Abstract
Background
This double-blind study assessed immunogenicity, lot consistency, and safety of recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine (rVSVΔG-ZEBOV-GP).
Methods
Healthy adults (N = 1197) were randomized 2:2:2:2:1 to receive 1 of 3 consistency lots of rVSVΔG-ZEBOV-GP (2 × 107 plaque-forming units [pfu]), high-dose 1 × 108 pfu, or placebo. Antibody responses pre-/postvaccination (28 days, 6 months; in a subset [n = 566], months 12, 18, and 24) were measured. post hoc analysis of risk factors associated with arthritis following vaccination was performed.
Results
ZEBOV-GP enzyme-linked immunosorbent assay (ELISA) geometric mean titers (GMTs) increased postvaccination in all rVSVΔG-ZEBOV-GP groups by 28 days (>58-fold) and persisted through 24 months. The 3 manufacturing lots demonstrated equivalent immunogenicity at 28 days. Neutralizing antibody GMTs increased by 28 days in all rVSVΔG-ZEBOV-GP groups, peaking at 18 months with no decrease through 24 months. At 28 days, ≥94% of vaccine recipients seroresponded (ZEBOV-GP ELISA, ≥2-fold increase, titer ≥200 EU/mL), with responses persisting at 24 months in ≥91%. Female sex and a history of arthritis were identified as potential risk factors for the development of arthritis postvaccination.
Conclusions
Immune responses to rVSVΔG-ZEBOV-GP persisted to 24 months. Immunogenicity and safety results support continued rVSVΔG-ZEBOV-GP development.
Clinical Trials Registration
Keywords: Ebola, clinical trial, immunogenicity, rVSVΔG-ZEBOV-GP, vaccine
Immune responses to rVSVΔG-ZEBOV-GP, measured by ZEBOV-GP immunoglobulin G enzyme-linked immunosorbent assay and plaque reduction neutralization test, were robust and persisted up to 24 months. Together with the favorable safety profile, the immunogenicity results support continued rVSVΔG-ZEBOV-GP development.
Ebola viruses (EBOVs) are members of the Filoviridae family that cause sporadic outbreaks of hemorrhagic fever with high mortality [1–4]. Until the 2014–2016 outbreak of Zaire EBOV (ZEBOV), most outbreaks were small and located in isolated rural areas. The 2014–2016 outbreak centered in Sierra Leone, Liberia, and Guinea, and was notable for its spread to urban areas and the resulting large number of cases and deaths; >28 600 cases and >11 300 deaths were reported [3].
Several ZEBOV vaccines were under development at the onset of the epidemic, but few had progressed to clinical trials. The size and scope of the epidemic led to an unprecedented international collaborative effort to accelerate the development of candidate ZEBOV vaccines. To date, no ZEBOV vaccine is licensed for use outside of China and Russia.
One vaccine that has progressed through phase 1–3 clinical trials, including an efficacy trial, is the recombinant vesicular stomatitis virus–ZEBOV envelope glycoprotein vaccine (rVSVΔG-ZEBOV-GP). This vaccine was originally developed by the Public Health Agency of Canada [5] and uses rVSV, Indiana Strain, as a vector to elicit an immune response against the ZEBOV by replacing the gene encoding VSV surface G protein with the gene encoding ZEBOV GP (Kikwit strain) [6–8]. rVSVΔG-ZEBOV-GP was shown to be safe, immunogenic, and efficacious in rodents and nonhuman primates prior to testing in clinical trials [5, 8–14]. In the face of the epidemic, rVSVΔG-ZEBOV-GP was demonstrated to be generally well tolerated and immunogenic in 8 coordinated phase 1 clinical trials conducted in North America, Europe, and countries in Africa not impacted by the 2014–2016 outbreak [15–18]. Arthritis was initially identified as an important reactogenicity event following vaccination with rVSVΔG-ZEBOV-GP at a World Health Organization (WHO) site in Geneva, where 35 participants were vaccinated with 1 × 107 plaque-forming units (pfu) and 16 were vaccinated with 5 × 107 pfu [17, 19]. However, such a high proportion of participants reporting arthritis postvaccination was not observed in other phase 1 studies or at other WHO sites [15, 16, 18, 19]. rVSVΔG-ZEBOV-GP was also evaluated and generally well tolerated in phase 2 and/or 3 studies in West African countries most impacted by the outbreak [20–22]. The vaccine was demonstrated to be efficacious in one phase 3 open-label, cluster-randomized clinical trial during the epidemic in Guinea [20].
Lot consistency is a regulatory requirement for licensure, and the current study was designed to test the lot-to-lot clinical consistency, immunogenicity, and safety of 3 standard-dose lots of rVSVΔG-ZEBOV-GP and to explore the immunogenicity and safety of a high-dose formulation to inform manufacturing limitations for the vaccine. Safety outcomes from this study at 6 months postvaccination were previously reported [23] and showed that the vaccine (standard- and high-dose formulations) was generally well tolerated. rVSVΔG-ZEBOV-GP recipients reported higher rates of injection-site pain, erythema, and swelling, as well as higher rates of fever, headache, arthralgia, pain, chills, and fatigue than placebo recipients; the majority of the reported adverse events (AEs) were mild to moderate in severity. No serious AEs (SAEs) attributed to the vaccine were reported. Here, we report the immunogenicity of rVSVΔG-ZEBOV-GP through 24 months postvaccination, and comment on continued SAE monitoring from 6 to 24 months and a post hoc analysis of risk factors associated with arthritis following vaccination with rVSVΔG-ZEBOV-GP.
METHODS
Study Design and Population
Study V920-012 (NCT02503202) was a randomized, double-blind, placebo-controlled, clinical trial undertaken at 40 sites in the United States and 1 site each in Canada and Spain between 17 August 2015 and 29 September 2017. Safety and reactogenicity data in the base study (6 months) were previously reported [23]; we report SAEs, risk factors for arthritis, and immunogenicity up to 24 months.
Details of the study design, randomization and blinding methods, and participant eligibility criteria have been reported [23]. Briefly, healthy adults (18–65 years of age; planned N = 1125) were randomized in a 2:2:2:2:1 ratio to receive 1 of 3 consistency lots of rVSVΔG-ZEBOV-GP “standard dose” (nominal 2 × 107 pfu), a “high-dose” rVSVΔG-ZEBOV-GP (nominal 1 × 108 pfu), or placebo. Participants were either not of childbearing potential or were advised to avoid pregnancy by effective birth control for 2 months following vaccination (complete inclusion and exclusion criteria were previously reported [23]); see Supplementary Methods for additional study design details.
At the 6-month postvaccination visit (the last visit in the base study), all participants in all treatment groups enrolled at trial centers in the United States that agreed to participate (n = 23/40) were asked to participate in a trial extension to evaluate the durability of the antibody responses to rVSVΔG-ZEBOV-GP and SAEs through 24 months postvaccination. The study sites enrolling participants remained blinded to treatment-group assignments at the time the extension study was implemented. Upon unblinding, the enrollment was evenly distributed across the 5 treatment groups. A total of 600 participants were targeted for enrollment in the study extension, equaling approximately 50% of the original cohort size, to account for those who may decline participation. Of 1197 participants randomized in the base study, 566 continued in the extension, during which additional blood samples for immunogenicity were collected at 12, 18, and 24 months postvaccination. Any SAEs were also reported during this time period.
The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies; all participants provided written informed consent prior to initiation of the study as well as at entry into the 24-month extension.
Vaccine Administration and Study Procedures
Participants received a single vaccination with 1.0 mL of 1 of 3 consistency lots (standard dose; 2 × 107 pfu nominal dose), a high-dose lot (1 × 108 pfu nominal dose) of rVSVΔG-ZEBOV-GP (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ), or placebo (0.9% normal saline) as an intramuscular injection [23].
Randomization was accomplished centrally with an interactive voice response system, stratified by age (18–45 and 46–65 years of age) [23]. Vaccine and placebo were prepared and dispensed by an unblinded pharmacist or qualified site personnel who had no other role in the study. The participants, investigators, and all other study personnel involved in vaccine administration or data collection were blinded.
Immunogenicity Assessments
Serum was collected by venipuncture prior to vaccination (at day 1), and at 28 days and 6 months postvaccination. A subset of participants (US sites only) had additional serum specimens collected at 12, 18, and 24 months.
Antibody response to rVSVΔG-ZEBOV-GP was measured by a ZEBOV-GP immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ZEBOV-GP ELISA) and an rVSVΔG-ZEBOV-GP plaque reduction neutralization test (PRNT) as described previously [16]. Details are given in the Supplementary Methods. Both assays were performed by Q2 Solutions Vaccines (formerly Focus Diagnostics; San Juan Capistrano, CA) using validated assays. See Supplementary Methods for details of the ZEBOV-GP ELISA and PRNT.
Safety Assessments
As reported previously [23], arthralgia and arthritis were prompted on the vaccine report card to be recorded from days 1 through 42 postvaccination by the participant. From end of day 42 through month 6, only recurrence of events of previously reported arthralgia and arthritis were collected. Participants who experienced arthralgia or arthritis at any point through 42 days postvaccination were instructed to immediately contact the investigative site. Based on the symptoms, participants were recalled for unscheduled examinations, which may have included rheumatology consultation or specimen collection for detection of vaccine virus using real-time polymerase chain reaction.
A uni- and multivariable post hoc analysis of risk factors associated with arthritis (arthritis, monoarthritis, polyarthritis, osteoarthritis, joint swelling, or joint effusion) following vaccination with rVSVΔG-ZEBOV-GP evaluated the following factors: treatment dose, body mass index, age (18–45 vs 46–65 years), sex, medical history of arthritis (same as outcome variables), and race.
Statistical Analysis
The immunogenicity analysis was performed on the per-protocol immunogenicity population, which comprised all participants who satisfied the inclusion/exclusion criteria, were seronegative at baseline, and did not have an important protocol deviation that could have substantially affected the immunogenicity analysis.
The primary study objective was to determine whether vaccination with rVSVΔG-ZEBOV-GP from 3 consistency lots resulted in equivalent immunogenicity as measured by ZEBOV-GP ELISA at 28 days. The criterion for lot consistency required that the 2-sided 95% confidence interval (CI) on the pairwise lot-to-lot comparison of the ZEBOV-GP ELISA geometric mean titer (GMT) ratio be >0.5-fold but ≤2.0-fold. Demonstrating lot consistency using alternate criteria (2-sided 95% CI on pairwise lot-to-lot comparison of ZEBOV-GP ELISA GMT ratio >0.67-fold but ≤1.5-fold) was a secondary objective. Three pairwise comparisons of lots (each consisting of 2, 1-sided tests of equivalence at the α = 0.025 level) were performed. This procedure controls the overall type I error at the 2-sided, 5% level. Summary statistics for the 3 consistency lots and hypothesis testing of the pairwise comparisons were based on an analysis of variance model, including consistency lot and age group as covariates.
Secondary immunogenicity objectives (at 28 days) included estimating the GMTs of anti–ZEBOV-GP antibody measured by ZEBOV-GP ELISA in 3 consistency lots and the high-dose group, and GMTs of neutralizing antibodies (PRNT) in 3 consistency lots and the high-dose group. No formal hypotheses were tested for these objectives. Anti–ZEBOV-GP ELISA GMTs and neutralizing antibody GMTs through 24 months postvaccination in a subset of vaccine recipients were estimated as a prespecified additional objective.
For the uni- and multivariable post hoc analysis of risk factors associated with arthritis, 3 methods were used to determine the association between the covariates and arthritis: cross-tabulations of counts and percentages, multivariate logistic regression, and multivariate logistic regression with random effects. For multivariate logistic regression analyses, estimate ratios and associated 95% CIs were provided.
A total of 1125 participants were planned to enroll in the study, with 250 participants in each of the consistency-lot groups, providing approximately 99% power to demonstrate equivalent immunogenicity across the 3 consistency lots using the primary criteria (0.5-fold to 2.0-fold equivalence margins).
RESULTS
Participants
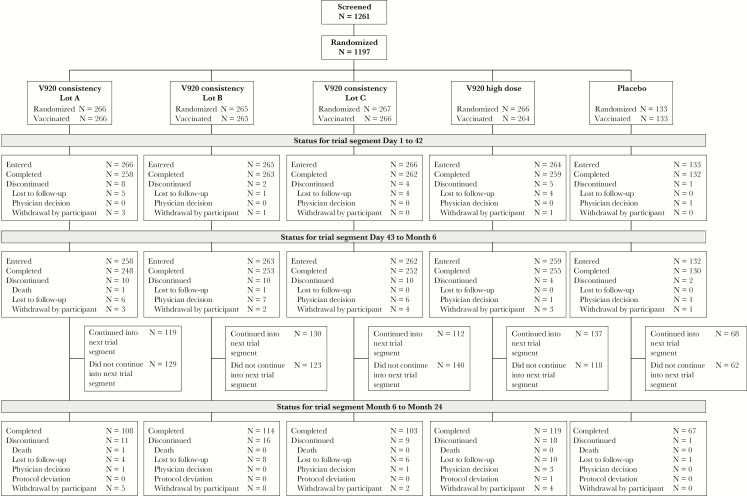
A total of 1261 participants were screened, 1197 were randomized, and 1194 were vaccinated. Of the vaccinated participants, 1138 (95.1%) completed the 6-month follow-up (Figure 1). The most common reason for nonrandomization was screen failure (63 [98.4%] nonrandomized participants). A total of 1039 (86.8%) vaccinated participants were included in the per-protocol analysis. Reasons for exclusion from the analyses included vaccine temperature excursion, lack of baseline clinical laboratory results, and participants being positive for ZEBOV by ZEBOV-GP ELISA at baseline. A total of 112 participants discontinued the study. Reasons for discontinuation included lost to follow-up (n = 64), withdrawal by participant (n = 38), physician decision (n = 6), death (n = 3), and protocol violation (n = 1). A cohort of 566 participants were enrolled at US sites for antibody measurement at 12, 18, and 24 months postvaccination.
Figure 1.
Participant disposition.
The vaccination groups were similar at baseline for age, gender, and racial distribution, and most (94.7%) were enrolled at sites in the United States (Table 1). Participants in the extension were similar to those in the base study (Supplementary Table 1).
Table 1.
Participant Baseline Characteristics (Base Study)
| rVSVΔG-ZEBOV-GP | |||||
|---|---|---|---|---|---|
| Lot Aa | Lot Ba | Lot Ca | High Doseb | Placebo | |
| Participants in population, n | 266 | 265 | 267 | 266 | 133 |
| Female sex, n (%) | 143 (53.8) | 135 (50.9) | 138 (51.7) | 149 (56.0) | 72 (54.1) |
| Age, y, mean (SD) | 41.3 (13.4) | 41.5 (12.4) | 40.9 (13.1) | 41.7 (13.4) | 41.1 (13.7) |
| Race, n (%) | |||||
| American Indian or Alaska Native | 2 (0.8) | 2 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Asian | 0 (0.0) | 1 (0.4) | 3 (1.1) | 2 (0.8) | 3 (2.3) |
| Black | 78 (29.3) | 70 (26.4) | 82 (30.7) | 83 (31.2) | 37 (27.8) |
| Multiple | 3 (1.1) | 4 (1.5) | 7 (2.6) | 2 (0.8) | 1 (0.8) |
| Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 1 (0.8) |
| White | 183 (68.8) | 188 (70.9) | 175 (65.5) | 177 (66.5) | 90 (67.7) |
| BMI, kg/m2, mean (SD)c | 30 (8) | 29 (7) | 31 (8) | 29 (7) | 30 (7) |
Abbreviations: BMI, body mass index; pfu, plaque-forming units; rVSVΔG-ZEBOV-GP, recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine. (used with permission from Oxford University Press [23]).
a Lots A, B, C = nominal 2 × 107 pfu.
b High-dose = nominal 1 × 108 pfu.
c Participants with data: Lot A, n = 263; Lot B, n = 264; Lot C, n = 265; high-dose, n = 264; placebo, n = 133.
Immunogenicity
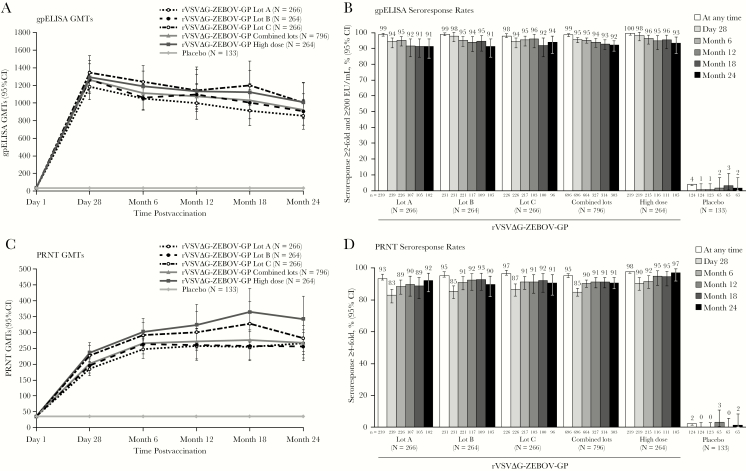
ZEBOV-GP ELISA GMTs increased after vaccination for each vaccine group by 28 days and persisted through 24 months postvaccination (Figure 2A and Supplementary Table 2). The immunogenicity of the 3 manufacturing lots was demonstrated to be equivalent at 28 days postvaccination (Table 2). A secondary analysis using more stringent definitions for equivalence (0.67 for the lower bound and 1.5 for the upper bound) also demonstrated equivalence for the 3 manufacturing lots (data not shown).
Figure 2.
Antibody responses through 24 months postvaccination by ZEBOV-GP ELISA (A and B) and PRNT (C and D) GMTs (A and C) and seroresponse rates (B and D) in the per-protocol immunogenicity population. N = Number of participants with serology data at 1 or more timepoints according to the treatment to which they were randomized. n = Number of participants contributing to the analysis. Values below the LLOQ (ZEBOV-GP ELISA: <36.11; PRNT: <35) were replaced with ½ LLOQ in GMT calculations. Abbreviations: CI, confidence interval; GMT, geometric mean titer; LLOQ, lower limit of quantification; PRNT, plaque reduction neutralization test; rVSVΔG-ZEBOV-GP, recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein vaccine; ZEBOV-GP ELISA, Zaire Ebola virus envelope immunoglobulin G glycoprotein enzyme-linked immunosorbent assay.
Table 2.
Consistency of ZEBOV-GP ELISA GMTs Between Lots of rVSVΔG-ZEBOV-GP at 28 Days
| Comparison Group A | Comparison Group B | Estimated Fold Difference | P Value for Equivalence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparison Group A vs Comparison Group B | N | n | Estimated GMTa (EU/mL) | N | n | Estimated GMTa (EU/mL) | Group A/Group B (95% CI) | Lower Boundb | Upper Boundc |
| Lot A vs Lot B | 266 | 239 | 1183.9 | 264 | 231 | 1266.0 | 0.94 (0.77–1.14) | <.001 | <.001 |
| Lot A vs Lot C | 266 | 239 | 1183.9 | 266 | 226 | 1346.0 | 0.88 (0.71–1.09) | <.001 | <.001 |
| Lot B vs Lot C | 264 | 231 | 1266.0 | 266 | 226 | 1346.0 | 0.94 (0.77–1.15) | <.001 | <.001 |
Abbreviations: ANOVA, analysis of variance; CI, confidence interval; EU, ELISA unit; GMT, geometric mean titer; rVSVΔG-ZEBOV-GP, recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein vaccine; ZEBOV-GP ELISA, Zaire Ebola virus envelope glycoprotein immunoglobulin G enzyme-linked immunosorbent assay.
N = Number of participants with serology data at 1 or more timepoints according to the treatment to which they were randomized; n = number of participants contributing to the analysis.
a Based on an ANOVA model with a response of the natural log of individual titers and fixed effects for lots and age group (18–45 and 46–65 years).
b P value for the comparison of the GMT ratio to the lower bound (0.5).
c P value for the comparison of the GMT ratio to the upper bound (2.0).
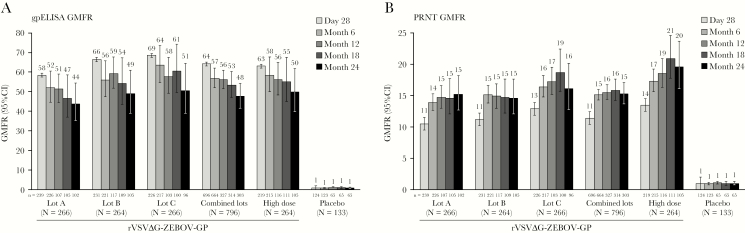
At 28 days postvaccination, ZEBOV-GP ELISA geometric mean fold rises (GMFRs) of ≥58-fold from baseline were observed across all standard-dose (combined, 64.2; 95% CI, 59.3–69.4 at 28 days) and high-dose (63.1; 95% CI, 54.8–72.6) rVSVΔG-ZEBOV-GP groups (Figure 3A and Supplementary Table 3). The GMFR responses were durable over 24 months, with GMFRs of at least 43-fold from baseline observed at all timepoints and in all vaccine groups. At 24 months postvaccination, GMFRs were 47.6 (95% CI, 42.2–53.7) and 49.8 (95% CI, 40.5–61.1) in the combined standard- and high-dose lots, respectively.
Figure 3.
ZEBOV-GP ELISA (A) and PRNT (B) GMFR by vaccination group in the per-protocol immunogenicity population. Error bars represent 95% CI. The per-protocol immunogenicity population includes all participants who were compliant with the protocol, received vaccination, were seronegative at day 1, and had a serum sample at 1 or more timepoints collected within an acceptable day range. N = Number of participants with serology data at 1 or more timepoints according to the treatment to which they were randomized. n = Number of participants contributing to the analysis. Values below the LLOQ (ZEBOV-GP ELISA: <36.11; PRNT: <35) were replaced with ½ LLOQ in GMT calculations. Abbreviations: CI, confidence interval; GMFR, geometric mean fold rise; GMT, geometric mean titer; LLOQ, lower limit of quantification; PRNT, plaque reduction neutralization test; rVSVΔG-ZEBOV-GP, recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine; ZEBOV-GP ELISA, Zaire Ebola virus envelope glycoprotein immunoglobulin G enzyme-linked immunosorbent assay.
ZEBOV-GP ELISA seroresponse, defined as a ≥2-fold increase in antibody over baseline and antibody titer ≥200 EU/mL, was observed in >94% of participants in each vaccine group by 28 days postvaccination, and most (>91%) remained seropositive through 24 months (Figure 2B and Supplementary Table 4). At 28 days, 95.4% (95% CI, 93.6–96.8) of the combined standard-dose vaccine recipients and 98.2% (95% CI, 95.4–99.5) of high-dose recipients had a seroresponse. At 24 months, 92.1% (95% CI, 88.4–94.9) of combined standard-dose and 93.3% (95% CI, 86.7–97.3) of high-dose recipients continued to meet the seroresponse criteria. In contrast, only 4.0% (95% CI, 1.3–9.2; n = 5) of placebo recipients met the ZEBOV-GP ELISA seroresponse criteria at any time during the study. Seroresponse results, when defined as a ≥4-fold increase in antibody over baseline, were similar (Supplementary Table 4).
Neutralizing antibody titers by PRNT increased by 28 days postvaccination in all rVSVΔG-ZEBOV-GP groups. Titers continued to increase after 28 days, with a peak at 18 months and no decrease at 24 months (Figure 2C and Supplementary Table 5). In recipients of standard-dose vaccine lots (combined), the GMFR from baseline was approximately 11 by 28 days, peaked at approximately 16-fold at 18 months postvaccination, and remained elevated by >15-fold from baseline at 24 months postvaccination (Figure 3B and Supplementary Table 6). Results were generally consistent across the individual standard-dose lot and high-dose groups (Figure 2C, Figure 3B, and Supplementary Table 5 and Table 6).
Based on PRNT, 84.9% (95% CI, 82.0–87.5) of standard-dose recipients and 90.4% (95% CI, 85.7–94.0) of high-dose recipients met seroresponse criteria at 28 days (Figure 2D and Supplementary Table 7). At 24 months, seroresponse rates remained high, with 90.7% (95% CI, 86.9–93.8) and 97.1% (95% CI, 91.9–99.4) of standard- and high-dose recipients, respectively, meeting seroresponse criteria. Only 2.4% (95% CI, 0.5–7.0) of placebo recipients met the PRNT seroresponse criteria at any time during the study.
Safety
Safety outcomes in the 6 months postvaccination have been reported previously [23]. During the full 24-month study, 35 (4.4%) participants in the combined standard-dose groups, 8 (3.1%) participants in the high-dose group, and 4 (3.0%) participants in the placebo group reported SAEs, none of which were considered related to vaccine. Three participants died, all in the standard-dose vaccine groups. Two of the deaths occurred during the base study (1 due to a craniocerebral injury after a fall 152 days postvaccination and 1 due to hepatic failure 76 days postvaccination), as reported previously [23]. In addition, a 51-year-old man died due to a motor vehicle accident during the study extension, 688 days after vaccination. None of the deaths were considered vaccine related. There were no discontinuations due to AEs.
As previously reported [23], the proportion of participants with specific AEs of arthralgia or arthritis from days 1 to 42 are summarized in Supplementary Table 8. Overall, these AEs were mild to moderate in intensity, with severe arthralgia reported by 0.8% (combined-lots group) and 3.1% (high-dose group) of participants, and severe arthritis reported by 0.4% of participants (both combined-lots and high-dose groups) of participants. The median duration of arthralgia was 3.0 days each for the combined-lots, high-dose, and placebo groups, and the median duration of arthritis was 7.0 and 5.0 days for the combined-lots and high-dose groups, respectively. All incidences of severe AEs resolved.
Multivariate logistic regression analysis of risk factors for arthritis (from day 1 through 42 postvaccination) identified an association with female sex and a medical history of arthritis as potential risk factors for the development of arthritis postvaccination (odds ratio [OR], 2.2 [95% CI, 1.1–4.1] to 2.8 [95% CI, 1.3–6.2], respectively). Treatment dose (OR, 1.5; 95% CI, 1.0–2.3), body mass index (OR, 1.0; 95% CI, 1.0–1.1), age (OR, 1.6; 95% CI, .9–3.0), and race (OR, 0.5; 95% CI, .2–1.0) did not emerge as significant risk factors.
Discussion
The results of this study demonstrate the lot-to-lot manufacturing consistency of the rVSVΔG-ZEBOV-GP vaccine and provide first-time immunogenicity data using validated assays over 24 months postvaccination. The 3 manufacturing lots met the preset equivalence criteria, as well as a secondary analysis using more stringent criteria. A higher-dose formulation had generally similar immunogenicity at the timepoints measured. Immune responses to rVSVΔG-ZEBOV-GP were durable through 24 months, with most (≥90%) participants meeting ZEBOV-GP ELISA and PRNT seroresponse criteria at 24 months. Consistent with the safety profile observed during the 6 months postvaccination [23], there were no vaccine-related SAEs or deaths during the 24-month study.
The results provide confirmatory evidence from previous phase 1 and 2 studies that utilized nonvalidated assays. In those studies, the rVSVΔG-ZEBOV-GP vaccine had elicited ZEBOV-GP ELISA responses in nearly all participants measured 1 month postvaccination [15–17, 19] with persistence of antibody levels through the latest timepoint studied (6 months [15], 12 months [16], or 24 months [24] postvaccination). The results of the ZEBOV ZEBOV-GP ELISA were further supported by the viral PRNT results, which demonstrated slightly different kinetics from the ZEBOV-GP ELISA. Whereas the ZEBOV-GP ELISA GMTs peaked at 28 days, PRNT titers continued to increase through 18 months. This result may be due to avidity maturation and selection of higher-avidity, better-functioning neutralizing antibodies. These results are consistent with the persistent PRNT response reported by Heppner et al [16]. Other neutralizing assays such as the pseudovirion neutralization assay (PsVNA) have been reported to decrease by 6 months. The difference between the PRNT and the PsVNA may be due to the use of different challenge viruses in the assays or to interassay variability of the unqualified PsVNA [16].
Post hoc multivariate analyses that control for covariates were performed during the extension phase of the trial and demonstrated that female sex and a positive medical history of arthritis were independent baseline variables associated with a 2.2- to 2.8-fold higher risk of developing postvaccination arthritis, with 95% CI lower bounds of 1.1 and 1.3, respectively. Postvaccination arthritis has been described with live-attenuated vaccines such as rubella [25, 26]. An association was found between the stage of the menstrual cycle and joint manifestations, suggesting that hormonal factors may play a role in the pathophysiology involving joint signs and symptoms after HPV-77 rubella vaccine administration.
While this is one of the largest studies of immunogenicity and tolerability of the rVSVΔG-ZEBOV-GP vaccine, there are several limitations. Participants were recruited from North American sites (as well as a single site in Spain), but EBOV affects primarily African populations. Although most of the phase 1 and 2 studies of rVSVΔG-ZEBOV-GP have been performed in Europe and North America, a number of phase 2/3 studies have been undertaken in West Africa and provide important data that expand on the initial results obtained in the earlier-phase studies. Although the “Ebola ça Suffit!” trial conducted in Guinea did not assess immunogenicity [20, 27], other studies in the affected regions assessed immunogenicity during the end of the 2014–2016 ZEBOV outbreak [21, 22, 28]. The PREVAIL I ZEBOV-GP ELISA results demonstrated slightly lower postvaccination ZEBOV-GP ELISA GMTs compared with those reported in the current clinical study, which utilized a validated ZEBOV-GP ELISA. The PREVAIL I study also resulted in markedly lower ZEBOV-GP ELISA GMFRs [21]. The lower GMFRs are likely explained by higher baseline ELISA titers in the Liberian population. Immunogenicity assessments of the Front Line Worker, PREVAIL, and STRIVE trials using validated ELISA and PRNT are ongoing.
The importance of ZEBOV-GP ELISA and PRNT as immune correlates of protection has not yet been firmly established. Preclinical data in nonhuman primates suggest that neutralizing as well as nonneutralizing antibodies may correlate with protection [29, 30]; however, this has yet to be confirmed in humans. Future studies may assess immune correlates of protection following vaccination with rVSVΔG-ZEBOV-GP in nonhuman primates at the individual level, as well as in humans at the population level.
CONCLUSION
Three manufacturing lots of rVSVΔG-ZEBOV-GP vaccine were demonstrated to be equivalent by ZEBOV-GP ELISA at 28 days postvaccination. Immune responses as measured by ZEBOV-GP ELISA were robust and persisted through 24 months. There were no vaccine-related SAEs or deaths over the 24-month study, consistent with the previously reported tolerability profile up to 6 months postvaccination [23]. The data from this trial, taken with the demonstrated efficacy of rVSVΔG-ZEBOV-GP vaccine in a ring vaccination, cluster-randomized “Ebola ça Suffit!” trial that utilized the same nominal dose of 2 × 107 pfu [20, 27], provide further evidence of the potential for rVSVΔG-ZEBOV-GP vaccine to be used to prevent Ebola outbreaks.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. The authors thank all of the study participants who made this trial possible.
Additional contribution. Medical writing assistance was provided by Erin M. Bekes, PhD, of CMC AFFINITIY, a division of McCann Health Medical Communications Inc., San Francisco, CA, funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
Financial support. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ (sponsor); and the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract number HHSO100201500002C).
Potential conflicts of interest. R. D., M. T. O., K. L., J. M., R. J. G.-K., B.-A. C., F. A. H., and J. K. S. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and may own stock or stock options in Merck & Co., Inc., Kenilworth, NJ. R. N. is a consultant to NewLink Genetics. S. A. H. reports no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. S. A. H., R. D., M. T. O., K. L., J. M., R. J. G.-K., R. N., B.-A. C., F. A. H., and J. K. S. have contributed to, seen, and approved the final, submitted version of the manuscript.
Presented in part: American Society of Tropical Medicine and Hygiene, 66th Annual Meeting, 5–9 November 2017, Baltimore, MD, and the American Society of Tropical Medicine and Hygiene, 67th Annual Meeting, 28 October-1 November 2018, New Orleans, LA.
References
- 1. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011; 377:849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Ebola Response Team. After Ebola in West Africa—unpredictable risks, preventable epidemics. N Engl J Med 2016; 375:587–96. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Ebola virus disease. http://www.who.int/ebola/en/. Accessed 17 April 2019. [Google Scholar]
- 4. Centers for Disease Control and Prevention. History of Ebola virus disease. https://www.cdc.gov/vhf/ebola/outbreaks/history/summaries.html. Accessed 17 April 2019. [Google Scholar]
- 5. Garbutt M, Liebscher R, Wahl-Jensen V, et al. . Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 2004; 78:5458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marzi A, Ebihara H, Callison J, et al. . Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J Infect Dis 2011; 204(Suppl 3):S1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Expert Rev Vaccines 2014; 13:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mire CE, Miller AD, Carville A, et al. . Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl Trop Dis 2012; 6:e1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldmann H, Jones SM, Daddario-DiCaprio KM, et al. . Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geisbert TW, Daddario-Dicaprio KM, Lewis MG, et al. . Vesicular stomatitis virus-based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog 2008; 4:e1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones SM, Feldmann H, Ströher U, et al. . Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 2005; 11:786–90. [DOI] [PubMed] [Google Scholar]
- 12. Jones SM, Stroher U, Fernando L, et al. . Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis 2007; 196(Suppl 2):S404–12. [DOI] [PubMed] [Google Scholar]
- 13. Marzi A, Robertson SJ, Haddock E, et al. . Ebola Vaccine. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 2015; 349:739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu X, Fernando L, Alimonti JB, et al. . Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong Ebola GP-specific immune responses. PLoS One 2009; 4:e5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ElSherif MS, Brown C, MacKinnon-Cameron D, et al. ; Canadian Immunization Research Network. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ 2017; 189:E819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heppner DG Jr, Kemp TL, Martin BK, et al. ; V920-004 study team. Safety and immunogenicity of the rVSV∆G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis 2017; 17:854–66. [DOI] [PubMed] [Google Scholar]
- 17. Huttner A, Dayer JA, Yerly S, et al. ; VSV-Ebola Consortium. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Regules JA, Beigel JH, Paolino KM, et al. ; rVSVΔG-ZEBOV-GP Study Group. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 2017; 376:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agnandji ST, Huttner A, Zinser ME, et al. . Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374:1647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henao-Restrepo AM, Camacho A, Longini IM, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kennedy SB, Bolay F, Kieh M, et al. ; PREVAIL I Study Group. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samai M, Seward JF, Goldstein ST, et al. ; STRIVE Study Team. The Sierra Leone trial to introduce a vaccine against Ebola: an evaluation of rVSV∆G-ZEBOV-GP vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis 2018; 217:S6–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halperin SA, Arribas JR, Rupp R, et al. ; V920-012 Study Team. Six-month safety data of recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis 2017; 215:1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huttner A, Agnandji ST, Combescure C, et al. ; VEBCON; VSV-EBOVAC; VSV-EBOPLUS Consortia. Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect Dis 2018; 18:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swartz TA, Klingberg W, Goldwasser RA, Klingberg MA, Goldblum N, Hilleman MR. Clinical manifestations, according to age, among females given HPV-77 duck rubella vaccine. Am J Epidemiol 1971; 94:246–51. [DOI] [PubMed] [Google Scholar]
- 26. Weibel RE, Stokes J Jr, Buynak EB, Hilleman MR. Influence of age on clinical response to HPV-77 duck rubella vaccine. JAMA 1972; 222:805–7. [PubMed] [Google Scholar]
- 27. Henao-Restrepo AM, Longini IM, Egger M, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386:857–66. [DOI] [PubMed] [Google Scholar]
- 28. Juan-Giner A, Tchaton M, Jemmy JP, et al. . Safety of the rVSV ZEBOV vaccine against Ebola Zaire among frontline workers in Guinea [published online ahead of print 25 September, 2018]. Vaccine doi: 10.1016/j.vaccine.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 29. Marzi A, Engelmann F, Feldmann F, et al. . Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A 2013; 110:1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gunn BM, Yu WH, Karim MM, et al. . A role for Fc function in therapeutic monoclonal antibody-mediated protection against Ebola virus. Cell Host Microbe 2018; 24:221–33.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.