Abstract
Objectives
This study examined the antibacterial and antibiofilm properties of conventional glass-ionomer cement (GIC) modified by the addition of magnesium oxide (MgO) nanoparticles.
Materials and methods
MgO nanoparticles were characterised by XRD, FTIR, and SEM analysis and tested for its activity against Streptococcus mutans and Streptococcus sobrinus. MgO nanoparticles were incorporated into GIC powder (Ketac Molar Easymix) at different concentrations and the antibacterial and antibiofilm activity was evaluated using agar disk diffusion and biofilm-CFU counting assays. ANOVA and Tukey's post hoc tests were used for the analysis, and the level of significance was set at p < 0.05.
Results
MgO nanoparticles showed antibacterial activity against both microorganisms (MIC = 500 μg/ml and MBC = 1000 μg/ml). A significant difference in the zones of inhibition was detected (p < 0.005). The effect was evident in the 2.5% MgO nanoparticle modified GIC while the CFU counting biofilm assay showed the effect of the added nanoparticles from 1% with a significant difference between the tested material groups (p < 0.005).
Conclusions
The MgO nanoparticle modified GIC showed effective antibacterial and antibiofilm activity against two cariogenic microorganisms and could be considered for further development as a biocompatible antibacterial dental restorative cement.
Keywords: Dentistry, Materials science, Glass-ionomer cements, Dental caries, Antibacterial material, Magnesium oxide nanoparticles, Streptococcus mutans, Nanoparticles, Antimicrobial, Biofilms, Dental materials
1. Introduction
Dental caries is a dietary, biofilm-induced oral disease with Streptococcus mutans contributing a significant role in the development of cariogenic biofilms [1] and the reduction in the bacterial load of the oral cavity is an essential aspect in the prevention of the disease [2]. The use of some antimicrobial compounds, such as chlorhexidine, and fluoride-containing products have resulted in a decline in the prevalence of dental caries [3, 4]. However, it is essential to note that some reports suggested that fluoride may not be very effective [5], and the development of bacterial resistance with the effect of antibiotics and chemical bactericides on the normal microbial flora of the digestive tract may limit their application in developing new antimicrobial dental materials [6]. In atraumatic restorative treatment (ART), as well as the traditional treatment of dental caries, microorganisms may be left in the residual carious dentine, can survive under the filling, and can cause recurrent caries that will eventually cause the failure of the restoration [7]. Furthermore, the modern scientific procedure involving only the removal of the infected or softened dentine from the carious lesion is sufficient to decrease the risk of pulp exposure, arrest the carious process, and maintain pulp vitality [8]. An alternative restorative treatment procedure may be beneficial when circumstances do not permit traditional restoration treatment such as for infants, children, adolescents, elderly patients, and those with special care needs [9].
Mutans streptococci are the primary causative microorganisms for dental caries. Streptococcus mutans and Streptococcus sobrinus are the two most commonly isolated species from the human oral cavity, and these microorganisms are recognised as the main cariogenic micro-organisms [10, 11, 12]. Among both microorganisms, Streptococcus mutans is the most common, and Streptococcus sobrinus occurs in approximately 10%–14% of carious lesions [13]. It has been reported that the latter is associated with the severest form of clinical dental caries, specifically in children [14].
Glass-ionomer cement, created by A.D Wilson et al. in 1969 [15], has been clinically used in many forms because of its desirable properties in dentistry [16]. GIC is a tooth-coloured, water-based cement that is set by an acid-base reaction between aluminosilicate glass powder and polyacrylic acid [17]. It has excellent biocompatibility [18], is adhesive to natural tooth structures [19], has fluoride release and rechargeability properties [20], and is better than resin composite materials for the remineralisation of caries affected dentin [21]. Glass-ionomer cement is the preferable material for restorations performed according to minimally invasive dentistry, including ART, as it can be applied in both the very early and late stages of caries development [22]. Although GICs have fluoride-releasing capabilities, evidence suggests that the fluoride released from GICs is not adequate over a long time duration [23]. Enhancing the antibacterial potential of glass-ionomer cement with the addition of antimicrobial substances is of a therapeutic advantage and may contribute to the elimination of any residual microorganisms underneath the restorative cement [24].
An increasing number of studies recently reported that nanomaterials can provide novel preventive and therapeutic strategies for dental caries, notably for the reduction and control of dental plaque biofilms, improving the antibacterial properties of dental materials, and remineralisation of initial dental caries lesions [25]. Magnesium oxide (MgO) nanoparticles have attracted interest for use in biomedical applications due to their antimicrobial properties [26]. They are reported to have antimicrobial activity against both Gram-positive and Gram-negative bacteria, spores, and viruses, and can be prepared from economic precursors [27]. Recent studies reported the antibacterial effect and biofilm formation of chemically synthesised magnesium oxide nanoparticles and Zein-based magnesium oxide nanoparticles on some oral microorganisms, including Streptococcus mutans [28, 29]. This paper explores the antibacterial and antibiofilm activity of MgO nanoparticle modified glass-ionomer cement against Streptococcus mutans and Streptococcus sobrinus.
2. Materials and methods
2.1. Materials
Magnesium oxide nanoparticles (20 nm) were acquired from Nanjing Nanotechnology Co. (Nanjing, China). X-ray powder diffraction (XRD) was used to investigate the structure of the nanoparticles via an X-ray diffractometer (PANalytical X'Pert PRO, Malvern Panalytical Ltd, Malvern, UK) operating at 45 kV with a current of 40 mA using CuKα radiation (λ = 1.5406Å) in the range of 30∘-90∘. FTIR spectrophotometry was recorded on a PerkinElmer spectrometer (PerkinElmer Inc., Waltham, MA, USA) in the range of 400–4000 cm−1. The morphology and dispersion of the MgO nanoparticles were analysed via a CS 3200 LV CamScan scanning electron microscope (CamScan Electron Optics Ltd, Waterbeach, UK) accelerated at 25 kV. The GIC used was 3M ESPE Ketac Molar Easymix Glass Ionomer Filling Material A3 Shade (3M Deutschland GmbH, Neuss, Germany) in a powder/liquid form. Chlorhexidine diacetate powder (C6143 Sigma-Aldrich, St. Louis, MO, USA) was used as the positive control additive material.
2.2. Bacterial strains and culture conditions (isolation and identification of the mutans streptococci species)
The isolation and identification of the mutans streptococci species were performed according to a protocol developed and reported by Villhauer et al. (2017). Briefly, the protocol includes preliminary identification based on colony morphology on selective agar media (SB-20M) followed by polymerase chain reaction (PCR) confirmation for species identification using primers targeting specific regions of the glucosyltransferase (gtf) genes of Streptococcus mutans and Streptococcus sobrinus. This method of isolation and identification is reported to be highly accurate and more rapid than previous methods [30]. The primer sets selected were reported by Oho et al. (2000). They performed better with minimal false positive or false negative results [30]: Streptococcus mutans (gtfB-F:5′-ACTACACTTTCGGGTGGCTTGG-3′ and gtfB-R:5′-CAGTATAAGCGCCAGTTTCATC-3′) with a 517 bp amplicon size and a 55 °C annealing temperature and Streptococcus sobrinus (gtfI-IN-F:5′-TGGTATCGTCCAAAATCAATCC-3′ and gtfI-IN-R:5′-AGATTTGCAGTTGGTCAGCATC-3′) with a 664 bp amplicon size and a 55 °C annealing temperature [31].
2.3. Determination of the antibacterial activity of the MgO nanoparticles
The minimum inhibitory concentration (MIC) is the lowest concentration of each antimicrobial agent that inhibits the growth of the microorganisms being tested and is detected by a lack of turbidity matching against a negative control. The minimum bactericidal concentration (MBC) is defined as the lowest concentration of an agent killing the majority of bacterial inoculums [32]. The MIC was determined through a broth dilution method. The MBC was determined by culturing all clear (negative) tubes through serially diluting 100 μl in a 9.9 ml broth to 10−3 fold and plating a 100 μl volume from this dilution on BHI agar (incubated for 24 h), then observing for a <50 CFU/ml colony count (that is, a 99.9% reduction in the bacterial count).
2.4. Antibacterial activity testing for GIC (agar disk diffusion assay)
Glass-ionomer cement disks were prepared using PTFE moulds (7 mm in diameter and 1 mm thick). MgO nanoparticles were added to the glass-ionomer powder, and the powder/liquid ratio was maintained per the manufacturer's instructions (P/L ratio: 2.9:1). A total of 120 specimens were prepared (10 disks for each group of material per microorganism tested). The positive control group was prepared by mixing chlorhexidine diacetate powder at 1% (w/w) to the GIC powder. A 1% concentration of chlorhexidine diacetate was selected based on results reported that the incorporation of 1% CHX diacetate is the optimal concentration to provide appropriate antibacterial properties without compromising the physical and bonding properties of the glass-ionomer cement [24]. The MgO nanoparticles were added to the GIC powder at 0%, 1%, 2.5%, 5%, and 10% concentrations.
Antibacterial activity was determined via an agar disk diffusion method using BHI agar. After the 24-h incubation period, suspensions of McFarland 0.5 were prepared in a standard saline solution (0.85% NaCl). Using a sterile swab, a 100 μl aliquot was spread onto BHI agar plates and left to dry at room temperature for 30 min. Then the GIC specimens were placed onto a BHI agar plate inoculated with each bacterial strain. After the agar plates were incubated at 37 °C for 48 h, the diameters of the zones of inhibition produced around the specimens were measured using a digital calliper at three different points. The sizes of the inhibition zones around the GIC disks were calculated by subtracting the diameter of the specimen (7 mm) from the average of three measurements of the halo around the GIC disks [24].
2.5. GIC biofilm assessment through the colony-forming unit (CFU) counts
Six GIC disks (7 mm in diameter and 1 mm thick) per group per microorganism were used for the biofilm assessment. The GIC discs were incubated in a test tube containing 1 ml of 108 CFU/ml. After 3 days of incubation, the disks were removed, gently dipped into sterile PBS, and transferred into Eppendorf tubes with 1 mL of BHI broth. The biofilms were detached, dispersed, and harvested by vortexing for 5 min at the maximum speed. The resulting bacterial suspensions were serially diluted and plated on BHI agar plates and then incubated for 24–48 h. The total CFU/mL on each disk was calculated from the number of colonies along with the dilution factor [33].
2.6. Statistical analysis
The statistical analysis was performed using SPSS software (IBM SPSS Statistics Version 16), and the level of significance was set at p < 0.05. The GIC agar disk diffusion and CFU biofilm counting assay data were submitted for an analysis of the distribution and homoscedasticity using the Shapiro-Wilk test. The reported data were presented as means and standard deviations. One-way ANOVA and Tukey's HSD post hoc test were used for the analysis of the glass-ionomer disk diffusion and the CFU/mL counting of the biofilm assay. The CFU/mL values were log-transformed (base 10) for the statistical analysis. The results of the MIC and MBC for the MgO nanoparticles were only expressed numerically without analysis.
3. Results
3.1. MgO nanoparticle characterisation and antibacterial activity
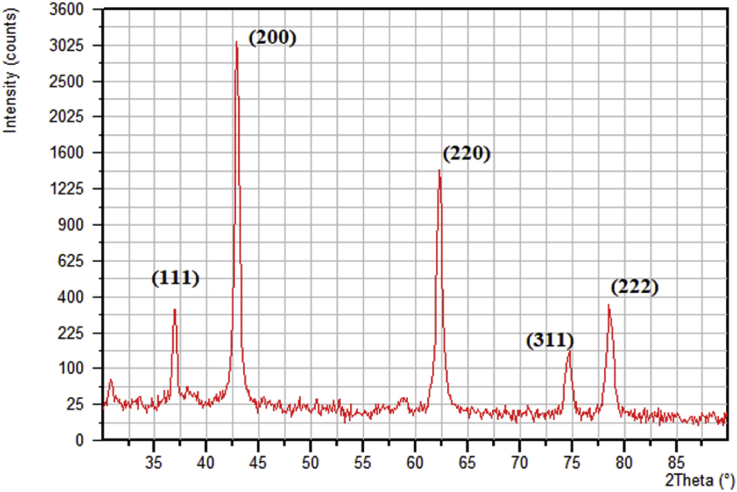
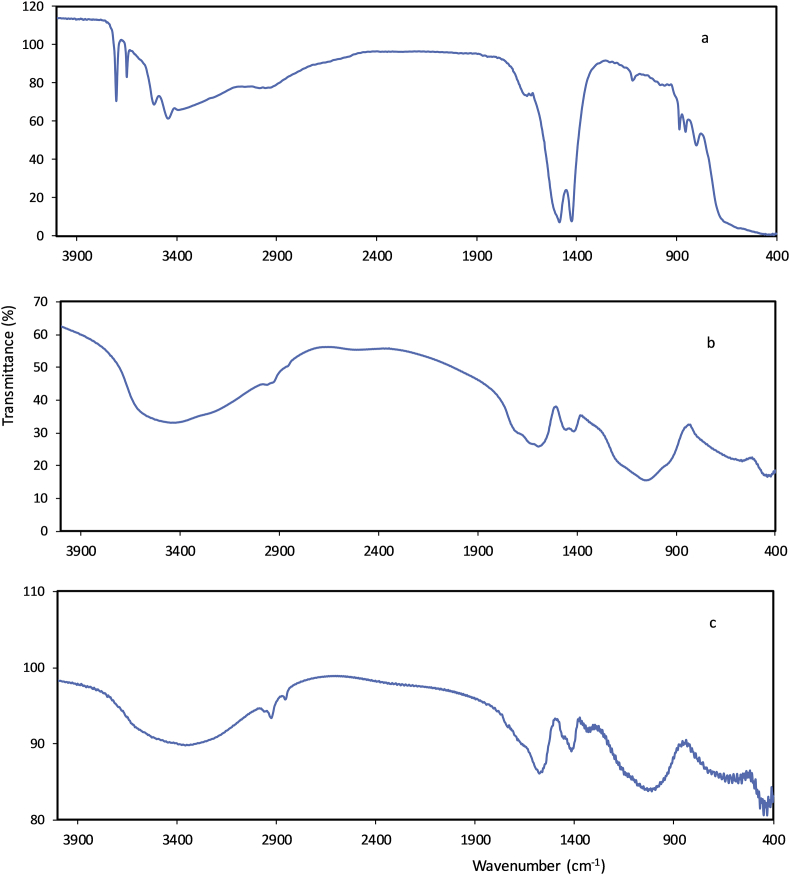
The MgO nanoparticle powder was a fine white powder, and the MgO nanoparticle suspensions had an alkaline pH (pH = 10.6). The powder XRD pattern of the MgO nanoparticles is shown in Fig. 1. All of the diffraction peaks matched well with the face-centred cubic structure of periclase MgO (JCPDS No. 87-0653). The major peaks at 2θ values of 36.9°, 42.9°, 62.3°, 74.8°, and 78.6° were indexed to the lattice planes of (111), (200), (220), (311), and (222), respectively. The sharp diffraction peaks perfectly matched the crystalline structure of the MgO with high purity [34]. The average nanoparticle size was calculated from the diffraction peaks using the Debye-Scherrer equation [35], and it was approximately 20.8 nm. SEM images of the MgO nanoparticles are shown in Fig. 2. White spot agglomerates of the MgO nanoparticles were due to the hygroscopic nature of the material [35]. The Fourier transform infrared (FTIR) spectra of the MgO nanoparticles are shown in Fig. 3a. The observed bands in the 3400-3700 cm−1 range were due to the O-H stretching bond vibrations from the absorbed water molecules [36]. The peaks at 1424 cm−1 and 1485 cm−1 were attributed to stretching of the carbonate ion and CO32- species and the bending vibration of the water molecules [37]. Bands at 581 cm−1, 850 cm−1, and 890 cm−1 corresponded to v1 and v2 stretching vibrations of the metal-oxygen bond [38, 39], which corresponded to the presence of the MgO nanoparticles [35]. The FTIR spectra of both the control GIC and MgO nanoparticles modified GIC are shown in Fig. 3b and c. The v1 and v2 stretching vibrations of the metal oxides were present in the FTIR spectra of the modified GIC, indicating successful embedding of the nanoparticles in the GIC matrix (Fig. 3c). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for both microorganisms were determined at 500 μg/ml and 1000 μg/ml, respectively.
Fig. 1.
Powder X-ray diffraction (XRD) pattern of the MgO nanoparticles.
Fig. 2.
SEM image of the MgO nanoparticles.
Fig. 3.
FTIR spectra of the (a) MgO nanoparticles, (b) GIC, and (c) MgO modified GIC.
3.2. Antibacterial activity of the MgO modified GIC
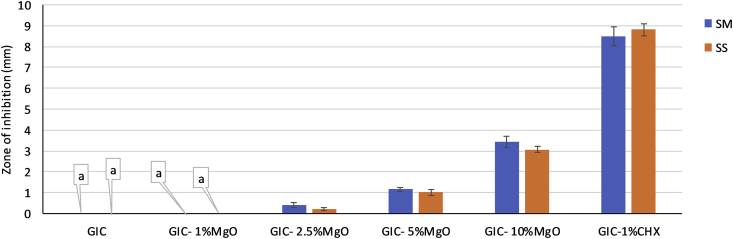
The data were normally distributed. One-way ANOVA (Table 1) followed by the post-hoc Tukey honest significant difference (HSD) test showed that the produced size of the inhibition zones differed significantly among all of the experimental groups for both of the tested microorganisms (p < 0.000) (Fig. 4). The mean values (mm) and standard deviations of the growth inhibition zones for the control and experimental groups are shown in Fig. 4 for both Streptococcus mutans and Streptococcus sobrinus. GIC alone and GIC with 1% MgO nanoparticles exhibited no inhibition zones. However, the size of the inhibition zones was dependent upon the amount of MgO nanoparticles incorporated into the experimental GIC. The size of the inhibition zones of Streptococcus mutans was slightly larger than those of Streptococcus sobrinus, and GIC with 1% chlorhexidine (positive control) had the largest inhibition zones.
Table 1.
Results of one-way analysis of variance (ANOVA) for the size of the inhibition in the agar disk diffusion test.
| Size of inhibition | Sum of squares | DF | Mean square | F-value | Significance (p-value) | |
|---|---|---|---|---|---|---|
| Streptococcus mutans | Between groups | 557.111 | 5 | 111.422 | 2073 | 0.000 |
| Within groups | 2.903 | 54 | 0.054 | |||
| Total | 560.014 | 59 | ||||
| Streptococcus sobrinus | Between groups | 526.516 | 5 | 105.303 | 4483 | 0.000 |
| Within groups | 1.268 | 54 | 0.023 | |||
| Total | 527.785 | 59 | ||||
Fig. 4.
The mean values (standard deviation) of the zone of inhibition in the agar disk diffusion test for all of the groups (groups with the same small letter callouts show no statistical difference using Tukey's HSD test (p > 0.05). SM: Streptococcus mutans, SS: Streptococcus sobrinus.
3.3. GIC biofilm assessment through the colony-forming unit (CFU) counts
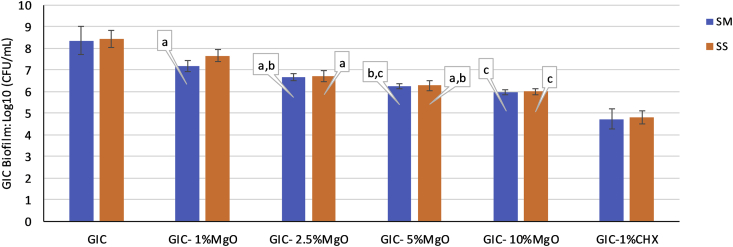
The log10 (CFU/mL) values were normally distributed and the one-way ANOVA test analysis (Table 2) followed by the post-hoc Tukey honest significant difference (HSD) test demonstrated significant differences in the number of adhered bacterial cells between the original GIC material and the experimental groups (p < 0.000) (Fig. 5). The mean values and standard deviations of the log10 (CFU/mL) values for the tested GIC groups are summarised in Fig. 5. The unmodified GIC cement had the highest number of adhered cells in the biofilm that formed on the disks. Some differences occurred between the experimental groups with different nanoparticle concentrations for both of the microorganisms, and the number of adhered cells decreased with the increasing nanoparticle content in the GIC as shown in Fig. 5.
Table 2.
Results of one-way analysis of variance (ANOVA) for the log10 (CFU/mL) values in the GIC biofilm test.
| Log10 (CFU/mL) | Sum of squares | DF | Mean square | F-value | Significance (p-value) | |
|---|---|---|---|---|---|---|
| Streptococcus mutans | Between groups | 44.446 | 5 | 8.889 | 71.530 | 0.000 |
| Within groups | 3.728 | 30 | .124 | |||
| Total | 48.174 | 35 | ||||
| Streptococcus sobrinus | Between groups | 48.515 | 5 | 9.703 | 123.552 | 0.000 |
| Within groups | 2.356 | 30 | .079 | |||
| Total | 50.871 | 35 | ||||
Fig. 5.
The mean values (standard deviation) of the log10 (CFU/mL) values in the GIC biofilm test for all of the groups (groups with the same small letter callouts show no statistical difference using Tukey's HSD test (p > 0.05). SM: Streptococcus mutans, SS: Streptococcus sobrinus.
4. Discussion
The field of GICs is an important topic of interest in healthcare because of their unique properties, especially controlled antibacterial release behaviour [16]. One of the fundamental concepts for long-lasting dental materials is to control the growth of microorganisms, such as bacteria [16, 40]. Although developed primarily for use in less-developed regions, ART is also applied within the minimum intervention dentistry approach in dental clinics in other countries [41, 42]. Early caries lesions remain challenging for dental research and public health, particularly for individuals with a high risk of developing caries [25]. A biocompatible antibacterial glass-ionomer cement is a primary required material for dental practitioners endorsing minimum intervention dentistry and alternative restorative technique philosophy in their daily practise routine.
The application of nanoparticles as local preventive measures against cariogenic microorganisms has attracted increasing interest over the last two decades, and targeted therapy and reduced adverse effects are the advantages of local drug delivery in dental caries prevention and treatment [43]. Several studies reported the potential role of nanomaterials in dentistry, but their biosafety remains a significant concern for the medical field and the accumulation of nonbiodegradable nanoparticles in different body organs, including the brain, can result in unpleasant effects in the biological tissues [43]. In this context, the application of nanoparticles with safe and biocompatible degradable compounds may be of more significant benefit. Magnesium oxide nanoparticles have the advantage of nontoxicity, high thermal stability, biocompatibility, and low cost of production [44]. MgO nanoparticles are superior to other metal oxide nanoparticles due to their biocompatibility and degraded by-products and have been recognised as harmless materials by the US Food and Drug Administration (21CFR184.1431) and are attracting increased interest for biomedical applications (such as implants, bone surgery, antimicrobial agents, etc.) due to their biodegradable, nontoxic products (namely magnesium ion), which is an essential element in the human body [45].
Nanoparticles can affect microorganisms in numerous ways, and the microbes are less likely to develop resistance against nanoparticles because most of the antibiotic resistance mechanisms are irrelevant for nanoparticles [46]. Along with membrane disruption and reactive oxygen species generation, MgO nanoparticles also inhibit the essential enzymes of bacteria [47]. The high pH (alkaline pH) of MgO nanoparticles may also play a role in their antibacterial action [48]. The alkaline characteristic of MgO nanoparticle suspensions may be of interest for combating the acidogenic microorganisms responsible for the development of dental caries and may also favour the surrounding environment for enamel remineralisation [49].
Recent studies in the combat against dental caries focus on both Streptococcus mutans and Streptococcus sobrinus and their biofilms, as they play crucial roles in the initiation and development of dental caries [50, 51]. The minimal inhibitory concentration for MgO nanoparticles was found to be 500 μg/mL against both Streptococcus mutans and Streptococcus sobrinus, and similar results were also reported against Escherichia coli [26]. Previous studies with different research methods and in different application situations reported varying degrees of antibacterial and antibiofilm activity of MgO nanoparticles against only Streptococcus mutans [28, 29]. In our results, the MgO nanoparticles showed similar antimicrobial activity against both Streptococcus mutans and Streptococcus sobrinus. Although Streptococcus mutans is the most common species studied, several studies reported that the presence of both species together is associated with higher prevalence and higher caries activity in children [13, 14]. Microorganisms in the biofilm are more resistant to antimicrobial agents; for example, biofilms can survive antimicrobial agent levels up to 1000 times the concentration required to kill planktonic microorganisms [52]. Hence, it is crucial that any antibacterial agent developed to combat cariogenic microorganisms should be able to pose its effect, not only on the planktonic cells but also on the cells in a biofilm status.
In vitro studies reported that the release of minimal amounts of fluoride from GIC is not sufficient to prevent biofilm formation around the filling material, possibly due to the low amount of fluoride ions released form these materials [53]. Hence, there is a need for an alternative biocompatible additive that has the potential to enhance the antibacterial effects of GIC without compromising the physical properties [54]. Protective mechanisms of GICs from biofilm attacks can be improved through several strategies including enhancing fluoride release and the addition of other antimicrobial substances [55].
Previous studies have tried to improve the antibacterial properties of glass-ionomer cement by adding antimicrobial substances such as propolis, chlorhexidine, Salvadora persica (miswak) extracts, casein phosphopeptide-amorphous calcium phosphate, nanoparticles, and antibiotics to GICs [54, 56, 57]. Within the limitations of this study, the results showed that the addition of MgO nanoparticles could enhance the antibacterial and antibiofilm properties of GIC material and the magnitude of the effect is dependent on the percentage of the nanoparticles added. Due to the biocompatible nature of MgO nanoparticles and their degradable by-products, MgO nanoparticles could be excellent candidates for further research and development of GICs and other preventive and restorative dental materials. Nevertheless, its crucial to investigate other properties that are required for any clinically applied dental restorative material (for example, the setting time, mechanical and adhesive properties, biocompatibility of the mixture of nanoparticles with GIC, etc.).
5. Conclusions
MgO nanoparticles are promising antibacterial agents for dental applications due to their unique properties, including antibacterial activity against cariogenic microorganisms. MgO nanoparticle modified GIC showed effective antibacterial and antibiofilm activity against cariogenic microorganisms.
Declarations
Author contribution statement
Arass J. Noori: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fadil A. Kareem: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors extend their gratitude to the technical support and advice provided by the laboratory staff of both the Microbiology Lab (QC Department at Pioneer Co. for Pharmaceutical Industries, Sulaymaniyah, Iraq) and MegaGene Lab (Harem Private Hospital, Sulaymaniyah, Iraq).
References
- 1.Jeon J.-G.G., Rosalen P.L., Falsetta M.L., Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 2011;45:243–263. doi: 10.1159/000327250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassel M.O., Khattab M.A. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 2017;8:387–392. doi: 10.1016/j.jare.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinar Erdem A., Sepet E., Kulekci G., Trosola S.C., Guven Y. Effects of two fluoride varnishes and one fluoride/chlorhexidine varnish on Streptococcus mutans and Streptococcus sobrinus biofilm formation in vitro. Int. J. Med. Sci. 2012;9:129–136. doi: 10.7150/ijms.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baehni P.C., Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003;9(Suppl 1):23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. http://www.ncbi.nlm.nih.gov/pubmed/12974527 [DOI] [PubMed] [Google Scholar]
- 5.Featherstone J.D.B. The continuum of dental caries—evidence for a dynamic disease process. J. Dent. Res. 2004;83:39–42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 6.Järvinen H., Tenovuo J., Huovinen P. In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob. Agents Chemother. 1993;37:1158–1159. doi: 10.1128/aac.37.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weerheijm K.L., Kreulen C.M., de Soet J.J., Groen H.J., van Amerongen W.E. Bacterial counts in carious dentine under restorations: 2-year in vivo effects. Caries Res. 1999;33:130–134. doi: 10.1159/000016506. [DOI] [PubMed] [Google Scholar]
- 8.De Castilho A.R.F., Duque C., Negrini T.D.C., Sacono N.T., De Paula A.B., Costa C.A.D.S., Spolidório D.M.P., Puppin-Rontani R.M. In vitro and in vivo investigation of the biological and mechanical behaviour of resin-modified glass-ionomer cement containing chlorhexidine. J. Dent. 2013;41:155–163. doi: 10.1016/j.jdent.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatric Dentistry Clinical guideline on pediatric restorative dentistry. Pediatr. Dent. 2004;26:106–114. [PubMed] [Google Scholar]
- 10.Loesche W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanzer J.M., Livingston J., Thompson A.M. The microbiology of primary dental caries in humans. J. Dent. Educ. 2001;65:1028–1037. http://www.ncbi.nlm.nih.gov/pubmed/11699974 [PubMed] [Google Scholar]
- 12.Nurelhuda N.M., Al-Haroni M., Trovik T.A., Bakken V. Caries experience and quantification of Streptococcus mutans and Streptococcus sobrinus in saliva of Sudanese schoolchildren. Caries Res. 2010;44:402–407. doi: 10.1159/000316664. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Acedo M., Montiel-Company J.-M., Dasí-Fernández F., Almerich-Silla J.-M. Streptococcus mutans and Streptococcus sobrinus detection by polymerase chain reaction and their relation to dental caries in 12 and 15 year-old schoolchildren in Valencia (Spain) Med. Oral Patol. Oral Cir. Bucal. 2013;18:e839–e845. doi: 10.4317/medoral.18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada M. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J. Med. Microbiol. 2005;54:661–665. doi: 10.1099/jmm.0.46069-0. [DOI] [PubMed] [Google Scholar]
- 15.Wilson A.D., Kent B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972;132:133–135. doi: 10.1038/sj.bdj.4802810. [DOI] [PubMed] [Google Scholar]
- 16.Hafshejani T.M., Zamanian A., Venugopal J.R., Rezvani Z., Sefat F., Saeb M.R., Vahabi H., Zarrintaj P., Mozafari M. Antibacterial glass-ionomer cement restorative materials: a critical review on the current status of extended release formulations. J. Control. Release. 2017;262:317–328. doi: 10.1016/j.jconrel.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 17.Crisp S., Ferner A.J., Lewis B.G., Wilson A.D. Properties of improved glass-ionomer cement formulations. J. Dent. 1975;3:125–130. doi: 10.1016/0300-5712(75)90063-9. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu S.K., Schmalz G. The biocompatibility of glass-ionomer cement materials. A status report for the American Journal of Dentistry. Am. J. Dent. 2001;14:387–396. http://www.ncbi.nlm.nih.gov/pubmed/11949800 [PubMed] [Google Scholar]
- 19.Yoshida Y., Van Meerbeek B., Nakayama Y., Snauwaert J., Hellemans L., Lambrechts P., Vanherle G., Wakasa K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J. Dent. Res. 2000;79:709–714. doi: 10.1177/00220345000790020301. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.Y., Dong D.R., Huang H.M., Shih Y.H. Fluoride ion diffusion from a glass-ionomer cement. J. Oral Rehabil. 2000;27:576–586. doi: 10.1046/j.1365-2842.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- 21.Aykut-Yetkiner A., Simşek D., Eronat C., Ciftçioğlu M. Comparison of the remineralisation effect of a glass ionomer cement versus a resin composite on dentin of primary teeth. Eur. J. Paediatr. Dent. 2014;15:119–121. http://www.ncbi.nlm.nih.gov/pubmed/25102459 [PubMed] [Google Scholar]
- 22.Ericson D., Kidd E., McComb D., Mjör I., Noack M.J. Minimally invasive dentistry--concepts and techniques in cariology. Oral Health Prev. Dent. 2003;1:59–72. [PubMed] [Google Scholar]
- 23.Weng Y., Guo X., Gregory R., Xie D. A novel antibacterial dental glass-ionomer cement. Eur. J. Oral Sci. 2010;118:531–534. doi: 10.1111/j.1600-0722.2010.00770.x. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y., Imazato S., Kaneshiro A.V., Ebisu S., Frencken J.E., Tay F.R. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent. Mater. 2006;22:647–652. doi: 10.1016/j.dental.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Hannig M., Hannig C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010;5:565–569. doi: 10.1038/nnano.2010.83. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamoorthy K., Manivannan G., Kim S.J., Jeyasubramanian K., Premanathan M. Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J. Nanoparticle Res. 2012;14:1063. [Google Scholar]
- 27.Beyth N., Houri-Haddad Y., Domb A., Khan W., Hazan R. Alternative antimicrobial approach: nano-antimicrobial materials. Evid. Based. Complement. Alternat. Med. 2015;2015:246012. doi: 10.1155/2015/246012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naguib G.H., Hosny K.M., Hassan A.H., Al Hazmi F., Al Dharrab A., Alkhalidi H.M., Hamed M.T., Alnowaiser A.M., Pashley D.H. Zein based magnesium oxide nanoparticles: assessment of antimicrobial activity for dental implications. Pak. J. Pharm. Sci. 2018;31:245–250. http://www.ncbi.nlm.nih.gov/pubmed/29386150 [PubMed] [Google Scholar]
- 29.Haghshenas L., Amini A., Bashir Bahati A., Rahimi G. In vitro antibacterial biofilm effect of magnesium oxide nanoparticles on Streptococcus mutans. Micro Nano Biomed. 2016;1:21–27. [Google Scholar]
- 30.Villhauer A.L., Lynch D.J., Drake D.R. Improved method for rapid and accurate isolation and identification of Streptococcus mutans and Streptococcus sobrinus from human plaque samples. J. Microbiol. Methods. 2017;139:205–209. doi: 10.1016/j.mimet.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oho T., Yamashita Y., Shimazaki Y., Kushiyama M., Koga T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol. Immunol. 2000;15:258–262. doi: 10.1034/j.1399-302x.2000.150408.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahrari F., Eslami N., Rajabi O., Ghazvini K., Barati S. The antimicrobial sensitivity of streptococcus mutans and streptococcus sangius to colloidal solutions of different nanoparticles applied as mouthwashes. Dent. Res. J. 2015;12:44–49. doi: 10.4103/1735-3327.150330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim M.A., Meera Priyadarshini B., Neo J., Fawzy A.S. Characterization of chitosan/TiO2 nano-powder modified glass-ionomer cement for restorative dental applications. J. Esthetic Restor. Dent. 2017;29:146–156. doi: 10.1111/jerd.12282. [DOI] [PubMed] [Google Scholar]
- 34.Rad A.M., Mokhtary M. Efficient one-pot synthesis of pyrido[2,3-d]pyrimidines catalyzed by nanocrystalline MgO in water. Int. Nano Lett. 2015;5:109–123. [Google Scholar]
- 35.Bdewi S.F., Abdullah O.G., Aziz B.K., Mutar A.A.R. Synthesis, structural and optical characterization of MgO nanocrystalline embedded in PVA matrix. J. Inorg. Organomet. Polym. Mater. 2016;26:326–334. [Google Scholar]
- 36.Foster M., Furse M., Passno D. An FTIR study of water thin films on magnesium oxide. Surf. Sci. 2002;502–503:102–108. [Google Scholar]
- 37.Selvam N.C.S., Kumar R.T., Kennedy L.J., Vijaya J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloy. Comp. 2011;509:9809–9815. [Google Scholar]
- 38.Kumar A., Kumar J. On the synthesis and optical absorption studies of nano-size magnesium oxide powder. J. Phys. Chem. Solids. 2008;69:2764–2772. [Google Scholar]
- 39.Al-Hazmi F., Alnowaiser F., Al-Ghamdi A.A.A.A., Al-Ghamdi A.A.A.A., Aly M.M.M., Al-Tuwirqi R.M., El-Tantawy F. A new large - scale synthesis of magnesium oxide nanowires: structural and antibacterial properties. Superlattice Microstruct. 2012;52:200–209. doi: 10.1016/j.spmi.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L., Shen H., Suh B.I. Antibacterial dental restorative materials: a state-of-the-art review. Am. J. Dent. 2012;25:337–346. http://www.ncbi.nlm.nih.gov/pubmed/23409624 [PubMed] [Google Scholar]
- 41.Honkala E., Behbehani J., Ibricevic H., Kerosuo E., Al-Jame G. The atraumatic restorative treatment (ART) approach to restoring primary teeth in a standard dental clinic. Int. J. Paediatr. Dent. 2003;13:172–179. doi: 10.1046/j.1365-263x.2003.00455.x. [DOI] [PubMed] [Google Scholar]
- 42.Burke F.J.T., McHugh S., Shaw L., Hosey M.-T., Macpherson L., Delargy S., Dopheide B. UK dentists’ attitudes and behaviour towards Atraumatic Restorative Treatment for primary teeth. Br. Dent. J. 2005;199:365–369. doi: 10.1038/sj.bdj.4812696. discussion 353; quiz 372. [DOI] [PubMed] [Google Scholar]
- 43.Ahmadian E., Shahi S., Yazdani J., Maleki Dizaj S., Sharifi S. Local treatment of the dental caries using nanomaterials. Biomed. Pharmacother. 2018;108:443–447. doi: 10.1016/j.biopha.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamoorthy K., Moon J.Y., Hyun H.B., Cho S.K., Kim S.-J. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J. Mater. Chem. 2012;22:24610. [Google Scholar]
- 45.Di D.-R., He Z.-Z., Sun Z.-Q., Liu J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012;8:1233–1241. doi: 10.1016/j.nano.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slomberg D.L., Lu Y., Broadnax A.D., Hunter R.A., Carpenter A.W., Schoenfisch M.H. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces. 2013;5:9322–9329. doi: 10.1021/am402618w. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto O., Fukuda T., Kimata M., Sawai J., Sasamoto T. Antibacterial characteristics of MgO-mounted spherical carbons prepared by carbonization of ion-exchanged resin. J. Ceram. Soc. Japan. 2001;109:363–365. [Google Scholar]
- 49.Tang Z.X., Lv B.F. MgO nanoparticles as antibacterial agent: preparation and activity. Braz. J. Chem. Eng. 2014;31:591–601. [Google Scholar]
- 50.Singla D., Sharma A., Sachdev V., Chopra R. Distribution of Streptococcus mutans and Streptococcus sobrinus in dental plaque of Indian pre-school children using PCR and SB-20M agar medium. J. Clin. Diagn. Res. 2016;10:ZC60–ZC63. doi: 10.7860/JCDR/2016/19256.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam M.K., Zheng L., Liu R., Papagerakis S., Papagerakis P., Geyer C.R. Synthetic antigen-binding fragments (Fabs) against S. mutans and S. sobrinus inhibit caries formation. Sci. Rep. 2018;8:10173. doi: 10.1038/s41598-018-28240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng L., Zhang K., Weir M.D., Melo M.A.S., Zhou X., Xu H.H.K. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine. 2015;10:627–641. doi: 10.2217/nnm.14.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Naimi O.T., Itota T., Hobson R.S., McCabe J.F. Fluoride release for restorative materials and its effect on biofilm formation in natural saliva. J. Mater. Sci. Mater. Med. 2008;19:1243–1248. doi: 10.1007/s10856-006-0023-z. [DOI] [PubMed] [Google Scholar]
- 54.Debnath A., Kesavappa S.B., Singh G.P., Eshwar S., Jain V., Swamy M., Shetty P. Comparative evaluation of antibacterial and adhesive properties of chitosan modified glass ionomer cement and conventional glass ionomer cement: an in vitro study. J. Clin. Diagn. Res. 2017;11:ZC75–ZC78. doi: 10.7860/JCDR/2017/25927.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheetham J.J. The future of glass-ionomers. In: Sidhu S.K., editor. Glas. Dent. Springer International Publishing; Cham: 2016. pp. 125–148. [Google Scholar]
- 56.Kabil N.S., Badran A.S., Wassel M.O. Effect of the addition of chlorhexidine and miswak extract on the clinical performance and antibacterial properties of conventional glass ionomer: an in vivo study. Int. J. Paediatr. Dent. 2016 doi: 10.1111/ipd.12273. [DOI] [PubMed] [Google Scholar]
- 57.Garcia P.P.N.S., Cardia M.F.B., Francisconi R.S., Dovigo L.N., Spolidório D.M.P., de Souza Rastelli A.N., Botta A.C. Antibacterial activity of glass ionomer cement modified by zinc oxide nanoparticles. Microsc. Res. Tech. 2017;80:456–461. doi: 10.1002/jemt.22814. [DOI] [PubMed] [Google Scholar]