Abstract
Background
Impaired cognitive flexibility has been implicated in the genetic basis of obsessive-compulsive disorder (OCD). Recent endophenotype studies of OCD showed neural inefficiency in the cognitive control network and interference by the limbic network of the cognitive control network. Exploring the relationship between the functional brain network and impaired cognitive flexibility may provide novel information about the neurobiological basis of OCD.
Methods
We obtained resting-state functional magnetic resonance imaging (rsfMRI) scans and measured the cognitive flexibility of 37 medication-free OCD patients and 40 healthy control (HC) participants using the Wisconsin Card Sorting Test (WCST). We explored the difference between OCD and HC groups in the functional brain network related to impaired cognitive flexibility from the amygdala and dorsal striatal regions of interest (ROIs) by using a seed-based approach.
Results
Significant differences between the OCD and HC groups were identified in the resting state functional network from the dorsal caudate. Increased functional connectivity from the dorsal caudate to the dorsal anterior cingulate cortex (dACC) and anterior insula (AI) was associated with poorer cognitive flexibility in the OCD group, but better cognitive flexibility in the HC group.
Conclusions
These results provide evidence that the impaired cognitive flexibility of OCD may be associated with dysfunctions of the brain network from the dorsal caudate (DC) to important nodes of the salience network. Our results extend the neuropsychological model of OCD by showing intrinsically different associations between OCD and HC in functional network and cognitive flexibility.
Keywords: Obsessive-compulsive disorder, Cognitive flexibility, Salience network, Dorsal caudate, Functional connectivity, Resting state functional MRI
Highlights
-
•
Increased functional connectivity from the dorsal caudate to the dorsal anterior cingulate cortex and anterior insula was associated with poorer cognitive flexibility in the OCD group, but better cognitive flexibility in the HC group.
-
•
Our results may suggest that the dysfunction from DC to SN is associated with impaired cognitive flexibility of OCD.
-
•
These findings could provide additional insights into the important role of cooperative interactions between the dorsal striatum and the large-scale intrinsic brain networks in human cognitive function.
1. Introduction
Obsessive-compulsive disorder (OCD) is characterized by persistent intrusive thoughts and repetitive actions. Conventionally, evidence of brain alterations in OCD has been presented under a cortico-striato-thalamo-cortical circuit (CSTC) model that includes the frontal cortex, striatum, and thalamus as core implicated structures (Saxena and Rauch, 2000). While it has undergone some modifications (Menzies et al., 2008; Milad and Rauch, 2012), this model is still considered to be a conventional neuropsychological model of OCD. Persistent clinical symptoms are considered to arise from cognitive dysfunction that reflects the neural basis of this model. Recently, it has gradually become clear that the limbic network also could interfere with cognitive function in OCD patients and the relatives of the patients with OCD (De Vries et al., 2014; van Velzen et al., 2015). Several neuropsychological studies support the hypothesis that the cognitive dysfunction of OCD relies on these CSTC and limbic networks, especially focusing on the dorsal striatum (dorsal caudate and putamen) and amygdala (De Vries et al., 2014; Milad and Rauch, 2012; Pauls et al., 2014; Vaghi et al., 2017; van Velzen et al., 2015).
A number of functional neuroimaging studies have shown the importance of large-scale intrinsic brain networks to understand human brain function (Dosenbach et al., 2008; Fox and Raichle, 2007). The important finding is that these resting-state functional brain networks can be associated with cognitive processes and can predict many cognitive functions (Keller et al., 2015; Rosenberg et al., 2016; Yamashita et al., 2015). The salience network (SN) and frontoparietal network (FPN) are representative large-scale intrinsic brain networks for top-down control over goal-directed behavior (Dosenbach et al., 2008; Menon, 2011). Additionally, striatal subregions are functionally coupled with these large-scale functional networks (Choi et al., 2012), and dynamic cooperative and competitive interactions between cortical large-scale networks and the striatum may realize appropriate cognitive control and behavior (Cocchi et al., 2014; Rieckmann et al., 2018). There is much evidence of involvement of the dorsal anterior cingulate cortex (dACC) and anterior insula (AI), both of which are central nodes of SN, in OCD based on mega-analysis of structural changes (De Wit et al., 2014). In recent meta-analyses using rsfMRI, OCD patients exhibited aberrant functional connectivity from FPN to SN, striatum, and thalamus (Gursel et al., 2018).
Genetic and endophenotype studies have shown that OCD patients and their unaffected relatives share a common genetic linkage and neural basis (Cavedini et al., 2010; Grados et al., 2003; Wendland et al., 2009). According to Gottesman, an endophenotype is defined as “a measurable trait along the path between phenotype and distal genotype, reflecting a simpler clue to the genetic basis of a disorder than the syndrome itself” (Gottesman and Gould, 2003). A few cognitive dysfunctions and underlying brain functions, for example in working memory, cognitive flexibility, and response inhibition, and their neural bases, shared by OCD patients and their relatives are considered to be a candidate endophenotype of OCD (Bey et al., 2018; Cavedini et al., 2010; Chamberlain et al., 2007; De Vries et al., 2014; De Wit et al., 2012; van Velzen et al., 2015). A recent neurocognitive model of OCD considers that inflexibility and difficulties of inhibition are key characteristics of OCD (Fineberg et al., 2014; Robbins et al., 2012), and that unaffected relatives of OCD patients also could have these deficits (Cavedini et al., 2010; Chamberlain et al., 2007; Rajender et al., 2011). Although OCD patients have many cognitive dysfunctions (Abramovitch et al., 2013), impaired cognitive flexibility might be one of the important key traits to understand the neural basis of the disorder. Cognitive flexibility is a multidimensional cognitive function that includes salience detection, working memory, attention, and inhibition, and is controlled by important nodes of the large-scale brain network (Dajani and Uddin, 2015). The Wisconsin Card Sorting Test (WCST) has been one of the most commonly used tests of cognitive flexibility (set-shifting). A previous task-fMRI study using WCST showed that the dorsal caudate and dACC were activated specifically when receiving negative feedback that signals a need for set-shifting (Monchi et al., 2001). Furthermore, AI and dACC, as central nodes of SN, play a critical role in detecting salience (Seeley et al., 2007) and modulating large-scale network cooperation (Menon, 2011), both of which are also essential processes to achieve cognitive flexibility. In healthy individuals, temporal flexibility of SN predicted the performance of cognitive flexibility (Chen et al., 2016), and increased functional connectivity of SN was positively correlated with cognitive flexibility (Muller et al., 2015). Recent meta-analyses of rsfMRI showed that OCD patients exhibited general dysconnectivity from FPN to SN, striatum, and thalamus (Gursel et al., 2018). Unaffected relatives of OCD patients also exhibited increased activity of SN (de Vries et al., 2017) and of the brain network including dorsal caudate (Hou et al., 2014). Cognitive dysfunctions as an endophenotype of OCD may be related to altered function of these brain networks.
In OCD, hyperactivation during many cognitive tasks has been suggested in previous studies (Henseler et al., 2008; van den Heuvel et al., 2005; Yücel et al., 2007). Additionally, both OCD patients and their relatives showed hyperactivation of cognitive control networks (DLPFC, parietal cortex, dACC, and pre-supplementary motor cortex, constituting FPN and SN) even in low load cognitive processing, which is thought to reflect neural inefficiency as a genetic vulnerability of OCD (De Vries et al., 2014). Furthermore, recent task-fMRI studies of OCD patients and their unaffected relatives reported increased functional connectivity between those cognitive networks and the amygdala (De Vries et al., 2014; van Velzen et al., 2015). In healthy individuals, the importance of cognitive-emotional interactions in the brain has been suggested (Okon-Singer et al., 2015; Pessoa, 2017), and a previous study using task-fMRI showed a negative correlation between cognitive flexibility and amygdala activation during down-regulation of negative emotions (Zaehringer et al., 2018). These previous studies suggested that neural inefficiency in the cognitive control networks and interference from the amygdala to these networks could be a genetic vulnerability of OCD. Recently, it is increasingly clear that there is a strong relationship between task-evoked functional connectivity and resting-state functional connectivity, and that the characteristics of a functional network during a task are shaped by a functional network in the resting state (Cole et al., 2014). Therefore, unlike healthy individuals, OCD patients with the cognitive dysfunction may have a distinctive neural basis in the resting state, which could reflect neural inefficiency of the cognitive control networks or abnormal recruitment of the amygdala during cognitive tasks. The importance of the approach to investigate the brain-behavior relationship (for example, between dysfunction in neural circuits and cognitive dysfunction) has been proposed to understand mental illness as a dysfunction of brain networks (Insel et al., 2010). A varying approach could be useful to clarify the dysfunction in biological system, and there have been important findings from the studies focusing the correlation between cognitive function and patterns of brain network in psychosis related disorder (Kristensen et al., 2019) or major depressive disorder (Albert et al., 2019). In OCD, despite the importance of the relationship between the functional network during rest and impaired cognitive flexibility as a genetic vulnerability, few studies have directly investigated these neural correlates. It remains unclear whether cognitive inflexibility has different neural bases in healthy individuals and patients with OCD.
In this study, we focused on the impaired cognitive flexibility (set-shifting impairments) measured by WCST that might be implicated in OCD patients and their unaffected relatives. So far, there is some evidence that the dorsal striatum and amygdala play an important role in the cognitive dysfunction of OCD (De Vries et al., 2014; Vaghi et al., 2017). While previous studies have examined differences in functional connectivity between diagnostic groups, few have examined the differences in the relationship between functional brain networks and the cognitive function. It still remains unclear whether cognitive inflexibility in OCD and healthy individuals has different neural bases during the resting state. Exploring the relationship between cognitive inflexibility and resting state brain network may provide novel information about the neurobiological basis of OCD. We hypothesized that, reflecting neural inefficiency, the OCD group would have abnormal relationships (non- or lower degree negative correlation) between the poorer performance of cognitive flexibility and the functional connectivity from the dorsal striatum to SN. Additionally, we also hypothesized that, reflecting aberrant interference from the limbic network, the OCD group would have abnormal relationships (positive correlation) between the poorer performance of cognitive flexibility and functional connectivity from the amygdala to SN.
2. Methods
2.1. Subjects
A total of 79 participants were recruited for this study, including 38 drug-free OCD patients and 41 healthy controls (HC) matched for age and sex. All OCD patients were diagnosed primarily using the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID) and fulfilled DSM-IV criteria. We ensured that none of them met the criteria for any current comorbid Axis I disorder and that all of them also fulfilled DSM-5 criteria. No OCD participant had taken any psychiatric medication for at least 4 weeks, and nine patients were drug-naïve.
HC subjects were recruited from the local community and interviewed according to the Structured Clinical Interviewed for DSM-IV non-patient Edition (SCID-NP). None of them had ever suffered any psychiatric disorder.
Candidates who had a lifetime history of significant head injury, seizure disorder, or intellectual disability were excluded. All of the participants were medication-free for at least 4 weeks. All participants completed the MRI scan, clinical assessment, and neuropsychological test within a few hours on the same day. This study was approved by the Kyushu University Ethics Committee. All participants provided written informed consent prior to study commencement.
2.2. Clinical assessment
To assess the global severity of OCD symptoms, we used the Japanese version of the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (Nakajima et al., 1995). No OCD patient was excluded due to Y-BOCS severity scores. The Hamilton Ratings Scales for Anxiety (HAM-A) (Hamilton, 1959) and Depression (HAM-D, 17-item version) (Hamilton, 1960) were also used to quantify the degree of anxiety and depression. The Japanese version of the National Adult Reading test (JART) (Matsuoka et al., 2006) was administered to estimate a participant's verbal intelligence quotient (IQ) (Table 1). Four trained and experienced clinical psychiatrists performed all the clinical assessments and neuropsychological tests. Demographic and clinical data were statistically analyzed using χ2, Student's t-test, and the Mann-Whitney U test to detect group differences between OCD and HC.
Table 1.
Demographic and clinical characteristics of the participants
| Variable | OCD (n = 37) | HC (n = 40) | Statistics |
||||
|---|---|---|---|---|---|---|---|
| χ2 | t | u | df | p Value | |||
| Demographic and clinical characteristics | |||||||
| Sex, male/female | 16/21 | 17/23 | 0.004 | 1 | .948 | ||
| Handedness, right/left | 33/4 | 39/1 | 2.186 | 1 | .139 | ||
| Age, years | 33.49 (11.41) | 35.48 (11.11) | −0.765 | 75 | .447 | ||
| Estimated verbal IQa | 103.56 (8.90)b | 107.83 (10.03) | −1.928 | 74 | .580 | ||
| HAM-D-17 | 4.41 (4.37) | 0.23 (0.58) | 5.694 | 37.13 | .000⁎ | ||
| HAM-A | 4.68 (5.18) | 0.28 (0.75) | 5.052 | 37.36 | .000⁎ | ||
| Y-BOCS total | 24.57 (6.11) | – | |||||
| Y-BOCS obsessions | 12.22 (3.60) | – | |||||
| Y-BOCS compulsions | 12.35 (3.32) | – | |||||
| Onset, years | 20.76 (8.03) | – | |||||
| Duration of disease, years | 13.73 (12.91) | – | |||||
| Stanford Sleepiness Scale | 3.44 (1.54) | 3.13 (1.38) | 0.942 | 74 | .349 | ||
| WCST-%PE | 15.79 (14.3) | 7.415 (15.84) | 419.000 | .001⁎ | |||
WCST-%PE is expressed as median (interquartile range:IQR). Other variables are expressed as mean (standard deviation: SD), or n/n, as appropriate.
HAM-A, Hamilton Anxiety Scale; HAM-D, Hamilton Depression Scale; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale; WCST, Wisconsin Card Sorting Test.
Estimated verbal IQ was measured with the Japanese version of National Adult Reading Test (JART).
One participant did not complete JART.
p < .01.
2.3. Neuropsychological assessment
Cognitive flexibility, which is the ability to modulate action and thought according to the situation, was tested using the Wisconsin Card Sorting Test (WCST) (Alvarez and Emory, 2006) adopted by many previous studies. Previous studies report that not only individuals with OCD but also their relatives have a high percentage of perseverative errors indicating a lack of mental flexibility. Thus, impaired cognitive flexibility is thought to be one of the endophenotypes as a genetic basis of OCD symptoms (Cavedini et al., 2010; Rajender et al., 2011). To elucidate the functional network associated with the cognitive inflexibility, we used a Japanese computerized version of the Wisconsin Card Sorting Test, the Keio Version, which is standardized and commonly used in Japan (Hori et al., 2006; Kimura et al., 2015; Takahashi et al., 2008). Four kinds of cards different from each other in color, number, and shape shown are shown in the upper part of the computer screen, while there is one card in the lower part of the computer screen. Participants were instructed to match the card following the three possible criteria: color, number, or shape. The correct criteria on how to match the card was not known to the participants before the pairing selection. The computer provides feedback whether their choice was right or wrong after each pairing selection, and all sets of cards change after every trial. In this study, we selected the percentage of perseverative errors of the Nelson type (%PE), which is the percentage of incorrect responses by selecting the same category chosen immediately before in spite of the negative feedback, as the main outcome.
2.4. Image data acquisition and preprocessing
All participants underwent MRI scanning on a 3.0-Tesla MRI scanner (Achieva TX, Phillips Healthcare, Best, The Netherlands) equipped with standard phased array head coils. A T2*-weighted gradient-echo echo-planar imaging (EPI) sequence (echo time (TE), 30 ms; repetition time (TR), 2500 ms; field of view (FOV), 212 × 212 mm; matrix, 64 × 64; slice thickness, 3.2 mm; flip angle, 80°) was acquired from each participant. During a 10-min real scan after an initial 10-s dummy scan, we completed 240 real scans. During a resting-state fMRI scan, participants were instructed to relax with their eyes open and keep watching a grey cross on the screen. High-resolution T1-weighted anatomical images were also acquired (TE = 3.8 ms; TR = 8.2 ms; FOV 240 × 240 mm; flip angle 8°; slice thickness, 1 mm; inversion time = 1026 ms) after each EPI image scan. After acquisition of all MRI image data, we used the Stanford-Sleepiness Scale to check the arousal level during the scan of all participants (Table 1).
We used the CONN toolbox 17.f (http://www.nitrc.org/projects/conn) running on MATLAB R2016b version 9.1.0 (MathWorks, Inc., Natick, MA, USA) on MacOS 10.12.6 to analyze functional connectivity. After discarding the first four volumes, the remaining 236 volumes were preprocessed using the CONN toolbox default preprocessing parameters. Functional images were slice timing corrections based on the slice order, and realigned and normalized in accordance with the standard Montreal Neurological Institute (MNI) template. The six rigid-body parameters (translational and rotational) were estimated for each subject. The ART scrubbing procedure (https://www.nitrc.org/projects/artifact_detect/) was applied to exclude image artifacts due to head movement using the 97th percentile in a normative sample (with thresholds for motion = 0.9 mm and global signal z = 5). Signal noise from the white matter and cerebrospinal fluid were discerned. Next, fMRI data were band-pass filtered at 0.008–0.09 Hz, and all functional images were smoothed using a Gaussian kernel of 6-mm full width at half-maximum.
During image acquisition, one OCD patient was excluded due to extreme artifacts.
There was no significant difference between OCD and HC groups in motion parameters (max FD [t = 1.45 p = .149] and mean FD [t = 0.90 p = .368]).
2.5. Data analysis
We used predefined spherical seed regions-of-interest (ROIs) of the dorsal caudate (DC) and posterior putamen (PUT) as described in the literature (Vaghi et al., 2017). An ROI was created in each hemisphere as a 3 mm radius sphere (diameter = 6 mm), and MNI coordinates were centered at DC (±12,6,14), PUT (±24,0,3). Amygdala ROIs were created from the AAL Harvard-Oxford atlas supplied by the CONN toolbox.
Following the preprocessing steps, the blood‑oxygen-level-dependent (BOLD) signal time series correlation was calculated between each pair of sources for each participant across the resting-state time series, and then a Fisher z transformation was applied. Seed-based connectivity maps were generated from each seed ROI for each participant.
Then, to test our hypothesis, we examined the difference between the OCD and HC groups in functional connectivity associated with %PE by using an analysis of covariance (ANCOVA) model (using non-parametric analyses (permutation/randomization analyses), statistical significance was set at a voxel height threshold of p < .001, and the cluster-size threshold of p < .05 false discovery rate (FDR) corrected) implemented in the CONN toolbox (https://web.conn-toolbox.org). We compared the functional connectivity associated with %PE between OCD and HC, while controlling for age, gender, and verbal IQ (these variables are commonly controlled for when examining the relationship of a brain network and behavior (Albert et al., 2019; Cha et al., 2015; Posner et al., 2017)). Connectivity values for significantly different clusters between groups were examined to determine the direction and coefficient of correlation with %PE for each group. Supplementarily, to examine whether these networks were specifically associated with impaired cognitive flexibility or overlapped with a brain network associated with other cognitive deficits, we performed additional ANCOVA to explore functional networks associated with non-PE related scores in WCST. Following the previous literatures (Aizawa et al., 2012; Nyhus and Barcelo, 2009; Wang et al., 2018), difficulties of maintenance set (DMS: reflect response consistency associated with memory and sustained attention), efficient errors (reflect efficiency of try and error process of hypothesis testing necessary to successfully execute the task) and numbers of response cards until the first category was achieved (NUCA: reflects conceptual ability) were used as covariates of interest in additional ANCOVA. We also controlled for age, gender, verbal IQ, and %PE scores in these additional ANCOVA. Statistical significance was set at a voxel height threshold of p < .001, and the cluster-size threshold of p < .05 false discovery rate (FDR) was corrected by using non-parametric analyses (permutation/randomization analyses).
3. Results
3.1. Clinical characteristics
One OCD patient was excluded due to extreme artifacts during image acquisition, and one HC participant was excluded for an outlier %PE score (above the third quartile +1.5 × interquartile range (IQR)). Therefore, this study included 37 Japanese obsessive-compulsive disorder (OCD) patients (age 33.49 ± 11.41 (mean ± SD), 16 men and 21 women) and 40 HC participants (age 35.48 ± 11.00 (mean ± SD), 17 men and 23 women). OCD groups did not differ from HC groups in terms of age, gender, handedness, or estimated verbal IQ (Table 1). All participants had been medication-free for at least 4 weeks, and nine OCD patients were drug-naïve. The mean (SD) scores of Y-BOCS of the OCD group were 24.57 ± 6.11 (mean ± SD), showing a moderate degree of severity in OCD symptoms. During the acquisition of MRI data, no participant fell asleep, and there was no significant difference between OCD and HC groups in arousal level (Table 1).
3.2. Between-group differences in WCST
We found a significant difference in the %PE of the OCD group compared with the HC group [u = 419.000, p = .001] (Table 1) on WCST. OCD patients performed worse than HC in set-maintenance (DMS) [u = 502.500, p = .012], conceptual ability (NUCA) [u = 487.500, p = .010] and efficiency of try and error process (efficient errors) [u = 423.500, p = .001]. To check the effects of severity of psychiatric symptoms on the WCST performance of the OCD group, we analyzed the correlation between %PE and Y-BOCS total scores, HAM-D scores, and HAM-A scores. Consequently, there was no significant association between %PE and these clinical scores (all p > .09).
3.3. Between-group differences in functional network associated with cognitive flexibility
We found group differences in the association between the functional connectivity from DC to AI and dACC and the impaired cognitive flexibility (a voxel height threshold of p < .001, and the cluster-size threshold of p < .05 FDR corrected) (Table 2, Fig. 1). In association with DMS, NUCA and efficient errors, the pattern of functional connectivity was not similar to that of %PE (Data in Brief: Supplemental Table S1).
Table 2.
Group difference in functional connectivity associated with impaired cognitive flexibility (%PE).
| Seed | Region | Ke | x | y | z | Direction | Effect size |
|---|---|---|---|---|---|---|---|
| L DC | R Dorsal anterior cingulate cortex | 227 | 00 | 32 | 28 | OCD > HC | 0.12 |
| R Anterior-insula | 232 | 46 | 24 | −6 | OCD > HC | 0.11 | |
| R DC | – | – | – | – | – | – | |
| L PUT | – | – | – | – | – | – | |
| R PUT | – | – | – | – | – | – | |
| L Amygdala | – | – | – | – | – | – | |
| R Amygdala | – | – | – | – | – | – |
Peak coordinates are given in MNI space. PUT, putamen; DC, dorsal caudate; L, left; R, right.
⁎ Cluster size after applying voxel height threshold of p < .001; cluster-corrected threshold of p < .05 false discovery rate (FDR) corrected.
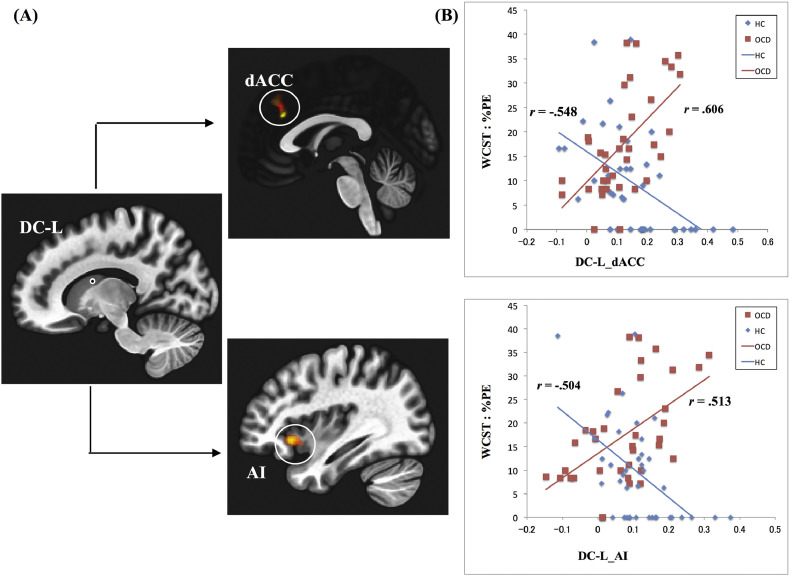
Fig. 1.
Resting state functional network from dorsal caudate and impaired cognitive flexibility in OCD patients and HC.
(A) Group difference in resting state functional network from the left dorsal caudate (L-DC) associated with impaired cognitive flexibility (cluster size corrected significance p < .05 FDR corrected, after applying a per-voxel height threshold of p < .001). L-DC, left dorsal caudate; dACC, dorsal anterior cingulate cortex; AI, anterior insula.
(B) Functional connectivity from DC and scores of cognitive flexibility (%PE) in the OCD and HC groups. r = Spearman's rank-order correlation coefficient.
In the OCD group, greater functional connectivity from DC to AI and dACC was associated with higher %PE (Fig. 1). Meanwhile, the HC group showed the inverse functional connectivity pattern associated with higher %PE (Fig. 1). After controlling for the DMS, NUCA and efficient errors in second-level ANCOVA analyses, these results remained unchanged. Complementarily, we examined between-group differences in functional connectivity from DC by using a two-sample t-test, but found no significant difference between OCD and HC (a voxel height threshold of p < .001, and the cluster-size threshold of p < .05 FDR corrected).
4. Discussion
OCD patients exhibited a more profound impairment of cognitive flexibility (set shift impairments) than HC. These results are consistent with previous findings (Cavedini et al., 2010; Chamberlain et al., 2007; Shin et al., 2014). The main findings of this study were that OCD patients have different associations between the abilities of cognitive flexibility and resting state functional networks from DC to dACC and AI when compared with HC (Table 2). Increased functional connectivity from DC to these brain regions was associated with poorer performance of cognitive flexibility in the OCD group, but better performance in the HC group (Table 2, Fig. 1). Controlling for non-PE related scores did not alter these results. As far as we know, this is the first study to show a different association between functional connectivity from DC to the central nodes of SN and impaired cognitive flexibility in HC and medication-free OCD patients.
Participants of this study exhibited no significant group differences in functional connectivity from DC between diagnostic groups, in line with a previous rsfMRI study of drug-free OCD (Sakai et al., 2011). These results are in contrast to a part of previous studies of OCD using rsfMRI. Several previous studies reported increased (Posner et al., 2014) or decreased functional connectivity (Vaghi et al., 2017) between DC and some cortical regions. This discrepancy may be due to a few reasons. It could be due to methodological differences in seed ROI placement and in the threshold of correction of multiple comparisons. Another factor could be due to confusion of medicated subjects, which could have widespread effects on functional connectivity (Posner et al., 2013). Additionally, different OCD symptom dimensions could have different functional connectivity patterns (Harrison et al., 2013). These factors could confuse the findings in the simple between-group comparisons of a part of previous studies.
Different from our hypothesis, there was no significant difference in the association between the patterns of functional connectivity from the amygdala and cognitive inflexibility in OCD compared to HC. Previous task fMRI studies of OCD reported increased amygdala activation during working memory and response inhibition tasks (De Vries et al., 2014; De Wit et al., 2012). However, aberrant activity in the limbic network might be especially apparent when experiencing OCD symptoms or during specific cognitive tasks, therefore, that influence may not appear in the resting state (van den Heuvel et al., 2016). Additionally, a previous rsfMRI study reported that alteration of the limbic network was especially associated with the aggression symptom dimension (Harrison et al., 2013). It is a possibility that the heterogeneity of our OCD patients and the characteristics of the cognitive flexibility task could have affected our results. Further research to explore the role of the amygdala during rest and during cognitive processing in the pathophysiology of many cognitive deficits in OCD is needed.
In our results, there were inverse relationships between patients and HC groups in resting state functional connectivity from DC and impaired cognitive flexibility. It is hypothesized that DC is more selectively associated with working memory and executive function; in contrast, PUT is more selectively associated with motor and response inhibition (Milad and Rauch, 2012; Robbins et al., 2012). In our HC, increased functional connectivity from DC to AI and dACC as central nodes of SN was associated with better performance (Table 2, Fig. 1). It is known that AI and dACC are thought to play an important role in detection of the external or internal saliency, error detection, conflict monitoring, modulating behavior, and coordinating dynamic neural network interactions (Ide et al., 2013; Jiang et al., 2015; Klein et al., 2013; Uddin, 2015; Uddin et al., 2011). A previous task-fMRI study using WCST also showed that DC and dACC are selectively activated when receiving negative feedback (Monchi et al., 2001). Furthermore, our HC results may agree with previous studies showing that stronger task-related functional connectivity of the CSTC circuit, including the dorsal striatum, was associated with better cognitive flexibility (Berry et al., 2018), and that functional connectivity of SN was positively correlated with cognitive flexibility (Muller et al., 2015). In contrast to the HC group, increased functional connectivity from DC to SN was associated with poorer performance of cognitive flexibility in the OCD group (Table 2, Fig. 1). A previous study on OCD showed that increased caudate activity was positively correlated with the errors on WCST (Lucey et al., 1997). Furthermore, altered brain networks including the caudate nucleus (Hou et al., 2014) and hyperactivation of cognitive control networks, even in low load cognitive processing (De Vries et al., 2014), could reflect neural inefficiency as a genetic vulnerability of OCD. It has been shown that SN modulates other large-scale intrinsic networks in a hierarchical setting (Zhou et al., 2018), especially that the right AI has a causal effect on important nodes of DMN and FPN (Uddin et al., 2011). It is a possibility that increased connectivity to SN has a different effect on the cognitive functions of healthy individuals and individuals with a genetic vulnerability to cognitive inflexibility. Based on our results, the increased connectivity in healthy individuals may reflect appropriate cooperation between cognitive control networks to improve cognitive flexibility during task processing. In contrast, brain dysfunction due to genetic vulnerability of OCD may disable the function of these compensatory mechanisms that target restoring network cooperation, and contrarily, may lead to an imbalance between other cognitive control functional networks that have a negative effect on cognitive flexibility as multidimensional cognitive functions. Additionally, many other abnormalities of large-scale brain networks in OCD (Gursel et al., 2018) may also lead to an imbalance in the context of these changes. In cognitive processes, these dysfunctions may affect especially the salience detection and error-related behavioral modules, because both of them are essential elements to guide cognitive flexibility (Fig. 2). However, we can't conclude whether our findings represent a genetic neural basis of the disorder or not. Further studies including not only OCD patients but also their first-degree unaffected relatives are needed to clarify whether these brain networks associated with cognitive flexibility represent a genetic basis of OCD.
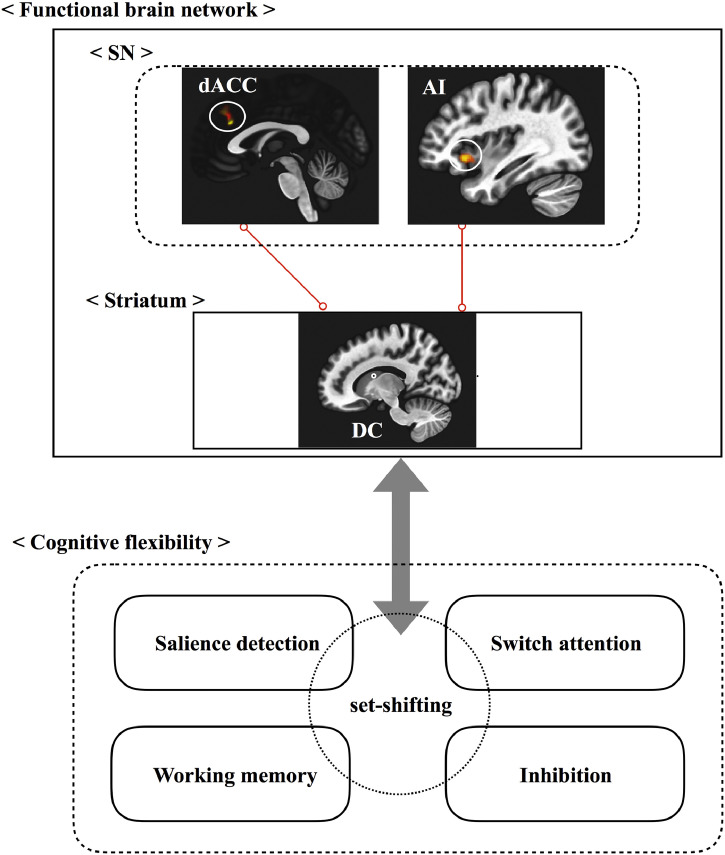
Fig. 2.
Resting state functional network and cognitive flexibility of OCD.
Increased functional connectivity from DC to SN may be associated with the set-shift impairments of OCD. Red line indicates increased connectivity. SN, salience network; DC, dorsal caudate; dACC, dorsal anterior cingulate cortex; AI, anterior insula.
The traditional CSTC circuit model associates certain pathways in the cooperation of direct and indirect pathways based on the cytoarchitecture of the striatum receiving projections from many cortical regions with a role in action selection and action inhibition (Benarroch, 2016). Beyond these classical parallel CSTC pathway models, it is gradually becoming clear that dynamic cooperative and competitive interactions of large-scale functional cortical networks and subcortical regions might realize appropriate cognitive control and behavior (Cerliani et al., 2015; Cocchi et al., 2013; Rieckmann et al., 2018). This is the first study to show different associations between functional connectivity from DC as the central node of dorsal cognitive circuits to SN and impaired cognitive flexibility in HC and medication-free OCD. This study could extend understanding of the mental flexibility necessary to generate complex human behaviors in response to external situations.
Several limitations are present in our study. First, though we measured cognitive flexibility by WCST, which mainly measures the set-shifting ability, WCST potentially includes various kinds of cognitive functions, such as working memory, inhibition, and attention. Therefore, it has been criticized for its non-specificity (Meiran et al., 2011). Moreover, a recent meta-analysis revealed a possibility that previous neuropsychological findings were not adequate to infer specific cognitive flexibility (Fradkin et al., 2018). In consideration of the difficulty of measuring cognitive flexibility, future work using the many types of cognitive functions that compose cognitive flexibility is needed. Second, we did not consider OCD symptom heterogeneity. Previous studies showed that different OCD symptom dimensions could cause different neuropsychological deficits (Hashimoto et al., 2011; Kashyap et al., 2017) and biological substrates (Harrison et al., 2013; Mataix-Cols et al., 2004). To identify the biological heterogeneity of symptomatology, we should examine the resting state network with a larger subject sample. Finally, we focused on impaired cognitive flexibility from previous endophenotype studies, but our study did not include unaffected siblings. Further studies including unaffected relatives are needed to clarify whether these brain characteristics underlying cognitive flexibility represent a genetic basis of OCD or are the result of a compensatory mechanism for OCD symptoms. Despite these limitations, our results extend the understanding of the neural bases of human mental flexibility. Future work should extend our findings by using a larger sample and other types of neurocognitive assessments.
5. Conclusion
In summary, we found that impaired cognitive flexibility in OCD patients and HC have different neural bases in the functional network from DC to SN. Our results may suggest that the dysfunction from DC to SN may represent a genetic vulnerability to OCD. These findings extend the neuropsychological model of OCD by showing that intrinsically different neural bases underlie the differences in cognitive flexibility between OCD patients and HC. These findings could provide additional insights into the important role of cooperative interactions between the dorsal striatum and the large-scale intrinsic brain networks in human cognitive function.
Declaration of competing interest
All authors declare no conflicts of interests.
Acknowledgments
Acknowledgement
We would like to thank Mayumi Tomita from Kurume University and Aikana Ohno and Sae Tsuruta from Kyushu University for their helpful advice on the statistical methods. Katherine Ono provided helpful advice with language. We would like to thank for advice on analysis of imaging data by JSPS KAKENHI Grant Number JP16H06280. This work was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant Number (C)16K10253, (C)19K08076).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.102004.
Appendix A. Supplementary data
Supplementary material
References
- Abramovitch A., Abramowitz J.S., Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin. Psychol. Rev. 2013;33:1163–1171. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Aizawa E., Sato Y., Kochiyama T., Saito N., Izumiyama M., Morishita J., Kanazawa M., Shima K., Mushiake H., Hongo M., Fukudo S. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology. 2012;143:1188–1198. doi: 10.1053/j.gastro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- Albert K.M., Potter G.G., Boyd B.D., Kang H., Taylor W.D. Brain network functional connectivity and cognitive performance in major depressive disorder. J. Psychiatr. Res. 2019;110:51–56. doi: 10.1016/j.jpsychires.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J.A., Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. Intrinsic circuits of the striatum: complexity and clinical correlations. Neurology. 2016;86:1531–1542. doi: 10.1212/WNL.0000000000002599. [DOI] [PubMed] [Google Scholar]
- Berry A.S., Shah V.D., Jagust W.J. The influence of dopamine on cognitive flexibility is mediated by functional connectivity in young but not older adults. J. Cogn. Neurosci. 2018;30:1330–1344. doi: 10.1162/jocn_a_01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey K., Kaufmann C., Lennertz L., Riesel A., Klawohn J., Heinzel S., Grutzmann R., Kathmann N., Wagner M. Impaired planning in patients with obsessive-compulsive disorder and unaffected first-degree relatives: evidence for a cognitive endophenotype. J. Anxiety Disord. 2018;57:24–30. doi: 10.1016/j.janxdis.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Cavedini P., Zorzi C., Piccinni M., Cavallini M.C., Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol. Psychiatry. 2010;67:1178–1184. doi: 10.1016/j.biopsych.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Cerliani L., Mennes M., Thomas R.M., Di Martino A., Thioux M., Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiatry. 2015;72 doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J., Fekete T., Siciliano F., Biezonski D., Greenhill L., Pliszka S.R., Blader J.C., Roy A.K., Leibenluft E., Posner J. Neural correlates of aggression in medication-naive children with ADHD: multivariate analysis of morphometry and tractography. Neuropsychopharmacology. 2015;40:1717–1725. doi: 10.1038/npp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S.R., Fineberg N.A., Menzies L.A., Blackwell A.D., Bullmore E.T., Robbins T.W., Sahakian B.J. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am. J. Psychiatr. 2007;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Cai W., Ryali S., Supekar K., Menon V. distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.Y., Yeo B.T., Buckner R.L. The organization of the human striatum estimated by intrinsic functional connectivity. J. Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L., Zalesky A., Fornito A., Mattingley J.B. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn. Sci. 2013;17:493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cocchi L., Halford G.S., Zalesky A., Harding I.H., Ramm B.J., Cutmore T., Shum D.H., Mattingley J.B. Complexity in relational processing predicts changes in functional brain network dynamics. Cereb. Cortex. 2014;24:2283–2296. doi: 10.1093/cercor/bht075. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Bassett D.S., Power J.D., Braver T.S., Petersen S.E. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani D.R., Uddin L.Q. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends Neurosci. 2015;38:571–578. doi: 10.1016/j.tins.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries F.E., De Wit S.J., Cath D.C., Van Der Werf Y.D., van der Borden V., van Rossum T.B., van Balkom A.J.L.M., van der Wee N.J.A., Veltman D.J., van den Heuvel O.A. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol. Psychiatry. 2014;76:878–887. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- de Vries F.E., de Wit S.J., van den Heuvel O.A., Veltman D.J., Cath D.C., van Balkom A.J.L.M., van der Werf Y.D. Cognitive control networks in OCD: a resting-state connectivity study in unmedicated patients with obsessive-compulsive disorder and their unaffected relatives. World J. Biol. Psychiatry. 2017:1–13. doi: 10.1080/15622975.2017.1353132. [DOI] [PubMed] [Google Scholar]
- De Wit S.J., De Vries F.E., Van Der Werf Y.D., Cath D.C., Heslenfeld D.J., Veltman E.M., Van Balkom A.J.L.M., Veltman D.J., van den Heuvel O.A. Presupplementary motor area hyperactivity during response inhibition- a candidate endophenotype of obsessive-compulsive disorder. Am. J. Psychiatr. 2012;169:1100–1108. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- De Wit S.J., Alonso P., Schweren L., Mataix-Cols D., Lochner C., Menchón J.M., Stein D.J., Fouche J.P., Soriano-Mas C., Sato J.R., Hoexter M.Q., Denys D., Nakamae T., Nishida S., Kwon J.S., Jang J.H., Busatto G.F., Cardoner N., Cath D.C., Fukui K., Jung W.H., Kim S.N., Miguel E.C., Narumoto J., Phillips M.L., Pujol J., Remijnse P.L., Sakai Y., Shin N.Y., Yamada K., Veltman D.J., Van Den Heuvel O.A. Multicenter voxel-based morphometry mega- analysis of structural brain scans in obsessive-compulsive disorder. Am. J. Psychiatr. 2014;171:340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg N.A., Chamberlain S.R., Goudriaan A.E., Stein D.J., Vanderschuren L.J., Gillan C.M., Shekar S., Gorwood P.A., Voon V., Morein-Zamir S., Denys D., Sahakian B.J., Moeller F.G., Robbins T.W., Potenza M.N. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectrums. 2014;19:69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fradkin I., Strauss A.Y., Pereg M., Huppert J.D. Rigidly applied rules? Revisiting inflexibility in obsessive compulsive disorder using multilevel meta-analysis. Clin. Psychol. Sci. 2018;6:481–505. [Google Scholar]
- Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatr. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grados M.A., Walkup J., Walford S. Genetics of obsessive-compulsive disorders: new findings and challenges. Brain Dev. 2003;25:S55–S61. doi: 10.1016/s0387-7604(03)90010-6. [DOI] [PubMed] [Google Scholar]
- Gursel D.A., Avram M., Sorg C., Brandl F., Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2018;87:151–160. doi: 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Cardoner N., Deus J., Alonso P., Lopez-Sola M., Contreras-Rodriguez O., Real E., Segalas C., Blanco-Hinojo L., Menchon J.M., Soriano-Mas C. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol. Psychiatry. 2013;73:321–328. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Nakaaki S., Omori I.M., Fujioi J., Noguchi Y., Murata Y., Sato J., Tatsumi H., Torii K., Mimura M., Furukawa T.A. Distinct neuropsychological profiles of three major symptom dimensions in obsessive–compulsive disorder. Psychiatry Res. 2011;187:166–173. doi: 10.1016/j.psychres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Henseler I., Gruber O., Kraft S., Krick C., Reith W., Falkai P. Compensatory hyperactivations as markers of latent working memory dysfunctions in patients with obsessive-compulsive disorder: an fMRI study. J. Psychiatry Neurosci. 2008;33:209–215. [PMC free article] [PubMed] [Google Scholar]
- Hori H., Noguchi H., Hashimoto R., Nakabayashi T., Omori M., Takahashi S., Tsukue R., Anami K., Hirabayashi N., Harada S., Saitoh O., Iwase M., Kajimoto O., Takeda M., Okabe S., Kunugi H. Antipsychotic medication and cognitive function in schizophrenia. Schizophr. Res. 2006;86:138–146. doi: 10.1016/j.schres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Hou J.M., Zhao M., Zhang W., Song L.H., Wu W.J., Wang J., Zhou D.Q., Xie B., He M., Guo J.W., Qu W., Li H.T. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J. Psychiatry Neurosci. 2014;39:304–311. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Shenoy P., Yu A.J., Li C.R. Bayesian prediction and evaluation in the anterior cingulate cortex. J. Neurosci. 2013;33:2039–2047. doi: 10.1523/JNEUROSCI.2201-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jiang J., Beck J., Heller K., Egner T. An insula-frontostriatal network mediates flexible cognitive control by adaptively predicting changing control demands. Nat. Commun. 2015;6:8165. doi: 10.1038/ncomms9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap H., Kumar J.K., Kandavel T., Reddy Y.C. Relationships between neuropsychological variables and factor-analysed symptom dimensions in obsessive compulsive disorder. Psychiatry Res. 2017;249:58–64. doi: 10.1016/j.psychres.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Keller J.B., Hedden T., Thompson T.W., Anteraper S.A., Gabrieli J.D., Whitfield-Gabrieli S. Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex. 2015;64:271–280. doi: 10.1016/j.cortex.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Tanaka S., Kushima I., Koide T., Banno M., Kikuchi T., Nakamura Y., Shiino T., Yoshimi A., Oya-Ito T., Xing J., Wang C., Takasaki Y., Aleksic B., Okada T., Ikeda M., Inada T., Iidaka T., Iwata N., Ozaki N. Association study of BCL9 gene polymorphism rs583583 with schizophrenia and negative symptoms in Japanese population. Sci. Rep. 2015;5 doi: 10.1038/srep15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T.A., Ullsperger M., Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Front. Hum. Neurosci. 2013;7:14. doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T.D., Mandl R.C.W., Raghava J.M., Jessen K., Jepsen J.R.M., Fagerlund B., Glenthoj L.B., Wenneberg C., Krakauer K., Pantelis C., Nordentoft M., Glenthoj B.Y., Ebdrup B.H. Widespread higher fractional anisotropy associates to better cognitive functions in individuals at ultra-high risk for psychosis. Hum. Brain Mapp. 2019 doi: 10.1002/hbm.24765. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey J.V., Burness C.E., Costa D.C., Gacinovic S., Pilowsky L.S., Ell P.J., Marks I.M., Kerwin R.W. Wisconsin Card Sorting Task (WCST) errors and cerebral blood flow in obsessive-compulsive disorder (OCD) Br. J. Med. Psychol. 1997;70:403–411. doi: 10.1111/j.2044-8341.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D., Wooderson S., Lawrence N., Brammer M.J., Speckens A., Phillips M.L. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Uno M., Kasai K., Koyama K., Kim Y. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (Kanji) compound words: Japanese version of national adult reading test. Psychiatry Clin. Neurosci. 2006;60:332–339. doi: 10.1111/j.1440-1819.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- Meiran N., Diamond G.M., Toder D., Nemets B. Cognitive rigidity in unipolar depression and obsessive compulsive disorder: examination of task switching, stroop, working memory updating and post-conflict adaptation. Psychiatry Res. 2011;185:149–156. doi: 10.1016/j.psychres.2010.04.044. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menzies L., Chamberlain S.R., Laird A.R., Thelen S.M., Sahakian B.J., Bullmore E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O., Petrides M., Petre M., Worsley K., Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J. Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller V.I., Langner R., Cieslik E.C., Rottschy C., Eickhoff S.B. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Struct. Funct. 2015;220:2401–2414. doi: 10.1007/s00429-014-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Nakamura M., Taga C., Yamagami S., Kiriike N., Nagata T., Saitoh M., Kinoshita T., Okajima Y., Hanada M. Reliability and validity of the japanese version of the yale–brown obsessive-compulsive scale. Psychiatry Clin. Neurosci. 1995;49:121–126. doi: 10.1111/j.1440-1819.1995.tb01875.x. [DOI] [PubMed] [Google Scholar]
- Nyhus E., Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H., Hendler T., Pessoa L., Shackman A.J. The neurobiology of emotion cognition interactions: fundamental questions and strategies for future research. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls D.L., Abramovitch A., Rauch S.L., Geller D.A. Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014;15:410–424. doi: 10.1038/nrn3746. [DOI] [PubMed] [Google Scholar]
- Pessoa L. A network model of the emotional brain. Trends Cogn. Sci. 2017;21:357–371. doi: 10.1016/j.tics.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Hellerstein D.J., Gat I., Mechling A., Klahr K., Wang Z., McGrath P.J., Stewart J.W., Peterson B.S. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Marsh R., Maia T.V., Peterson B.S., Gruber A., Simpson H.B. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2014;35:2852–2860. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Song I., Lee S., Rodriguez C.I., Moore H., Marsh R., Blair Simpson H. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2017;38:678–687. doi: 10.1002/hbm.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajender G., Bhatia M.S., Kanwal K., Malhotra S., Singh T.B., Chaudhary D. Study of neurocognitive endophenotypes in drug-naive obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr. Scand. 2011;124:152–161. doi: 10.1111/j.1600-0447.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- Rieckmann A., Johnson K.A., Sperling R.A., Buckner R.L., Hedden T. Dedifferentiation of caudate functional connectivity and striatal dopamine transporter density predict memory change in normal aging. Proc. Natl. Acad. Sci. U. S. A. 2018;115:10160–10165. doi: 10.1073/pnas.1804641115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W., Gillan C.M., Smith D.G., de Wit S., Ersche K.D. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Rosenberg M.D., Finn E.S., Scheinost D., Papademetris X., Shen X., Constable R.T., Chun M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 2016;19:165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Narumoto J., Nishida S., Nakamae T., Yamada K., Nishimura T., Fukui K. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur. Psychiatry. 2011;26:463–469. doi: 10.1016/j.eurpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Saxena S., Rauch S.L. Functional nenroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr. Clin. N. Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.Y., Lee T.Y., Kim E., Kwon J.S. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol. Med. 2014;44:1121–1130. doi: 10.1017/S0033291713001803. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Kato M., Takano H., Arakawa R., Okumura M., Otsuka T., Kodaka F., Hayashi M., Okubo Y., Ito H., Suhara T. Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J. Neurosci. 2008;28:12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghi M.M., Vertes P.E., Kitzbichler M.G., Apergis-Schoute A.M., van der Flier F.E., Fineberg N.A., Sule A., Zaman R., Voon V., Kundu P., Bullmore E.T., Robbins T.W. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol. Psychiatry. 2017;81:708–717. doi: 10.1016/j.biopsych.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel O.A., Veltman D.J., Groenewegen H.J., Cath D.C., van Balkom A.J., Hartskamp J.v., Barkhof F., Dyck R.v. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2005;62:301–309. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- van den Heuvel O.A., van Wingen G., Soriano-Mas C., Alonso P., Chamberlain S.R., Nakamae T., Denys D., Goudriaan A.E., Veltman D.J. Brain circuitry of compulsivity. Eur. Neuropsychopharmacol. 2016;26:810–827. doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- van Velzen L.S., de Wit S.J., Curcic-Blake B., Cath D.C., de Vries F.E., Veltman D.J., van der Werf Y.D., van den Heuvel O.A. Altered inhibition-related frontolimbic connectivity in obsessive-compulsive disorder. Hum. Brain Mapp. 2015;36:4064–4075. doi: 10.1002/hbm.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Jing J., Igarashi K., Fan L., Yang S., Li Y., Jin Y. Executive function predicts the visuospatial working memory in autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res. 2018;11:1148–1156. doi: 10.1002/aur.1967. [DOI] [PubMed] [Google Scholar]
- Wendland J.R., Moya P.R., Timpano K.R., Anavitarte A.P., Kruse M.R., Wheaton M.G., Ren-Patterson R.F., Murphy D.L. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2009;66:408–416. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Kawato M., Imamizu H. Predicting learning plateau of working memory from whole-brain intrinsic network connectivity patterns. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep07622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M., Harrison B.J., Wood S.J., Fornito A., Wellard R.M., Pujol J., Clarke K., Phillips M.L., Kyrios M., Velakoulis D., Pantelis C. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Zaehringer J., Falquez R., Schubert A.L., Nees F., Barnow S. Neural correlates of reappraisal considering working memory capacity and cognitive flexibility. Brain Imaging Behav. 2018;12:1529–1543. doi: 10.1007/s11682-017-9788-6. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Friston K.J., Zeidman P., Chen J., Li S., Razi A. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb. Cortex. 2018;28:726–737. doi: 10.1093/cercor/bhx307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material