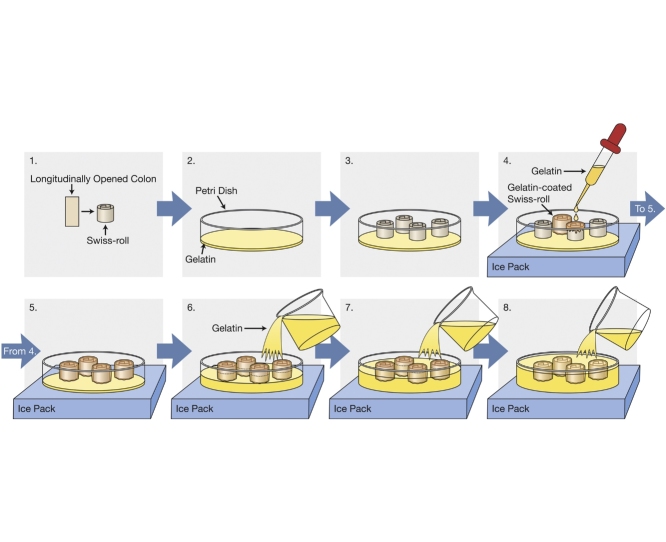
Graphical abstract
Method name: Gelatin embedded Swiss-rolls
Keywords: Longitudinal histologic exam, Colon, Gelatin embedded sections
Abstract
The appropriate methodological approach for intestinal preparation enables researchers to create representative histological and immunostaining images which validate their biochemical data. The Swiss-roll technique was first introduced by Reilly and Kirsner in 1965. Later, Moolenbeek and Ruitenberg described a detailed procedure to longitudinally study the rodent intestine in 1981 [1]. In this publication, our slightly different approach for co-embedding four different Swiss-rolls in a gelatin block provides a full-length overview of cross-sectional colons on a single slide. This protocol allows for longitudinal histologic examination of multiple tissue samples on a single slide simultaneously. In this method, antigenicity is retained for immunohistology. In addition, the accessibility of samples during the prolonged hardening time required of the gelatin matrix allows the tissue samples to be adjusted/re-adjusted to provide the desired orientation and spatial arrangement for ideal cross sections with similar planes of section and optimum space utilization for slide mounting. Although not the focus of this protocol, the room temperature stability of the gelatin matrix and the ability to contain numerous tissue samples in a block allows the flexibility of performing thicker sections for free-floating tissue staining and ease of mounting a single gelatin sheet rather than individual tissue sections. This is a convenient approach for allowing precise preparation of multi-tissue blocks and simultaneous sectioning, staining, and slide mounting of tissue for subsequent comparisons.
-
•
A single gelatin block is prepared by simultaneously embedding at least four different intestinal Swiss-rolls.
-
•
The tissue orientation can be adjusted for each sample as desired which facilitates the comparison of different colon samples on a single gelatin section.
-
•
The gelatin sections containing tissue samples are stable at least overnight at room temperature for staining.
Specification Table
| Subject Area: | Neuroscience |
| More specific subject area: | Longitudinal preparation of intestinal tissue using gelatin based matrix instead of paraffin embedding to investigate the colon histology and immunohistochemistry changes. |
| Method name: | Gelatin embedded Swiss-rolls |
| Name and reference of original method: | 1.Moolenbeek, C. and E.J. Ruitenberg, The "Swiss roll": a simple technique for histological studies of the rodent intestine. Lab Anim, 1981. 15(1): p. 57-9. 2.Whittem, C.G., A.D. Williams, and C.S. Williams, Murine Colitis modeling using Dextran Sulfate Sodium (DSS). J Vis Exp, 2010(35). 3.Nagamoto-Combs, K., et al., An improved approach to align and embed multiple brain samples in a gelatin-based matrix for simultaneous histological processing. J Neurosci Methods, 2016. 261: p. 155-60. 4.Puig, K.L., et al., Overexpression of mutant amyloid-β protein precursor and presenilin 1 modulates enteric nervous system. J Alzheimers Dis, 2015. 44(4): p. 1263-78. |
| Resource availability: | NA |
Method details
Materials
-
•
15% gelatin (Sigma, G2500-1KG) in PBS + 0.02% Na Azide or 1:500 (from 10% stock)
-
•
4% paraformaldehyde (PFA)
-
•
15% and 30% sucrose in PBS + 0.02% Na Azide or 1:500 (from 10% stock)
-
•
Swiss-roll intestines
-
•
Immunohistochemistry (IHC) solution containing 0.5% bovine serum albumin (BSA, Equitech-Bio, Inc.), 0.1% Triton X-100 (Sigma-Aldrich), 5% normal goat serum (NGS, Equitech-Bio, Inc.), and 0.02% Na Azide in PBS
-
•
Mice were euthanized followed by cardiac perfusion. Animal protocol was reviewed and approved by the UND Institutional Animal Care and Use Committee (UND IACUC). The investigation conforms to the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals (8th edition). After euthanizing a mouse, use forceps to pull up the abdomen at the midline and make a V-shaped incision using surgical scissors.
-
•
Extend the incision by cutting the skin along with peritoneum from the pelvis toward the anus.
-
•
Locate the colon and use surgical scissors to carefully cut it out from the cecum toward the distal colon.
-
•
Remove the colon from the peritoneal cavity and use surgical scissors to remove the cecum.
-
•
Remove surrounding fat tissue from the remaining colon using forceps.
-
•
Gently wash the feces from the colon in a proximal to distal direction using a gavage needle and syringe filled with cold PBS.
-
•
Hold the colon using fine-tipped forceps and cut it longitudinally from the distal to the proximal end using fine-tipped scissors.
-
•
Keep the luminal side upward and pick up the distal end via the fine-tipped forceps and wrap it around one end of a toothpick as modified from Moolenbeek and Ruitenberg [1].
-
•
Wrap the colon around the toothpick from the distal to the proximal end by holding both ends of the toothpick with your fingers and gently rolling.
-
•
Once the roll is finished, add a few drops of PBS onto the fine end of the toothpick and gently slide the roll toward the fine end of the toothpick to release the Swiss-roll.
-
•
Hold both sides of the roll with blunt end forceps and insert a 27G1/2 needle through the roll.
-
•
Fix the roll in place by bending the sharp end of the needle using blunt end forceps. This method was obtained from Whittem et al with some modifications [2].
-
•
Transfer the Swiss-roll to a 5 mL microcentrifuge tube filled with 4% PFA and store at 4°C for 5 days.
-
•
Replace the PFA with 30% sucrose on the 5th day for 5 days. Repeat this step one more time for four more days. Store the tube at 4°C in 30% sucrose until ready to make the gelatin block.
-
•
Once the roll is ready, proceed with embedding it into a gelatin block [3].
-
•
Add 15% gelatin with 0.02% Na Azide in pre-warmed PBS (30–40°C) and dissolve it at 40–42°C in a shaking incubator set on high speed until it goes into solution completely.
-
•
Label the bottom of a petri dish using a permanent Sharpie marker. Fill 1/3 of a petri dish with the gelatin solution and let it cool down to room temperature for 10 min. Transfer it to 4°C for 10–20 min to completely solidify. This thin layer of gelatin in the petri dish provides a level stage to stick multiple (4–6) different samples upright in order to have roughly identical sections/regions of all tissues on a single slide for future comparison.
-
•
Take the Swiss-rolls out of the sucrose and gently remove the needle using blunt end forceps to straighten the tip to allow the needle to be removed.
-
•
Gently dab the Swiss-roll on a Kimwipe to remove any extra sucrose.
-
•
Soak the rolls in a well (e.g. use a 6 or 12 well plate) containing the 15% fresh gelatin solution and let them equilibrate in the oven at 40°C for 20–30 min.
-
•
Bring the petri dish to room temperature and transfer the Swiss-rolls using blunt end forceps from the gelatin solution to the petri dish. Place the flat side of the roll onto the gelatin in the petri dish. 4 or more Swiss-rolls can be used per gelatin block.
-
•
Allow the rolls to stick onto the gelatin surface for 2–3 min at room temperature, adjusting tissue orientation as needed.
-
•
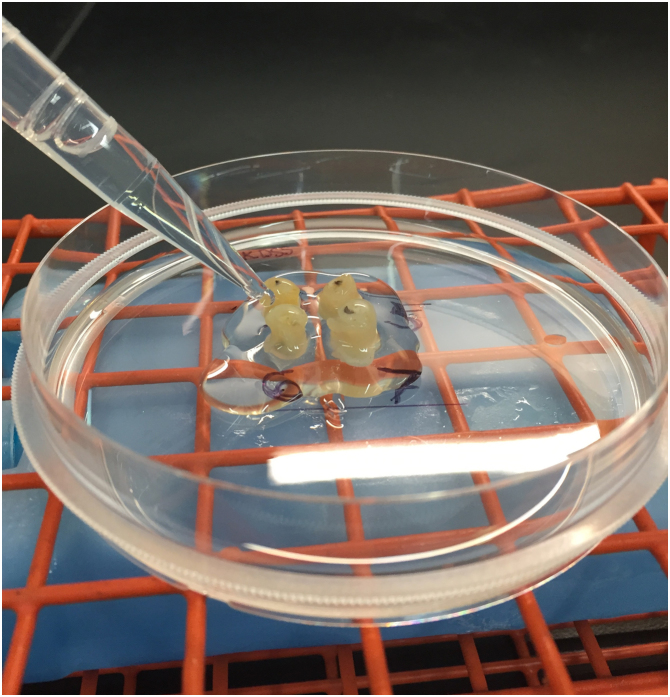
Transfer the petri dish onto the top of a flat rack, which previously was pre-cooled at −20°C, containing an ice pack for approximately 5 min. The gelatin block making procedure is done on a laboratory bench at room temperature. Applying a cold plate would also have the same effect. The optimum temperature for a cold plate is 10–15 °C and the petri dish can be directly placed on it. The cold surface cools down the gelatin-soaked samples, keeps them upright and attached to the gelatin bottom layer, and prevents the bottom gelatin layer from melting and releasing the Swiss-rolls as they are covered with warm gelatin solution in the following step. Remove the gelatin solution from the oven to cool down a bit for about 5 min prior to adding to the rolls. Carefully, add gelatin solution dropwise onto each roll to just cover them (Fig. 1). Let the gelatin solution solidify for ∼2 min, again adjusting tissue orientation as needed.
-
•
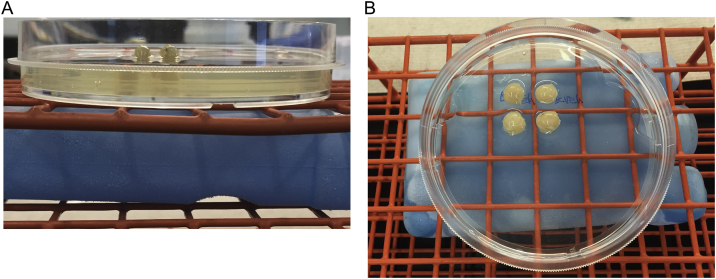
Gently pour warm gelatin solution on top of the rolls until they are covered to almost half of their height without making bubbles (Fig. 2).
-
•
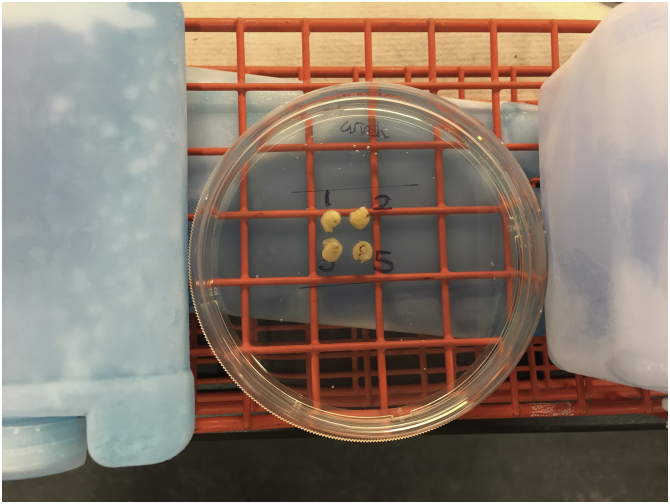
After approximately 2 min, carefully cover the rolls with additional gelatin solution and place ice packs on both sides of the petri dish and let it cool for about 20 min (Fig. 3). If a cold plate is used, it is also recommended that ice packs be placed on the sides of the petri dish, especially if the additional gelatin that covers the Swiss-rolls is still hot. The cold surface cools down the bottom of the petri dish. The ice packs on both sides of the petri dish help cool down the newly added gelatin layer. An optimal cold environment around the petri dish accelerates the solidification of the block and prevents the fixed Swiss-rolls from becoming detached during the addition of warm gelatin. However, if the area around the petri dish is too cold, subsequent gelatin layers cannot appropriately merge into a single layer.
-
•
Transfer the petri dish to 4 °C for 30 min to 1 h to fully solidify.
-
•
The cooled gelatin block is ready to trim when solidified. Use a spatula to pry out the solidified gelatin from the petri dish. Place the block onto a smooth surface and carefully trim away extra gelatin using a long blade and a slide (25 × 75 × 1 mm) to make the required block size (Fig. 4).
-
•
Fix the trimmed gelatin block in 4% PFA for 24 h. The fixative solution should cover the gelatin block completely. Replace PFA with 15% sucrose for another 24 h. The block needs to be stored at 4°C. The block can be stored in a plastic specimen container or a collection cup which is slightly larger (approximately 5× as large as the block) than the gelatin block.
-
•
Keep the gelatin block in sucrose at 4°C until it sinks to the bottom of the 15% sucrose container.
-
•
Replace the 15% sucrose with 30% sucrose for another 48 h. Replace with one more 30% sucrose incubation for an additional 48 h until it sinks. The fully cryoprotected block is now ready to be sectioned.
-
•
In order to section the block using a chambered cryostat (e.g. LEICA CM1850), gently dry the gelatin block on a Kimwipe to remove any excess sucrose solution and transfer it to a styrofoam container containing crushed dry ice and 2-Methylbutane (Fisher Chemical, 95% minimum) to freeze the block. To avoid cracks from forming in the block it is preferable to not keep it in the dry ice-2-Methylbutane mixture for greater than 10–20 s depending on the size of the gelatin block.
-
•
Once the block is frozen evenly, use optimal cutting temperature (OCT) compound to adhere the block onto the cutting stage at −20°C inside a cryostat.
-
•
Proceed to section the frozen block using a microtome knife (e.g. flat back permanent knife 16 cm- profile c- steel, Leica Biosystems Nussloch GmbH, Order No. 14 0216 07100, made in Germany) at −27°C to −35°C for 10 microns thick sections and mount them onto double-subbed slides (25 × 75 × 1 mm) right away. To prevent the thin gelatin section from rolling on the knife, place a small paint brush at the edge of the newly sectioned tissue and hold on to it while sectioning is being completed. This helps keep the thin gelatin section containing the tissue to stay stretched out and facilitates mounting the section onto the slide. A traditional chambered cryostat is recommended for sectioning 10 microns due to its adjustable temperature which keeps the section frozen during the sectioning and mounting steps. A sliding microtome (e.g. LEICA SM2000R) can be used for thicker sectioning (40μm) which is appropriate for free-floating tissue staining as in our prior work using brains [3]. We have also used the Swiss-roll gelatin matrix approach to make mouse heart and lung blocks for mounted staining.
-
•
The hardened gelatin blocks are stable at 4 °C. Although we typically store the mounted slides at −20 °C, they are stable overnight at room temperature before starting the staining.
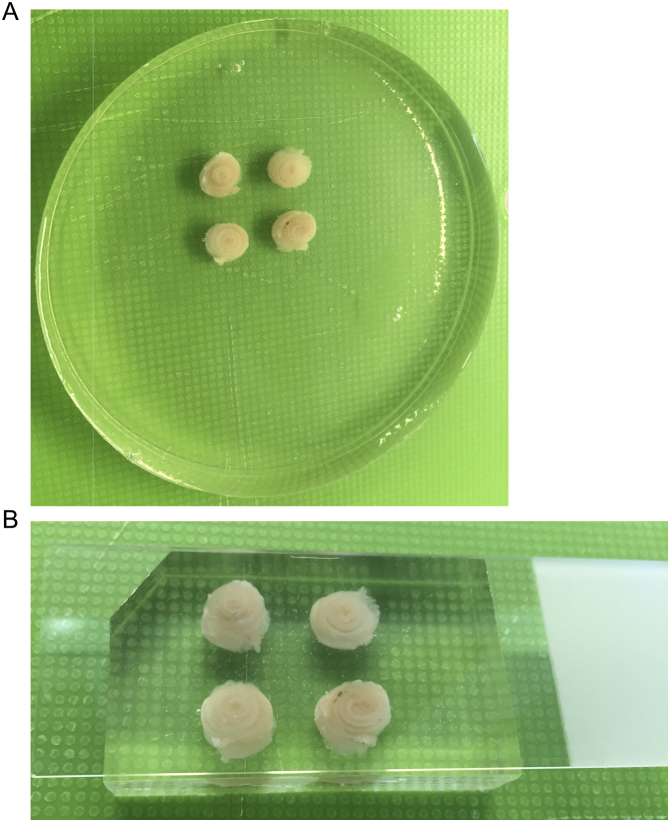
Fig. 1.
Four colon Swiss-rolls were placed into a petri dish containing a thin layer of 15% gelatin solution sitting atop an ice pack chilled surface. After 2–3 min, additional gelatin solution was added dropwise to gradually cover each roll.
Fig. 2.
Gelatin solution was added gently to the petri dish atop the ice pack chilled surface until the rolls were covered halfway. A) Side and B) overhead images are shown.
Fig. 3.
Additional gelatin solution was added until all four Swiss-rolls were completely covered and allowed to partially solidify for 20 min. atop the ice pack chilled surface. The petri dish was transferred to 4°C for 30 min to allow the gelatin solution to fully solidify.
Fig. 4.
The solidified gelatin was A) removed from the petri dish and B) trimmed to the same size as a double-subbed 25 × 75 × 1 mm slide.
Histologic and immunostaining
-
1
For H&E and Alcian blue staining, the slides were first removed from −20°C and allowed to dry at room temperature or in front of a fan for at least 1 h or 30 min, respectively. The tissues were then hydrated by successive incubations in 95%, 75% alcohol, and distilled water, 5–7 dips each.
-
2
To demonstrate utility of the gelatin embedded section for histologic stains we performed H&E staining. The slides were stained with Hematoxylin (HARRIS HEMATOXYLIN, American MasterTech Scientific) for 5 min, rinsed in tap water, followed by 2 dips in hydrochloric acid 1 N solution (Fisher Chemical) for differentiation of the color, and a final rinse in tap water. The sections were then blued in the 28–30% ammonium hydroxide (NEWCOMERSUPPLY) for 5–10s and rinsed thoroughly with tap water. The slides were then rinsed in 75% alcohol for a minute followed by 30–50s in Eosin (AQUEOUS EOSIN Y, American MasterTech Scientific) as a counterstain. The slides were dehydrated with incubations in 95% alcohol, 100% alcohol (×2) for 3–5 dips each, and xylene (×3) for 1 min each followed by coverslipping using Permount (Fisher Chemical) (Fig. 5).
-
3
Alcian blue staining was performed by placing the slides in 3% aqueous acetic acid for 3 min followed by 1% aqueous Alcian blue (NEWCOMERSUPPLY kit) pH 2.5 for 20–30 min. The excess stain was removed by rinsing the slides in tap water then distilled water. Then, the slides were counterstained in Nuclear Fast Red stain (Kernechtrot) for 2–3 min and rinsed in distilled water thoroughly. The slides were dehydrated in 95% alcohol and 100% alcohol 15–30s (2 dips) each and three times in xylene for 1 min each before being coverslipped using Permount (Fisher Chemical) (Fig. 5).
-
4
In order to validate that antigenicity was maintained in gelatin embedded tissue sections, immunohistochemistry (IHC) was performed. The dried slides were placed in a ProHisto antibody amplifier plate (PHI-AA1-1), a plate which is divided into 12 boxes (1 slid per well), and rinsed twice with PBS, 5 min each. Any type of plastic or glass microscope slide staining jars would work and no rocking is needed for this staining. In order to block endogenous peroxidase activity, tissue slides were incubated in PBS containing 0.3% H2O2 (30% Hydrogen Peroxide in water, Fisher BioReagents) for 5 min at room temperature. After incubation in IHC solution for 30 min to 1 h at room temperature to block non-specific antibody binding, the primary antibody was added. The tissue slides were immunostained using an anti-CD68 (rat anti-mouse antibody, 1:1000 dilution in IHC solution, MCA1957, BIO-RAD) antibody overnight at 4 °C. This macrophage marker antibody demonstrates robust intestinal immunoreactivity in our hands [4]. On the following day, the primary antibody was removed and the slides were rinsed in IHC solution 4–5 times, 5–10 min each. The biotinylated secondary antibody (rabbit anti-rat IgG antibody mouse adsorbed, 1:2000 dilution in IHC solution, BA-4001, Vector Laboratories, Inc.) was added for 2 h at room temperature. VECTASTAIN Avidin-Biotin Complex (ABC, Vector Laboratories, Inc.) kit was used to increase the detection sensitivity of the biotinylated secondary antibody. The AB solution (1:500 dilutions each) was prepared in PBS and incubated for 30 min at room temperature before use. After removing the secondary antibody, the slides were rinsed 2 times in IHC solution and 2 times in PBS, 5 min each. The AB solution was added for 2.5 h at room temperature followed by 4 rinses in PBS, 5 min each. To visualize the immunoreactivity, the Vector VIP Peroxidase (HRP) Substrate kit (SK-4600, Vector laboratories, Inc., Burlingame, CA) was used according to manufacture protocol. Vector VIP Substrate develops a purple color after reacting with peroxidase (HRP) enzyme. The images were taken using an upright Leica DM1000 microscope and Leica DF320 digital camera system (Fig. 6) [4]. As can be seen, it is feasible to simultaneously stain and mount a minimum of 4–6 different tissues in a single block as well as mount 2 different blocks containing the same or different treatments on a single standard microscope slide (25 × 75 × 1 mm). This simultaneous IHC processing allows us to decrease reagent use, reduce variability in staining intensity across samples, and improve tissue comparisons across samples during subsequent analysis.
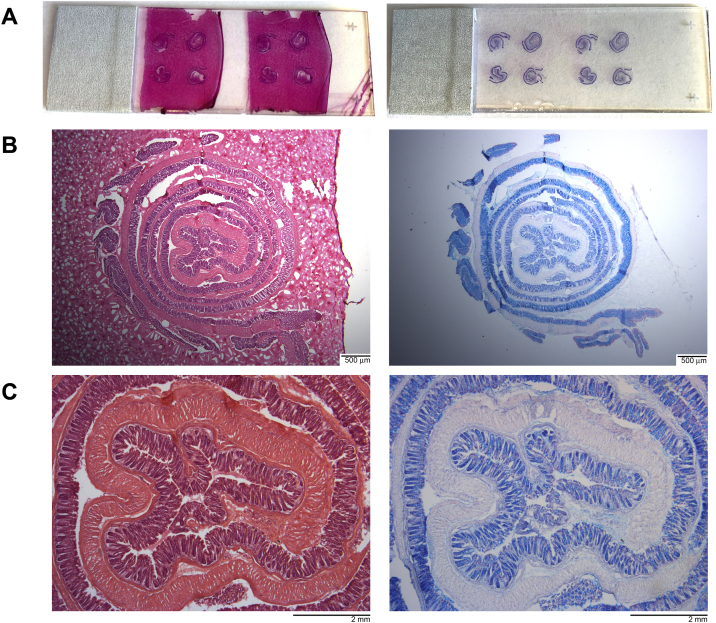
Fig. 5.
Representative H&E and Alcian blue staining of gelatin embedded tissue sections are shown with digital images of the A) entire slides, B) 1.25× magnification, and C) 4× magnification to demonstrate the whole length of cross-sectioned colon obtained by cutting the prepared blocks.
Fig. 6.
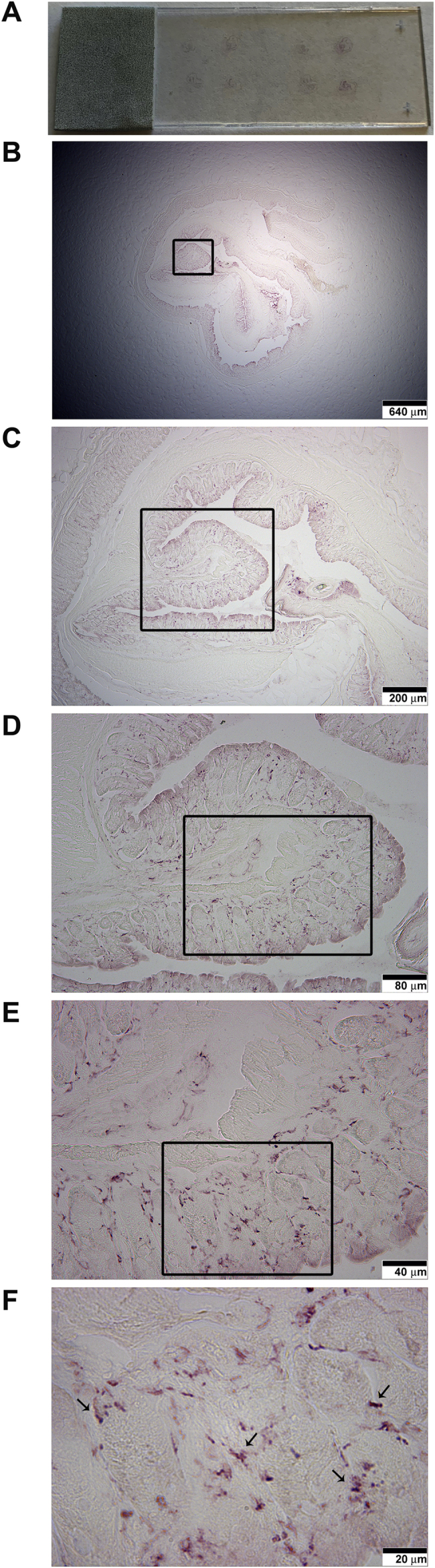
Representative CD68 immunoreactivity of gelatin embedded tissue sections are shown with digital images of A) a whole slide containing two gelatin sections, 4 samples each, B) 1.25× magnification, C) 4× magnification, D) 10× magnification, E) 20× magnification, and F) 40× magnification images to confirm that our protocol does not attenuate CD68 antigenicity. Boxes demarcate image areas displayed in higher magnification images.
Acknowledgements
The authors would like to express their gratitude to John Lee, the publication coordinator at information resources of the University of North Dakota School of Medicine & Health Sciences, for his assistance in the graphical abstract preparation. This research was supported by NIH, USA grants RO1AG048993, P20GM113123, and P20GM103442. We are grateful for the assistance of Hongyan Wang, the histology technologist at the department of pathology, with H&E and Alcian blue staining.
Contributor Information
Mona Sohrabi, Email: mona.sohrabi@und.edu.
Colin K. Combs, Email: colin.combs@med.und.edu.
References
- 1.Moolenbeek C., Ruitenberg E.J. The "Swiss roll": a simple technique for histological studies of the rodent intestine. Lab. Anim. 1981;15(1):57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 2.Whittem C.G., Williams A.D., Williams C.S. Murine colitis modeling using dextran sulfate sodium (DSS) J. Vis. Exp. 2010;(35) doi: 10.3791/1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagamoto-Combs K. An improved approach to align and embed multiple brain samples in a gelatin-based matrix for simultaneous histological processing. J. Neurosci. Methods. 2016;261:155–160. doi: 10.1016/j.jneumeth.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puig K.L. Overexpression of mutant amyloid-β protein precursor and presenilin 1 modulates enteric nervous system. J. Alzheimers Dis. 2015;44(4):1263–1278. doi: 10.3233/JAD-142259. [DOI] [PMC free article] [PubMed] [Google Scholar]