanodevices which are used in various practical applications [9].
Nanotechnology is a science that deals with manipulation of materials within size range of 0.1–100 nm. Nanomaterials are known to possess unique properties such as electrical conductance, chemical reactivity, magnetism, optical effects and physical strength, which differentiate them from bulk materials. The unique properties possessed by nanomaterials are as a result of their small size [10]. Nanoparticles are the basic building blocks in Nano devices which are useful in various applications. Nanoparticles have unique characteristics such as large surface to volume ratio and definite quantum confinement effect among other biological, physical and chemical properties which give them an edge over bulk materials [11].
Production of nanoparticles can be achieved through physical, chemical and biological methods. Of these three methods, chemical method is the most common method which involves the use of toxic chemicals in the production of nanoparticles. Some of the physical and chemical methods include reduction, arc discharge, electrochemical and reduction processes. The desire of nanotechnology in the production of eco-friendly nanoparticles which is different from the previous ones has given rise to the biosynthesis of nanoparticles [12]. The wide use of synthesized nanoparticles in human activities create the need for a method which will not pose threat to the environment and humans as a result of their exposure to the nanoparticles, hence the biological method [13, 14].
The use of metallic nanoparticles in modern technologies is based on their unique characteristics such as surface Plasmon features, morphologies and interesting physicochemical properties among others [15]. Silver nanoparticles compare to other metallic nanoparticles as a result of their outstanding antimicrobial properties such as broad spectrum and surface Plasmon resonance are of great importance in modern nanoscience and nanotechnology [16, 17]. Unlike standard antimicrobial agents, low doses of silver nanoparticles are needed in the treatment of diseases [18]. The interaction of silver nanoparticles with microorganisms gives rise to the release of silver ions which are potential destroyers of microbial cells due to their ability to deactivate enzymes in microbial cell and membrane permeability disruption leading to lysis and apoptosis [19, 20]. Silver nanoparticles also possess antiinflammatory, antiplatelets and antiangiogenesis potentials among other thus making their application in medical field paramount [21]. The use of green algae in the production of silver nanoparticles have been reported to be efficient due to the composition of the microalgae as well as little or no toxic effect of the synthesized nanoparticles and outstanding features of the nanoparticles biosynthesized [22]. This work aims at biosynthesizing silver nanoparticles from methanol extract of the green alga; Oscillatoria sp. and characterizing the biosynthesized silver nanoparticles.
2. Materials and methods
All chemicals were analytical grade products purchased from Sigma –Aldrich (St.Louis, MO, USA).
2.1. Sample collection and extraction of the bioactive compound from the algae
The blue green algae (Oscillatoria sp.) sample was collected from the wall of the walkway of the Department of Theatre Art, University of Ibadan, Ibadan, Nigeria (Latitude 03°434̍ 43̍ E and longitude 07°26̍ 41̋ N) with sterile scalpel from where thick mass was visually observed and transported to the laboratory, Botany Department, University of Ibadan for further analysis. The scraped algal sample was washed under running tap water to remove sand particles adhering to the sample and thereafter rinsed with sterile distilled water. The washed algal biomass was dried at room temperature for one week, ground into powder using blender and stored at room temperature for further analysis.
The test pathogens; S. aureus ATCC29213, E. coli ATCC 11775, E. coli ATCC 35218, P. aeruginosa ATCC27853, Citrobacter sp., S. typhi ATCC 14028 and Bacillus cereus were obtained from the Department of Microbiology and Department of Pharmacy, University of Ibadan, Ibadan Oyo state. Brine shrimp eggs were obtained from the Biochemistry Department, University of Ibadan and the Sea water was obtained from the Atlantic Ocean in Lagos, Nigeria. The seawater was 29.4 °C, pH 8.2, Salinity 309 + 0.03PSU and Turbidity 15.5 NTU.
For the extraction of the bioactive compound in the algae samples, the algae were washed with distilled water to remove extraneous materials, dried properly and grind into a fine powder. Soxhlet extraction of 200 grams of the dried sample was done using 300 mL methanol as solvent. The marc was filtered and solvents were removed under reduced pressure in a rotary evaporator. Dark brown pastes obtained were weighed and stored in a refrigerator at 4 °C.
2.2. Biosynthesis of silver nanoparticles
The silver nanoparticles were biosynthesized by adding 10 mL of 1 g/mL of the methanol extract of Oscillatoria sp. into 90 mL of 0.1 mM of AgNO3 solution [23]. The mixture was kept in the dark at room temperature for 72 h. The samples were used for further analysis.
2.3. Characterization of the biosynthesized OsSNPs
Visual detection of the greenly synthesized OsSNPs was done by observing the mixture for a change in colour in comparison to the control samples. UV–Visible spectrophotometric analysis of the OsSNPs solutions were determined at room temperature using UV–Vis spectrophotometer (a Lambda 25-Perkin Elmer, Waltham, MA, USA) with a resolution of 0.5 nm. The absorbance of the sample was read at the wavelengths of 200–800 nm [24]. The chemical structure of the OsSNPs samples was analyzed using FTIR spectroscopy (Shimadzu). Two milligram of the dried samples was taken ground with KBr salt at 25 °C and pressed into a mold to form pellet. The spectra were recorded at a wave range of 500–4000 cm-1 and at resolution of 4 cm−1 [25].
The morphological structure of the OsSNPs was analyzed by Scanning Electron Microscopy (SEM). The dried samples were coated with gold using a coater (JEOL, Akishima-shi, Japan, and Model number JFC-1600). The images of SNPs were taken in a SEM (ZEISS EVO-MA 10, Oberkochen, Germany). X ray diffraction patterns (XRD) were obtained in a Siemens Kristalloflex diffractometer using nickel filtered CuKα radiation from 4 to 70° (2θ angle). Thermogravimetry (TG) analysis was carried out using dried OsSNPs in SDT 2960 device from TA Instruments. The nanoparticles were heated in open alumina pans from 40 to 600 °C, under an oxidant atmosphere (O2), flux of 50 mL/min and a heating rate of 10 °C/min were used. The estimation of the silver content in OsSNPs was done using the residue at 600 °C.
The elemental composition of OsSNPs was determined using an Energy Dispersion X-ray (EDX). The dried OsSNPs powder was used for the analysis and the EDX analysis software was sourced from Oxford instruments analytical. All measurements were performed at accelerated voltage of 10 KV.
Particle size analyzer (Zetasizer Nano ZS, Malvern Instruments Limited, Worcestershire, United Kingdom) was used to determine the particle size distribution and surface charge of the OsSNPs. The analysis was done at 25 °C with 90° detection angle and 633 nm. Hydrodynamic diameter and polydispersity index were measured as a function of time. Prior to the analysis, the OsSNPs was suspended in sterile water and sonicated for 15 min.
2.4. Antibacterial potential of the OsSNPs
The antibacterial potential of the OsSNPs was evaluated using the Agar Well Diffusion method [26] using S. aureus ATCC29213, E. coli ATCC 11775, E. coli ATCC 35218, P. aeruginosa ATCC27853, Citrobacter sp., S. typhi ATCC 14028 and Bacillus cereus as the test pathogens. Escherichia coli ATCC 11775 is a reference type strain which was first isolated from urine sample of a Danish patient's in 1941 [27]. Escherichia coli ATCC 35218 is a strain recommended by NCCLS for used as a quality control organism for the assay of betalactam antibiotics, evaluation of Mueller Hinton agar and the quality control strain for susceptibility testing [28, 29]. The isolate was cultured overnight in peptone water. 18 hrs old culture of the isolate was seeded on Mueller - Hinton Agar (Lab M Ltd., UK) plates. Uniform wells were cut on the dried agar plate using a sterile cork -borer of diameter 7 mm. Each well was filled with 20 μL of the biosynthesized OsSNPs. AgNO3 solution (1 mM) and the methanol extract of Oscillatoria sp. were put into respective wells as negative controls. The inoculated plates were incubated at 37 °C for 24 h. After incubation the plates were observed for zones of inhibition (ZOI) around the wells. ZOI diameters (mm) greater than 1 mm were considered positive [30].
2.5. Antibiofilm activity of OsSNPs
The antibiofilm activity of the biosynthesized SNPs was determined according to the method as described by [31] with slight modification. The inoculum was added to 200 μL of already prepared Tryptic Soy Broth (TSB) in a 96 well microtiter plate and incubation was done at 37 °C for 48 h. The non-adherent bacterial cells were removed by discarding the mixture carefully and rinsing the microtiter plate with sterile water three times. The adherent cells were fixed with 95% ethanol for 5 min before rinsing with sterile water. Aliquot of 200 μL crystal violet was added to the fixed cells for 15 min, rinsed with sterile water and air-dried. For biofilm inhibition, 150 μL of TSB was added to microtiter wells and 50 μL of OsSNPs were added. Same procedure was used for biofilm inhibition process. Quantification of the biofilm formation and inhibition were done at the absorbance of 560 nm (OD560) using ELISA reader (BIOTEX model: ELx800, Biotex Instruments, USA). Percentage of biofilm inhibition was calculated as:
| % biofilm inhibition = ODcontrol – ODtreatment / ODcontrol × 100 | (1) |
2.6. In vitro cytotoxicity assay of OsSNPs against brine shrimp
Brine shrimp lethality test for larvae nauplii was used to determine the toxicity of OsSNPs [32]. The Brine shrimp eggs hatching were done in a dish using the sea water. The active free floating phototropic nauplii were collected from bright illumination with pipette after 48 h and were used for the assay. The nauplii were dispensed into a sterile well plate contain 2 mL of sea water and suspension of yeast under illuminated condition. Different concentrations (100, 500, 1000, 2000, 3000 and 4000 μg/mL) of silver nanoparticles was added into the each wells that contains 10 nauplii and incubated at room temperature in the dark for 24 h. Sea water without silver nanoparticles was used as control. Macroscopic count of the survivors’ nauplii every 3 h for 24 h was done and percentage of mortality, LD50 for the tested concentration of OsSNPs were determined using probit analysis [33].
3. Results and discussion
The methanol extract of Oscillatoria sp. was able to bio-reduced the AgNO3 to biosynthesized OsSNPs. The greenly biosynthesized nanoparticles was characterized.
3.1. Visual Observation of the nanoparticles biosynthesis
Formation of silver nanoparticles through reduction of silver nitrate into silver ion by the reducing agent is known to be associated with colour change (Fig. 1). The reaction mixture of AgNO3 and the methanol extract of Oscillatoria sp. turned brown indicating formation of silver nanoparticles. The intensity of the colour increases after 24 h of incubation.
Fig. 1.
Visual Observation of SNPs formation: (a) Oscillatoria sp. extract; (b) Silver nitrate solution and (c) SNPs.
Silver nanoparticles formation was identified visually by colour change. The immediate colour change as observed in formation of silver nanoparticles biosynthesized using Oscillatoria sp. extract was due to the excitation of Surface Plasmon Resonance (SPR) of nanoparticles in the reaction mixture. This was also reported by Rajeshkumar et al. [23] who observed colour change in the reaction mixture during silver nanoparticles formation biosynthesized from Padina tetrastromatica.
3.2. UV-vis spectrophotometry analysis
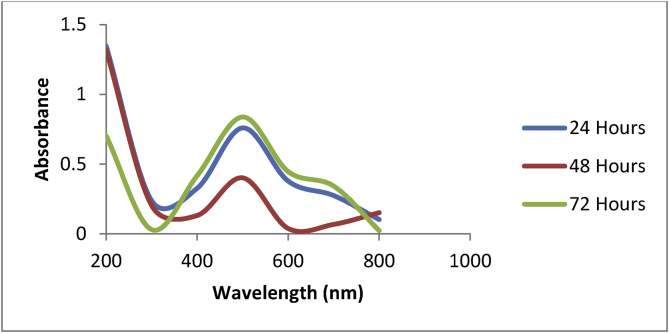
The biosynthesized OsSNPs were characterized at different incubation hours using UV-Vis Spectrophotometer. At different hours of incubation Surface Plasma Resonance (SPR) peak was observed at 500 nm and broad spectrum range was at 400–600 nm (Fig. 2). The results obtained from the UV-Vis spectra suggested the formation of silver nanoparticles.
Fig. 2.
UV-Vis spectra of silver nanoparticle biosynthesized at different hours of incubation.
3.3. Fourier transform infrared (FTIR)
The FTIR spectra of the biosynthesized OsSNPs showed 16 major bands each of which suggested the presence of different functional groups of compounds present (Fig. 2).
The UV-vis spectroscopy is a technique used to confirm the formation and stability of nanoparticles based on the optical properties of the nanoparticles and it also serves as an indirect method used to determine the reduction of silver nitrate to silver nanoparticles in the aqueous solution. The optical property of silver nanoparticles is dependent on size and shape [34]. The result obtained as shown by the UV-vis spectra of the biosynthesized silver nanoparticles was formed after 24 h of incubation, a drop in the surface Plasmon peak was observed at 48 h and maximum increase in the resonance was observed after 72 h of incubation. This shows that complete nanoparticles formation occurred after 72 h of incubation and it implies that nanoparticles formation is associated with incubation time. This is not in agreement with the work of Singh et al. [21] who reported consistent increase in the peak after 24, 48, 72, till 168 h of incubation while working on silver nanoparticles biosynthesized from Acinetobacter calcoaceticus. Mie's theory states that only a single surface plasmon resonance band is expected in the absorption spectra of spherical metal nanoparticles whereas anisotropic particles can give rise to two or more surface plasmon resonance bands, depending on the shape of the particles [35, 36]. From the UV-vis spectra of silver nanoparticles synthesized from Oscillatoria sp. methanol extract, a single SPR peak was observed which suggests that the biosynthesized silver nanoparticles are spherical in shape and this was confirmed by the scanning electron micrograph. Broadening of the surface plasmon resonance can be attributed to the electron surface scattering which can be enhanced for small aggregates [37]. Silver nanoparticles formed in such enhanced process are usually stable and their absorption spectra can be unaltered even after six months at room temperature [21].
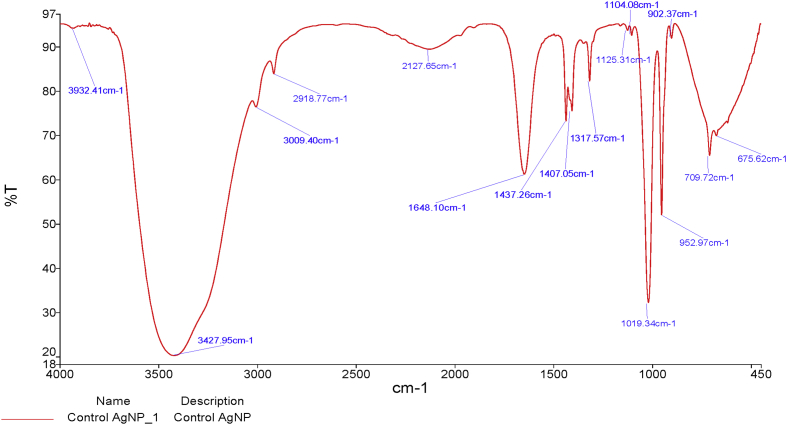
The FTIR spectrum of the nanoparticles is shown in Fig. 3. The peak at 3427.95 cm−1 was more prominent than other peaks and it showed the presence of hydroxyl (OH) stretch of alcohol. The peak 1648.10 cm−1 suggested the presence of benzene. The peaks 1019.34 cm-1and 952.97 cm−1 indicate the presence of hetero oxy compounds in which 1019.34 cm−1 is an aliphatic phosphate with P–O–C stretch while 952.97 cm−1 is an aromatic phosphate compound. However, the peak 902.37 cm−1 is also an aromatic phosphate compound but not a hetero oxy compound. Peak 709.72 cm−1 is a hydroxyl compound that was out of plane bend and peak 675.62 cm−1 indicates the presence of an aryl thioether compound with C–S stretch that is a sulphate ion.
Fig. 3.
FTIR spectrum of the biosynthesized silver nanoparticles.
3.4. Scanning electron microscopy (SEM)
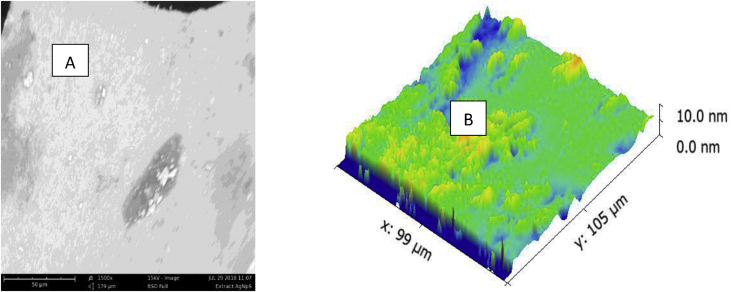
Morphological identification of the biosynthesized silver nanoparticles was determined using scanning electron microscopy. The scanning electron micrograph confirmed the shape of the biosynthesized OsSNPs to be spherical and 10 nm in size (Fig. 4).
Fig. 4.
a & b. SEM and 3D image of the biosynthesized silver nanoparticles.
3.5. Thermogravimetry (TGA)
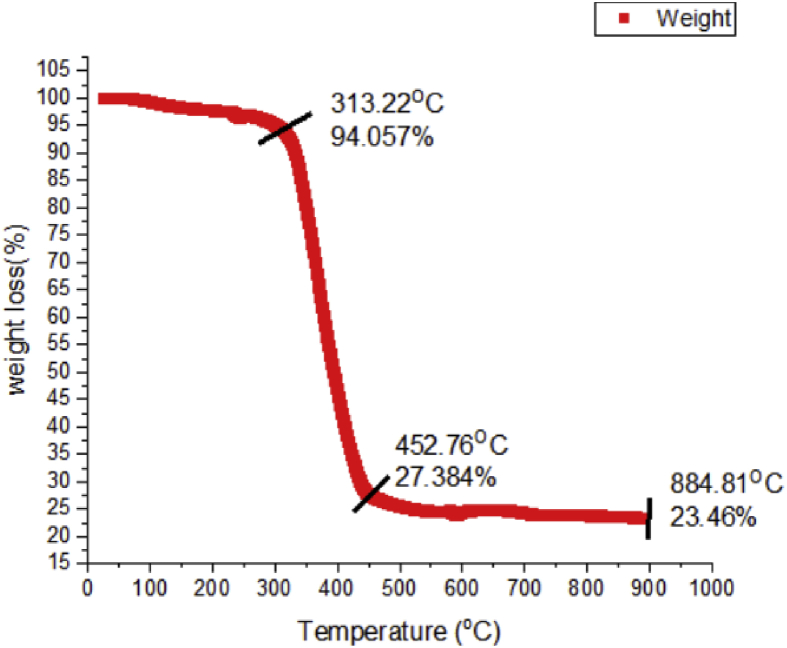
Thermal stability in relative to weight of the biosynthesized silver nanoparticles was assessed using Thermogravimetry (TGA). It was observed from the TGA curve that dominant weight loss of the SNPs of Oscillatoria sp. extract occurred in temperature region between 350 °C and 450 °C (Fig. 5). There was little weight loss below 350 °C and above 450 °C.
Fig. 5.
TGA thermograph of the biosynthesized silver nanoparticles.
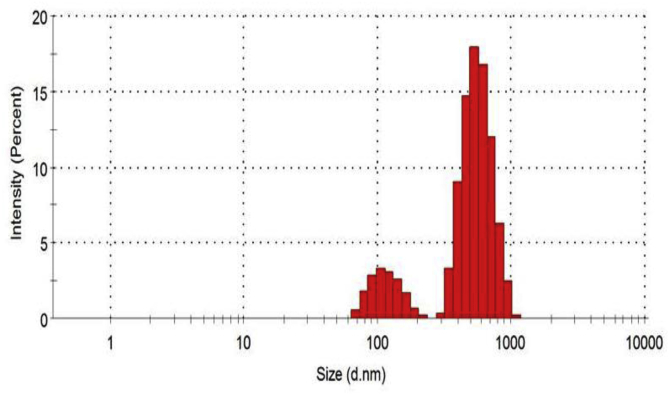
3.6. Particle size and distribution of the OsSNPs
The DLS is used to determine the diameter of nanoparticles dispersed in liquid. It also determines the size of particles and their distribution in physiological solutions [38]. The size distribution histogram of dynamic light scattering (DLS) analysis of the OsSNPs shows the particle diameter average of 0.000 nm and 558.1 nm with a polydispersity index of 0.580 (Fig. 6).
Fig. 6.
Particle size distribution of the biosynthesized SNPs by DLS.
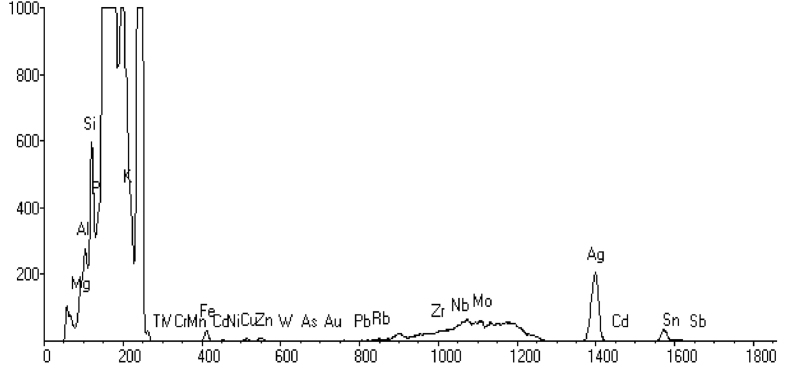
3.7. Energy dispersive X-ray analysis of the OsSNPs
The weight percentage of silver was 0.0132 from the EDX spectrum of OsSNPs (Fig. 7). The EDX analysis revealed strong signals in the silver region and confirmed the formation of silver nanoparticles.
Fig. 7.
EDX micrograph of the biosynthesized SNPs.
The micrograph showed that the biosynthesized silver nanoparticles are spherical in shape and the 3D-image revealed that the size of the nanoparticles is 10 nm. Raza et al. [38] reported the effect of size and shape of silver nanoparticles on the antibacterial activity of the silver nanoparticles and it was stated that small size spherical silver nanoparticles are more active against microorganisms than larger spherical silver nanoparticles likewise that spherical silver nanoparticles are more effective against microbes than triangular shaped silver nanoparticles.
The behavioural pattern of the silver nanoparticles biosynthesized from Oscillatoria sp. methanol extract when subjected to high temperature. It can be generally attributed to the evaporation of water and organic components. The thermograph showed the relative weight of the nanoparticles with respect to heat. The biosynthesized OsSNPs was able to withstand heat to a certain extent before the significant weight lost.
The DLS determines the size of particles and their distribution in physiological solutions [39]. Some distribution at lower range of particle size indicates that the synthesized particles are also in lower range of particle size. The size of biosynthesized SNPs obtained from DLS in this work is larger than SEM and this is in agreement with the report of Zhang et al. [40]. It was reported that the larger size of DLS than SEM is as a result of Brownian motion influence.
There were other peaks for other elements suggesting that they are mixed precipitates present in the methanol extract of Oscillatoria sp. This agrees with the work of Khan et al. [41] who also confirmed SNPs formation in plant extract and observed the presence of other elements in the plant (Ziziphus nummularia) extract used in biosynthesis of SNPs.
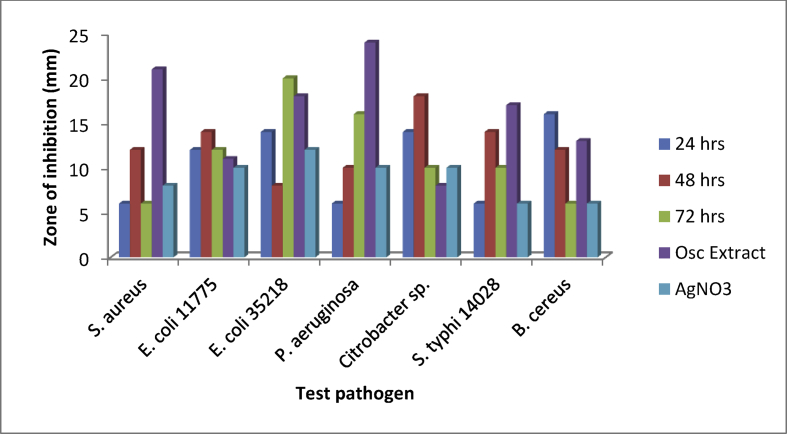
3.8. Antibacterial potential of the OsSNPs
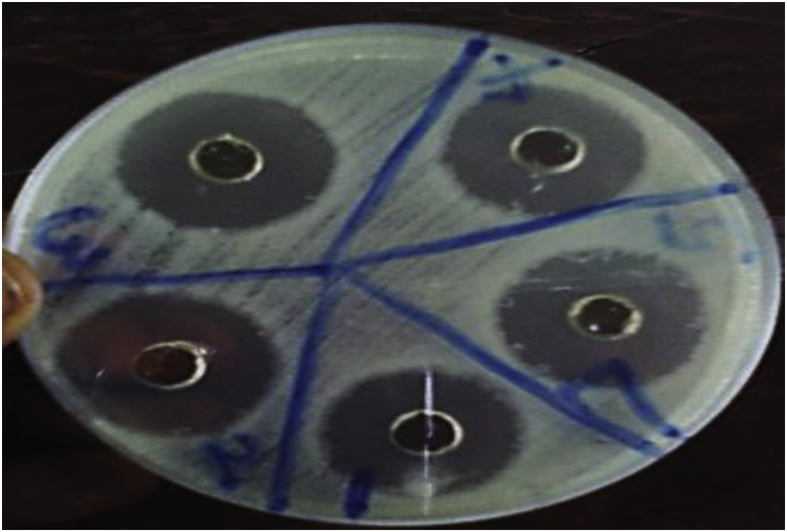
The antibacterial activity of the biosynthesized OsSNPs was tested against seven clinical pathogenic bacteria. All the test pathogens were susceptible to the nanoparticle as shown Fig. 8. At 24 and 48 h Bacillus cereus and Citrobacter sp. had the highest susceptibility with zone of inhibition 16.0 mm and 18.0 mm respectively. At 72 h Escherichia coli ATCC 35218 had the highest susceptibility to OsSNPs with 20.00 mm zone of inhibition while Bacillus cereus was least susceptible to the OsSNPs with lowest zone of inhibition (6.00 mm). Reduction in the zone of inhibition after 48 h may be due to production of enzyme by Escherichia coli ATCC 35218. The isolated has been reported as a beta lactamase producing strains [29]. Osc. sp. extract had antibacterial activity against the pathogen, the antibacterial activity ranged from 13.0 – 24.0 mm. The highest activity was against P. aeruginosa. The extract exhibited higher antibacterial activity against some of the pathogens compared to the nanoparticles and AgNO3 solution AgNO3 solution had antibacterial activity against the test pathogens. The antibacterial activity ranged from 6.0 – 12.0 mm, the highest activity was against E. coli 35218. Fig. 9 shows the antibacterial activity of the nanoparticles on the test pathogen on plates.
Fig. 8.
Antibacterial activity of the biosynthesized SNPs.
Fig. 9.
The antibacterial activity of OsSNPs against E. coli ATCC 35218.
3.9. Antibiofilm activity of the OsSNPs
3.9.1. Visual observation of biofilm formation
The biofilm formation was assessed visually by observing formation of ring at the bottom of the test tube and at the meniscus level of the test tubes after staining with the crystal violet. 90 % of the test pathogens were biofilm formers (Table 1).
Table 1.
The assessment of biofilm formation by test pathogens.
| Test pathogen | Biofilm Intensity |
|---|---|
| S. aureus ATCC 29213 | ++++ |
| E. coli ATCC 35218 | +++ |
| P. aeruginosa ATCC27853 | ++ |
| Citrobacter sp. | +++ |
| S. typhi ATCC 14028 | + |
| E. coli ATCC 11775 | + |
| Bacillus cereus | - |
Key: + = slightly formed, ++ = partially formed, +++ = formed, ++++ = strongly formed, - = not formed.
3.9.2. Antibiofilm potential of the OsSNPs
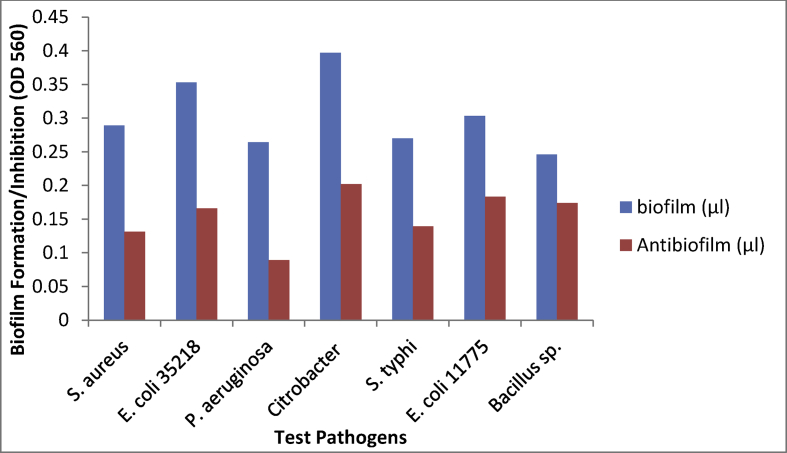
The antibiofilm activity of the biosynthesized silver nanoparticles shows reduction in the biofilm formation by all test pathogens that were used thus implied that the biosynthesized silver nanoparticles is active against bacterial biofilm. The highest inhibition was against Pseudomonas aeruginosa ATCC 27853 while the lowest inhibition was against Citrobacter sp. (Fig. 10).
Fig. 10.
Antibiofilm Activity of the biosynthesized SNPs.
The OsSNPs showed more activity than silver nitrate solution which was used as control. The mechanism of antibacterial activity of silver nanoparticles is not well understood but some mechanisms have been proposed which include the release of silver ions when silver nanoparticles come in contact with bacterial cell, these ions prevent DNA replication in such bacteria by deactivating the synthesis of enzymes that take part in DNA synthesis and ATP synthesis [15, 42]. Rai et al. [43] reported that silver nanoparticles can act as potential antimicrobial agents against different microorganisms. Raza et al. [44] reported that the antibacterial efficacy of silver nanoparticles is size and shape dependent while working on silver nanoparticles of different sizes and shapes. The antibacterial activity of silver nanoparticles from this research showed that Escherichia coli ATCC 25318 was more susceptible to the silver nanoparticles while Bacillus sp. was least susceptible to the biosynthesized silver nanoparticles. The result obtained from this research showed that the biosynthesized SNP is effective against all the selected microorganisms used in this experiment. Reduction in the zone of inhibition after 48 h may be due to production of enzyme by Escherichia coli ATCC 35218. The isolated has been reported as a beta lactamase producing strains [29].
The inhibition of biofilm formation by nanoparticles has been reported previously by Lee et al. [45], Seil and Webster, [46]. This research reports the biofilm inhibition of the biosynthesized silver nanoparticles with the highest inhibition observed in Pseudomonas aeruginosa ATCC 27853 and the least inhibition in Citrobacter sp. Lee et al. [45] in their research reported the inhibition of formation of biofilm of Pseudomonas aeruginosa by zinc oxide nanoparticles dose dependently. Vijayan et al. [47] also reported reduction in the formation of biofilm by silver nanoparticles. Here we report the reduction in biofilm formation of selected gram positive and gram negative bacteria by silver nanoparticles biosynthesized using methanol extract of Oscillatoria sp.
3.10. Cytotoxicity of OsSNPs
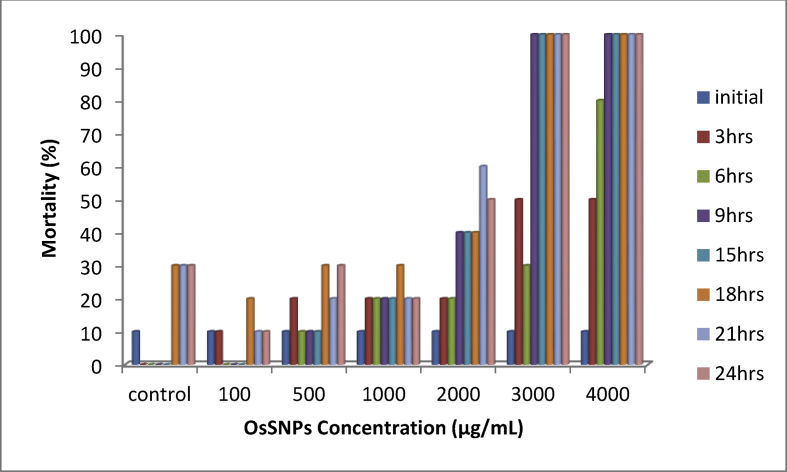
The Artemia cytotoxicity (Brine Shrimp Assay) is one of the reliable methods to screen the cytotoxicity of products [44]. It was observed that the increase in mortality rate is dose dependent (Fig. 11).
Fig. 11.
Mortality rate of Artemia salina treated with the biosynthesized SNPs.
From the result, it was observed that the increase in mortality rate is dose dependent and this is in agreement with the work of Vijayan et al. [47] who also reported same thing while working on silver nanoparticles synthesized from the extract of seaweed Turbinaria conoides. Arulvasu et al. [48] in their work on the toxicity effect of silver nanoparticles in brine shrimp Artemia reported that Artemia possesses nonselective filter feeding behaviour which enables them to feed on all silver nanoparticles that are less than 50 μm in size. They also reported that the aggregation amount of silver nanoparticles in the gut of the Artemia is dependent on the concentration amount of nanoparticles and the amount of nanoparticles consumed by the Artemia. Silver nanoparticles biosynthesized in this work are less than 50 microns as confirmed by the SEM result thus suggesting the aggregation of the biosynthesized SNPs in the gut of the Artemia thereby leading to the mortality rate obtained in this work.
Methanol extract of Oscillatoria sp. contain some bioactive phytochemicals with some unique functional groups which act as capping and stabilizing agent for AgNO3 bio-reduction for silver nanoparticles biosynthesis.
4. Conclusion
This present research has been able to biosynthesize silver nanoparticles in an eco-friendly manner using methanol extract of Oscillatoria sp. The nanoparticles were thermostable to an extent, spherical shape of 10 nm size and exhibited a strong antibacterial and antibiofilm activity against the test pathogens and low cytotoxicity against brim shrimp. This makes it a promising nanomaterial to be used in any nanoproduct as an antibacterial agent in the production of heat resistant nanoproducts and in the production of medical devices such as nanocatheters among others.
Declarations
Author contribution statement
Bukola Adebayo-Tayo: Conceived and designed the experiments, Analyzed and interpreted the data, Wrote the paper.
Adebayo Salaam: Conceived and designed the experiments, Contributed reagents, materials, Analysis tools or data.
Adebola Ajibade: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kesarwani S., Tandon R., Tiwari G.L. The genus Oscillatoria Vaucher (Cyanoprokaryota) from India. J. Phycol. Soc. India. 2015;45(1):18–29. [Google Scholar]
- 2.Conlo B.P., Rowe S.E., Lewis K. Persister cells in biofilm associated infections. Biofilm-Based Healthcare-Assoc. Infect. 2015;1–9 doi: 10.1007/978-3-319-09782-4_1. Sprng. [DOI] [PubMed] [Google Scholar]
- 3.Salas-Orozco M., Niño-Martínez N., Martínez-Castañón G. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: a literature review. J. Nanomater. 2019:1–11. [Google Scholar]
- 4.Ayrapetya M., Williams T.C., Oliver J.D. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 2015;23(1):7–13. doi: 10.1016/j.tim.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Lewi K. Persister cells. Annu. Rev. Microbiol. 2010;64(1):357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 6.Cohen M.L. Nanotubes, nanoscience, and nanotechnology. Math. Sci. Eng. 2001;15(1-2):1–11. [Google Scholar]
- 7.Rajabi S., Ramazani A., Hamidi M. Artemia salina as a model organism in toxicity assessment of nanoparticles. J. Pharm. Sci. 2015;2:20. doi: 10.1186/s40199-015-0105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samanta H.S., Da R., Bhattachajee C. Influence of nanoparticles for wastewater treatment: a short review. Austin Chem. Eng. 2016;3(3):1036. [Google Scholar]
- 9.Takeshima T., Tada Y., Sakaguchi N. DNA/Ag nanoparticles as antibacterial agents against gram-negative bacteria. Nanomaterials. 2015;5:284–297. doi: 10.3390/nano5010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikalje A.P. Nanotechnology and its application in medicine. Med. Chem. 2015;5:81–89. [Google Scholar]
- 11.Takeshima T., Tada Y., Sakaguchi N., Watari F., Fugetsu B. DNA/Ag nanoparticles as antibacterial agents against gram-negative bacteria. Nanomaterials. 2015;5:284–297. doi: 10.3390/nano5010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massironi A., Morelli A., Grassi L. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019;203:310–321. doi: 10.1016/j.carbpol.2018.09.066. [DOI] [PubMed] [Google Scholar]
- 13.Prasad T.N., Elumalai E.K. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011;1(6):439–442. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair B., Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Grth. Des. 2002;(4):293–298. [Google Scholar]
- 15.Agnihotri S., Mukherji S., Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5 – 100 nm using the same protocol and their antibacterial efficacy. Res. Adv. 2014;2014:3974–3983. [Google Scholar]
- 16.Franci G., Falang A., Galdiero S. Silver nanoparticles as potential antibacterial agents. Mol. 2015;20(5):8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jana S., Pal T. Synthesis, characterization and catalytic application of silver nanoshell coated functionalized polystyrene beads. J. Nanosci. Nanotechnol. 2007;7:2151–2156. doi: 10.1166/jnn.2007.785. [DOI] [PubMed] [Google Scholar]
- 18.Sharma G., Jasuja N.D., Rajgovind R. Synthesis, characterization and antimicrobial activity of Abelia grandiflora assisted AgNPs. J. Microb. Biochem. Technol. 2014;6(5):274–278. [Google Scholar]
- 19.Feng Q.L., Wu J., Chen G.Q. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Choi O., Deng K.K., Kim N.J. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008;42:3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Singh R., Wagh P., Wadhwani S. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013;8:4277–4290. doi: 10.2147/IJN.S48913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firdhouse M.J., Lalitha P. Green synthesis of silver nanoparticles using the aqueous extract of Portulaca oleracea (L.) Asian J. Pharmaceut. Clin. Res. 2012;6(1):92–94. [Google Scholar]
- 23.Rajeshkumar S., Malarkodi C., Venkat-Kumar S. Synthesis and characterization of silver nanoparticles from marine Brown seaweed and its antifungal efficiency against clinical fungal pathogens. Asian J. Pharmaceut. Clin. Res. 2017;10(2):190–193. [Google Scholar]
- 24.Ashokkumar T., Vijayaraghavan K. Brown seaweed-mediated biosynthesis of gold nanoparticles. J. Environ. Biotech. Res. 2016;2(1):45–50. [Google Scholar]
- 25.Bhat R., Deshpande R., Ganachari S.V. Photo-irradiated bio-synthesis of silver nanoparticles using edible mushroom Pleurotus florida and their antibacterial activity studies. Bioinorgan. Chem. Appl. 2011;36:168–171. doi: 10.1155/2011/650979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivashankar M., Premkumari B., Chandan N. Biosynthesis, partial characterization and antimicrobial activities of silver nanoparticles from Pleurotus species. Intl. J. Integr. Sci. Innov. Technol. 2013;2(3):13–23. [Google Scholar]
- 27.Meier-Kolthoff J.P., Hahnke R.L., Petersen J., Scheuner C., Michael V., Fiebig A., Rohde C., Rohde M., Fartmann B., Goodwin L.A., Chertkov O., Reddy T.B.K., Pati A., Ivanova N.N., Markowitz V., Kyrpides N.C., Woyke T., Göker M., Klenk H.P. Complete genome sequence of DSM 30083T, the type strain (U5/41T) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genomic. Sci. 2014;9:2. doi: 10.1186/1944-3277-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards . National Committee for Clinical Laboratory Standards; Wayne, Pa: 1997. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. NCCLS publication no. M7-A4. [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards . National Committee for Clinical Laboratory Standards; Wayne, Pa: 1997. Performance Standards for Antimicrobial Disk Susceptibility Tests. NCCLS publication no. M2-A6. [Google Scholar]
- 30.Prabhu S.S., Mohan R.K., Sanhita P. Production of bacteriocin and biosynthesis of silver nanoparticles by lactic acid bacteria isolated from yoghurt and its antibacterial activity. Scrut. Int. Res. J. Microbiol. Biotechnol. 2014;1(3):7–14. [Google Scholar]
- 31.Alves E., Faustino M., Neves M.G., Tome J., Almeida A. An insight on bacterial cellular targetsof photodynamic inactivation. Future Med. Chem. 2014;6:141–164. doi: 10.4155/fmc.13.211. [DOI] [PubMed] [Google Scholar]
- 32.Finney D.J. third ed. Cambridge University press; Camb Lond: 1971. Probit Analysis. [Google Scholar]
- 33.Sosa I.O., Noguez C., Barrera R.G. Optical properties of metal nanoparticles with arbitrary shapes. J. Phys. Chem. 2003;107(26):6269–6275. [Google Scholar]
- 34.He R., Qian X., Yin J. Preparation of polychrome silver nanoparticles in different solvent. J. Mater. Chem. 2002;1:3783–3786. [Google Scholar]
- 35.McLaughlin J.L., Chang C., Smith D.L. Simple bench-top bioassays (brine-shrimp and potato discs) for the discovery of plant antitumour compounds. In: Kinghorn A.D., Balandrin M.F., editors. Human Medicinal Agents from Plants. Am. Chem. Soc.; Washington DC: 1993. pp. 112–137. [Google Scholar]
- 36.Novak J.P., Feldheim D.L. Assembly of phenylacetylene bridged silver and gold nanoparticle arrays. J. Am. Chem. Soc. 2000;12:3979–3980. [Google Scholar]
- 37.Link S., El-Sayed M.A. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003;54:331–366. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- 38.Raza M.A., Kanwal Z., Rauf A. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials. 2016;6:74. doi: 10.3390/nano6040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan F.A., Zahoor M., Jalal A. Green synthesis of silver nanoparticles by using Ziziphus nummularia leaves aqueous extract and their biological activities. J. Nanomater. 2016:1–8. [Google Scholar]
- 40.Murdock R.C., Braydich-Stolle L., Schrand A.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 2008;101:239–253. doi: 10.1093/toxsci/kfm240. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X., Liu Z., Shen W. Silver nanoparticles: synthesis, Characterization,Properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pal S., Tak Y.K., Song J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.H., Kim Y.G., Cho M.H. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol. Res. 2014;169:888–896. doi: 10.1016/j.micres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Seil J.T., Webster T.J. Reduced Staphylococcus aureus proliferation and biofilm formation on zinc oxide nanoparticle PVC composite surfaces. Acta Biomater. 2011;7:2579–2584. doi: 10.1016/j.actbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Gutierrez F., Boegli L., Agostinho A. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling. 2013;29(6):651–660. doi: 10.1080/08927014.2013.794225. [DOI] [PubMed] [Google Scholar]
- 47.Vijayan S.R., Santhiyagu P., Singamuthu M. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014:1–10. doi: 10.1155/2014/938272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arulvasu C., Jennifer S., Prabhu D. Toxicity effect of silver nanoparticles in Brine Shrimp Artemia. Sci. World J. 2014:1–10. doi: 10.1155/2014/256919. [DOI] [PMC free article] [PubMed] [Google Scholar]