Abstract
Background:
Breast cancer is the most common malignancy among women. Chronic pain after breast surgeries is a well-known entity and is mainly neuropathic in nature. The primary aim of this study was to assess the effect of pregabalin given as preventive analgesic on the incidence of chronic postmastectomy pain.
Methods:
A randomized control trial (RCT) was performed on 80 patients. Patients were allocated into two groups. Group 1 received pregabalin (Lyrica, Pfizer) 75 mg. BD starting from the morning of surgery and continued for 1 week. Group 2 received placebo capsules at identical time intervals. Patients were followed up for 3 months postoperatively. Incidence, severity, and location of chronic pain were recorded. The primary objective was to evaluate the effect of perioperative oral pregabalin on the incidence of chronic postmastectomy pain (at 3 months postoperatively).
Results:
Of the 80 patients enrolled, 71 patients completed the study and were assessed for final outcomes. Incidence of chronic pain was comparable in both groups, with 16 out of 35 patients in Group 1 (44.7%) and 20 out of 36 patients in Group 2 (55.6%) reported chronic pain (P = 0.407). There was no difference between the severity of chronic pain (numeric rating scale ≥ 4) in both groups (P = 0.307). Incidence of adverse effects was comparable in both groups.
Conclusion:
This RCT shows that perioperative pregabalin may not have a role in the prevention of chronic pain after breast surgeries.
Keywords: Chronic postmastectomy pain, pregabalin, preventive analgesia
INTRODUCTION
Breast cancer is the most common malignancy among women in India[1] and worldwide.[2] Surgical resection with axillary dissection is one of the main treatment modalities for breast cancer. Chronic pain after breast surgeries is a well-known entity and is known to decrease the quality of life of patients.[3]
Chronic pain after breast surgery is defined as persistent pain beyond the normal healing time of 3 months.[4] It is mainly neuropathic in nature and varies from scar pain and tenderness, the sensation of phantom breast or phantom breast pain to pain in the shoulder, axilla, and chest wall.[5] Incidence of chronic pain after breast surgery varies widely in the literature from 25% to 60%.[5,6,7,8,9] Thus, postmastectomy chronic pain is a widespread problem in the surgical population.
There is a paucity of literature and successful randomized control trials (RCTs) on the prevention of this debilitating problem.[10] The concept of preventive analgesia has been tried with limited success for the prevention of chronic pain after breast surgery.[11] Gabapentinoids (gabapentin and pregabalin) have shown to decrease acute pain after mastectomy.[12,13] Pregabalin is a gabapentinoid which has better pharmacokinetic and dosage profile than gabapentin.[14] It is approved among the first-line drugs for the treatment of neuropathic pain.[15] Pregabalin has been shown to reduce chronic postoperative pain at 3 months after cardiac surgeries,[16] lumbar disc fusion,[17] and total knee arthroplasty.[18]
The aim of this placebo-controlled RCT was to assess the efficacy of pregabalin given as preventive analgesic starting from the morning of surgery, given till 1 week postoperatively on the incidence of chronic postmastectomy pain (at 3 months postoperatively).
METHODS
This parallel group double-blind, randomized pilot trial was conducted at a tertiary care teaching hospital and cancer center. The study was approved by the Institutional Ethics Committee (ref no – IECPG/448/27-7-2016. RT 04/29.08.2016.) and was registered before patient enrollment with the Clinical Trials Registry India (CTRI) (number CTRI/2017/11/010390). Patients were recruited from November 7, 2017, and follow-up was completed till April 2018.
Female patients in the age group of 18–65 years, American Society of Anesthesiologists (ASA) physical status 1 or 2, body mass index <30 kg/m2 with carcinoma breast posted for modified radical mastectomy were included in the study. Exclusion criteria were refusal to participate in the study, previous or current use of gabapentinoids, history of epilepsy, patient on anti-anxiety drugs, or antianxiety drugs given preoperatively, patients with chronic pain on analgesics, history of drug or alcohol abuse, impaired kidney or liver functions, bilateral mastectomy, history of previous breast surgery on the same side (excluding diagnostic biopsy), and cancer recurrence.
Written informed consent was obtained before randomization and 11-point numeric rating scale (NRS) for pain was explained on the preoperative visit. Patients were randomly allocated in one of the two groups using a computer-generated random number table in 1:1 ratio. The numbers were concealed in an opaque envelope, to be opened after recruitment. Group 1 received pregabalin (Lyrica, Pfizer) 75 mg. BD starting from the morning of surgery and continued for 1 week. Group 2 received placebo capsules at identical time intervals. Drugs were started at 2 h before surgery and were given by a staff nurse who was not involved in further follow-up of the patient.
The primary objective was to evaluate the effect of perioperative oral pregabalin on the incidence of chronic postmastectomy pain (at 3 months postoperatively).
Secondary objectives included: – (1) the assessment of the severity of chronic postmastectomy pain, (2) the evaluation of the effect of perioperative pregabalin on acute postoperative pain and analgesic requirements and; and (3) the assessment of adverse effects such as dizziness, somnolence, nausea, and vomiting due to pregabalin use.
The technique of general anesthesia was standardized in all patients and the anesthesiologist performing the general anesthesia was blinded to the group allocation. No anxiolytic premedication was used in either group. Premedication with dexamethasone 8 mg was given to each patient to prevent postoperative nausea and vomiting. Anesthesia was induced with intravenous (IV) fentanyl 2 mcg/kg, propofol (1–2 mg/kg), and atracurium 0.5 mg/kg followed by insertion of I-gel laryngeal mask airway (size depending on the weight of the patient). After induction paracetamol (15 mg/kg) IV, was given and thereafter repeated every 6 h until the patient was allowed oral intake. Anesthesia was maintained with oxygen, air, and desflurane (0.8–1 minimum alveolar concentration). Fentanyl (0.5 mcg/kg) was given if heart rate or arterial blood pressure increased by >20% of the baseline values and total intraoperative fentanyl consumption was recorded.
After surgery, patients were transferred to the postanesthesia care unit. In the postoperative period pain, assessment and data collection were done by an independent anesthesiologist blinded to group allotment and not involved in the intraoperative management of the patient. NRS scores for pain were assessed at 1, 2, 6, 12, and 24 h. From the second postoperative day till discharge, pain assessment was made once a day in the morning before giving pregabalin or placebo.
Paracetamol was the standard analgesic in all cases, given as 15 mg/kg IV (rounded off to 500 or 1000 mg) repeated every 6 h. Rescue analgesia with diclofenac 1.5 mg/kg IV (rounded off to 50 mg or 75 mg) was given if NRS ≥4. A maximum of three doses of diclofenac at eight hourly intervals was given in a day.
Once the patients were started orally, analgesics were converted to oral doses, i.e., paracetamol tablet 15 mg/kg (rounded off to 500 or 1000 mg), every sixth hourly to be taken for 1 week. Rescue analgesic was diclofenac tablet (50 or 75 mg), to be taken if NRS ≥4, with a maximum of 3 doses per day with 8 h difference between each dose. Pregabalin or placebo was continued as scheduled twice day doses till 1 week postoperatively. Patients were explained this analgesic regime at discharge and advised to maintain a log of a number of rescue analgesic doses of diclofenac needed at home.
Sedation scores were assessed using the Ramsay Sedation Score (RSS), at day 1 and 2. A score of ≥3 was considered a significant sedation. RSS scores were assessed along with pain scores in the morning. Incidence of dizziness, nausea, and vomiting (if any) was recorded in the postoperative period.
Patients were followed up at 1 week (first visit), 1 month (second visit), and 3 months (third visit). For the follow-up visits, patients were contacted by phone by the same investigator who observed the patients for acute pain. On the first postoperative visit, the need for rescue analgesics and number of doses of diclofenac taken throughout the week were noted. On the second- and third-visit postoperatively, the following data were collected: pain if any at rest and on 90° abduction and location of pain (surgical site/ipsilateral arm/shoulder). NRS score (0–10) was used to assess the intensity of pain, NRS of ≥4 was considered as severe pain.
Sample size estimation and drug dosage
Incidence of chronic postmastectomy pain varies in the literature from 25% to 60%.[5,6,7,8,9] We did a pilot RCT as could not find any study where pregabalin had been given continuously in the perioperative period.
Drug dosage was decided on the basis of a study Hetta et al.,[19] where three doses of pregabalin (75 mg, 150 mg, and 300 mg) were compared with a placebo in patients undergoing modified radical mastectomy. The 300 mg pregabalin group had significantly higher adverse effects as compared to other groups. A total of 150 mg pregabalin was concluded to be the optimal dose for the reduction of acute postoperative pain. Thus, we gave 150 mg pregabalin per day, in divided doses of 75 mg twice daily for 7 days starting from the day of surgery.
Statistical analysis
Continuous data are expressed as a mean ± standard deviation, and qualitative data are expressed as a number of events (%). Nonnormally distributed data are expressed as the median (interquartile range). Quantitative variables were compared with the Student's t-test; nominal variables were compared using the Chi-squared test or Fisher's exact test, when appropriate. Results with P < 0.05 were considered statistically significant. The IBM SPSS (Statistical package for social sciences) version 16. (IBM, Armonk, NY, United States of America) was used to perform statistical analysis.
RESULTS
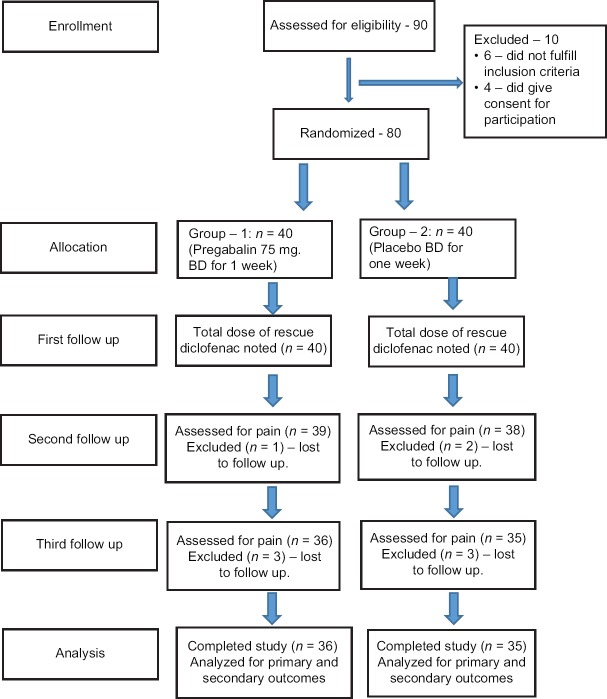
A total of 90 patients were assessed for eligibility, 80 patients were included in the trial (40 in each group). Of the 80 patients enrolled, 71 patients completed the study and were assessed for outcomes. The flow of the patients through different stages of the trial is summarized in the CONSORT diagram shown in Figure 1.
Figure 1.
CONSORT diagram summarizing the flow of patients in the study
Baseline patient characteristics, duration of surgery, and intraoperative fentanyl consumption were comparable in both groups (P > 0.05). A number of patients that received neoadjuvant chemotherapy, or had ongoing chemotherapy or radiotherapy to the surgical site at the time of follow-up at 3 months were also comparable in both groups [Table 1].
Table 1.
The comparison between demographic parameters, duration of surgery, American Society of Anesthesiologists physical status, preoperative pain, and intraoperative fentanyl consumption in the two groups
| Variable | Group 1 (n=35) | Group 2 (n=36) | P |
|---|---|---|---|
| Age (years)* | 48.54±10.03 | 50.28±10.43 | 0.478 |
| BMI* | 26.56±3.42 | 26.79±5.17 | 0.829 |
| ASA PS - 1/2 | 17/18 | 21/15 | 0.529 |
| Preoperative pain at surgical site | 9/35 | 8/36 | 0.73 |
| Duration of surgery (min)* | 98.53±25.98 | 95.42±25.33 | 0.614 |
| Intra operative fentanyl consumption (mg)^ | 30 (15) | 27.5 (15) | - |
| Neoadjuvant chemotherapy | 18/35 | 13/36 | 0.193 |
| Ongoing chemotherapy at 3 months | 23/35 | 25/36 | 0.737 |
| Ongoing radiotherapy at 3 months | 6/35 | 7/36 | 0.802 |
*Data expressed as mean±SD, ^Data are expressed as median (IQR). SD: Standard deviation, ASA PS: American Society of Anesthesiologists physical status, IQR: Interquartile range, BMI: Body mass index
Incidence of chronic pain at 3 months was comparable in both groups. The percentage of patients experiencing severe chronic pain (i.e., NRS > 4) was also similar in both groups [Table 2].
Table 2.
A comparison between the incidence and severity of chronic pain at 3 months after surgery
| Group 1 (n=35), n (%) | Group 2 (n=36), n (%) | P | |
|---|---|---|---|
| Incidence | 16 (45.71) | 20 (55.56) | 0.407 |
| Severity (NRS ≥4) | 7 (20) | 11 (30.56) | 0.307 |
Values are expressed as numbers and percentages. NRS: Numeric rating scale (0-10)
Out the 36 patients experiencing chronic pain, the location of pain was mainly described at shoulder or axilla (23 out of 36) other sites of pain were drain site, surgical scar. However, the majority of patients did not specify a single site of chronic pain and described the pain as being present around the shoulder, axilla, and anterior chest wall [Table 3].
Table 3.
The site of pain in patients having chronic pain
| Site | Number of patients (%) |
|---|---|
| Surgical scar/anterior chest wall | 5 (13.88) |
| Shoulder | 2 (5.55) |
| Axilla | 2 (5.55) |
| Drain site | 3 (8.33) |
| Shoulder + axilla | 9 (25) |
| Shoulder + anterior chest wall | 7 (19.44) |
| Anterior chest wall + drain site | 1 (2.77) |
| Anterior chest + shoulder + axilla | 7 (19.44) |
| Total | 36 (100) |
With regard to pain at 1 month after surgery (2nd follow-up) 18 patients in Group 1 and 20 patients in Group 2 reported pain at the 1st month which was comparable and statistically insignificant (P = 0.727).
The analysis of acute postoperative pain and analgesic showed that significantly fewer patients required rescue analgesia in Group 1 (P = 0.00). The total amount of diclofenac consumed as rescue analgesic in the first postoperative week was also significantly smaller in Group 1 (P = 0.00) [Table 4].
Table 4.
The number of patients requiring rescue analgesics in the first 24 h and the total rescue analgesic (diclofenac) consumed in 1 week
| Group 1 (n=35) | Group 2 (n=36) | P | |
|---|---|---|---|
| Need for rescue analgesic in first 24 h (n) | 10 | 24 | 0.001 |
| Total diclofenac consumed in 1 week (mg) | 94.29±58.5 | 189.58±92.26 | 0.00 |
Four patients in Group 1 and 3 patients in Group 2 complained of dizziness during hospital stay, also none of the patients had a Ramsay Sedation Score (RSS) of >3. Incidence of nausea and vomiting was minimal and comparable between both the groups (5 patients in Group 1 and 3 patients in Group 2).
DISCUSSION
The results of our RCT show an overall incidence of chronic postmastectomy pain of 51%, which is in accordance with the internationally reported incidence of chronic postmastectomy pain. The Danish study group of Gärtner et al. in a cross-sectional study on 3253 patients reported an incidence of chronic postmastectomy pain of 47% 2–3 years of surgery.[9]
We could not find any significant difference in the incidence of chronic pain after giving pregabalin as a preventive analgesic in the perioperative period. About 55% of patients in the control group and 45% of patients in the pregabalin group developed chronic pain (P = 0.407).
The literature on the preventive effects of gabapentinoids on chronic postsurgical pain has varied over the years. In a meta-analysis published by Clarke et al.[20] studying the effect of gabapentin or pregabalin on persistent postsurgical pain showed a beneficial effect of gabapentinoids in reducing chronic postsurgical pain.
However, the role of gabapentinoids in the prevention of chronic postmastectomy pain has been questioned in a recent meta-analysis published in 2017 by Rai et al.[21] These authors included 12 trials (12 gabapentin and 4 pregabalin) studying the effect of perioperative gabapentinoids on chronic postmastectomy pain. The authors concluded that neither gabapentin nor pregabalin affected the development of chronic postmastectomy pain.
Our RCT adds to the evidence that using pregabalin as a preventive analgesic may not reduce the incidence of chronic postmastectomy pain.
With regard to the location of chronic pain, the majority of our patients reported overlapping pain at two or more areas [Table 2]. As per the classification of chronic pain after breast surgeries by Jung et al.;[5] chronic pain may be due to damage to the intercostobrachial nerve, medial and lateral pectoral nerves, long thoracic nerve, thoracodorsal nerve, or intercostal nerves. This damage may lead to pain in the distribution of these nerves, i.e., anterior chest wall, axilla, shoulder, or medial upper arm. As seen in the study by Gärtner et al.[9] out of the 1573 patients reporting chronic pain only 18% reported pain in a single area the rest had pain in two, three, or all four areas (breast, axilla, chest, and medial upper arm). Thus, pain following breast surgery may be due damage to multiple nerves resulting in pain at multiple areas.
We found minimal adverse effects due to pregabalin use in our patients. Incidence of dizziness and somnolence (RSS > 3) were similar in both the groups. This could be due to the fact that we gave 150 mg in two divided doses of 75 mg each 12 h apart. Furthermore, we decided on the dose keeping in mind the unwanted adverse effects of pregabalin seen at doses >50 mg.[18]
We have made a simple attempt to assess the incidence of chronic postmastectomy pain in the Indian surgical population and the role of pregabalin in its prevention. Our results may be important as the incidence of pain may vary in different ethnicities, socioeconomic groups and health-care systems around the world. Furthermore, the results from this pilot study may be used to design adequately powered large sample size studies on prevention of chronic postmastectomy pain in the Indian surgical population.
However, our results have some limitations, the negative result, i.e., no difference in the incidence of chronic pain between the intervention and control group may be due to the inadequate sample size being a pilot study. We also acknowledge that we could assess the incidence and severity of chronic pain only at a single point, i.e., 3 months after surgery due to time constraints to complete the study. As shown by an adequately powered and large sample-sized study published recently by Reyad et al.[22] pregabalin when given as a preventive analgesic was shown to reduce the incidence and severity of chronic postmastectomy pain at 4, 12, and 24 weeks. The severity of chronic pain can change with time and attempts should be made to follow patients at multiple points of time. Finally, we did not use a specific diagnostic tool or questionnaire to classify the type of chronic pain as neuropathic pain. Future studies in this field may work to overcome these drawbacks and get more accurate data on chronic pain after breast surgery.
The field of preventive analgesia is ever evolving; there are no fixed guidelines on dose and duration of pregabalin to be given as preventive analgesic to prevent chronic pain after breast surgeries. Attempts should be made in future studies to try higher doses or increase the duration drug administration of pregabalin to evaluate its effects on chronic postmastectomy pain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Part of the study was presented as a poster in The 25th Annual Conference of the Indian Association of Palliative Care (IAPCON 2018) with subsequent abstract publication in Indian Journal of Palliative care (IJPC).
REFERENCES
- 1.Statistics of Breast Cancer in India: Trends in India. [Last accessed on 2018 May 26]. Available from: http://www.breastcancerindia.net/statistics/trends.html .
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80:39–48. doi: 10.1023/A:1024435101619. [DOI] [PubMed] [Google Scholar]
- 4.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–7. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: Proposed classification and research update. Pain. 2003;104:1–3. doi: 10.1016/s0304-3959(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 6.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–46. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Alves Nogueira Fabro E, Bergmann A, do Amaral E Silva B, Padula Ribeiro AC, de Souza Abrahão K, da Costa Leite Ferreira MG, et al. Post-mastectomy pain syndrome: Incidence and risks. Breast. 2012;21:321–5. doi: 10.1016/j.breast.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Variawa ML, Scribante J, Perrie H, Chetty S. The prevalence of chronic postmastectomy pain syndrome in female breast cancer survivors. S Afr J Anaesth Analg. 2016;22:108–13. [Google Scholar]
- 9.Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–92. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 11.Brennan TJ, Kehlet H. Preventive analgesia to reduce wound hyperalgesia and persistent postsurgical pain: Not an easy path. Anesthesiology. 2005;103:681–3. doi: 10.1097/00000542-200510000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Dirks J, Fredensborg BB, Christensen D, Fomsgaard JS, Flyger H, Dahl JB. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology. 2002;97:560–4. doi: 10.1097/00000542-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Song JW, Park B, Park S, An YJ, Shim YH. Pregabalin reduces post-operative pain after mastectomy: A double-blind, randomized, placebo-controlled study. Acta Anaesthesiol Scand. 2011;55:290–6. doi: 10.1111/j.1399-6576.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- 14.Gajraj NM. Pregabalin: Its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–15. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 15.Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132:237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Pesonen A, Suojaranta-Ylinen R, Hammarén E, Kontinen VK, Raivio P, Tarkkila P, et al. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: A randomized placebo-controlled trial. Br J Anaesth. 2011;106:873–81. doi: 10.1093/bja/aer083. [DOI] [PubMed] [Google Scholar]
- 17.Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg. 2010;110:1180–5. doi: 10.1213/ANE.0b013e3181cf949a. [DOI] [PubMed] [Google Scholar]
- 18.Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: A prospective, randomized, controlled trial. Anesth Analg. 2010;110:199–207. doi: 10.1213/ANE.0b013e3181c4273a. [DOI] [PubMed] [Google Scholar]
- 19.Hetta DF, Mohamed MA, Mohammad MF. Analgesic efficacy of pregabalin in acute postmastectomy pain: Placebo controlled dose ranging study. J Clin Anesth. 2016;34:303–9. doi: 10.1016/j.jclinane.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: A combined systematic review and meta-analysis. Anesth Analg. 2012;115:428–42. doi: 10.1213/ANE.0b013e318249d36e. [DOI] [PubMed] [Google Scholar]
- 21.Rai AS, Khan JS, Dhaliwal J, Busse JW, Choi S, Devereaux PJ, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg. 2017;70:1317–28. doi: 10.1016/j.bjps.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 22.Reyad RM, Omran AF, Abbas DN, Kamel MA, Shaker EH, Tharwat J, et al. The possible preventive role of pregabalin in postmastectomy pain syndrome: A double-blinded randomized controlled trial. J Pain Symptom Manage. 2019;57:1–9. doi: 10.1016/j.jpainsymman.2018.10.496. [DOI] [PubMed] [Google Scholar]