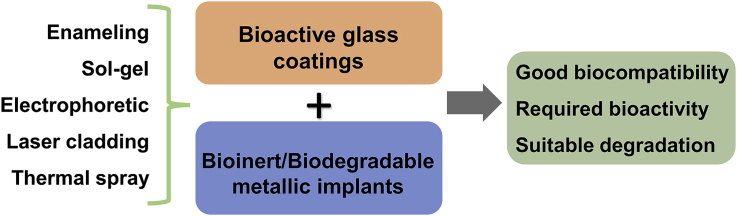
Abstract
Metallic implant materials possess adequate mechanical properties such as strength, elastic modulus, and ductility for long term support and stability in vivo. Traditional metallic biomaterials, including stainless steels, cobalt-chromium alloys, and titanium and its alloys, have been the gold standards for load-bearing implant materials in hard tissue applications in the past decades. Biodegradable metals including iron, magnesium, and zinc have also emerged as novel biodegradable implant materials with different in vivo degradation rates. However, they do not possess good bioactivity and other biological functions. Bioactive glasses have been widely used as coating materials on the metallic implants to improve their integration with the host tissue and overall biological performances. The present review provides a detailed overview of the benefits and issues of metal alloys when used as biomedical implants and how they are improved by bioactive glass-based coatings for biomedical applications.
Keywords: Bioactive glass coating, Metallic biomaterials, Biodegradation, Biocompatibility, Bioactivity. contents
Graphical abstract
Highlights
-
•
Coating necessity for different metallic implant materials.
-
•
Comparison of bioactive glass coating methods.
-
•
Summary of bioactive glass and composite coatings.
-
•
Bioactive glass coating criteria and evaluation routines.
1. Introduction
A biomaterial is a natural or synthetic material engineered to interact with the biological system and used in tissue repair and the creation of implants. Many types of metallic materials including stainless steel 316L, cobalt-chromium alloy, and titanium (Ti) and its alloy (Ti–6Al–4V) are used as implant biomaterials [1]. Through the replacement and restoration of traumatized or degenerated tissues or organs, these implant biomaterials aim to improve the life quality of patients [2]. In order to be considered as an appropriate biomaterial, bioactivity is one of the most significant characteristics. For example, orthopedic implants should be able to facilitate bone induction and cell proliferation, i. e., osteoinduction; cardiovascular implants should be able to induce the growth of blood vessels i. e., angiogenesis; it is also beneficial for implant surfaces that can kill or prevent the growth of bacteria – antimicrobial activity. These characteristics are closely related to the implant surface properties and the interface actions between implants and tissue. When the implant surface could not integrate well with the host tissues, fibrous tissue would develop at the interface, and this leads to loosening and eventual failure of the implant.
Surface coating is one of the most conventional and widely adopted methods to improve the surface biocompatibility and bioactivity of the biomaterials [[3], [4], [5], [6]]. Compared to the other materials, bioactive glasses are highly biocompatible and have a greater chance of integrating with human tissue than the metal implants stated above, making them a good option for improving the biocompatibility and bioactivity of these metals. Bioactive glass offers the following benefits: replacing damaged bone and tissue that will integrate well with the body's environment, facilitating tissue regeneration, and degrading at a similar rate of tissue regeneration [7]. As a coating material, bioactive glasses can facilitate better integration of the metal implants to the host tissue by forming apatite at the interfaces. Furthermore, they can regulate or inhibit corrosions of the implant metals in biological environments.
There are several review papers published in the related area previously. For example, Sola et al. summarized the bioactive glass coating [7] and Bellucci et al. specifically summarized the thermal expansion coefficient of different bioactive glasses [8]. Baino et al. [9] focused on the orthopedic and other biomedical applications of bioactive glass-coated materials. In the present study, a comprehensive review of the bioactive glass coating on the metallic implants was conducted. Additionally, beside bioactive glasses, glass-ceramics and glass/polymer composites are also commonly used as coating materials for the benefit of mechanical strength, bioactivity, adhesion strength, chemical stability and surface functionality [[10], [11], [12], [13], [14]]. Therefore, these materials were also summarized in this study.
2. Metallic implants
2.1. Traditional metallic implants
Stainless steel (SS), titanium (Ti) and its alloys, and cobalt-based alloys are the top three Traditional metallic implant materials. Table 1 shows a list of commonly used metallic implants and their physical and mechanical properties [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]].
Table 1.
Physical and mechanical properties of common used metallic implants compared with bone tissue.
| Material | Young's Modulus, E (GPa) | Yield Strength (MPa) | Tensile Strength (MPa) | Density (g/cm3) | Thermal Expansion coefficient (10−6/°C) | Ref. |
|---|---|---|---|---|---|---|
| Cortical Bone | 15–30 | 30–70 | 70–150 | 1.75 | 27.5 | [15,16] |
| Stainless Steel 316L | 200 | 221–1213 | 586–1351 | 8.03 | 19.5 | [17] |
| Pure Titanium (Ti) -Grade 1 | 110 | 485 | 760 | 4.51 | 8.5 | [19] |
| Ti–6Al–4V | 101–120 | 795–1034 | 860–1103 | 4.43 | 8.7 | [19] |
| Cobalt–Chromium | 210–253 | 448–1606 | 655–1869 | 8.3 | 15.1 | [18] |
| Pure Magnesium | 45 | 20 | 90–190 | 1.74 | 26 | [15,20] |
| AZ31 | 45 | 171–303 | 241–365 | 1.78 | 26 | [21] |
| Pure Iron | 211 | 150 | 210 | 7.87 | 12 | [22] |
| Fe–30Mn | 201 | 239 | 518 | 7.64 | 8.8 | [[22], [23], [24]] |
| Pure Zinc | 90–100 | 10–85 | 20–170 | 7.14 | 30–35 | [25] |
| Zn–1Mg | – | 180 | 340 | – | – | [26] |
| Zn-0.7Li | – | 476 | 568 | – | – | [27] |
SS is one of the most commonly used materials in making implants that apply to treatments such as hard tissue repair, fabrication of cardiovascular stents and valves, and orthopedic prosthesis [1], dentistry, craniofacial surgery, and otorhinology applications [28]. SS-316L is especially known for its good fatigue properties, ductility, and work hardenability [29]. Bekmurzayeva et al. acknowledges a common functionality that limits the use of SS-316L for different implant applications to be lack of bio-functionality, and goes further into investigating different pre-treatments in the form of surface modifications to increase its anti-fouling properties [1]. Another downfall that poses an issue for the use of SS-316L in these biomedical applications includes its inability to consistently integrate with the bodily tissue and low blood compatibility. SS tends to corrode in the body at regions where there is not enough oxygen to maintain the passive film and in areas where crevices are formed. This can easily lead to the failure by fracture of common applications like the femoral components.
Commercially pure Ti and its Ti–6Al–4V alloy are known in the biomedical field for their high strength, low density, excellent corrosion resistance, and are therefore quite capable of manufacturing lightweight implants with high strength [30]. Therefore, they present as and are the primary material type to prepare dental implants [19,31,32]. Titanium and its alloys are bioinert; they have excellent biocompatibility as they are able to form very stable oxides on their surfaces [32,33]. However, titanium and its alloy maintain a relatively low hardening coefficient, making it quite difficult to improve their mechanical properties by common strengthening mechanisms such as work hardening [30]. Additionally, it is known that titanium alloy Ti–6Al–4V, when corroded, produces high toxicity in the body when it enters the bloodstream [30].
Cobalt-chromium alloy is quite popular in biomedical engineering applications such as metal-on-metal hip resurfacing joints because of their superiority ion corrosion resistance, low wear performance, and biocompatibility. As a metal alloy, they have relatively good mechanical properties, however, they often fall short with their ability to be easily fabricated which limits them to specialized fabrication methods [34]. Previous research has also indicated the leaching of chromium and cobalt into the bloodstream when the metals are in direct contact with each other in implantation [[35], [36], [37]].
Haynes et al. conducted an in vivo experiment using rats to compare the difference in toxicity and release of metal on metal implants using titanium grade 5 and cobalt-chromium implants [38]. Results concluded that the titanium-aluminum-vanadium alloy implant leached particles induced a much larger release of prostaglandin E2 in comparison to the cobalt-chromium particles. Additionally, it was noted that the presence of the titanium alloy particles also increased the interleukin-1, interleukin-6, and tumor necrosis factor. Whereas, the chromium-cobalt alloy particles reduced the release of prostaglandin E2 and interleukin-6, while it had little effect on the release of interleukin-1 and tumor necrosis factor [[39], [40], [41], [42], [43], [44]]. Therefore, it is safe to say that cobalt-chromium is a superior choice over titanium and its alloys for large joint replacement applications.
2.2. Biodegradable metallic implants
Compared to the traditional implants, biodegradable implants can be gradually degraded and replaced by newly formed tissue. Ideally, the biodegradation rate should match the new tissue forming rate. There are three main kinds of biodegradable metals: iron (Fe), magnesium (Mg), and zinc (Zn) [45,46]. In addition to their in vivo biodegradation characteristics in body fluids, the common benefits of these three kinds of biodegradable metals are their presence of natural ionic content that may contribute to functional roles in physiological systems [[47], [48], [49], [50]]. As shown in Table 1, Fe and its alloys have much higher strengths and lower degradation rates than the other two metals, while Zn alloys own a mild degradation behavior and their mechanical strength could also meet the requirement after appropriate alloying and post-treatments [[50], [51], [52], [53], [54]]. Fe and Zn possess higher densities than bone tissue, which makes them better candidates as vascular stents or bone scaffolds than bone plates [22,[55], [56], [57]]. Mg has physical and mechanical properties quite similar or within range to cortical bone, including density, elastic moduli and compressive strength [58,59]. They are known to be biocompatible, bioactive, and biodegradable materials, able to form scaffolds that can be used in loadbearing applications [47]. The downfall of the use of Mg-based alloys is that they have relatively high degradation rates. Therefore, surface modification with different coatings is critical for its clinical applications [59].
3. Bioactive glass coating
The metallic materials as mentioned above could be improved by three main routines to suit for biomedical applications: alloying design, novel structure design and surface modification [45,60]. Alloying design is the main and best routine to improve the mechanical property, while novel structures including porous structure, nanostructure and glassy structure provide multiple choices for different biomedical applications. Compared to these two routines, surface modification is more conventional and widely adopted to modify the surface topology, chemical composition, and wetting property. Through the suitable surface modifications, the surface biocompatibility and bioactivity of the biomaterials can be improved [[3], [4], [5], [6]]. Compared to the other surface modifications, it is more effective and efficient to use bioactive materials as coating on the metallic substrates. Polymers and ceramics are the two main bioactive materials to be used as coating materials. Although bioactive glasses are not as common-used as polymers for coating materials, their unique properties could provide multiple functions. Based on the coating composition, the bioactive glass and its composite coatings are introduced as follows:
3.1. Bioactive glass coating
45S5 Bioglass® (46.1SiO2-24.4Na2O-26.9CaO-2.6P2O5 in mol%) was one of the first bioactive glasses discovered by Prof. Larry Hench about five decades ago and is still one of the most studied bioactive glass compositions [61,62]. The glass matrix can accommodate various dopants while maintaining the glass character and basic physical and chemical properties [63]. Many new glass compositions have been proposed and found several biomedical applications from dental filling, drug delivery, coating to load-bearing metal implants, to tissue engineering [64]. The composition flexibility enables large design space and the capability to introduce additional functionality such as enhancement of osteo-growth by Sr2+ [[65], [66], [67]], angiogenesis by Cu2+ [[68], [69], [70]], and antibacterial by Ag+ in bioactive glasses [71]. Table 2 summarizes the commonly used bioactive glass coatings with their components and thermal properties [[72], [73], [74], [75], [76]].
Table 2.
Commonly used bioactive glass coatings and their thermal properties.
| Bioactive glasses | Composition (wt%) | Thermal properties | Thermal expansion coefficient (10−6/K) | Ref. |
|---|---|---|---|---|
| Bioglass | 45SiO2-24.5Na2O-24.5CaO-6.0P2O5 | Tg (511 °C), Ts (557 °C) | 15.1 | [72] |
| 6P44 | 44.2SiO2-17.0Na2O-4.6K2O-18.0CaO-10.2MgO-6.0P2O5 | Tg (516 °C), Ts (560 °C) | 13.0 | |
| 6P50 | 49.8SiO2-15.5Na2O-4.2K2O-15.6CaO-8.9MgO-6.0P2O5 | Tg (522 °C), Ts (560 °C) | 12.2 | |
| 6P55 | 54.5SiO2-12.0Na2O-4.0K2O-15.0CaO-8.5MgO-6.0P2O5 | Tg (548 °C), Ts (602 °C) | 11.0 | |
| 6P61 | 61.1SiO2-10.3Na2O-2.8K2O-12.6CaO-7.2MgO-6.0P2O5 | Tg (564 °C), Ts (624 °C) | 10.2 | |
| 6P68 | 67.7SiO2-8.3Na2O-2.2K2O-10.1CaO-5.7MgO-6.0P2O5 | Tg (565 °C), Ts (644 °C) | 8.8 | |
| 7Na2O–50CaO–3TiO2–40P2O5 (mol%) | Tc (540–670 °C) | 18 | [73] | |
| 7Na2O–60CaO–3TiO2–30P2O5 (mol%) | Tc (600–730 °C) | 12 | ||
| H12 | 7.5SiO2-8.0Na2O-40.0B2O3-40.0CaO-2.0Al2O3-2.5P2O5 | Tg (565 °C), Td (590 °C), Tx (755 and 780 °C), | 9.7 ± 0.3 | [74] |
| B18 | 6.5SiO2-12.5Na2O-41.5B2O3-35.0CaO-3.5Al2O3-1.0P2O5 | Tg (510 °C), Td (547 °C), Tx (700 and 750 °C), | 10.1 ± 0.3 | |
| 0Sr | 49.96SiO2-7.25MgO-3.30Na2O-3.30K2O-3.00ZnO-1.07P2O5-32.62CaO (mol%) | Tg (604 °C), Tc (808 °C) | [75] | |
| 10Sr | 49.96SiO2-7.25MgO-3.30Na2O-3.30K2O-3.00ZnO-1.07P2O5-29.36CaO-3.26SrO (mol%) | Tg (580 °C), Tc (818 °C) | ||
| 50Sr | 49.96SiO2-7.25MgO-3.30Na2O-3.30K2O-3.00ZnO-1.07P2O5-16.31CaO-16.31SrO (mol%) | Tg (590 °C), Tc (807 °C) | ||
| 100Sr | 49.96SiO2-7.25MgO-3.30Na2O-3.30K2O-3.00ZnO-1.07P2O5-32.62SrO (mol%) | Tg (587 °C), Tc (753 °C) | ||
| QM5 | 41.70SiO2-36.31CaO-7.82MgO-3.13ZnO-5.20Na2O-1.00K2O-4.70P2O5 (mol%) | Tg (629 °C), Tc (811 °C) | 11.6 | [76] |
| QM8 | 41.70SiO2-30.00CaO-14.00MgO-3.13ZnO-5.20Na2O-1.00K2O-4.70P2O5 (mol%) | Tg (584 °C), Tc (813 °C) | 11.4 | |
| QM10 | 41.70SiO2-26.00CaO-18.00MgO-3.13ZnO-5.20Na2O-1.00K2O-4.70P2O5 (mol%) | Tg (606 °C), Tc (815 °C) | 11.3 |
3.2. Bioactive glass-ceramics composite coating
Glass-ceramics are materials that consist of the ability to achieve controlled devitrification and the evolution of variable proportions of crystalline and glassy phases [77]. This allows for these materials to overcome the lacunae that are usually encountered in glasses. Two commonly used glass-ceramic coating material available for the benefit of improving mechanical strength such as apatite-wollastonite (A-W) containing glass-ceramic (BGC) [78], and modified early-stage surface reactivity such as 45S5 sintered bioactive glass [79]. The A-W glass-ceramic system was developed by Professor Tadashi Kokubo and his colleagues and has been proven to exhibit good mechanical, physical and biological properties when its crystallization behavior was extensively evaluated [80]. Bioactive glass-ceramics serve as adequate options for coating applications because of their ability to form hydroxyapatite layer. Fig. 1 shows different bioactive glass-ceramic composite coatings on Ti6Al4V and Mg–Ca alloys, respectively [81,82]. Through a sol-gel process, bioactive glass, glass-ceramic or hydroxyapatite particles with methyl-triethoxisilane (MTES) and tetraethilorthosilicate (TEOS) were used as a coating on titanium alloy, as shown in Fig. 1 a-c, respectively [81]. Fig. 1 d-e shows the surface and cross-sectional morphology of wollastonite and hydroxyapatite composite coatings prepared on Mg–Ca alloy substrates by pulsed laser deposition method. The corrosion resistance of the Mg alloy was improved after the coating treatment (Fig. 1 f) [82].
Fig. 1.
Different bioactive glass-ceramic composite coatings on (a-c)Ti6Al4V and (d–f) Mg–Ca alloys, respectively: Sol-gel coating with (a) bioglass particles, (b) hydroxyapatite-wollastonite composite particles, and (c) hydroxyapatite particles [81]; (d–e) Pulsed laser deposited hydroxyapatite-wollastonite composite coating on Mg–Ca alloy, and (f) the electrochemical corrosion behavior with potential dynamic polarization curves [82].
3.3. Bioactive glass-polymer composite coating
Beside bioactive glasses and glass-ceramics, glass/polymer composites are commonly used as coating materials for the benefit of bioactivity, adhesion strength, chemical stability and surface functionality [14,83]. Chitosan is a natural cationic polymer which is derived from the natural occurring chitin through N-deacetylation [28]. Chitin can be found in the crust and shells of e.g. crabs, lobster or butterflies [29] and also in cell walls of fungi [30]. The polysaccharide chitosan is highly considered for biomedical applications due to its biodegradability, biocompatibility and antibacterial activity [19,20,29,31]. Furthermore, it can promote cell ingrowth [29] and chelate metal ions [32]. Chitosan has also been widely used to prepare coatings on metallic substrates by electrophoretic deposition (EPD) [23,25,27,28,33], and some studies have been carried out to investigate the deposition of chitosan on Mg alloys, as recently reviewed [6]. Most of the research investigating the application of chitosan/Bioglass as a composite coating are carried out using the Taguchi technique, which has been a statistical tool designed to optimize texture coefficient based on three factors: precursor concentration, annealing temperature, and annealing time [[84], [85], [86]].
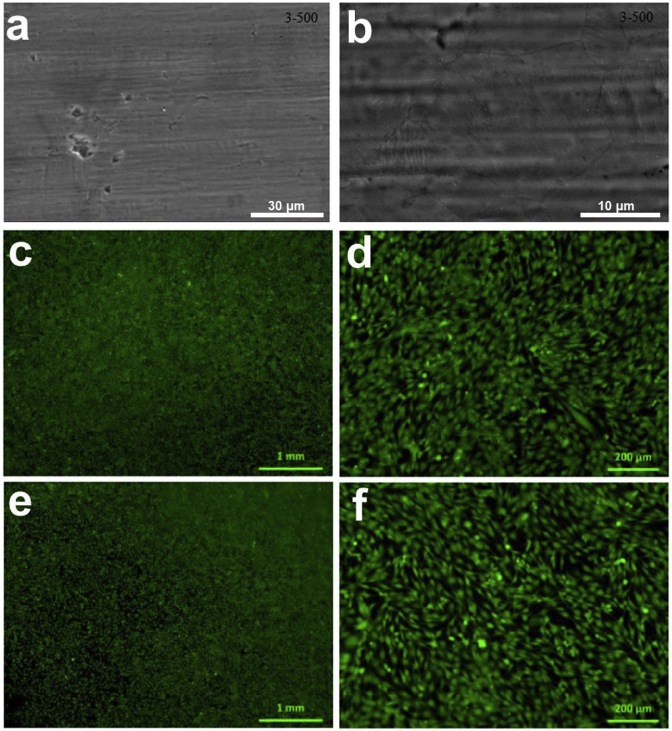
In other studies, the reduction of brittleness in glass composites, and the improvement of its elasticity are accomplished by the incorporation of a synthetic polymer known as polycaprolactone (PLC). When incorporated into the glass composite mixture, PLC acts as an organic material due to its biocompatibility and biodegradation and exhibits qualities similar to a natural polymer present in bone, collagen, primarily known to improve elasticity and cellular adhesion. Studies determined that the PLC-Bioactive glass composite containing 75 wt% bioactive glass, produced a promising composite capable of participating in bone tissue engineering application [87]. Fig. 2 shows a polysiloxane/bioactive glass composite coating on the SS316 surface and the human osteoblast-like cells (MG-63) morphology on the uncoated and coated surfaces [88]. The in vitro cell culture study indicated the composite coating possesses a good cytocompatibility.
Fig. 2.
(a–b) Polysiloxane/bioactive glass composite coating morphology on the SS316 surface and (c–f) human osteoblast-like cells (MG-63) morphology when cultured with the (c–d) coated surface and (e–f) uncoated SS316 surface for 48 h [88].
4. Bioactive glass coating methods
4.1. Pretreatments of alloys
Dissolution or corrosion in metals occurs via the electrochemical redox reactions. Therefore, pretreatment is important in limiting or somewhat controlling the corrosion rate. For instance, the pretreatment of bioactive Ti and its alloys via simple chemical surface treatment can improve corrosion resistance [89]. Surface pretreatment could also increase the implant surface area and thus beneficial for its surface activity. For example, after NaOH and heat treatment, there was an apatite layer formed on the titanium surface, which made the metal implant more conducive to tissue bonding. Cell media pretreatment and alkali heat treatment have also been used to promote the cell adhesion, hydroxyapatite formation, and overall biological performance of implant performance [[90], [91], [92]]. In addition to the chemical methods, mechanical methods are also commonly used to increase or decrease the surface roughness. The mechanical grinding and polishing are beneficial to increase the coating adhesion for the successive coating treatment [93]. Shot blasting or peening processes are also well known to produce nanocrystal thin layers on the metal surfaces [94].
4.2. Coating methods
Coating methods are crucial for utilizing the benefits from both biocompatible metal and bioactive glass, which are designed to bind the bioactive glass and the metal substrate to provide a more compatible biomaterial. The main purpose of applying a coating is to extend the life of materials used as biomedical components. Bioactive glass or ceramics are typically used to coat implant materials to improve the attachment and interaction between the metals and the surrounding tissue. With this type of improvement, the overall life of the implant or the coated material is extended. Additionally, the coating protects the material from leaching, corrosion, and degradation that often occurs over time when in the presence of the human's bodily environment. Popular methods of coating include sol-gel technique, enameling, electrophoretic deposition, thermal spraying, and laser cladding. Table 3 provides a summary of bioactive glass coating techniques and their advantages and disadvantages.
Table 3.
Pros and cons of commonly used coating methods.
| Methods | Pros | Cons | Ref. |
|---|---|---|---|
| Enameling | Simple, cheap, versatile, large range of thickness | Compositional gradient, glass crystallization, metal degradation, formation of chemical by-product, thermal residual stress | [95,96] |
| Sol-gel | Controlled composition and homogeneity, large compositional range of bioactive glasses, versatile, porous microstructure, multilayer coating | Post heat treatment for setting introduces internal stress due to difference in CTE between coating and substrate | [[97], [98], [99]] |
| Electrophoretic deposition | Consistent, cheap, coating objects with a complex shape, easy thickness control | Substrate must be conductive | [85,100,101] |
| Laser cladding | Flat coating on surfaces with curved geometry | Need surface pre-treatment, lack of uniformity | [[39], [40], [41], [42], [43], [44],102] |
| Thermal spray | Wide range of coating materials, small probability to compromise glass bioactivity | Poor adhesion between glass and substrate | [105,106] |
Enameling is a type of traditional surface treatment that involves the fusing of a layer of glass frit [95]. However, the development of a lower melting frit can be used depending on the metal substrate that is being coated. Advantages of enameling include low cost of processing, ease of operation, and the possibility of optimization by changing the parameters [96]. The enameling method along with tailoring the composition of the glass is known to be a possible option to fabricate a layer of bioactive glasses onto bioinert metal substrates with adequate thermal expansion coefficient, good adherence, and bioactivity.
With the advantage of low-cost, high purity, high accommodation for compositions, relatively good adhesion on complex substrate and uniformity of coating layer, sol-gel becomes one of the most popular coating methods for bioactive glass or glass-ceramic composite [97]. It is also convenient to combine sol-gel with other different coating technologies. Despite the convenience, the sol-gel process requires high temperature of post-heat treatment to fix the bioactive coating upon the metallic substrate [98], the high temperature involves the mismatch of coefficient of thermal expansion between substrate and coating layer, the accumulation of residual stress on interface or the compositional change of the coated glasses [99]. Since these issues largely weaken the biocompatibility, interfacial adhesion, and corrosion resistance, the recent researches used various methods, including, protective layer coating, substrate etching or polymer-assistance procedure, to optimize the sol-gel process.
Electrophoretic deposition (EPD) is known to be a highly convenient method that can produce a variety of coatings such as ceramic polymers and composite for numerous applications. The process of EPD consists of the deposition of particles (e.g. bioactive glass particles) that are suspended in a stable solvent [100]. Electrophoresis occurs via the movement of charged particles or molecules while deposition consists of the coagulation and precipitation of these particles and molecules on the electrode surface. Benefits of this process include its simplicity in procedure, the ability to fabricate coatings on complex-shaped substrates, ability to deposit temperature sensitive coatings at room temperature, achieving homogeneous and high purity coating, the possibility of achieving porous and textured coatings, its short processing time, and the capability of manipulating the coating thickness [85,100,101].
Comparing to the conventional surface modification techniques, laser technique not only conducting fast surface modification with high resolution but also taking care of advantages as mention above [[39], [40], [41], [42], [43], [44]]. Moreover, considering short laser beam-material interaction times (milliseconds to few seconds), Krzyzanowski et al. predicted the evolution of thermokinetic conditions and thermal stresses during the laser-assisted synthesis of bioactive glass coating on Ti-based alloys using a comprehensive computational model and primary experimental observations [102]. It is easy to produce micro-cracks within the coating during the laser coating operation. It is mainly attributed to rapid cooling cycle during laser treatments. The porosity produced within the coated layers was regarded to be beneficial for cell attachment when used in biomedical applications [103].
Thermal spray, also referred to as plasma spray or suspension plasma spray, is the only technology commercially approved by U.S. Food and Drug Administration (FDA), according to the minimum requirements for ASTM F1854-15 [104], as shown in Table 4. In the coating process, molten particles are created and deposited onto the substrate in the form of flattened drops that pile on each other to produce a layered coating. It allows for in-situ deposition of the coating while preserving the amorphous nature of the feedstock. It has been shown in a previous study that the bioactive glass coating doped with MgO leads to the formation of an Mg-rich apatite layer [105]. Despite the overall aspect that plasma spray has been considered a successful technique for biomedical application, it indicates that the coating tends to experience cracking overtime. This was regarded as a result of high dissolution rate, low mechanical strength, and low chemical stability [106].
Table 4.
| Property | Specification |
|---|---|
| Thickness | Not specific |
| Interface particle and void | <100 μm (void < 50 μm) |
| Tensile strength | >22 MPa (>24.1 MPa with polymeric adhesive) |
| Shear strength | >20 MPa (stable within 107 cycle) |
| Abrasion | <65 mg (after 100 cycle); <50% deformation |
5. Coating criteria and evaluations
There are several key criteria for a successful coating. First, the coefficient of thermal expansion (CTE) of glass and metallic substrates should be close to prevent crack or delamination during sintering process. For instance, CTE of 45S5 is reported 15.1 × 10−6 K−1, which is dependent on the measurement technique and testing parameters [8], while for Ti and Ti alloys, the most commonly used metallic implants, have a CTE of ~8–10 × 10−6 K−1 [8]. In a case like this, some oxides such as SiO2, MgO or K2O concentration must be increased in order to decrease the CTE of 45S5 in order to have a matching CTE [8]. Additionally, inducing a bond-coat in between glass and metallic substrate could overcome this drawback [107].
Secondly, the sintering process should not degrade the properties of both metals and glasses. For instance, α to β transformation of Ti and its alloys (885–950 °C for unalloyed Ti, depending on the impurity content, and 955–1010 °C for Ti6Al4V) should be avoided to prevent degradation of the mechanical properties of the implant [108]. On the other hand, glasses have a wider processing window (the large difference between glass transition temperature and crystallization temperature) would benefit the coating application, since crystallization results in reduced bioactivity of glasses [109].
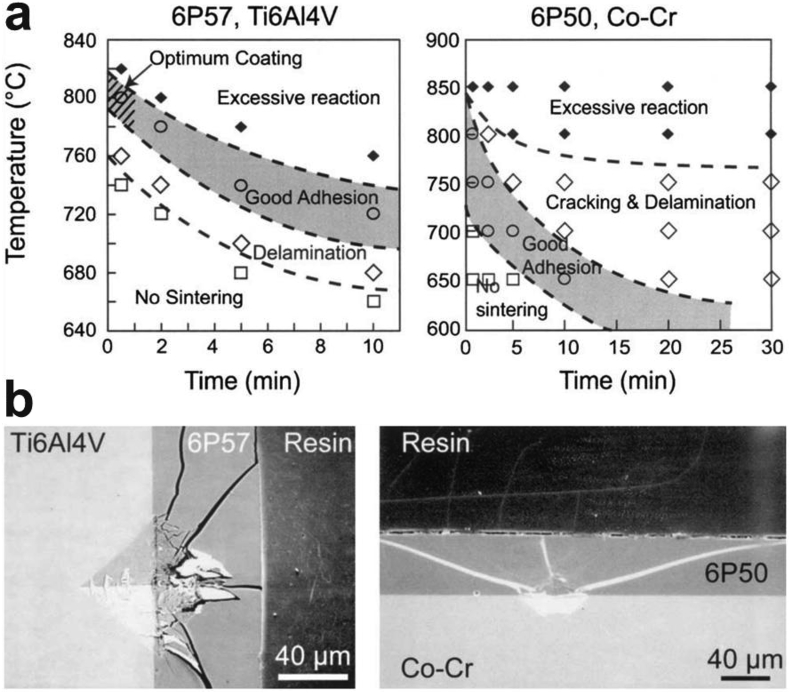
Thirdly, good adhesion should form at the interface between the glass and substrate. It was found that reactive plasma spraying coating technique offers a high quality of adhesion strength (40 MPa in shear stress and 70 MPa in tensile stress) [110]. It has shown that the sintering temperature and time could significantly change the coating/substrate interface reactions and thus influence the coating adhesion property, as shown in Fig. 3 a-b [108]. Normally, high temperatures could create porosity in the inner part of a coating where the coating directly binds to the substrate, therefore resulting in a lower degree of densification which caused a reduction in the strength of the coating-substrate bonding [111]. It should be pointed out that more than 30 testing methods exist for adhesion evaluation of coatings and there exists no single ideal adhesion test; therefore, choosing an appropriate and economically feasible testing method or combination of methods should be considered for different products and applications and [110]. SEM can be used conveniently to determine the interfacial behavior of coating-substrate interaction. Fig. 3c–d illustrates one example to characterize the coating interface property through the cracking behavior after Vickers indentations [108]. The cracks were developed towards the glass instead of the interface direction, which indicates a good interface reaction and coating adhesion.
Fig. 3.
(a) Effects of sintering time and temperature on the coating adhesion of bioactive glass 6P57 on Ti6A14V and 6P50 on Co–Cr and (b) the corresponding interface behavior after Vickers indentations [108].
Moreover, corrosion resistance is an important factor to consider for the bioactive glasses based on their intended application. Corrosion study has been carried out in simulated body fluid (SBF). This process involves exposing the glass material to the SBF solution at allocated time segments and tracking their loss in mass and their potentiodynamic responses. In some cases, atomic absorption spectrophotometry has been used to obtain more precise results after such tests were carried out [112]. Additionally, in studies evaluating the corrosion rate of surface proteins and its influence on the behavior of bioactive glass in SBF, XPS was used to determine the absorption of proteins to the surface of an Mg-alloy coated with chitosan-BG [113].
6. Biomedical applications and evaluations
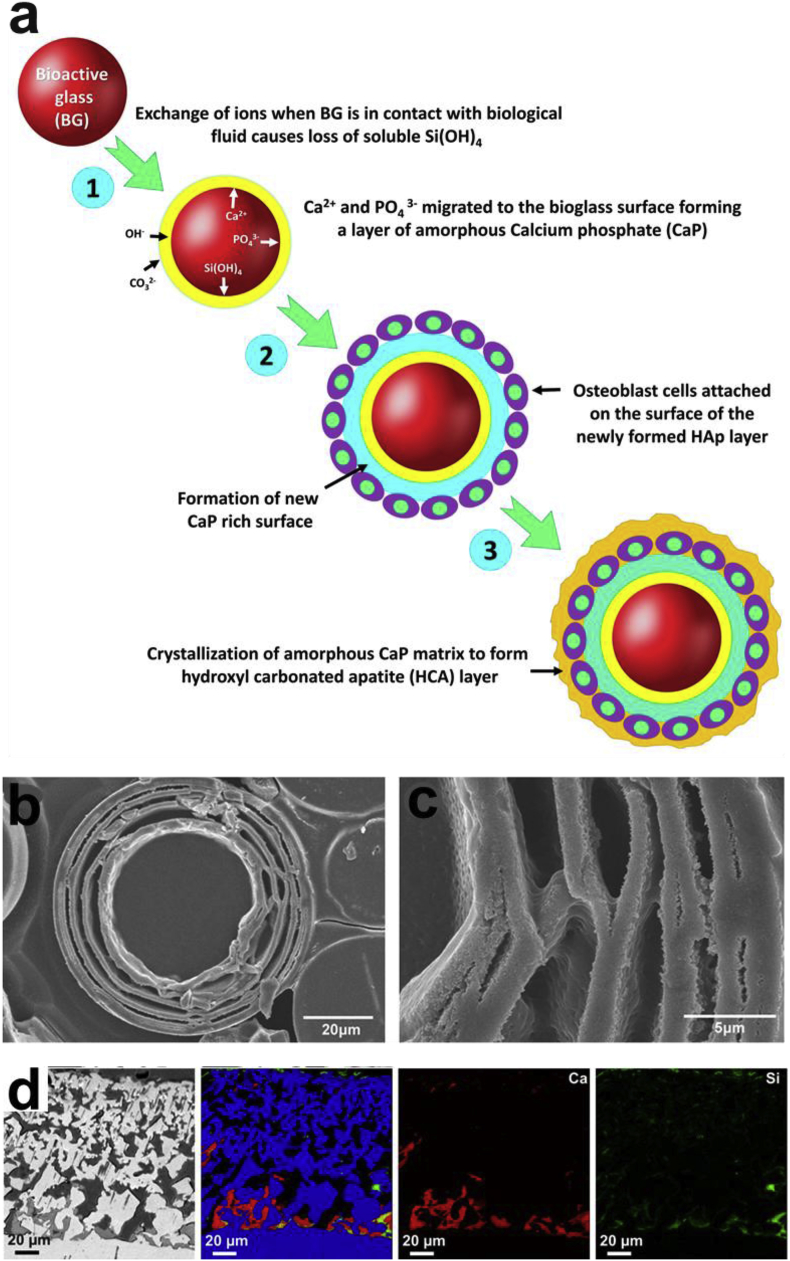
Fig. 4 shows the biological performance of bioactive glass in vitro and in vivo. When bioactive glass comes into contact with tissues in the body, its biocompatibility is determined by a series of reactions. Ions leave the glass material complex of the implant surface to enter the body fluid while hydrogen leaves the body fluids to form a network with silicon in the glass coating. This network attracts the movement of ions to the surface of the SiO2 rich layer, resulting in the formation of an amorphous (ACP) layer or hydroxyl-carbonate-apatite (HCA) layer when crystallized, as shown in Fig. 4 a-c [69,114,115]. This layer is very important because it provides a link between the tissue and the glass material. The success of these reactions confirms the biocompatibility of the implant material. Otherwise, the body would reject the implant by interpreting it as a foreign entity, forming a fibrous capsule around it. With the avoidance of rejection, the implant would then be able to achieve a long-term application.
Fig. 4.
In vitro and in vivo bioactivity of bioactive glass. (a) Scheme of apatite formation on the surface of bioactive glass [114]. (b–c) SEM images of the layer-structured hydroxyapatite microspheres converted from 2Na2O–2CaO–6B2O3 bioactive glass in vitro [69]. (d) BSE-SEM images and elemental mappings for Ti (blue), Ca (red), Si (green) of sol–gel bioactive glass coated porous Ti after 4 weeks of implantation in a rabbit tibia defect [121].
Based on the above interactions between the bioactive glass coating and the tissue, especially the HCA formation, it is classically used in dental and orthopedic applications. The first in vivo test on 45S5 was in rat femurs [61,116]. Subsequent in vivo studies indicate 45S5 regenerates bone better than commercial bioceramics [[117], [118], [119]]. It has been found that the bioactive glass could induce the formation of vascular and bone tissues in the rabbit tibia [120]. Fig. 4d shows the mineralized tissue formation on the bioactive coating after 4 weeks of implantation in a rabbit tibia defect [121]. Comparative studies have been conducted between the bioactive glass-coated and hydroxyapatite-coated titanium dental implants, and bioactive glass coating materials showed similar or better performance than hydroxyapatite coating in achieving osseointegration in the human jaw bone [122,123]. Recent studies have indicated that the application of bioactive glass has surpassed the traditional hard tissue bonding capabilities in dental and orthopedic implants, and advanced in soft tissue applications as nano-particles, including cardiac tissue regeneration, wound healing/dressing, nerve regeneration, gastrointestinal regeneration, urinary tract, lung tissue engineering, and laryngeal repair [124].
Although there have been many previous clinical studies about the bioactive glass coating on metallic implants [122,123,125,126], an issue with clinical trials is that results cannot be compared directly because of different patient and applications. In vivo animal study could be easier for comparisons and close to the clinical trials. As compared to in vivo testing as mentioned above, in vitro evaluation is more economical and budget-friendly, e.g., cell culture, immersion test in various solutions such as SBF. Nevertheless, it should be pointed out that bioactivity evaluated by in vitro method has some notable exceptions although it is generally agreeable with in vivo bioactivity [127,128]. Details of the experimental conditions can significantly alter the dissolution rate and apatite formation [[129], [130], [131], [132]]. Therefore, following an ISO standard test [133] is highly recommended. Macon et al. [134] proposed a testing method based on an ISO standard procedural for a better evaluation for glasses that have very different specific surface areas. Due to the different tolerance of each element in different host tissue (e.g., the limited concentration of silicon is 0.6 μg/mL for serum and 41 μg/mL for muscle [64]), dynamic tests can be more accurate for mimicking element released concentration. Studies by Marquardt et al. [135] and Modglin et al. [136] discovered that even though released boron ions exhibit toxicity in static experiments in vitro, the negative effect can be minimized in a dynamic environment, which is close to the microvasculature from human body. Fu et al. [137] observed similar results in 13–93 bioactive glass with all silica substituted by boron oxide. It was found that the glass is toxic to osteogenic cells in vitro but did not show toxicity and support new tissue infiltration in vivo when implanted in rats.
7. Conclusions and perspectives
In summary, we acknowledge the solid growth and development in metallic implant materials and bioactive glasses and glass-ceramics, as well as the application of bioactive glass as a coating on metallic implants for better biocompatibility and various biomedical applications. The integration of metallic implants and bioactive glasses provide a combination of mechanical, corrosion and biological advantages. Moving forward, it is believed that the application of glass-based coatings on metallic implants could surpass the traditional hard tissue bonding capabilities in dental and orthopedic implants, and advance in soft tissue applications, and 3D coating application on porous scaffolds. However, there are still many difficulties to overcome before their clinical applications. Metals and glasses have distinctly different thermal expansion coefficients, leading to the coating stress and failure. Depending on the glass properties, metal substrates, and specific applications, different coating methods can be selected to improve the chance of achieving a coating with good adhesion while maintaining the bioactivity. Moreover, it is important to consider the vast dynamics of the human body, therefore, it is understandable to state that every different type of implant is subjected to a different environment.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgement
This work was funded by the National Institutes of Health (Grant number R01HL140562) and National Science Foundation DMR Ceramics Program (Grant number: 1508001).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jincheng Du, Email: Jincheng.Du@unt.edu.
Donghui Zhu, Email: Donghui.Zhu@stonybrook.edu.
References
- 1.Bekmurzayeva A., Duncanson W.J., Azevedo H.S., Kanayeva D. Surface modification of stainless steel for biomedical applications: revisiting a century-old material. Mater. Sci. Eng. C. 2018;93:1073–1089. doi: 10.1016/j.msec.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 2.Lemons J.E., Lucas L.C. Properties of biomaterials. J. Arthroplast. 1986;1:143–147. doi: 10.1016/s0883-5403(86)80053-5. [DOI] [PubMed] [Google Scholar]
- 3.Asri R.I.M., Harun W.S.W., Samykano M., Lah N.A.C., Ghani S.A.C., Tarlochan F., Raza M.R. Corrosion and surface modification on biocompatible metals: a review. Mater. Sci. Eng. C. 2017 doi: 10.1016/j.msec.2017.04.102. [DOI] [PubMed] [Google Scholar]
- 4.Minagar S., Berndt C.C., Wang J., Ivanova E., Wen C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012 doi: 10.1016/j.actbio.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Su Y., Cockerill I., Zheng Y., Tang L., Qin Y.-X., Zhu D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019;4 doi: 10.1016/j.bioactmat.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Y., Luo C., Zhang Z., Hermawan H., Zhu D., Huang J., Liang Y., Li G., Ren L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018;77 doi: 10.1016/j.jmbbm.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Sola A., Bellucci D., Cannillo V., Cattini A. Bioactive glass coatings: a review. Surf. Eng. 2011;27:560–572. [Google Scholar]
- 8.Bellucci D., Cannillo V., Sola A. Coefficient of thermal expansion of bioactive glasses: available literature data and analytical equation estimates. Ceram. Int. 2011;37:2963–2972. [Google Scholar]
- 9.Baino F., Verne E. 2017. Glass-based Coatings on Biomedical Implants: A State-Of-The-Art Review, Biomed. Glas. [Google Scholar]
- 10.Wang D.G., Chen C.Z., Ma Q.S., Jin Q.P., Li H.C. A study on in vitro and in vivo bioactivity of HA/45S5 composite films by pulsed laser deposition. Appl. Surf. Sci. 2013;270:667–674. [Google Scholar]
- 11.Demirkiran H., Mohandas A., Dohi M., Fuentes A., Nguyen K., Aswath P. Bioactivity and mineralization of hydroxyapatite with bioglass as sintering aid and bioceramics with Na3Ca6(PO4)5 and Ca5(PO4)2SiO4 in a silicate matrix. Mater. Sci. Eng. C. 2010;30:263–272. doi: 10.1016/j.msec.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Pang X., Zhitomirsky I. Electrodeposition of composite hydroxyapatite-chitosan films. Mater. Chem. Phys. 2005;94:245–251. [Google Scholar]
- 13.Farnoush H., Muhaffel F., Cimenoglu H. Fabrication and characterization of nano-HA-45S5 bioglass composite coatings on calcium-phosphate containing micro-arc oxidized CP-Ti substrates. Appl. Surf. Sci. 2015;324:765–774. [Google Scholar]
- 14.Seuss S., Chavez A., Yoshioka T., Stein J., Boccaccini A.R. Electrophoretic deposition of soft coatings for orthopaedic applications. Ceram. Trans. 2012;237:145–152. [Google Scholar]
- 15.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Biomaterials; 2006. Magnesium and its Alloys as Orthopedic Biomaterials: A Review. [DOI] [PubMed] [Google Scholar]
- 16.Ranu H.S. The thermal properties of human cortical bone: an in vitro study. Eng. Med. 1987 doi: 10.1243/emed_jour_1987_016_036_02. [DOI] [PubMed] [Google Scholar]
- 17.United Performance Metals, 316 Stainless Steel Sheet, Coil & Bar - AMS 5524, AMS 5507 - 316L S Stainless Supplier, ((n.d.)).
- 18.Cobalt Chrome Molybdenum Bar Stock - Chrome Moly Steel - ASTM F1537 Alloy 1, (n.d.).
- 19.Niinomi M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A. 1998 [Google Scholar]
- 20.Mordike B.L., Ebert T. Magnesium Properties - applications - potential. Mater. Sci. Eng. A. 2001 [Google Scholar]
- 21.Dziubińska A., Gontarz A., Horzelska K., Pieśko P. The microstructure and mechanical properties of AZ31 magnesium alloy aircraft brackets produced by a new forging technology. Procedia Manuf. 2015;2:337–341. [Google Scholar]
- 22.Hermawan H., Dubé D., Mantovani D. Degradable metallic biomaterials: design and development of Fe-Mn alloys for stents. J. Biomed. Mater. Res. A. 2010 doi: 10.1002/jbm.a.32224. [DOI] [PubMed] [Google Scholar]
- 23.Heiden M., Kustas A., Chaput K., Nauman E., Johnson D., Stanciu L. Effect of microstructure and strain on the degradation behavior of novel bioresorbable iron-manganese alloy implants. J. Biomed. Mater. Res. A. 2015 doi: 10.1002/jbm.a.35220. [DOI] [PubMed] [Google Scholar]
- 24.Lu X., Qin Z., Zhang Y., Wang X., Li F., Ding B., Hu Z. Study of the paramagnetic-antiferromagnetic transition and the γ → ε martensitic transformation in Fe-Mn alloys. J. Mater. Sci. 2000 [Google Scholar]
- 25.Li G., Yang H., Zheng Y., Chen X.-H., Yang J.-A., Zhu D., Ruan L., Takashima K. Challenges in the use of zinc and its alloys as biodegradable metals: perspective from biomechanical compatibility. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Mostaed E., Sikora-Jasinska M., Mostaed A., Loffredo S., Demir A.G., Previtali B., Mantovani D., Beanland R., Vedani M. Novel Zn-based alloys for biodegradable stent applications: design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016 doi: 10.1016/j.jmbbm.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S., McNamara C.T., Bowen P.K., Verhun N., Braykovich J.P., Goldman J., Drelich J.W. Structural characteristics and in vitro biodegradation of a novel Zn-Li alloy prepared by induction melting and hot rolling. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017 [Google Scholar]
- 28.Hermawan H., Ramdan D., Djuansjah J.R.P. Biomed. Eng. - from Theory to Appl. InTech; 2011. Metals for biomedical applications. [Google Scholar]
- 29.Zardiackas L.D. Wiley Encycl. Biomed. Eng. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2006. Stainless steels for implants. [Google Scholar]
- 30.Elias C.N., Fernandes D.J., de Souza F.M., Monteiro E. dos S., de Biasi R.S. Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J. Mater. Res. Technol. 2018 [Google Scholar]
- 31.Özkurt Z., Kazazoğlu E. Zirconia dental implants: a literature review. J. Oral Implantol. 2011 doi: 10.1563/AAID-JOI-D-09-00079. [DOI] [PubMed] [Google Scholar]
- 32.Saini M. Implant biomaterials: a comprehensive review. World J. Clin. Cases. 2015 doi: 10.12998/wjcc.v3.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.H. Tschernitschek, L. Borchers, W. Geurtsen, Nonalloyed titanium as a bioinert metal--a review., Quintessence Int.. 36 (n.d.) 523–530. [PubMed]
- 34.Marti A. Cobalt-base alloys used in surgery. Injury. 2000 doi: 10.1016/s0020-1383(00)80018-2. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs J.J., Skipor A.K., Doom P.F., Campbell P., Schmalzried T.P., Black J., Amstutz H.C. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin. Orthop. Relat. Res. 1996 doi: 10.1097/00003086-199608001-00022. [DOI] [PubMed] [Google Scholar]
- 36.Lhotka C., Szekeres T., Steffan I., Zhuber K., Zweymüller K. Four-year study of cobalt and chromium blood levels in patients managed with two different metal-on-metal total hip replacements. J. Orthop. Res. 2003 doi: 10.1016/S0736-0266(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 37.Schaffer A.W., Pilger A., Engelhardt C., Zweymueller K., Ruediger H.W. Increased blood cobalt and chromium after total hip replacement. J. Toxicol. Clin. Toxicol. 1999 doi: 10.1081/clt-100102463. [DOI] [PubMed] [Google Scholar]
- 38.Minoda Y., Kobayashi A., Iwaki H., Iwakiri K., Inori F., Sugama R., Ikebuchi M., Kadoya Y., Takaoka K. In vivo analysis of polyethylene wear particles after total knee arthroplasty: the influence of improved materials and designs. J. Bone Jt. Surgery-American. 2009;91:67–73. doi: 10.2106/JBJS.I.00447. [DOI] [PubMed] [Google Scholar]
- 39.Kuo P.H., Joshi S.S., Lu X., Ho Y.H., Xiang Y., Dahotre N.B., Du J. Laser coating of bioactive glasses on bioimplant titanium alloys. Int. J. Appl. Glass Sci. 2019 [Google Scholar]
- 40.Bechtel C.P., Gebhart J.J., Tatro J.M., Kiss-Toth E., Wilkinson J.M., Greenfield E.M. Particle-induced osteolysis is mediated by TIRAP/mal in vitro and in vivo. J. Bone Jt. Surg. 2016;98:285–294. doi: 10.2106/JBJS.O.00736. [DOI] [PubMed] [Google Scholar]
- 41.Shareghi B., Johanson P.-E., Kärrholm J. Wear of vitamin E-infused highly cross-linked polyethylene at five years. J. Bone Jt. Surg. 2017;99:1447–1452. doi: 10.2106/JBJS.16.00691. [DOI] [PubMed] [Google Scholar]
- 42.Witt F., Bosker B., Bishop N., Ettema H., Verheyen C.C.P., Morlock M. The relation between titanium taper corrosion and cobalt-chromium bearing wear in large-head metal-on-metal total hip prostheses. J. Bone Jt. Surgery-American. 2014;96:e157–1–9. doi: 10.2106/JBJS.M.01199. [DOI] [PubMed] [Google Scholar]
- 43.Swiontkowski M., Resnick L. Severe and strange consequences of arthroplasty component wear. JBJS Case Connect. 2015;5:e14. doi: 10.2106/JBJS.CC.O.00005. [DOI] [PubMed] [Google Scholar]
- 44.Lanting B., Naudie D.D.R., McCalden R.W. Clinical impact of trunnion wear after total hip arthroplasty. JBJS Rev. 2016;4:1. doi: 10.2106/JBJS.RVW.15.00096. [DOI] [PubMed] [Google Scholar]
- 45.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. [Google Scholar]
- 46.Li H., Zheng Y., Qin L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014 [Google Scholar]
- 47.Yazdimamaghani M., Razavi M., Vashaee D., Moharamzadeh K., Boccaccini A.R., Tayebi L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng. C. 2017;71:1253–1266. doi: 10.1016/j.msec.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 48.Zhu D., Su Y., Fu B., Xu H. Magnesium reduces blood-brain barrier permeability and regulates amyloid-β transcytosis. Mol. Neurobiol. 2018;55 doi: 10.1007/s12035-018-0896-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhu D., Su Y., Zheng Y., Fu B., Tang L., Qin Y.-X. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am. J. Physiol. Cell Physiol. 2018;314 doi: 10.1152/ajpcell.00279.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu D., Su Y., Young M.L., Ma J., Zheng Y., Tang L. Biological responses and mechanisms of human bone marrow mesenchymal stem cells to Zn and Mg biomaterials. ACS Appl. Mater. Interfaces. 2017;9 doi: 10.1021/acsami.7b06654. [DOI] [PubMed] [Google Scholar]
- 51.Witte F., Eliezer A. Degrad. Implant Mater. 2012. Biodegradable metals. [Google Scholar]
- 52.Su Y., Cockerill I., Wang Y., Qin Y.-X., Chang L., Zheng Y., Zhu D. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2019;37 doi: 10.1016/j.tibtech.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su Y., Yang H., Gao J., Qin Y.-X., Zheng Y., Zhu D. Interfacial zinc phosphate is the key to controlling biocompatibility of metallic zinc implants. Adv. Sci. 2019;6 doi: 10.1002/advs.201900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu D., Cockerill I., Su Y., Zhang Z., Fu J., Lee K.-W., Ma J., Okpokwasili C., Tang L., Zheng Y., Qin Y.-X., Wang Y. Mechanical strength, biodegradation, and in vitro and in vivo biocompatibility of Zn biomaterials. ACS Appl. Mater. Interfaces. 2019;11 doi: 10.1021/acsami.8b20634. [DOI] [PubMed] [Google Scholar]
- 55.Francis A., Yang Y., Virtanen S., Boccaccini A.R. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015 doi: 10.1007/s10856-015-5473-8. [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Jahr H., Lietaert K., Pavanram P., Yilmaz A., Fockaert L.I., Leeflang M.A., Pouran B., Gonzalez-Garcia Y., Weinans H., Mol J.M.C., Zhou J., Zadpoor A.A. Additively manufactured biodegradable porous iron. Acta Biomater. 2018 doi: 10.1016/j.actbio.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Mostaed E., Sikora-Jasinska M., Drelich J.W., Vedani M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018 doi: 10.1016/j.actbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Virtanen S. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011. Biodegradable Mg and Mg alloys: corrosion and biocompatibility. [Google Scholar]
- 59.Agarwal S., Curtin J., Duffy B., Jaiswal S. Biodegradable magnesium alloys for orthopaedic applications: a review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C. 2016 doi: 10.1016/j.msec.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 60.Chen Q., Thouas G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015 [Google Scholar]
- 61.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971;5:117–141. [Google Scholar]
- 62.Hench L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 63.Rabiee S.M., Nazparvar N., Azizian M., Vashaee D., Tayebi L. Effect of ion substitution on properties of bioactive glasses: a review. Ceram. Int. 2015;41:7241–7251. [Google Scholar]
- 64.Jones J.R. Reprint of: review of bioactive glass: from Hench to hybrids. Acta Biomater. 2015;23:S53–S82. doi: 10.1016/j.actbio.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 65.O'Donnell M.D., Hill R.G., Donnell M.D.O., Hill R.G. Influence of strontium and the importance of glass chemistry and structure when designing bioactive glasses for bone regeneration. Acta Biomater. 2010;6:2382–2385. doi: 10.1016/j.actbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Tian M., Chen F., Song W., Song Y., Chen Y., Wan C., Yu X., Zhang X. In vivo study of porous strontium-doped calcium polyphosphate scaffolds for bone substitute applications. J. Mater. Sci. Mater. Med. 2009;20:1505–1512. doi: 10.1007/s10856-009-3713-5. [DOI] [PubMed] [Google Scholar]
- 67.Lakhkar N.J., Abou Neel E.A., Salih V., Knowles J.C. Strontium oxide doped quaternary glasses: effect on structure, degradation and cytocompatibility. J. Mater. Sci. Mater. Med. 2009;20:1339–1346. doi: 10.1007/s10856-008-3688-7. [DOI] [PubMed] [Google Scholar]
- 68.Raju K.S., Alessandri G., Ziche M., Gullino P.M. Ceruloplasmin, copper ions, and angiogenesis. J. Natl. Cancer Inst. 1982;69:1183–1188. [PubMed] [Google Scholar]
- 69.Rahaman M.N., Day D.E., Sonny Bal B., Fu Q., Jung S.B., Bonewald L.F., Tomsia A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011;7:2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stähli C., James-Bhasin M., Hoppe A., Boccaccini A.R., Nazhat S.N. Effect of ion release from Cu-doped 45S5 Bioglass® on 3D endothelial cell morphogenesis. Acta Biomater. 2015;19:15–22. doi: 10.1016/j.actbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Bellantone M., Coleman N.J., Hench L.L. Bacteriostatic action of a novel four-component bioactive glass. J. Biomed. Mater. Res. 2000;51:484–490. doi: 10.1002/1097-4636(20000905)51:3<484::aid-jbm24>3.0.co;2-4. AID-JBM24>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Vega J.M., Saiz E., Tomsia A.P., Oku T., Suganuma K., Marshall G.W., Marshall S.J. Novel bioactive functionally graded coatings on Ti6Al4V. Adv. Mater. 2000;12:894–898. AID-ADMA894>3.0.CO;2-4. [Google Scholar]
- 73.Kasuga T., Nogami M., Niinomi M. Calcium phosphate glass-ceramics for bioactive coating on a b-titanium alloy. Adv. Eng. Mater. 2003;5:498–501. [Google Scholar]
- 74.Peddi L., Brow R.K., Brown R.F. Bioactive borate glass coatings for titanium alloys. J. Mater. Sci. Mater. Med. 2008;19:3145–3152. doi: 10.1007/s10856-008-3419-0. [DOI] [PubMed] [Google Scholar]
- 75.Lotfibakhshaiesh N., Brauer D.S., Hill R.G. Bioactive glass engineered coatings for Ti6Al4V alloys: influence of strontium substitution for calcium on sintering behaviour. 12th Int. Conf. Phys. Non-Crystalline Solids (PNCS 12) 2010;356:2583–2590. doi: 10.1016/j.jnoncrysol.2010.05.017. [DOI] [Google Scholar]
- 76.Al-Noaman A., Rawlinson S.C.F., Hill R.G. The role of MgO on thermal properties, structure and bioactivity of bioactive glass coating for dental implants. J. Non-Cryst. Solids. 2012;358:3019–3027. [Google Scholar]
- 77.Thomas S., Balakrishnan P., Sreekala M.S., Mukhopadhyay S. Bioactive glass-ceramics. Fundam. Biomater. Ceram. 2018:129–152. [Google Scholar]
- 78.Kokubo T., Ito S., Shigematsu M., Sanka S., Yamamuro T. Fatigue and life-time of bioactive glass-ceramic A-W containing apatite and wollastonite. J. Mater. Sci. 1987;22:4067–4070. [Google Scholar]
- 79.Xie K., Zhang L.L., Yang X., Wang X., Yang G., Zhang L.L., He Y., Fu J., Gou Z., Shao H., He Y., Fu J., Gou Z. Preparation and characterization of low temperature heat-treated 45S5 bioactive glass-ceramic analogues. Biomed. Glas. 2015;1:80–92. [Google Scholar]
- 80.Hoppe A., Boccardi E., Ciraldo F.E., Boccaccini A.R., Hill R.G. 1.10 bioactive glass-ceramics. Compr. Biomater. II. 2017:235–243. [Google Scholar]
- 81.García C., Ceré S., Durán A. Bioactive coatings deposited on titanium alloys. J. Non-Cryst. Solids. 2006;352:3488–3495. [Google Scholar]
- 82.Rau J.V., Antoniac I., Fosca M., De Bonis A., Blajan A.I., Cotrut C., Graziani V., Curcio M., Cricenti A., Niculescu M., Ortenzi M., Teghil R. Glass-ceramic coated Mg-Ca alloys for biomedical implant applications. Mater. Sci. Eng. C. 2016 doi: 10.1016/j.msec.2016.03.100. [DOI] [PubMed] [Google Scholar]
- 83.Chern Lin J.H., Liu M.L., Ju C.P. Structure and properties of hydroxyapatite-bioactive glass composites plasma sprayed on Ti6Al4V. J. Mater. Sci. Mater. Med. 1994;5:279–283. [Google Scholar]
- 84.Ebrahimi M., Mobasherpour I., Bafrooei H.B., Bidabadi F.S., Mansoorianfar M., Orooji Y., Khataee A., Mei C., Salahi E., Ebadzadeh T. Taguchi design for optimization of structural and mechanical properties of hydroxyapatite-alumina-titanium nanocomposite. Ceram. Int. 2019;45:10097–10105. [Google Scholar]
- 85.Pishbin F., Simchi A., Ryan M.P., Boccaccini A.R. Electrophoretic deposition of chitosan/45S5 Bioglass® composite coatings for orthopaedic applications. Surf. Coat. Technol. 2011;205:5260–5268. [Google Scholar]
- 86.El khalidi Z., Hartiti B., Fadili S., Thevenin P. Nickel oxide optimization using Taguchi design for hydrogen detection. Int. J. Hydrogen Energy. 2018;43:12574–12583. [Google Scholar]
- 87.Soni R., Kumar N.V., Chameettachal S., Pati F., Narayan Rath S. Synthesis and optimization of PCL-bioactive glass composite scaffold for bone tissue engineering, mater. Today Proc. 2019;15:294–299. [Google Scholar]
- 88.Francis A., Detsch R., Boccaccini A.R. Fabrication and cytotoxicity assessment of novel polysiloxane/bioactive glass films for biomedical applications. Ceram. Int. 2016 [Google Scholar]
- 89.Kim H.-M., Miyaji F., Kokubo T., Nakamura T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J. Biomed. Mater. Res. 1996;32:409–417. doi: 10.1002/(SICI)1097-4636(199611)32:3<409::AID-JBM14>3.0.CO;2-B. AID-JBM14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 90.Su Y., Wang K., Gao J., Yang Y., Qin Y.-X., Zheng Y., Zhu D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su Y., Lu Y., Su Y., Hu J., Lian J., Li G. Enhancing the corrosion resistance and surface bioactivity of a calcium-phosphate coating on a biodegradable AZ60 magnesium alloy via a simple fluorine post-treatment method. RSC Adv. 2015;5 [Google Scholar]
- 92.Su Y., Li D., Su Y., Lu C., Niu L., Lian J., Li G. Improvement of the biodegradation property and biomineralization ability of magnesium-hydroxyapatite composites with dicalcium phosphate dihydrate and hydroxyapatite coatings. ACS Biomater. Sci. Eng. 2016;2 doi: 10.1021/acsbiomaterials.6b00013. [DOI] [PubMed] [Google Scholar]
- 93.Browne M., Gregson P. Effect of mechanical surface pretreatment on metal ion release. Biomaterials. 2000;21:385–392. doi: 10.1016/s0142-9612(99)00200-8. [DOI] [PubMed] [Google Scholar]
- 94.Bagheri S., Guagliano M. Review of shot peening processes to obtain nanocrystalline surfaces in metal alloys. Surf. Eng. 2009 [Google Scholar]
- 95.Conde A., de Damborenea J.J. Degradation of vitreous enamel coatings. Ref. Modul. Mater. Sci. Mater. Eng. 2016 [Google Scholar]
- 96.Chang J., Zhou Y.L., Zhou Y. Surface modification of bioactive glasses. Bioact. Glas. 2011:29–52. [Google Scholar]
- 97.Kaur G., Pandey O.P., Singh K., Homa D., Scott B., Pickrell G. A review of bioactive glasses: their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. A. 2014;102:254–274. doi: 10.1002/jbm.a.34690. [DOI] [PubMed] [Google Scholar]
- 98.Bryington M.S., Hayashi M., Kozai Y., Vandeweghe S., Andersson M., Wennerberg A., Jimbo R. The influence of nano hydroxyapatite coating on osseointegration after extended healing periods. Dent. Mater. 2013;29:514–520. doi: 10.1016/j.dental.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 99.Shen S., Cai S., Xu G., Zhao H., Niu S., Zhang R. Influence of heat treatment on bond strength and corrosion resistance of sol-gel derived bioglass-ceramic coatings on magnesium alloy. J. Mech. Behav. Biomed. Mater. 2015;45:166–174. doi: 10.1016/j.jmbbm.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Avcu E., Baştan F.E., Abdullah H.Z., Rehman M.A.U., Avcu Y.Y., Boccaccini A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: a review. Prog. Mater. Sci. 2019;103:69–108. [Google Scholar]
- 101.Pishbin F., Mouriño V., Gilchrist J.B., McComb D.W., Kreppel S., Salih V., Ryan M.P., Boccaccini A.R. Single-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nano-silver composite system. Acta Biomater. 2013;9:7469–7479. doi: 10.1016/j.actbio.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 102.Krzyzanowski M., Bajda S., Liu Y., Triantaphyllou A., Mark Rainforth W., Glendenning M. 3D analysis of thermal and stress evolution during laser cladding of bioactive glass coatings. J. Mech. Behav. Biomed. Mater. 2016;59:404–417. doi: 10.1016/j.jmbbm.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 103.Gough J.E., Notingher I., Hench L.L. Osteoblast attachment and mineralized nodule formation on rough and smooth 45S5 bioactive glass monoliths. J. Biomed. Mater. Res. A. 2004 doi: 10.1002/jbm.a.20075. [DOI] [PubMed] [Google Scholar]
- 104.ASTM . Astm; 2015. ASTM F1854-15: Standard Test Method for Stereological Evaluation of Porous Coatings on Medical. [Google Scholar]
- 105.Jallot E. Role of magnesium during spontaneous formation of a calcium phosphate layer at the periphery of a bioactive glass coating doped with MgO. Appl. Surf. Sci. 2003 [Google Scholar]
- 106.Garcia E., Miranzo P., Sainz M.A. Thermally sprayed wollastonite and wollastonite-diopside compositions as new modulated bioactive coatings for metal implants. Ceram. Int. 2018;44:12896–12904. [Google Scholar]
- 107.Kim C.Y., Jung W.L. Surface bio-modification of titanium implants by an enamel process. J. Ceram. Process. Res. 2005;6:338–344. [Google Scholar]
- 108.Lopez-Esteban S., Saiz E., Fujino S., Oku T., Suganuma K., Tomsia A.P.P. Bioactive glass coatings for orthopedic metallic implants. J. Eur. Ceram. Soc. 2003;23:2921–2930. doi: 10.1016/S0955-2219(03)00303-0. [DOI] [Google Scholar]
- 109.Filho O.P., Latorre G.P., Hench L.L., Peitl Filho O., Latorre G.P., Hench L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996;30:509–514. doi: 10.1002/(SICI)1097-4636(199604)30:4<509::AID-JBM9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 110.Schrooten J., Van Oosterwyck H., Vander Sloten J., Helsen J.A. Adhesion of new bioactive glass coating. J. Biomed. Mater. Res. 1999;44:243–252. doi: 10.1002/(sici)1097-4636(19990305)44:3<243::aid-jbm2>3.0.co;2-o. AID-JBM2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 111.Cañas E., Sanz V., Orts M.J., Sánchez E. Post-deposition heat treatment effect on microstructure of suspension plasma sprayed bioactive glass coatings. Surf. Coat. Technol. 2019;371:136–142. [Google Scholar]
- 112.Rai P., Rai A., Kumar V., Chaturvedi R.K., Singh V.K. Corrosion study of biodegradable magnesium based 1393 bioactive glass in simulated body fluid. Ceram. Int. 2019 [Google Scholar]
- 113.Höhlinger M., Christa D., Zimmermann V., Heise S., Boccaccini A.R., Virtanen S. Influence of proteins on the corrosion behavior of a chitosan-bioactive glass coated magnesium alloy. Mater. Sci. Eng. C. 2019;100:706–714. doi: 10.1016/j.msec.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 114.Mondal S., Hoang G., Manivasagan P., Moorthy M.S., Nguyen T.P., Vy Phan T.T., Kim H.H., Kim M.H., Nam S.Y., Oh J. Nano-hydroxyapatite bioactive glass composite scaffold with enhanced mechanical and biological performance for tissue engineering application. Ceram. Int. 2018 [Google Scholar]
- 115.Lin K.S.K., Tseng Y.H., Mou Y., Hsu Y.C., Yang C.M., Chan J.C.C. Mechanistic study of apatite formation on bioactive glass surface using31P solid-state NMR spectroscopy. Chem. Mater. 2005 [Google Scholar]
- 116.Hench L.L., Pantano C.G., Buscemi P.J., Greenspan D.C. Analysis of bioglass fixation of hip prostheses. J. Biomed. Mater. Res. 1977;11:267–282. doi: 10.1002/jbm.820110211. [DOI] [PubMed] [Google Scholar]
- 117.Oonishi H., Hench L.L., Wilson J., Sugihara F., Tsuji E., Kushitani S., Iwaki H. Comparative bone growth behavior in granules of bioceramic materials of various sizes. J. Biomed. Mater. Res. 1999;44:31–43. doi: 10.1002/(sici)1097-4636(199901)44:1<31::aid-jbm4>3.0.co;2-9. aid-jbm4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 118.Oonishi H., Hench L.L., Wilson J., Sugihara F., Tsuji E., Matsuura M., Kin S., Yamamoto T., Mizokawa S. Quantitative Comparison of Bone Growth Behavior in Granules of Bioglass, A-W glass-ceramic, and hydroxyapatite. 2000. pp. 4–13. [DOI] [PubMed] [Google Scholar]
- 119.Wheeler D.L., Eschbach E.J., Hoellrich R.G., Montfort M.J., Chamberland D.L. Assessment of resorbable bioactive material for grafting of critical-size cancellous defects. J. Orthop. Res. 2000;18:140–148. doi: 10.1002/jor.1100180120. [DOI] [PubMed] [Google Scholar]
- 120.Fu Q., Huang W., Jia W., Rahaman M.N., Liu X., Tomsia A.P. Three-dimensional visualization of bioactive glass-bone integration in a rabbit tibia model using synchrotron X-ray microcomputed tomography. Tissue Eng. A. 2011 doi: 10.1089/ten.tea.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Braem A., Chaudhari A., Vivan Cardoso M., Schrooten J., Duyck J., Vleugels J. Peri- and intra-implant bone response to microporous Ti coatings with surface modification. Acta Biomater. 2014 doi: 10.1016/j.actbio.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 122.Mistry S., Kundu D., Datta S., Basu D. Comparison of bioactive glass coated and hydroxyapatite coated titanium dental implants in the human jaw bone. Aust. Dent. J. 2011 doi: 10.1111/j.1834-7819.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 123.Mistry S., Roy R., Kundu B., Datta S., Kumar M., Chanda A., Kundu D. Clinical outcome of hydroxyapatite coated, bioactive glass coated, and machined Ti6Al4V threaded dental implant in human jaws: a short-term comparative study. Implant Dent. 2016 doi: 10.1097/ID.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 124.Miguez-Pacheco V., Hench L.L., Boccaccini A.R. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater. 2015 doi: 10.1016/j.actbio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Galliano P., De Damborenea J.J., Pascual M.J., Durán A. Sol-gel coatings on 316L steel for clinical applications. J. Sol. Gel Sci. Technol. 1998;13(1–3):723–727. [Google Scholar]
- 126.Marcacci M., Kon E., Moukhachev V., Lavroukov A., Kutepov S., Quarto R., Mastrogiacomo M., Cancedda R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007 doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 127.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 128.Zadpoor A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C. 2014;35:134–143. doi: 10.1016/j.msec.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 129.Jones J.R., Sepulveda P., Hench L.L. Dose-dependent behavior of bioactive glass dissolution. J. Biomed. Mater. Res. 2001;58:720–726. doi: 10.1002/jbm.10053. [DOI] [PubMed] [Google Scholar]
- 130.Manupriya, Thind K.S., Sharma G., Singh K., Rajendran V., Aravindan S. Soluble borate glasses: in vitro analysis. J. Am. Ceram. Soc. 2007;90:467–471. [Google Scholar]
- 131.Huang W., Day D.E., Kittiratanapiboon K., Rahaman M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006;17:583–596. doi: 10.1007/s10856-006-9220-z. [DOI] [PubMed] [Google Scholar]
- 132.Varila L., Fagerlund S., Lehtonen T., Tuominen J., Hupa L. Surface reactions of bioactive glasses in buffered solutions. J. Eur. Ceram. Soc. 2012;32:2757–2763. doi: 10.1016/j.jeurceramsoc.2012.01.025. [DOI] [Google Scholar]
- 133.J M. Jabatan Standards Malaysia; 2010. Standard, Implants for Surgery: in Vitro Evaluation for Apatite- Forming Ability of Implant Materials. ISO 23317:2007, IDT. [Google Scholar]
- 134.Macon A.L., Kim T.B., Valliant E.M., Goetschius K., Brow R.K., Day D.E., Hoppe A., Boccaccini A.R., Kim I.Y., Ohtsuki C., Kokubo T., Osaka A., Vallet-Regi M., Arcos D., Fraile L., Salinas A.J., Teixeira A.V., Vueva Y., Almeida R.M., Miola M., Vitale-Brovarone C., Verne E., Holand W., Jones J.R., Maçon A.L.B., Kim T.B., Valliant E.M., Goetschius K., Brow R.K., Day D.E., Hoppe A., Boccaccini A.R., Kim I.Y., Ohtsuki C., Kokubo T., Osaka A., Vallet-Regí M., Arcos D., Fraile L., Salinas A.J., Teixeira A.V., Vueva Y., Almeida R.M., Miola M., Vitale-Brovarone C., Verné E., Höland W., Jones J.R. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015;26:115. doi: 10.1007/s10856-015-5403-9. [DOI] [PubMed] [Google Scholar]
- 135.Marquardt L.M., Day D., Sakiyama-Elbert S.E., Harkins A.B. Effects of borate-based bioactive glass on neuron viability and neurite extension. J. Biomed. Mater. Res. A. 2014;102:2767–2775. doi: 10.1002/jbm.a.34944. [DOI] [PubMed] [Google Scholar]
- 136.Modglin V.C., Brown R.F., Jung S.B., Day D.E. Cytotoxicity assessment of modified bioactive glasses with MLO-A5 osteogenic cells in vitro. J. Mater. Sci. Mater. Med. 2013;24:1191–1199. doi: 10.1007/s10856-013-4875-8. [DOI] [PubMed] [Google Scholar]
- 137.Fu Q., Rahaman M.N., Fu H., Liu X., Bal B.S., Bonewald L.F., Kuroki K., Brown R.F., Fu H., Liu X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. A. 2010;95A:164–171. doi: 10.1002/jbm.a.32823. [DOI] [PubMed] [Google Scholar]