Abstract
The periodontal ligament (PDL) is a highly vascularized connective tissue surrounding the root of a tooth. In particular, the PDL is continuously exposed to mechanical stresses during the phases of mastication, and it provides physical, sensory, and trophic functions. It is known that the application of orthodontic force creates a change in periodontal structures. In fact, these forces generate a pressure on the ligament that closes the vessels. The aim of this study is to observe the modifications of vascular endothelial growth factor (VEGF) in the PDL and extracellular matrix proteins after application of a pre-calibrated and constant orthodontic force at different phases of treatment. We used a 50-g NiTi coiled spring and in vivo samples of PDL of maxillary and mandibular premolars of patients subjected to orthodontic treatment. These teeth were extracted at 1, 7, 14, 21, and 30 days, respectively, by application of force. The extraction of the PDL was effectuated by scarifying the radicular surface on the pressure and tension sides. The mechanical stress induced by the application of force caused an increase in the reactive type of metabolism of extracellular matrix proteins and modulation of neoangiogenesis until restoration.
Keywords: Anatomy, Cell biology, Dentistry, Periodontal ligament, Collagen type I, Fibronectin, VEGF, Immunofluorescence, Collagen type IV
1. Introduction
The periodontal ligament (PDL) is a narrow band of highly vascularized fibrous connective tissue that lies between the hard tissues of the alveolar bone and the root surface (Lindhe et al., 2006). This tissue provides physical, sensory, and trophic functions (Berkovitz, 2004). Under physiological conditions, the synthesis and the degradation of periodontal structures are at a low level to maintain tissue homeostasis (Bhaskar, 1990; Kimmel, 1993).
PDL and alveolar bone cells are exposed to physical forces in vivo in response to mastication and orthodontic tooth movement (OTM), but the compression applied by orthodontic forces induces remodelling of the PDL and gingival tissue, including oedema, gradual obliteration of blood vessels, and breakdown of the vein walls, with leakage of blood into the extravascular space (Kumar et al., 2015).
It is known that orthodontic forces produce pressure and tension regions in the PDL, and this strain modifies the vascularization and blood flow of affected tissues, generating a favourable microenvironment for tissue resorption (Kohno et al., 2002; Matarese et al., 2017). The “compression region” is the area where the orthodontic forces press the blood vessels. Therefore, blood flow and periodontal tissue changes may adapt to the compression force. At the pressure region, the PDL displays disorganization, decreased cell replication, and reduced fibre production as a result of vascular constriction (Rygh, 1973).
The “tension region” is characterized by a stretching of the PDL fibres, which determines the increment of cell replication (Tan et al., 2009). The blood vessels on the side of PDL tension become distended, and fibroblasts are rearranged in the direction of the strain. The stretched fibroblasts appear spindle-shaped in the middle of the PDL and spherical near the alveolar bone. Findings by Garant and Cho (1979) suggest that these fibroblasts secrete new Sharpey's fibres in the PDL simultaneously with the deposition of a new matrix on the adjacent alveolar bone socket wall. In this condition, PDL and alveolar bone are degraded to create space for the moving tooth, while new PDL is formed to maintain the attachment (Thilander et al., 2005).
The use of orthodontic forces produces three distinct phases: initial phase, lag phase, and post lag phase (Burstone, 1962). Recently, OTM was divided into four phases: initial, arrest, acceleration, and linear phases (Pilon et al., 1996). A close relationship exists between the vascular integrity of the PDL and the type of resorption resulting from an applied force (Gianelly, 1969).
The initial vascular response of OTM is the release of vasoactive neuropeptides by the stimulated sensory nerve ending. The changes on the pressure side are characterized by compression, occlusion, and partial disintegration of blood vessels. Immediately after force application, the bloods vessels respond with increase of permeability. In this phase, it a decrease of CD31, an adhesion molecule on endothelial cells, occurs, suggesting an increased potential for vascular permeability (Mendes et al., 2018). During orthodontic therapy, biological activity is characterized by an increase in cellular response (Baumrind, 1969; Storey, 1973). The different modifications of fibrillar and vascular components of PDL during OTM can be monitored by analysis of the expression of the different proteins that compose it (Ten Cate et al., 1976; Bumann et al., 1997; Bruckner and Van der Rest, 1994).
In our previous study, we analysed collagen type I, collagen type IV, and fibronectin behaviour in PDL during OTM in the early stages of treatment until 14 days, demonstrating that the OTM induced important hemodynamic and structural modifications of PDL (Anastasi et al., 2008; Feller et al., 2015). Moreover, the response of fibronectin to mechanical force during OTM was important in the mechanosignalling and transduction pathway, leading to extracellular matrix (ECM) remodelling (Engel et al., 1981). The mechanical stimulation of the PDL alters the production of collagen type I and fibronectin through the alteration of the synthesis of ECM by the fibroblasts of the PDL (Yamada, 1983).
On this basis, in the present report, we re-evaluate our previous results (Anastasi et al., 2008), extending the time of the application force up to 30 days, because at this stage, hyalinization exists in the region of compression (Zainal Ariffin et al., 2011) and the movement is stopped (Pilon et al., 1996). Moreover, considering our previous results regarding collagen type IV, indicating a loss of vascular component at 14 days (Anastasi et al., 2008), we also investigate the modifications in vascular components, monitoring the staining pattern of vascular endothelial growth factor (VEGF), since this protein is one of the most important regulators of angiogenesis (Ferrara, 1999, 2009; Ferrara et al., 2003), and it participates in the regulation of wound healing during OTM (Kaku et al., 2008; Hazan-Molina et al., 2013). All proteins were investigated 1, 7, 14, 21, and 30 days after orthodontic treatment.
Furthermore, to verify an effective angiogenesis and the integrity of the vascular wall, we performed double-localization reactions between VEGF and CD31 and between collagen type IV and CD31 only at 30 days, because in this phase, the movement is finished and the initial conditions are restored.
2. Materials and methods
2.1. Subjects
For this study, we analysed in vivo human samples of PDL of 72 first maxillary and mandibular premolars of 18 subjects, scheduled for orthodontic treatment, at the Department of Dentistry of Messina University, Italy.
All patients met the following criteria: 1) all 4 first premolars were extracted for orthodontic reasons; 2) the patient was in good general health, and the periodontium was healthy with no radiographic evidence of bone loss and no gingival inflammation; and 3) no antibiotics or anti-inflammatory drugs were used in the month before the study. All subjects received repeated oral hygiene instructions about how to use a toothbrush, dental floss, and interdental brush before the placement of the orthodontic appliance. The subjects, 11 females and 7 males (mean age 15.4 years; range, 13–18 years), not matched by age and sex, were randomly divided into 6 main groups; each group consisted of 3 subjects.
Group I: Twelve premolars from 3 subjects, not subjected to orthodontic force, were extracted, and the PDL samples were used as control.
Group II: Twelve premolars from 3 subjects were extracted 1 day after the application of orthodontic force, and each PDL sample was analysed on both the tension and pressure side.
Group III: Twelve premolars from 3 subjects were extracted 7 days after the application of orthodontic force, and each PDL sample was analysed on both the tension and pressure side.
Group IV: Twelve premolars from 3 subjects were extracted 14 days after the application of orthodontic force, and each PDL sample was analysed on both tension and pressure side.
Group V: Twelve premolars from 3 subjects were extracted 21 days after the application of orthodontic force, and each PDL sample was analyzed on both tension and pressure side.
Group VI: Twelve premolars from 3 subjects were extracted 30 days after the application of orthodontic force, and each PDL sample was analysed on both tension and pressure side.
The older subjects (18 years) gave their informed consent for inclusion before they participated in the study; for minors, informed consent was provided by their parents or legal guardians. All procedures were performed according to the Helsinki Declaration of 1975. The research protocols were approved by the Ethics Committee of Azienda Ospedaliera Universitaria Policlinico “G. Martino” of Messina (project identification code XX 18-18 07/03/2018).
2.2. Clinical procedures
For the application of orthodontic force during experimental tooth movement, we directly bonded 0.022 inc, LP tubes, and master brackets (American Orthodontics Sheboygan, Wisconsin, USA) to the buccal surfaces of the maxillary first premolars and molars. The premolars were subjected to a buccally directed tipping force (50 g) with a nickel titanium closed-coil spring (American Orthodontics). Bonding of the brackets, bending of the springs, and calibration of force with a strain gauge (Dentaurum, Ispringen, Germany) were performed by the same clinician. After the experimental period was established for each group, the premolars were extracted by the same surgeon with no surgical trauma to the root. The PDL sampling was obtained by scraping the radicular surface of the premolars. This scraping was carried out in the compression area of the ligament that corresponds to the direction of the buccal orthodontic force application and the contralateral area of tension.
2.3. Immunofluorescence
The PDL samples were fixed in 3% paraformaldehyde in 0.2 M phosphate buffer at pH 7.4. After numerous rinses in 0.2 M phosphate buffer and phosphate-buffered saline (PBS), the samples were infiltrated with 12% and 18% sucrose and frozen in liquid nitrogen. By cryostat, the samples were cut into 20-μm-thick sections collected on glass slides coated with 0.5% gelatine and 0.005% chromium potassium sulphate.
First, we performed a negative control on PDL, exclusively incubating the secondary antibody, for 1 h at room temperature in a dark room to verify the accuracy of the reactions.
On obtained sections, four different single-localization reactions were performed to mark the collagen type I, collagen type IV, fibronectin, and VEGF. To block nonspecific sites and to create permeable membranes, the sections were preincubated with 1% bovine serum albumin and 0.3% Triton X-100 in PBS at room temperature for 15 min. Finally, the sections were incubated with the primary antibodies for 2 h at room temperature (Anastasi et al., 2007; Di Mauro et al., 2009; Cutroneo et al., 2015). The following primary antibodies were used: mouse monoclonal anti-collagen type I antibody, diluted 1:400 (Sigma Aldrich, S. Louis, Missouri, USA); mouse monoclonal anti-collagen type IV antibody, diluted 1:400 (Sigma Aldrich); mouse monoclonal anti-fibronectin antibody, diluted 1:400 (Sigma Aldrich); and mouse monoclonal anti-VEGF antibody, diluted 1:400 (Novus Biologicals, Littleton, USA). All primary antibodies were stained with fluorescein isothiocyanate (FITC)–conjugated immunoglobulin G (IgG) anti-mouse, diluted 1:100 (Jackson ImmunoResearch Laboratories, West Grove, PA); the fluorochrome was applied for 1 h at room temperature in a dark room. In the same reactions, 4′,6-diamidino-2-phenylindole (DAPI; Sigma Chemicals) was used for nuclear staining diluted 1:1.000 in PBS for 10 min.
For double-localization reactions between collagen type IV and CD31 and between VEGF and CD31, after incubation of the secondary antibody conjugated with FITC IgG anti-mouse, the rabbit polyclonal antibody anti-CD31 was applied diluted at 1:50 at 4 °C overnight (Thermo Fisher Scientific, Waltham, Ma, USA). The primary rabbit polyclonal antibody was stained with Texas Red–conjugated IgG anti-rabbit (Jackson ImmunoResearch Laboratories), applied for 1 h at room temperature in a dark room (Anastasi et al., 2006).
Sections were then observed and photographed using a Zeiss LSM 5 DUO (Carl-Zeiss, Jena, Germany) tested using a META scanning module. For the image detection, an argon laser (458 and 488 nm) was used. All images were digitized at a resolution of 8 bits into an array of 2048 × 2048 pixels.
Optical sections of fluorescence specimens were obtained using HeNe laser (543 nm) and argon laser (458) at a 1-min 2-sec scanning speed with up to 8 averages. Thick sections (1.50 μm) were obtained using a pinhole of 250. Contrast and brightness were established by examining the most brightly labelled pixels and choosing a setting that allowed clear visualization of structural details while keeping the highest pixel intensities near 200. Settings used for all images were the same as those obtained from other samples that had been processed in parallel.
To measure the intensity of the immunofluorescence of all tested proteins, we used the “Histo” function of the confocal laser scanning microscope. In detail, we observed 10 optical sections for each group of patients and 20 microscopic fields for each section; all of these fields had 1.048.576 pixels and an area of 50.652 μm2. The “Histo” function allowed us to convert each single pixel to obtain a numerical value that indicates the intensity of immunofluorescence excluding any type of error. Furthermore, we observed all samples of recruited subjects to demonstrate that the images shown in this report are representative of all remaining analysed samples.
Digital images were cropped, and figures were prepared using Adobe Photoshop 7.0.
2.4. Statistical analysis
For statistical analysis, we used the confocal laser scanning microscopy function, called “Histo” (previously described), using numerical values in order to calculate the distribution and to obtain the real mean intensity of fluorescent pixels for each acquired image.
For each group of premolars, the following independent variables were considered: type I collagen, fibronectin, type IV collagen, and VEGF. Their measurements were performed on both the pressure and the tension side. The numerical data are expressed as mean and standard deviation (SD).
Examined variables did not present normal distribution, as verified by Kolmogorov-Smirnov test; consequently, the nonparametric approach was used.
The Kruskal-Wallis test was used to compare measurements of the stained area of type I collagen, fibronectin, type IV collagen, and VEGF in the control group and after 1, 7, 14, 21, and 30 days. The significance level was set at P < 0.05. The Bonferroni method was applied for the correction of the type I error in the presence of multiple tests on the same data (α divided by the number of the hypotheses considered).
Statistical analyses were performed using the SPSS 25.0 (IBM SPSS Statistics, New York, USA) for Window package.
3. Results
Immunofluorescence reactions were performed to verify the immunostaining patterns of collagen type I, fibronectin, collagen type IV, and VEGF in human PDL in both normal conditions and after 1, 7, 14, 21, and 30 days from orthodontic treatment on both the tension and the pressure side. The sections were analysed using a stack of 16 sections (0.8 μm of scan step) and carried out on 20-μm-thick cryosections of PDL.
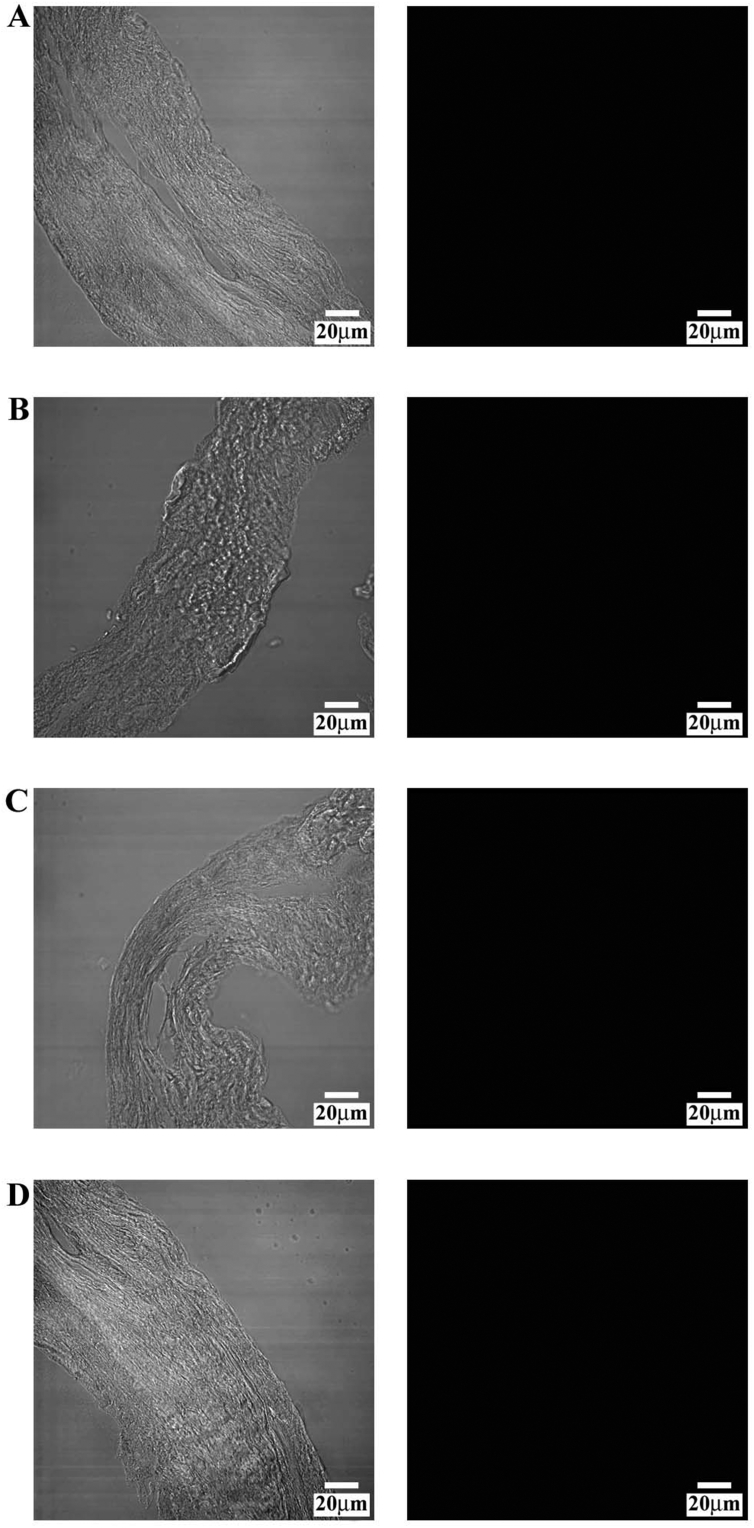
Initially, we performed a negative control on PDL, omitting the primary antibodies for collagen type I (Fig. 1A), fibronectin (Fig. 1B), collagen type IV (Fig. 1C), and VEGF (Fig. 1D); the black image allows us to attest that the fluorochrome did not link to the secondary antibody, demonstrating the accuracy of the reaction. The transmitted light for each negative control was shown to demonstrate the presence of microscopic fields.
Fig. 1.
Compound panel showing immunohistochemical findings in periodontal ligament omitting the primary antibodies for collagen type I (A), fibronectin (B), collagen type IV (C), and VEGF (D); the black image confirms that the fluorochrome did not link to the secondary antibody, demonstrating the accuracy of the reaction. The transmitted light for each negative control is shown to demonstrate the presence of microscopic fields.
3.1. Collagen type I
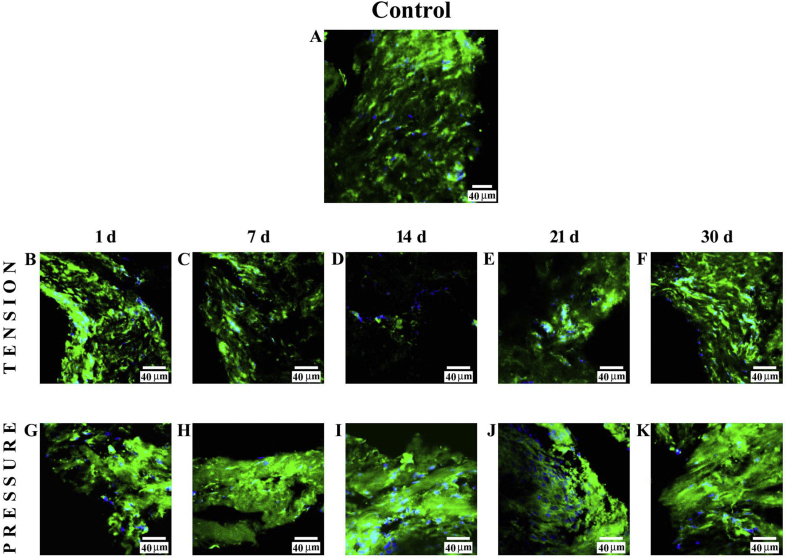
3.1.1. Tension side
Immunofluorescence reactions performed on PDL from control subjects showed a clearly detectable staining pattern for collagen type I (Fig. 2A). The fluorescence pattern for this protein, 1 day (B) after treatment, was increased with respect to observations on PDL obtained from control subjects. Its staining pattern was reduced at 7 days (C) and more severely reduced at 14 days (D); the fluorescence pattern was notably increased at 21 days (E) and 30 days (F) in comparison with the previous stage. In all images, the DAPI staining (blue channel) confirmed the presence of collagen type I in cells.
Fig. 2.
Compound panel showing immunohistochemical findings on samples immunolabelled with antibody against collagen type I. The staining pattern for this protein show clearly detectable fluorescence in the periodontal ligament from control subjects (A). On the tension side, after 1 day of treatment, it was gradually increased (B); at 7 days (C) and severely at 14 days (D), the collagen type I staining pattern was reduced, whereas the fluorescence pattern was notably increased at 21 (E) and 30 days (E). On the pressure side, the staining pattern for collagen type I slightly increased after 1 (G) and 7 days (H) of treatment, whereas at 14 (I), 21 (J), and 30 days (K) of treatment, it notably increased. The blue fluorescence represents nuclear-staining DAPI confirming the presence of the cells.
3.1.2. Pressure side
The staining pattern for collagen type I, 1 day (G) after treatment was increased as compared with PDL obtained from control subjects. At 7 days (H), the staining pattern for this protein slightly increased, whereas it notably increased at 14 (I), 21 (J), and 30 days (K) of treatment. In all images, the DAPI staining (blue channel) showed specific staining for collagen type I in cells.
3.2. Fibronectin
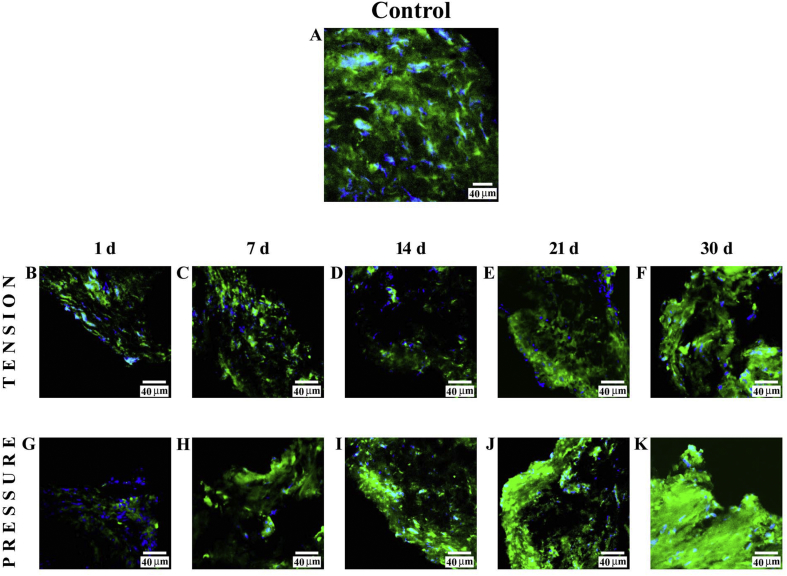
3.2.1. Tension side
Immunofluorescence reaction performed on PDL from control subjects showed a clearly detectable staining pattern for fibronectin (Fig. 3A). The staining pattern for this protein, after 1 day (B), showed low fluorescence as compared with control findings. After 7 days (C) and 14 days (D), it was possible to observe a decrease for this staining pattern, and at 21 days (E) and 30 days (F), this pattern increased. In all images, the DAPI staining (blue channel) confirmed the presence of fibronectin in cells.
Fig. 3.
Compound panel showing immunohistochemical findings on samples immunolabelled with antibody against fibronectin. The staining pattern for this protein shows a clearly detectable fluorescence in the periodontal ligament from control subjects (A). On the tension side, the staining pattern for this protein shows a very low staining pattern at 1 day (B), whereas at 7 (C) and 14 days (D), it shows a decrease. At 21 (E) and 30 days (F) of treatment, the staining pattern increases. On the pressure side, the results show a low fluorescence at 1 day (G), whereas at 7 (H), 14 (I), and 21 (J) days, it increases gradually. At 30 days (K), it is possible to observe a strong increase in staining pattern for this protein. The blue fluorescence represents nuclear-staining DAPI confirming the presence of the cells.
3.2.2. Pressure side
The results of the staining pattern for fibronectin showed low fluorescence at 1 day (G) of treatment, whereas at 7 (H), 14 (I), and 21 days (J), it increased gradually, and at 30 days (K), the fluorescence pattern for this protein still increased considerably. In all images, the DAPI staining (blue channel) confirmed the presence of fibronectin in cells.
3.3. Collagen type IV
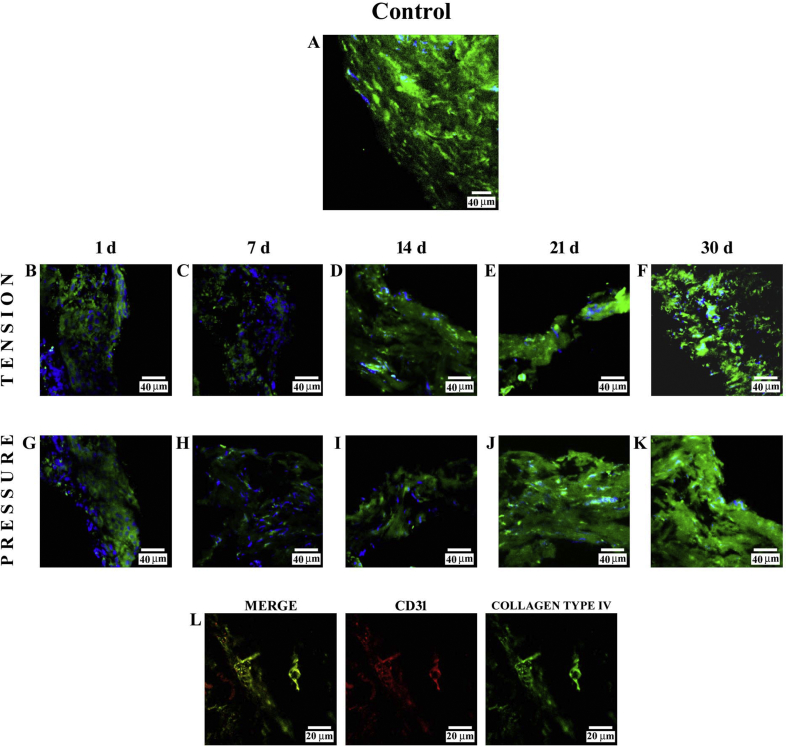
3.3.1. Tension side
The immunofluorescence reaction performed on PDL from control subjects showed a clearly detectable staining pattern for collagen type IV (Fig. 4A). Immunofluorescence results for this protein demonstrated a decrease in staining pattern at 1 day (B); after 7 days (C), the fluorescence slightly decreased, but it became more evident at 14 (D), 21 (E), and 30 days (F) of treatment. Furthermore, at 30 days of treatment, the collagen type IV staining pattern was mainly localized around the blood vessels. In all images, the DAPI staining (blue channel) confirmed the presence of collagen type IV in cells.
Fig. 4.
Compound panel showing immunohistochemical findings on samples immunolabelled with antibody against collagen type IV. The staining pattern for this protein shows a clearly detectable fluorescence in the periodontal ligament from control subjects (A). On the tension side, immunofluorescence results showed a decrease in staining pattern for collagen type IV at 1 day (B); after 7 days (C), this staining pattern slightly increased, becoming more evident at 14 (D), 21 (E) and 30 (F) days. On the pressure side, the staining pattern for collagen type IV showed a low fluorescence at 1 day (G), whereas at 7 (H), 14 (I), 21 (J), and 30 (K) days, the staining pattern is gradually increased. The blue fluorescence represents nuclear-staining DAPI confirming the presence of the cells. The double-localization reaction between collagen type IV (green channel in L) and CD31 (red channel in L) at 30 days on the tension side shows a yellow fluorescence due to overlap of CD31 on collagen type IV, demonstrating a co-localization between two proteins.
3.3.2. Pressure side
The results of the staining pattern for collagen type IV showed a low fluorescence at 1 day (G) as compared with control subjects. After 7 days (H), this protein showed an evident decrease, whereas at 14 (I), 21 (J), and 30 days (K), it became gradually more evident. In all images, the DAPI staining (blue channel) confirmed the presence of collagen type IV in cells. A double-localization reaction between collagen type IV (green channel in L) and CD31 (red channel in L), performed at 30 days, showed a yellow fluorescence due to overlap of CD31 on collagen type IV, demonstrating a co-localization between two proteins; furthermore, these proteins had a similar staining pattern distributed around the vessels.
3.4. VEGF
3.4.1. Tension side
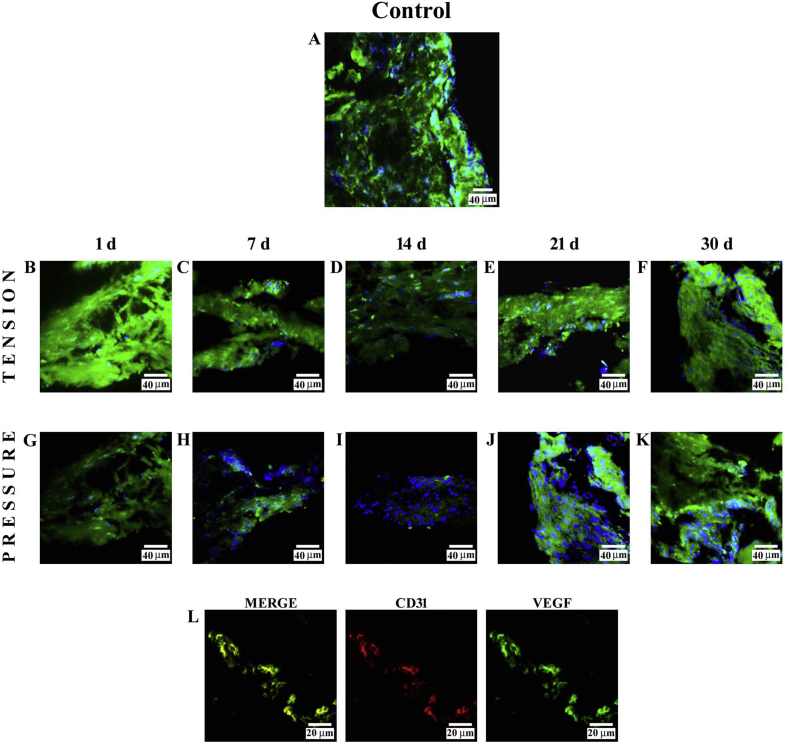
Immunofluorescence reaction performed on PDL from control subjects showed a clearly detectable staining pattern for VEGF (Fig. 5A). Immunofluorescence findings for this protein on the tension side showed a strong fluorescence pattern at 1 day (B), which decreased from 7 (C) to 14 days (D) and increased at 21 (E) and 30 days (F) of treatment. In all images, the DAPI staining (blue channel) confirmed the presence of VEGF in cells.
Fig. 5.
Compound panel showing immunohistochemical findings on samples immunolabelled with antibody against VEGF. The staining pattern for this protein shows a clearly detectable fluorescence in the periodontal ligament from control subjects (A). Results on the tension side showed a strong fluorescence pattern at day 1 (B) that decreased from 7 (C) to 14 days (D) and increased from 21 (E) to 30 (F) days of treatment. On the pressure side, a weak fluorescence pattern is demonstrated at day 1 (F), 7 (G), and 14 (H) of treatment. Results after 21 (I) and 30 (J) days showed an increase in VEGF staining pattern, which was uniformly distributed along the tissue. The blue fluorescence represents nuclear-staining DAPI confirming the presence of the cells. The double-localization reaction between VEGF (green channel in L) and CD31 (red channel in L), performed at 30 days on the tension side, shows yellow fluorescence due to overlap of CD31 on VEGF, demonstrating a co-localization between the two proteins.
3.4.2. Pressure side
Results obtained on the pressure side showed a weak fluorescence pattern at 1 day (G) and a more severely reduced staining pattern at 7 (H) and 14 days (I) of treatment. Results after 21 (J) and 30 days (K) showed an increase in VEGF staining pattern. In all images, the DAPI staining (blue channel) confirmed the presence of VEGF in cells. The double-localization reaction between VEGF (green channel in L) and CD31 (red channel in L), performed at 30 days, showed a yellow fluorescence due to overlap of CD31 on VEGF, demonstrating a co-localization between the two proteins.
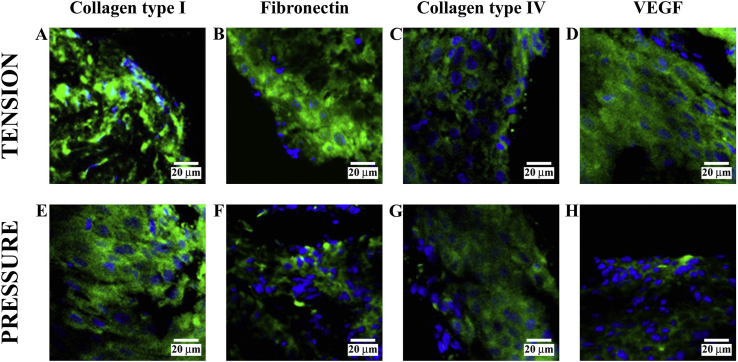
3.5. High magnifications
Performing higher magnification of any previous image, it is possible to demonstrate the specific staining of the cells (Fig. 6). In particular, on the tension side, we found collagen type I at 1 day (A), fibronectin at 21 days (B), collagen type IV at 1 day (C), and VEGF at 30 days (D); on the pressure side, we found collagen type I at 21 days (E), fibronectin at 1 day (F), collagen type IV at 1 day (G), and VEGF at 14 days (H). The same condition was observed for other stages of treatment (data not shown).
Fig. 6.
Higher magnifications of previous images demonstrated the specific staining of the cells. In particular, on the tension side is shown collagen type I at 1 day (A), fibronectin at 21 days (B), collagen type IV at 1 day (C), and VEGF at 30 days (D); on the pressure side is shown collagen type I at 21 days (E), fibronectin at 1 day (F), collagen type IV at 1 day (G), and VEGF at 14 days (H). The same condition was observed for other stages of treatment (data not shown).
3.6. Statistical analysis
The results obtained with confocal laser scanning microscopy were analysed and elaborated, and the values for collagen type I, fibronectin, collagen type IV, and VEGF in relation to the site of pressure/tension collection for each group are summarized in Table 1, indicating mean intensity and standard deviation.
Table 1.
Main intensity and standard deviation values of tested proteins on tension and pressure side of all treatment phases. Kruskal Wallis Test was used for pairwise comparisons between control group and orthodontically treated groups.
|
TENSION |
Groups (days after orthodontic treatment) |
|||||
|
Protein |
Control |
1 |
7 |
14 |
21 |
30 |
| Collagen type I | 180.88 ± 3.546 | 235.74 ± 3.557* | 175.89 ± 6.221 | 121.65 ± 4.058* | 176.44 ± 3.601 | 204.81 ± 3.161* |
| Fibronectin | 181.55 ± 4.804 | 164.01 ± 3.143* | 163.32 ± 3.564* | 146.02 ± 5.487* | 172.33 ± 1.748 | 182.03 ± 2.656 |
| Collagen type IV | 182.55 ± 5.136 | 145.75 ± 3.622* | 143.25 ± 4.022* | 145.62 ± 3.851* | 175.55 ± 2.617 | 176.03 ± 3.629 |
| VEGF |
181.22 ± 2.819 |
233.77 ± 3.422* |
174.21 ± 2.263 |
144.68 ± 3.757* |
174.48 ± 3.633 |
174.53 ± 3.364 |
|
PRESSURE |
||||||
|
Protein |
Control |
1 |
7 |
14 |
21 |
30 |
| Collagen type I | 180.88 ± 3.546 | 234.28 ± 3.934* | 238.47 ± 1.464* | 255.69 ± 3.497* | 250.54 ± 5.066* | 255.32 ± 3.429* |
| Fibronectin | 181.55 ± 4.804 | 144.34 ± 3.325 | 186.11 ± 3.445 | 213.22 ± 3.422* | 234.53 ± 3.822* | 264.11 ± 3.512* |
| Collagen type IV | 182.55 ± 5.136 | 138.29 ± 4.277* | 105.75 ± 3.059* | 115.10 ± 3.161* | 173.88 ± 3.341 | 183.66 ± 2.869 |
| VEGF | 181.22 ± 2.819 | 154.65 ± 3.819* | 134.40 ± 3.729* | 116.01 ± 3.289* | 174.66 ± 4.677 | 174.33 ± 3.725 |
*P < 0.05 vs control.
The Kruskal-Wallis test revealed a highly significant difference (P < 0.05) among the treated groups in association with the independent variables.
Analysing the multiple comparisons, it is possible to denote that collagen type I was significantly increased (P = 0.000) at 1 and 30 days after treatment and reduced at 14 days (P = 0.000) on the tension side as compared with control group, while immunofluorescence values at 7 and 21 days were not significantly modified (P = 1.000, 1.000, respectively) compared with the control group. On the pressure side, immunostaining for this protein was significantly increased at 1, 7, 14, 21, and 30 days after treatment (P = 0.001, 0.002, 0.000, 0.000, and 0.000, respectively) compared with the control group (Table 1, Fig. 2).
Fibronectin, on the tension side, was significantly reduced (P = 0.000) at 1, 7, and 14 days after treatment and not significantly modified after 21 and 30 days (P = 0.256, 1.000, respectively) compared with the control group. On the pressure side, after 14, 21, and 30 days, its immunostaining was significantly increased (P = 0.000) and not significantly modified after 1 and 7 days (P = 0.539, 1.000, respectively) compared with the control group (Table 1, Fig. 3).
Collagen type IV was significantly reduced compared with the control group, both on the tension (P = 0.000) and on the pressure side (P = 0.000) at 1, 7, and 14 days, while at 21 and 30 days, its immunofluorescence was not significantly modified compared with the control group for both the tension (P = 0.596, 0.787, respectively) and pressure sides (P = 0.350, 1.000, respectively; Table 1, Fig. 4).
VEGF was significantly increased compared with control group on the tension side at 1 day (P = 0.002), while its staining pattern was significantly reduced at 14 days (P = 0.000) compared with the control group. At 7, 21, and 30 days, its immunofluorescence was not significantly modified (P = 0.030, 0.041, 0,047, respectively). On the pressure side, this protein was significantly reduced (P = 0.000) at 1, 7, and 14 days compared with the control group, while at 21 and 30 days, its immunofluorescence was not significantly modified (P = 0.678, 0.597, respectively; Table 1, Fig. 5).
4. Discussion
The PDL consists of fibrous connective tissue containing cells, nerves, and blood vessels (Nemoto et al., 2010) and plays a key role in regulating the bone remodelling that occurs during tooth movement (Rygh, 1972; Roberts and Ferguson, 1984). It is known that the mechanical stress exerted on the teeth during OTM provokes changes in the structure of PDL. Moreover, the mechanical forces influence not only the PDL but also the state and composition of the crevicular fluid, playing a role in the development of periodontal disease (McDevitt et al., 2003; Fiorillo et al., 2018).
OTM has been defined as the result of a biologic response of the facial bones by an externally applied force, with interference in the integrity of the tissues (Anastasi et al., 2008; De Ponte et al., 2017). The tissues involved in OTM are alveolar bone and the periodontal tissues; moreover, the applied force causes the compression of the alveolar bone and the PDL on one side, while on the opposite side, the PDL is stretched (Masella and Meister, 2006; Isola et al., 2017).
Baumrind (1969) hypothesized that during pressure and tension, the PDL acts as a continuous hydrostatic system, suggesting that the forces are transmitted equally. Therefore, several studies have investigated the behaviour of PDL components during OTM, but much of the research has been conducted in on animal models (Maltha et al., 2004), in particular on rats (Rygh, 1973; Ren et al., 2004), and there are structural differences between animals and humans in terms of the arrangement of the periodontal fibres during experimental OTM (Reitan and Kvam, 1971; Miyoshi et al., 2001). Studies on human PDL tissues have been conducted in vitro on cell cultures (Kurol and Owman-Moll, 1998; Zhao et al., 2008). Moreover, other authors have shown the distribution of mechanical force in the PDL areas during OTM (Chen et al., 2014).
The orthodontic forces during OTM are transmitted to endothelial cells, promoting the migration of leukocytes, which secrete many signalling molecules, such as cytokines, chemokines, and growth factors, as well as inflammatory mediators that stimulate PDL and alveolar bone to remodel the ECM (Di Domenico et al., 2012; Mendes et al., 2018).
Moreover, the experimental data suggest that the intensity of the orthodontic force should not exceed 50 g, to induce tooth movement and promote cellular differentiation without vascular modification. This light force selected should induce frontal bone resorption with consequent tooth movement until the next activation (Kohno et al., 2002; Cheng et al., 2009).
Here, we have performed an in vivo study on human PDL components, including collagen type I, fibronectin, collagen type IV, and VEGF, over a period of 30 days by the application of pre-calibrated light orthodontic force on the maxillary and mandibular premolars.
Our results show that collagen type I increases on the pressure side after 21 days of treatment and it decreases on the tension side at 14 days. Relative to the pressure side, we can hypothesize that an increase in synthesis processes exists; these results are supported by previous studies suggesting that orthodontic force induces PDL remodelling, with an increase in collagen metabolism similar to wound-healing processes (Timpl et al., 1981). Relative to the tension side, our data suggest that an increase in degradation processes exists. These results seem to be in accordance with the literature demonstrating that the tension side is characterized by low proliferative activity; there is also an increase in the degradation processes during OTM (Zainal Ariffin et al., 2011). The most important results are that, on the tension side, a collagen type I staining pattern increases from 14 to 30 days, whereas on the pressure side, it increases from 7 to 30 days. Thus, we can assume that after 30 days of treatment, the staining pattern for collagen type I has been restored, hypothesizing that the production of this protein seems to be time dependent, as for collagen type IV (Oono et al., 1993).
The increase of collagen type I from 14 days to 30 days of treatment on the tension side suggests that, in the presence of tensional stimuli, the ECM is more synthetized; this is in accordance with reports that have hypothesized a potential increase in matrix synthesis associated with orthodontic forces (Timpl et al., 1981).
The positive trend of fibronectin from 1 to 30 days on the pressure side and from 14 to 30 days on the tension side suggests that when a force is applied, fibronectin could participate in reparative events to restore the PDL structure.
Our results show a low signal for collagen type IV at 1 day of force application, at both the tension and pressure sides. These data suggest that both tensional and compressive forces could affect basal lamina integrity. From 7 to 30 days, the staining pattern for collagen type IV increased at both the tension and pressure side; this suggests that the application of forces could promote angiogenesis processes order to restore vascularization. Moreover, the OTM leads to a decrease in blood flow, resulting in inflammation and hypoxia (which are closely related), regulating angiogenesis events (Feller et al., 2015). Finally, our results on the double-localization between collagen type IV and the CD31 staining pattern, showing these proteins around the vessels, confirmed that angiogenesis is finished at 30 days, when the vascular wall becomes impermeable.
Our results on VEGF, observed in both sides, are in accordance with reports demonstrating that the mechanical forces stimulate the production of VEGF (Kaku et al., 2001; Salomão et al., 2014). It has been reported that the application of orthodontic force produced significant changes in blood vessel number (Lew, 1989). From our results showing a decrease in VEGF staining pattern from 7 to 14 days at both pressure and tension sides, we can hypothesize that activity of this protein is reduced and consequently also that its role in bone remodelling and in promoting angiogenesis processes, during initial application of force, is reduced. However, from 14 to 30 days, the VEGF staining pattern increased, at both pressure and tension sides, suggesting that regenerative processes exist in this phase, with formation of new bone and vascular structures. On this basis, we suppose that during treatment, severe neoangiogenesis is established. The double-localization reaction between VEGF and CD31 confirm the integrity of vascular endothelium.
Our present results, obtained on human PDL, demonstrate that the application of light force allows orthodontic movement until 30 days, without damage to the protein architecture of the ligament.
Declarations
Author contribution statement
Angela Militi: Conceived and designed the experiments; Performed the experiments.
Giuseppina Cutroneo: Conceived and designed the experiments.
Angelo Favaloro: Conceived and designed the experiments; Wrote the paper.
Giovanni Matarese, Debora Di Mauro, Fabiana Nicita: Contributed reagents, materials, analysis tools or data.
Floriana Lauritano, Antonio Centofanti, Alessia Bramanti; Analyzed and interpreted the data. Gabriele Cervino, Giuseppina Rizzo: Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Anastasi G., Cutroneo G., Santoro G., Arco A., Rizzo G., Trommino C. Integrins, muscle agrin and sarcoglycans during muscular inactivity conditions: an immunohistochemical study. Eur. J. Histochem. 2006;50:327–336. [PubMed] [Google Scholar]
- Anastasi G., Cordasco G., Matarese G., Rizzo G., Nucera R., Mazza M. An immunohistochemical, histological, and electron-microscopic study of the human periodontal ligament during orthodontic treatment. Int. J. Mol. Med. 2008;21:545–554. [PubMed] [Google Scholar]
- Anastasi G., Cutroneo G., Rizzo G., Favaloro A. Sarcoglycan subcomplex in normal and pathological human muscle fibers. Eur. J. Histochem. 2007;51:15–19. [PubMed] [Google Scholar]
- Baumrind S.A. Reconsideration of the property of the pressure tension hypothesis. Am. J. Orthod. 1969;55:12–22. doi: 10.1016/s0002-9416(69)90170-5. [DOI] [PubMed] [Google Scholar]
- Berkovitz B.K. Periodontal ligament: structural and clinical correlates. Dent. Update. 2004;31:46–50. doi: 10.12968/denu.2004.31.1.46. [DOI] [PubMed] [Google Scholar]
- Bhaskar S.N. Mosby Year Book; St.Louis, MO: 1990. Periodontal Ligament. Orban's Oral Histology and Embryology; pp. 203–238. [Google Scholar]
- Bruckner P., van der Rest M. Structure and function of cartilage collagens. Microsc. Res. Tech. 1994;28:378–384. doi: 10.1002/jemt.1070280504. [DOI] [PubMed] [Google Scholar]
- Bumann A., Carvalho R.S., Schwarzer C.L., Yen E.H. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur. J. Orthod. 1997;19:29–37. doi: 10.1093/ejo/19.1.29. [DOI] [PubMed] [Google Scholar]
- Burstone C.J. The biomechanics of tooth movement. In: Kraus B.S., Riedel R.A., editors. Vistas in Orthodontics. Lea and Febiger; Philadelphia: 1962. [Google Scholar]
- Chen J., Li W., Swain M.V., Ali Darendeliler M., Li Q. A periodontal ligament driven remodeling algorithm for orthodontic tooth movement. J. Biomech. 2014;47:1689–1695. doi: 10.1016/j.jbiomech.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Cheng L.L., Turk T., Elekdag-Turk S., Jones A.S., Petrocz P., Darendeliler M.A. Physical properties of root cementum: part 13. Repair of root resorption 4 and 8 weeks after application of continuous light and heavy forces for 4 weeks: a microcomputed-tomography study. Am. J. Orthod. Dentofacial Orthop. 2009;136:320–321. doi: 10.1016/j.ajodo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Cutroneo G., Centofanti A., Speciale F., Rizzo G., Favaloro A., Santoro G., Bruschetta D., Milardi D., Micali A., Di Mauro D. Sarcoglycan complex in masseter and sternocleidomastoid muscles of baboons: an immunohistochemical study. Eur. J. Histochem. 2015;59:2509. doi: 10.4081/ejh.2015.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ponte F.S., Falzea R., Runci M., Siniscalchi E.N., Lauritano F., Bramanti E., Cervino G., Cicciu M. Histomorphological and clinical evaluation of maxillary alveolar ridge reconstruction after craniofacial trauma by applying combination of allogeneic and autogenous bone graft. Chin. J. Traumatol. 2017;20:14–17. doi: 10.1016/j.cjtee.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico M., D'apuzzo F., Feola A., Cito L., Monsurrò A., Pierantoni G.M., Berrino L., De Rosa A., Polimeni A., Perillo L. Cytokines and VEGF induction in orthodontic movement in animal models. J. Biomed. Biotechnol. 2012;201689:2012. doi: 10.1155/2012/201689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mauro D., Gaeta R., Arco A., Milardi D., Lentini S., Runci M., Rizzo G., Magaudda L. Distribution of costameric proteins in normal human ventricular and atrial cardiac muscle. Folia Histochem. Cytobiol. 2009;47:605–608. doi: 10.2478/v10042-009-0114-z. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J.A., Furthmay H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J. Mol. Biol. 1981;150:97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Feller L., Khammissa R.A., Schechter I., Thomadakis G., Fourie J., Lemmer J. Biological events in periodontal ligament and alveolar bone associated with application of orthodontic forces. Sci. World J. 2015 doi: 10.1155/2015/876509. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Gerber H.P., Lecouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2009;29:789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- Fiorillo L., Cervino G., Herford A.S., Lauritano F., D'Amico C., Lo Giudice R., Laino L., Troiano G., Crimi S., Cicciù M. Interferon crevicular fluid profile and correlation with periodontal disease and wound healing: a systemic review of recent data. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant P.R., Cho M.I. Autoradiographic evidence of the co-ordination of the genesis of sharpey’sfibres with new bone formation in the periodontium of the mouse. J. Periodontal. Res. 1979;14:107–114. doi: 10.1111/j.1600-0765.1979.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Gianelly A.A. Force-induced changes in the vascularity of the periodontal ligament. Am. J. Orthod. 1969;55:5–11. doi: 10.1016/s0002-9416(69)90169-9. [DOI] [PubMed] [Google Scholar]
- Hazan-Molina H., Reznick A.Z., Kaufman H., Aizenbud D. Assessment of IL-1β and VEGF concentration in a rat model during orthodontic tooth movement and extracorporeal shock wave therapy. Arch. Oral Biol. 2013;58:142–150. doi: 10.1016/j.archoralbio.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Isola G., Ramaglia L., Cordasco G., Lucchese A., Fiorillo L., Matarese G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017;66:1–8. doi: 10.23736/S0926-4970.16.03995-3. [DOI] [PubMed] [Google Scholar]
- Kaku M., Kohno S., Kawata T., Fujita T., Tokimasa C., Tsutsui K., Tanne K. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. J. Dent. Res. 2001;80:1880–1883. doi: 10.1177/00220345010800100401. [DOI] [PubMed] [Google Scholar]
- Kaku M., Motokawa M., Tohma Y., Tsuka N., Koseki H., Sunagawa H., Arturo Marquez Hernandes R., Ohtani J., Fujita T., Kawata T. VEGF and M-CSF levels in periodontal tissue during tooth movement. Biomed. Res. 2008;29:181–187. doi: 10.2220/biomedres.29.181. [DOI] [PubMed] [Google Scholar]
- Kimmel D.B. A paradigm for skeletal strength homeostasis. J. Bone Miner. Res. 1993;8:515–522. doi: 10.1002/jbmr.5650081317. [DOI] [PubMed] [Google Scholar]
- Kohno T., Matsumoto T., Kanno Z., Warita H., Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J. Orthod. 2002;29:129–135. doi: 10.1093/ortho/29.2.129. [DOI] [PubMed] [Google Scholar]
- Kumar A.A., Saravanan K., Kohila K., Kumar S.S. Biomarkers in orthodontic tooth movement. J. Pharm. BioAllied Sci. 2015;7:S325–330. doi: 10.4103/0975-7406.163437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurol J., Owman-Moll P. Hyalinization and root resorption during early orthodontic tooth movement in adolescents. Angle Orthod. 1998;68:161–165. doi: 10.1043/0003-3219(1998)068<0161:HARRDE>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Lew K.K. Orthodontically induced microvascular injuries in the tension zone of the periodontal ligament. J. Nihon Univ. Sch. Dent. 1989;31:493–501. doi: 10.2334/josnusd1959.31.493. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Karring T., Lang N.P. In: Parodontologia Clinica e Odontoiatria Implantare. Edi-Ermes, editor. 2006. [Google Scholar]
- Maltha J.C., Van Leeuwen E.J., Dijkman G.E., Kuijpers-Jagtman A.M. Incidence and severity of root resorption in orthodontically moved premolars in dogs. Orthod. Craniofac. Res. 2004;7:115–121. doi: 10.1111/j.1601-6343.2004.00283.x. [DOI] [PubMed] [Google Scholar]
- Masella R.S., Meister M. Current concepts in the biology of orthodontic tooth movement. Am. J. Orthod. Dentofacial Orthop. 2006;129:458–468. doi: 10.1016/j.ajodo.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Matarese G., Ramaglia L., Fiorillo, Cervino G., Lauritano F., Isola G. Implantology and periodontal disease: the panacea to problem solving? Open Dent. J. 2017;11:460–465. doi: 10.2174/1874210601711010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M.J., Russell C.M., Schmid M.J., Reinhardt R.A. Impact of increased occlusal contact, interleukin-1 genotype, and periodontitis severity on gingival crevicular fluid IL-1beta levels. J. Periodontol. 2003;74:1302–1307. doi: 10.1902/jop.2003.74.9.1302. [DOI] [PubMed] [Google Scholar]
- Mendes R.T., Nguyen D., Stephens D., Pamuk F., Fernandes D., Hasturk H., Van Dyke T.E., Kantarci A. Hypoxia-induced endothelial cell responses - possible roles during periodontal disease. Clin Exp Dent Res. 2018;4:241–248. doi: 10.1002/cre2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Igarashi K., Saeki S., Shinoda H., Mitani H. Tooth movement and changes in periodontal tissue in response to orthodontic force in rats vary depending on the time of day the force is applied. Eur. J. Orthod. 2001;23:329–338. doi: 10.1093/ejo/23.4.329. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Kajiya H., Tsuzuki T., Takahashi Y., Okabe K. Differential induction of collagens by mechanical stress in human periodontal ligament cells. Arch. Oral Biol. 2010;55:981–987. doi: 10.1016/j.archoralbio.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Oono T., Specks U., Eckes B., Majewski S., Hunzelmann N., Timpl, Krieg T. Expression of type VI collagen mRNA during wound healing. J. Investig. Dermatol. 1993;100:329–334. doi: 10.1111/1523-1747.ep12470022. [DOI] [PubMed] [Google Scholar]
- Pilon J.J., Kuijpers-Jagtman A.M., Maltha J.C. Magnitude of orthodontic forces and rate of bodily tooth movement. An experimental study. Am. J. Orthod. Dentofacial Orthop. 1996;110:16–23. doi: 10.1016/s0889-5406(96)70082-3. [DOI] [PubMed] [Google Scholar]
- Reitan K., Kvam E. Comparative behavior of human and animal tissue during experimental tooth movement. Angle Orthod. 1971;41:1–14. doi: 10.1043/0003-3219(1971)041<0001:CBOHAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ren Y., Maltha J.C., Kuijpers-Jagtman A.M. The rat as a model for orthodontic tooth movement-a critical review and a proposed solution. Eur. J. Orthod. 2004;26:483–490. doi: 10.1093/ejo/26.5.483. [DOI] [PubMed] [Google Scholar]
- Roberts W.E., Ferguson D. Cell kinetics of the periodontal ligament. In: Norton L.A., Burstone C.J., editors. The Biology of Tooth Movement. CRC Press; London: 1984. pp. 55–69. [Google Scholar]
- Rygh P. Ultrastructural changes in the periodontal fibers and their attachment in rat molar periodontium incident to orthodontic tooth movement. Scand. J. Dent. Res. 1973;81:467–480. doi: 10.1111/j.1600-0722.1973.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Rygh P. Ultrastructural vascular changes in pressure zones of rat molar periodontium incident to orthodontic tooth movement. Scand. J. Dent. Res. 1972;80:307–321. doi: 10.1111/j.1600-0722.1972.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Salomão M.F., Reis S.R., Vale V.L., Machado C.V., Meyer R., Nascimento I.L. Immunolocalization of FGF-2 and VEGF in rat periodontal ligament during experimental tooth movement. Dental Press J Orthod. 2014;19:67–74. doi: 10.1590/2176-9451.19.3.067-074.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey E. The nature of tooth movement. Am. J. Orthod. 1973;63:292–314. doi: 10.1016/0002-9416(73)90353-9. [DOI] [PubMed] [Google Scholar]
- Tan S.D., Xie R., Klein-Nulend J., Van Rheden R.E., Bronkers A.L., Kujjpers-Jagtman A.M. Orthodontic force stimulates eNOS and INOS in rat ostecytes. J. Dent. Res. 2009;88:255–260. doi: 10.1177/0022034508330861. [DOI] [PubMed] [Google Scholar]
- Ten Cate A.R., Deporter D.A., Freeman E. The role of fibroblast in the remodeling of periodontal ligament during physiologic tooth movement. Am. J. Orthod. 1976;69:155–168. doi: 10.1016/0002-9416(76)90194-9. [DOI] [PubMed] [Google Scholar]
- Thilander B., Rygh P., Reitan K. Tissue reaction in orthodontics. In: Graber T.M., Vanarsdall R., Vig K.W.L., editors. Vol. 4. Louid Elsevier; St: 2005. pp. 145–219. (Orthodontics: Current Principles and Techniques). [Google Scholar]
- Timpl R., Wiedemann H., Van Delden V., Furthmayr H., Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur. J. Biochem. 1981;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Yamada K.M. Cell surface interactions with extracellular materials. Annu. Rev. Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Zainal Ariffin S.H., Yamamoto Z., Zaionol Abidin I.Z., Megat Abdul Wahab R., Zainal Ariffin Z. Cellular and molecular changes in orthodontic tooth movement. ScientificWorldJournal. 2011;11:1788–1803. doi: 10.1100/2011/761768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Fan Y., Bai D., Wang J., Li Y. The adaptive response of periodontal ligament to orthodontic force loading – a combined biomechanical and biological study. Clin. Biomech. 2008;23:59–66. doi: 10.1016/j.clinbiomech.2007.10.016. [DOI] [PubMed] [Google Scholar]