Abstract
Rationale: Poor outcomes of adults surviving critical illness are well documented, but data in children are limited.
Objectives: To identify factors associated with worse postdischarge function and health-related quality of life (HRQL) after pediatric acute respiratory failure.
Methods: We assessed functional status at baseline, discharge, and 6 months after pediatric ICU discharge and HRQL 6 months after discharge in 2-week- to 17-year-olds mechanically ventilated for acute respiratory failure in the RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) trial. We assessed HRQL via Infant and Toddler Quality of Life Questionnaire-97 (<2 yr old) or Pediatric Quality of Life Inventory (≥2 yr old). We categorized patients with normal baseline function as having impaired HRQL if scores were greater than 1 SD below mean norms for Infant and Toddler Quality of Life Questionnaire-97 growth and development or Pediatric Quality of Life Inventory total score.
Measurements and Main Results: One-fifth (n = 192) of 949 patients declined in function from baseline to postdischarge; 20% (55/271) had impaired growth and development; 19% (64/343) had impaired HRQL. In multivariable analyses, decline in function was associated with baseline impaired function, prematurity, cancer, respiratory failure etiology, ventilation duration, and clonidine (odds ratio [OR] = 2.14; 95% confidence interval [CI] = 1.22–3.76). Independent predictors of impaired growth and development included methadone (OR = 2.27; 95% CI = 1.18–4.36) and inadequate pain management (OR = 2.94; 95% CI = 1.39–6.19). Impaired HRQL was associated with older age, non-white or Hispanic race, cancer, and inadequate sedation management (OR = 3.15; 95% CI = 1.74–5.72).
Conclusions: Postdischarge morbidity after respiratory failure is common and associated with admission factors, exposure to critical care therapies, and pain and sedation management.
Keywords: healthcare outcomes, pediatric, health-related quality of life, respiratory failure, functional status
At a Glance Commentary
Scientific Knowledge on the Subject
Adults undergoing mechanical ventilation with acute respiratory distress syndrome have well-documented physical limitations and diminished health-related quality of life. Recent studies of pediatric ICU populations found concerning rates of new physical morbidity at the time of hospital discharge and, at a single center, up to 3 years after discharge. There is a paucity of contemporary data, however, on postdischarge outcomes after pediatric acute respiratory failure.
What This Study Adds to the Field
In one of the largest studies to date of postdischarge function and health-related quality of life in children surviving acute respiratory failure, this study found postdischarge morbidity to be common and associated with admission factors, exposure to critical care therapies, and pain and sedation management. These data begin to address the current gap in the literature regarding the evolving phenomenon known as post–intensive care syndrome in pediatrics. These data identify children experiencing worse outcomes after pediatric ICU care, of use to inpatient providers to help parents understand their child’s risk for long-term sequelae and outpatient providers to refocus follow-up visits to potentially intervene to improve the 6-month trajectory.
Each year, more than 100,000 infants and children in the United States are supported on mechanical ventilation (1). Adults undergoing mechanical ventilation with acute respiratory distress syndrome (ARDS) have higher mortality rates than children, and survivors have well-documented physical limitations and diminished health-related quality of life (HRQL) (2–7). Recent studies of pediatric ICU (PICU) populations found concerning rates of new physical morbidity at hospital discharge (8, 9) and, at a single center, up to 3 years after discharge (10). There is a paucity of contemporary data, however, on postdischarge outcomes after pediatric acute respiratory failure.
We recently reported results of postdischarge functional status and HRQL in the RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) trial (11) of team-based, nurse-implemented, and goal-directed sedation versus usual care in children with acute respiratory failure (12). We found that the intervention, a sedation strategy allowing children to be more awake while intubated and exposing them to fewer sedative and analgesic medications, produced no long-term harm. However, postdischarge morbidity after acute respiratory failure was common in both treatment groups.
Here, we report an analysis of risk factors, known at the time of hospital discharge, associated with decreased postdischarge functional status and impaired HRQL among children in the RESTORE trial. Our objective was to identify sociodemographic factors and preexisting health status, features of the presenting acute illness, and hospital course variables associated with adverse long-term patient outcomes. We hypothesized that adverse outcomes would be associated with underlying disease, increased severity and duration of illness in the hospital, and greater exposure to opioid and sedative medications.
Methods
RESTORE was a cluster randomized trial enrolling 2,449 patients aged 2 weeks to 17 years at 31 U.S. sites from June 2009 to December 2013 (11). Patients were expected to require invasive mechanical ventilation for at least 24 hours for acute respiratory failure from lower airway or parenchymal disease. Patients were excluded if length of mechanical ventilation was unlikely to be altered by sedation management (e.g., patients with unrepaired cyanotic heart disease, a critical airway, or baseline ventilator dependence). We obtained written, informed consent for follow-up assessment from the parents or legal guardians of 87% of patients participating in the trial (see Figure E1 in the online supplement) (12). Patients were asked to provide assent when able, and adolescents turning 18 after enrollment were asked to provide consent. Institutional review board approval was obtained at each study site and the coordinating centers.
We conducted posthospital discharge assessments 6 months (±1 mo) after PICU discharge using mail, electronic mail, internet, and/or telephone on a random sample of consented patients stratified by age group and site. We interviewed parents/guardians to assess patients’ functional status and complete standardized HRQL questionnaires. For Spanish-speaking families, interviews were conducted in Spanish, and we used validated Spanish translations of all instruments. Patients and families were considered ineligible for follow-up if they lived outside the United States or could not understand English or Spanish, or if consenting parents/guardians no longer had custody of the patient.
We used the Pediatric Cerebral Performance Category (PCPC) and Pediatric Overall Performance Category (POPC) (13) to categorize baseline (before the acute illness), hospital discharge, and postdischarge functional status. Baseline and hospital discharge functional status were assessed by medical record review, and postdischarge functional status was assessed by telephone interview. We assessed HRQL at a single time point (postdischarge only) using two validated measures based on patient age and developmental status. In children under 2 years old, we used the Infant and Toddler Quality of Life Questionnaire-97 (ITQOL), which provides 12 domain-specific scores (14). In children 2 years of age and older, we primarily used the Pediatric Quality of Life Inventory, Version 4.0 Generic Core Scales (PedsQL) (15), which assesses four domains incorporated into a total score. If the parent of a child 2 to less than 6 years old had difficulty completing the PedsQL due to the child’s developmental impairment (e.g., children with PCPC ≥ 3), the ITQOL was used. We used the median household income of ZIP code of residence in 2011 (16) as an indicator of socioeconomic status, because the majority of families opted not to disclose their personal income (12).
We obtained follow-up data from 1,073 of 1,360 (79%) eligible patients, 30 of whom died between discharge and follow-up (Figure E1) (12). Of eligible survivors (n = 1,330), 72% (n = 960) provided interview data, and 63% (n = 838) provided HRQL data. The categories of the PCPC and POPC were developed to each represent distinct and clinically importantly different states of function, so the accepted minimally important clinical difference for the PCPC and POPC is one category of change. Therefore, we considered a patient to have an adverse functional outcome if they had a decline in functional status (worse score on either the PCPC or POPC) from baseline to postdischarge. For children assessed with the PedsQL, we categorized children as having impaired HRQL if their total score was greater than 1 SD below the mean of the reference population as per Varni and colleagues (17). For children assessed with the ITQOL, we used a similar method based on the score for the growth and development domain, because the ITQOL does not generate a total score. For the ITQOL and PedsQL, we did not assess baseline HRQL, so we could not assess change in HRQL.
Inadequate pain management was defined as a pain score greater than 4 (or pain assumed present if receiving neuromuscular blockade) on a 0–10 scale, with higher scores indicating more pain, for 2 consecutive hours. Inadequate sedation management was defined as State Behavioral Scale score greater than 0 (or agitation assumed present if receiving neuromuscular blockade) for 2 consecutive hours. Clinically significant iatrogenic withdrawal was defined as rescue therapy (an opioid or benzodiazepine bolus or an increase in opioid or benzodiazepine infusion) to manage an increase in withdrawal symptoms for patients weaning from 5 or more days of opioids. Pediatric ARDS severity was defined using the 2015 Pediatric Acute Lung Injury Consensus Conference criteria (18). Multiple organ dysfunction syndrome (MODS) was defined as respiratory dysfunction plus one or more extrapulmonary organ dysfunctions, with concurrent MODS defined by onset on Day 0/1 and new MODS by onset on Day 2 or later using nonpulmonary organ dysfunction criteria from the International Pediatric Sepsis Consensus Conference (19, 20).
Data Analysis
We used sociodemographic factors and preexisting health status, features of the presenting acute illness, and hospital course variables to predict adverse outcomes using logistic regression. In addition, answers to many of the HRQL questions are affected by physical and developmental status, so patients with disability will score lower. Therefore, we separately compared the HRQL scores of children with normal baseline function (PCPC = 1 and POPC = 1) and children with impaired baseline function with those of the reference populations (21, 22) using linear regression.
We used stepwise multivariable logistic regression to identify independent risk factors (P < 0.05) for adverse postdischarge outcomes among all patients for decline in functional status and among the subset of patients with normal baseline function for impaired HRQL. Adjusting for age group and Pediatric Risk of Mortality (PRISM III-12) score (23), preliminary multivariable models were generated considering risk factors present on admission with a P value less than 0.2 in univariate analyses, except parent education level due to missing data and median household income of residence ZIP code due to the nonspecificity of this variable. Building upon the preliminary models, final models were generated considering risk factors occurring during the course of hospitalization with a P value less than 0.2 in analyses adjusting for age group and PRISM III-12 score. We previously reported absence of treatment arm effects on postdischarge outcomes (12), and treatment arm was not a significant predictor in any multivariable model. Due to collinearity, PICU and hospital length of stay were not considered as potential covariates if duration of mechanical ventilation was considered. We assessed the area under the curve as a measure of discrimination for each multivariable model. All regression analyses accounted for PICU as a cluster variable using generalized estimating equations. Analyses were performed using SAS (Version 9.4; SAS Institute).
Results
Of the 960 patients whose parents/guardians were interviewed, nearly all had been discharged to home (91%; n = 873); 6% (n = 53) had been discharged to a rehabilitation or assisted-living/intermediate care facility. Approximately one-third of patients (34% [329/955]) were readmitted to a hospital after discharge, and 28% (267/950) received paid healthcare help at home.
Functional Status
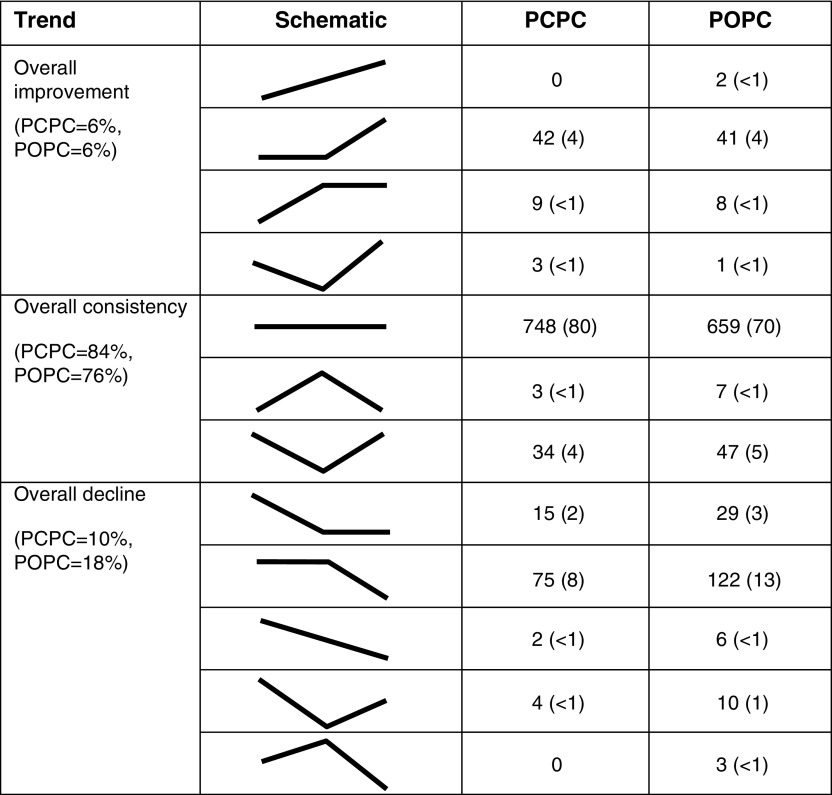
Of 949 patients with both baseline and interview data, 20% (n = 192) experienced a decline in functional status from baseline to follow-up. Among the 935 subjects with data at all three time points, more declined between hospital discharge and follow-up than from baseline to hospital discharge (Figure 1).
Figure 1.
Change in Pediatric Cerebral Performance Category (PCPC) or Pediatric Overall Performance Category (POPC) from baseline to hospital discharge to follow-up (n = 935). Values are number of patients (%).
Multiple factors were significantly associated with a decline in function from baseline to follow-up in univariate analyses (Table 1). Of sociodemographic factors and preexisting health status, decline in functional status was more common among those with history of prematurity or cancer. Decline in functional status was less common in children with normal functional status at baseline or a history of asthma. Of features of the presenting acute illness, decline in functional status was more common with greater illness severity or an admission diagnosis of sepsis-associated respiratory failure and less common in those admitted for bronchiolitis or asthma.
Table 1.
Factors Present at Admission and Hospital Course Variables according to Change in Functional Status from Baseline to Follow-up
| Variable | Unchanged or Improved Functional Status (n = 757) | Decline in Functional Status (n = 192) | Unadjusted P Value* | Adjusted P Value† |
|---|---|---|---|---|
| Factors present at hospital admission | ||||
| Sociodemographic factors and preexisting health status | ||||
| Age at PICU admission, n (%) | 0.06 | |||
| 2 wk to <2 yr | 400 (53) | 89 (46) | ||
| 2 to <6 yr | 142 (19) | 31 (16) | ||
| 6 to <18 yr | 215 (28) | 72 (38) | ||
| Female, n (%) | 357 (47) | 81 (42) | 0.23 | |
| Non-Hispanic white, n/total (%) | 395/755 (52) | 109/190 (57) | 0.20 | |
| Parent education, n (%) | 0.08 | |||
| Some high school | 72 (13) | 8 (6) | ||
| High school graduate/GED | 137 (25) | 33 (26) | ||
| Some college or technical school | 158 (29) | 47 (38) | ||
| College graduate/postgraduate | 177 (33) | 37 (30) | ||
| Unknown, n | 213 | 67 | ||
| Median household income of ZIP code of residence, n (%)‡ | 0.09 | |||
| <$40,000 | 156 (21) | 35 (18) | ||
| $40,000–$79,999 | 446 (59) | 128 (67) | ||
| ≥$80,000 | 155 (20) | 29 (15) | ||
| Normal functional status at baseline, n (%)§ | 561 (74) | 119 (62) | 0.0002 | |
| Any medical history, n (%) | ||||
| Prematurity (<36 wk postmenstrual age) | 99 (13) | 38 (20) | 0.004 | |
| Asthma (prescribed bronchodilators or steroids) | 114 (15) | 17 (9) | 0.006 | |
| Cancer (current or previous diagnosis) | 29 (4) | 22 (11) | 0.0002 | |
| Features of the presenting acute illness | ||||
| PRISM III-12 score, median (IQR)‖ | 7 (3–11) | 8 (3–14.5) | 0.003 | |
| Risk of mortality based on PRISM III-12 score, median (IQR), % | 2.9 (1.0–8.9) | 4.2 (1.3–20.1) | 0.0002 | |
| Primary diagnosis category, n (%) | <0.0001 | |||
| Bronchiolitis or asthma (or reactive airway disease) | 287 (38) | 42 (22) | ||
| Pneumonia or aspiration pneumonia | 319 (42) | 87 (45) | ||
| Acute respiratory failure related to sepsis | 80 (11) | 35 (18) | ||
| Other acute diagnoses¶ | 60 (8) | 17 (9) | ||
| Other chronic diagnoses¶ | 11 (1) | 11 (6) | ||
| Hospital course variables | ||||
| Moderate/severe PARDS based on worst OI or OSI during hospitalization, n (%)** | 535 (71) | 154 (80) | 0.03 | 0.04 |
| Early NMB (for the entire duration of Days 1 and 2), n (%) | 107 (14) | 33 (17) | 0.35 | 0.64 |
| HFOV, n (%) | 94 (12) | 33 (17) | 0.21 | 0.47 |
| ECMO, n (%) | 14 (2) | 7 (4) | 0.17 | 0.30 |
| Noninvasive ventilation before intubation, n (%) | 318 (42) | 77 (40) | 0.77 | 0.77 |
| Noninvasive ventilation after extubation, n (%) | 326 (43) | 96 (50) | 0.07 | 0.07 |
| Duration of mechanical ventilation, median (IQR), d | 5.9 (3.8–9.2) | 9.3 (5.0–15.5) | <0.0001 | <0.0001 |
| Duration of mechanical ventilation, n (%) | ||||
| <7 d | 455 (60) | 70 (36) | <0.0001 | <0.0001 |
| 7 to <14 d | 211 (28) | 65 (34) | ||
| 14 to <28 d | 64 (8) | 38 (20) | ||
| ≥28 d (including transfers by Day 28) | 27 (4) | 19 (10) | ||
| MODS (concurrent or new), n (%)†† | 534 (71) | 154 (80) | 0.02 | 0.16 |
| Extrapulmonary organ dysfunction during hospitalization, n (%) | ||||
| Cardiovascular | 326 (43) | 100 (52) | 0.06 | 0.46 |
| Neurologic | 358 (47) | 102 (53) | 0.26 | 0.57 |
| Hematologic | 112 (15) | 60 (31) | <0.0001 | 0.0001 |
| Renal | 40 (5) | 22 (11) | 0.002 | 0.02 |
| Hepatic | 146 (19) | 53 (28) | 0.02 | 0.17 |
| Number of organ dysfunctions, median (IQR) | 2 (1–3) | 3 (2–4) | 0.0003 | 0.02 |
| Mean daily opioid dose, median (IQR), mg/kg | 1.5 (0.7–2.5) | 2.0 (0.9–3.5) | 0.05 | 0.04 |
| Mean daily benzodiazepine dose, median (IQR), mg/kg | 1.3 (0.7–2.4) | 1.6 (0.7–3.5) | 0.0006 | 0.0002 |
| Synthetic primary opioid agent, n (%)‡‡ | 438 (58) | 106 (55) | 0.48 | 0.22 |
| Dexmedetomidine, n (%) | 269 (36) | 84 (44) | 0.009 | 0.008 |
| Clonidine, n (%) | 76 (10) | 46 (24) | <0.0001 | <0.0001 |
| Ketamine, n (%) | 193 (26) | 59 (31) | 0.26 | 0.35 |
| Barbiturates, n (%) | 110 (15) | 32 (17) | 0.43 | 0.35 |
| Methadone, n (%) | 147 (19) | 57 (30) | 0.02 | 0.01 |
| Antidelirium medication, n (%) | 12 (2) | 9 (5) | 0.01 | 0.07 |
| ≥4 sedative classes, n (%)§§ | 206 (27) | 79 (41) | 0.0007 | 0.0005 |
| Study days awake and calm (daily modal SBS score −1 or 0), median (IQR), % | 82 (60–100) | 78 (58–96) | 0.48 | 0.84 |
| Heavy sedation (daily modal SBS score ever −3), n (%) | 86 (11) | 26 (14) | 0.50 | 0.98 |
| Inadequate pain management, n (%)|||| | 101 (13) | 43 (22) | 0.004 | 0.008 |
| Inadequate sedation management, n (%)|||| | 160 (21) | 60 (31) | 0.0008 | 0.001 |
| Clinically significant iatrogenic withdrawal, n (%)¶¶ | 86 (11) | 31 (16) | 0.06 | 0.03 |
| Length of stay | ||||
| PICU, median (IQR), d | 8.9 (5.8–14.2) | 14.5 (7.9–25.0) | <0.0001 | <0.0001 |
| PICU, n (%) | ||||
| <7 d | 258 (34) | 34 (18) | <0.0001 | <0.0001 |
| 7 to <14 d | 300 (40) | 59 (31) | ||
| 14 to <28 d | 145 (19) | 60 (31) | ||
| ≥28 d | 54 (7) | 39 (20) | ||
| Hospital, median (IQR), d | 13 (9–22) | 24.5 (14–44) | <0.0001 | <0.0001 |
| Hospital, n (%) | ||||
| <7 d | 79 (10) | 9 (5) | <0.0001 | <0.0001 |
| 7 to <14 d | 316 (42) | 38 (20) | ||
| 14 to <28 d | 222 (29) | 60 (31) | ||
| ≥28 d | 140 (18) | 85 (44) | ||
| Opioids and/or benzodiazepines at hospital discharge, n (%) | 207 (27) | 76 (40) | 0.003 | 0.002 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; GED = Graduate Equivalency Degree; HFOV = high-frequency oscillatory ventilation; IQR = interquartile range; MODS = multiple organ dysfunction syndrome; NMB = neuromuscular blocking agent; OI = oxygenation index; OSI = oxygen saturation index; PARDS = pediatric acute respiratory distress syndrome; PICU = pediatric ICU; PRISM III-12 = Pediatric Risk of Mortality III score from first 12 hours in the PICU; SBS = State Behavioral Scale.
P values for comparison between groups were calculated using logistic regression accounting for PICU as a cluster variable using generalized estimating equations.
P values were calculated as above, adjusting for age group and PRISM III-12 score.
Median household income of ZIP code of residence in 2011 (16).
Normal functional status at baseline was defined as Pediatric Cerebral Performance Category (PCPC) = 1 and Pediatric Overall Performance Category (POPC) = 1. POPC must be greater than or equal to PCPC (13).
Severity of illness was defined by the PRISM III-12 score. The scale for the PRISM III-12 score ranges from 0 to 74, with higher scores indicating a higher risk of death (23).
Other acute primary diagnoses include pulmonary edema, thoracic trauma, laryngotracheobronchitis, pulmonary hemorrhage, pertussis, pneumothorax (nontrauma), pulmonary embolus, acute respiratory failure related to multiple blood transfusions, and chemical pneumonitis. Other chronic primary diagnoses include acute chest syndrome/sickle cell disease, acute respiratory failure after bone marrow transplantation, acute exacerbation lung disease (cystic fibrosis or bronchopulmonary dysplasia), and pulmonary hypertension (not primary).
PARDS severity was defined using the 2015 Pediatric Acute Lung Injury Consensus Conference criteria (18).
MODS was defined as respiratory dysfunction plus one or more extrapulmonary organ dysfunctions, with concurrent MODS defined by onset on Day 0/1 and new MODS by onset on Day 2 or later (19).
Synthetic primary opioid agent includes fentanyl, hydromorphone, and remifentanil.
§§Different sedative classes include opioids, benzodiazepines, α2-adrenergic agonists, propofol, barbiturates, ketamine, and chloral hydrate.
Inadequate pain management was defined as pain score >4 (or pain assumed present if receiving neuromuscular blockade) for 2 consecutive hours and inadequate sedation management as SBS score >0 (or agitation assumed present if receiving neuromuscular blockade) for 2 consecutive hours.
Clinically significant iatrogenic withdrawal was defined as rescue therapy (an opioid or benzodiazepine bolus or an increase in opioid or benzodiazepine infusion) to manage an increase in withdrawal symptoms for patients weaning from ≥5 days of opioids.
In univariate analyses of hospital course variables, decline in functional status was significantly associated with moderate or severe pediatric ARDS, 7 or more days of mechanical ventilation, 14 or more days of PICU and hospital stay, and many aspects of pain and sedation management, including receipt of specific types and amounts of medication and inadequate pain or sedation management (Table 1). Decline in function was also significantly more frequent with presence of MODS and increasing number of dysfunctional organs. Patients with decline in function were less likely to be discharged home (80% vs. 94%; P < 0.0001) and more likely to be readmitted to a hospital after discharge (54% vs. 29%; P < 0.0001) or to receive paid medical help at home (43% vs. 24%; P < 0.0001).
In multivariable analysis, decline in functional status was significantly independently associated with several sociodemographic factors and preexisting health status: baseline functional status (lower odds of decline among those with normal baseline function) or history of prematurity or cancer (Table 2). It was also significantly associated with admission diagnosis (higher odds of decline with those admitted for exacerbation of chronic disease causing respiratory failure vs. those with bronchiolitis or asthma). Decline in functional status was significantly independently associated with two hospital course variables: duration of mechanical ventilation (odds of decline increased with each increasing week) and receipt of clonidine (odds ratio [OR] = 2.14; 95% confidence interval [CI] = 1.22–3.76; P = 0.008).
Table 2.
Multivariable Model of Risk Factors to Predict Decline in Functional Status (n = 949)*
| Variable | No. Patients [n (%)] | OR (95% CI)† | P Value |
|---|---|---|---|
| Factors present at hospital admission | |||
| Sociodemographic factors and preexisting health status | |||
| Age at PICU admission | 0.63 | ||
| 2 wk to <2 yr | 489 (52) | 1.0 | |
| 2 to <6 yr | 173 (18) | 0.86 (0.47–1.55) | |
| 6 to <18 yr | 287 (30) | 1.13 (0.78–1.64) | |
| Normal functional status at baseline‡ | 680 (72) | 0.68 (0.49–0.94) | 0.02 |
| Premature (<36 wk postmenstrual age) | 137 (14) | 1.61 (1.02–2.54) | 0.04 |
| Cancer (current or previous diagnosis) | 51 (5) | 2.22 (1.15–4.28) | 0.02 |
| Features of the presenting acute illness | |||
| PRISM III-12 score (1-point increase) | 7 (3–12)§ | 1.01 (0.99–1.04) | 0.34 |
| Primary diagnosis category | 0.08 | ||
| Bronchiolitis or asthma (or reactive airway disease) | 329 (35) | 1.0 | |
| Pneumonia or aspiration pneumonia | 406 (43) | 1.40 (0.95–2.07) | |
| Acute respiratory failure related to sepsis | 115 (12) | 2.12 (0.94–4.76) | |
| Other acute diagnoses | 77 (8) | 1.72 (0.92–3.23) | |
| Other chronic diagnoses | 22 (2) | 4.55 (1.51–13.74) | |
| Hospital course variables | |||
| Duration of mechanical ventilation | <0.0001 | ||
| <7 d | 525 (55) | 1.0 | |
| 7 to <14 d | 276 (29) | 1.67 (1.10–2.53) | |
| 14 to <28 d | 102 (11) | 2.69 (1.55–4.66) | |
| ≥28 d (including transfers by Day 28) | 46 (5) | 3.42 (1.68–6.94) | |
| Clonidine | 122 (13) | 2.14 (1.22–3.76) | 0.008 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; PICU = pediatric ICU; PRISM III-12 = Pediatric Risk of Mortality III score from the first 12 hours in the PICU.
Area under the curve = 0.732.
OR > 1 indicates higher risk of decline in functional status. ORs were calculated using logistic regression accounting for PICU as a cluster variable using generalized estimating equations.
Normal functional status at baseline was defined as Pediatric Cerebral Performance Category (PCPC) = 1 and Pediatric Overall Performance Category (POPC) = 1. POPC must be greater than or equal to PCPC (13).
Median (interquartile range).
HRQL
ITQOL from patients <2 years old or 2–6 years old with substantial developmental impairment
RESTORE patients with normal baseline functional status (n = 273) scored significantly worse than U.S. norms (21) in the domains of physical abilities, growth and development, pain and discomfort, getting along with others, and general health perceptions (Table E1). They were significantly better in the domain of general behavior. Those with impaired baseline functional status (n = 63) scored significantly worse than U.S. norms in all domains except general behavior.
Among patients with normal baseline functional status, 20% (55/271) had impaired growth and development scores, of which 29% (14/49) had decline in functional status from baseline (vs. 8% [16/190] with decline in functional status from baseline among those without impaired growth and development; P < 0.0001). Multiple variables were significantly associated with impaired growth and development in univariate analyses (Table E2). In the final multivariable model, independent predictors of impaired growth and development included receipt of methadone (OR = 2.27 [95% CI = 1.18–4.36]; P = 0.01) and inadequate pain management (OR = 2.94 [95% CI = 1.39–6.19]; P = 0.005) (Table 3).
Table 3.
Multivariable Model of Risk Factors to Predict Impaired Health-related Quality of Life Based on Infant and Toddler Quality of Life Questionnaire-97 Growth and Development Score in Patients with Normal Functional Status at Baseline (n = 271)*
| Variable | No. Patients [n (%)] | OR (95% CI)† | P Value |
|---|---|---|---|
| Factors present at hospital admission | |||
| Sociodemographic factors and preexisting health status | |||
| Age at ITQOL | 0.71 | ||
| <1 yr | 127 (47) | 1.0 | |
| 1 to <6 yr | 144 (53) | 1.12 (0.62–2.00) | |
| Features of the presenting acute illness | |||
| PRISM III-12 score (1-point increase) | 5 (1–8)‡ | 1.01 (0.96–1.05) | 0.80 |
| Hospital course variables | |||
| Methadone | 57 (21) | 2.27 (1.18–4.36) | 0.01 |
| Inadequate pain management§ | 40 (15) | 2.94 (1.39–6.19) | 0.005 |
Definition of abbreviations: CI = confidence interval; ITQOL = Infant and Toddler Quality of Life Questionnaire, from patients <2 years old or 2–6 years old with substantial developmental impairment; OR = odds ratio; PRISM III-12 = Pediatric Risk of Mortality III score from the first 12 hours in the pediatric ICU.
Area under the curve = 0.594.
OR > 1 indicates higher risk of impaired health-related qualify of life. ORs were calculated using logistic regression accounting for pediatric ICU as a cluster variable using generalized estimating equations.
Median (interquartile range).
Inadequate pain management was defined as pain score >4 (or pain assumed present if receiving neuromuscular blockade) for 2 consecutive hours.
PedsQL from patients 2 years of age or older whose parents/guardians did not complete the ITQOL
Among the 343 RESTORE patients with normal baseline function, PedsQL scores were lower for emotional and school functioning than physical and social functioning. Compared with the reference population (22), patients with normal baseline function had similar total scores, significantly lower scores for the emotional functioning subscale, and significantly higher scores for the social functioning subscale (Table E3). Patients with impaired baseline function (n = 101) scored significantly lower than the reference population in total and in all subscales.
Of patients with normal baseline function, 19% (n = 64) had a total score indicating impaired HRQL, of which 49% (27/55) had decline in functional status from baseline (vs. 12% [30/247] with decline in functional status from baseline among those without impaired HRQL; P < 0.0001). In univariate analyses, multiple sociodemographic factors and preexisting health status were significantly associated with impaired HRQL (Table E4). Of features of the presenting acute illness, higher PRISM III-12 score was significantly associated with impaired HRQL. Multiple aspects of the hospitalization were also significantly associated with impaired HRQL, including inadequate pain or sedation management; longer durations of mechanical ventilation, PICU stay, and hospital stay; and receipt of opioid and/or benzodiazepines at hospital discharge.
In the final multivariable model, impaired HRQL was independently significantly associated with older age group, being non-white or Hispanic, and cancer diagnosis (Table 4). The only hospital course variable that independently predicted impaired HRQL was inadequate sedation management (OR = 3.15 [95% CI = 1.74–5.72]; P = 0.0002).
Table 4.
Multivariable Model of Risk Factors to Predict Impaired Health-related Quality of Life Based on Pediatric Quality of Life Inventory Total Score in Patients with Normal Functional Status at Baseline (n = 341)*
| Variable | No. Patients [n (%)] | OR (95% CI)† | P Value |
|---|---|---|---|
| Factors present at hospital admission | |||
| Sociodemographic factors and preexisting health status | |||
| Age at PedsQL | 0.0002 | ||
| 2–4 yr | 144 (42) | 1.0 | |
| 5–7 yr | 75 (22) | 3.37 (1.47–7.75) | |
| 8–12 yr | 60 (18) | 5.51 (2.06–14.77) | |
| 13–17 yr | 62 (18) | 4.76 (2.31–9.82) | |
| Non-Hispanic white | 186 (55) | 0.38 (0.20–0.72) | 0.003 |
| Cancer (current or previous diagnosis) | 27 (8) | 4.14 (1.80–9.50) | 0.0008 |
| Features of the presenting acute illness | |||
| PRISM III-12 score (1-point increase) | 8 (5–13)‡ | 1.01 (0.98–1.04) | 0.50 |
| Hospital course variables | |||
| Inadequate sedation management§ | 69 (20) | 3.15 (1.74–5.72) | 0.0002 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; PedsQL = Pediatric Quality of Life Inventory; PRISM III-12 = Pediatric Risk of Mortality III score from the first 12 hours in the pediatric ICU.
Area under the curve = 0.761.
OR > 1 indicates higher risk of impaired health-related qualify of life. ORs were calculated using logistic regression accounting for pediatric ICU as a cluster variable using generalized estimating equations.
Median (interquartile range).
Inadequate sedation management was defined as State Behavioral Scale score >0 (or agitation assumed present if receiving neuromuscular blockade) for 2 consecutive hours.
Discussion
In this large, multicenter cohort of pediatric patients with acute respiratory failure, postdischarge morbidity was common, with one-fifth of patients having a decline in functional status from baseline to follow-up and a similar proportion of patients who were functionally normal at baseline having impaired quality of life scores at follow-up. In multivariable analyses using information available at the time of hospital discharge, worse outcomes were strongly associated with sociodemographic factors and preexisting health status, features of the presenting acute illness, and aspects of the hospital course, particularly duration of mechanical ventilation, inadequate pain and sedation management, and receipt of medications used to facilitate weaning from prolonged use of sedatives and analgesics. In these analyses, the magnitude of effects related to the course of critical illness was comparable to having a severe underlying disease, such as cancer. These results could inform the development of a clinical stratification tool that could be used to guide postdischarge care.
The trajectory of change in functional status from baseline to hospital discharge to postdischarge varied considerably, from overall consistency in most to overall decline in some and overall improvement in only a small subset of patients. Although we cannot determine from these data the reason for the higher frequency of decline from hospital discharge to 6 months postdischarge (vs. from baseline to hospital discharge), progression of underlying disease or ongoing effects of the acute illness and hospitalization could contribute to postdischarge functional decline, and these findings are consistent with those of Pinto and colleagues (10) finding new morbidity up to 3 years after PICU discharge. Additional research is urgently needed to elucidate potentially modifiable factors affecting recovery in these different subgroups.
Decline in functional status from baseline to postdischarge was higher in children with greater baseline impairment and comorbidity (history of prematurity or cancer), as well as increasing duration of mechanical ventilation and receipt of clonidine. Comorbidity increases risk of critical illness, and may also place patients at higher risk of long-term detrimental effects after discharge, including greater functional impairment and decreased ability to recover from impairment induced by critical illness. Of children undergoing 1 week or more of mechanical ventilation, 29% had a decline in functional status, consistent with studies demonstrating that increased exposure to the ICU environment is independently associated with worse long-term outcome (24). The association of clonidine with worse outcome may have been related to its use to facilitate weaning from prolonged exposure to sedatives and analgesics. We cannot determine the extent to which this finding was an effect of prolonged medication exposure itself versus patient or illness characteristics that led to the need for prolonged sedation, although the finding occurred independent of measures of severity of illness, diagnostic category, and duration of mechanical ventilation.
Among patients under 2 years of age (or 2–6 yr old with substantial developmental impairment) whose parents completed the ITQOL, RESTORE patients scored better than U.S. norms in the general behavior domain. This domain includes questions related to whether doctors have suggested that the child’s behavior is a problem, parental concern about current and future behavior, and complaints from others about the child’s behavior. Although these scores may have been related to better child behavior after a life-threatening illness, they also may have been influenced by parent and family resilience and/or reprioritizing concerns by parents and others after such an experience.
We analyzed growth and development scores among patients with baseline normal function more extensively because that domain is the most general in the ITQOL, incorporating parental perceptions of child physical, emotional, language, cognitive, and social function. In multivariable analyses, the only factors independently associated with impairment were receipt of methadone and inadequate pain management, albeit in a model with a lower area under the curve, and hence more limited discrimination. Similar to findings related to functional decline and clonidine, patients who receive methadone usually have longer and more complicated courses of illness, with greater overall drug exposure, than patients who do not receive methadone. Findings related to inadequate pain management are consistent with research on neonates that found that pain itself can have detrimental effects on the developing brain (25, 26). This area has not been well explored in older children, and therefore warrants further study.
PedsQL scores among patients with normal baseline functional status were lower than the reference population in the emotional domain scores, but higher in the social domain scores. Multiple studies found that PICU hospitalization had extensive mental health impact on some children, with increased anxiety and depression (27). The relatively high social domain scores may have been due to patient resilience or increased social support related to the acute illness/hospitalization, and warrant further study to understand their impact on overall recovery.
Risk factors for impaired HRQL among those with normal function at baseline included being non-white or Hispanic, consistent with previous findings that economic and social strain affected patient and family resilience after the trauma of a PICU admission (28). Similar to our findings related to inadequate pain management and impaired growth and development, inadequate sedation management may have been due to patient-related factors or downstream effects of agitation. Regardless, patients experiencing inadequate pain or sedation management would benefit from further evaluation.
This study represents one of the largest evaluations of postdischarge function and HRQL in critically ill children. Although obtained primarily from patients in academic PICUs, the 31 U.S. PICUs in this study had a wide range of patient volume, and our data were collected to reflect a wide spectrum of acute respiratory illnesses seen in most well-resourced PICUs. Thus, we believe that our findings are generalizable, and these data begin to address the current gap in the literature regarding the evolving phenomenon known as post–intensive care syndrome in pediatrics (29–31). Although controversies remain regarding the impact of critical illness on developing children and the potential overlap between intensive care hospitalization and how critical illness may alter a child’s chronic illness trajectory, we provide much-needed data to identify children experiencing worse outcomes after PICU care. Inpatient providers can use these data to help parents understand their child’s risk for long-term sequelae, and out-patient providers can use these data to refocus follow-up visits to potentially intervene to improve the 6-month trajectory.
Our study has several limitations. We had incomplete data on family socioeconomic status, so we could not use those data in multivariable models. Estimation of baseline functional status was dependent upon parental recall and medical history, and was subject to recall bias. By relying on the PCPC and POPC, which assign functional status into broad categories, we cannot identify more subtle changes in function, nor details about impairment. We did not have baseline assessments of HRQL, so we do not know how postdischarge scores changed from those present before the critical illness, and scores can be influenced by functional status. To address this limitation, we focused HRQL analyses on patients with normal baseline functional status. For all outcomes, we relied on parent proxy-report, which, although frequently consistent with child self-report for external signs and symptoms (such as physical function), may be discrepant for internal factors (such as emotions) (22). We collected HRQL data at only a single postdischarge time point, so we have limited information about trajectory of recovery. We view our regression analyses as exploratory in nature, as we may be overfitting the data and overestimating the predictive ability of the models. Finally, unmeasured aspects of the hospital course may have had an impact on postdischarge outcomes.
Conclusions
Postdischarge morbidity in children with acute respiratory failure was common and associated with factors experienced over their course of critical illness. Even when controlling for sociodemographic factors and preexisting health status and features of the presenting acute illness, modifiable factors related to critical care were important, notably, duration of mechanical ventilation, receipt of medications used to facilitate weaning from prolonged use of sedatives and analgesics, inadequate pain management among younger patients with normal baseline function, and inadequate sedation management among older patients with normal baseline function. The extent to which these factors led to adverse outcomes and, if modified, would have led to better outcomes is unclear, but mandates further study. These factors identify populations at high risk of long-term adverse sequelae requiring careful evaluation and treatment after hospital discharge.
Supplementary Material
Acknowledgments
Acknowledgment
The authors are indebted to Caroline Pidro and Katya Swarts and the Long-Term Follow-Up Core at the University of Pittsburgh Clinical Research, Investigation, and Systems Modeling of Acute Illness Center for contacting families and conducting interviews for this study.
Footnotes
Supported by NIH NHLBI and National Institute of Nursing Research grants U01 HL086622 and U01 HL086649.
The study sponsor had no role in the design of the study, the collection, analysis, or interpretation of the data, or the writing or approval of the manuscript.
Author Contributions: R.S.W., L.A.A., D.W., and M.A.Q.C. made substantial contributions to the conception and design of the work, the analysis, and interpretation of data for the work, and drafted the initial manuscript; L.H. and G.K.B. made significant contributions in the acquisition of data; E.Y.K. made substantial contributions to the interpretation of data for the work; D.C.A. made substantial contributions to the conception of and interpretation of data for the work; all authors revised the manuscript for important intellectual content, approved the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201810-1881OC on April 29, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the RESTORE Study Investigators
References
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156:1120–1128. doi: 10.1164/ajrccm.156.4.9611047. [DOI] [PubMed] [Google Scholar]
- 3.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–360. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 4.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1389–1394. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 7.Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 8.Pollack MM, Holubkov R, Funai T, Clark A, Berger JT, Meert K, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15:821–827. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack MM, Holubkov R, Funai T, Berger JT, Clark AE, Meert K, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Simultaneous prediction of new morbidity, mortality, and survival without new morbidity from pediatric intensive care: a new paradigm for outcomes assessment. Crit Care Med. 2015;43:1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM. Long-term function after pediatric critical illness: results from the survivor outcomes study. Pediatr Crit Care Med. 2017;18:e122–e130. doi: 10.1097/PCC.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 11.Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, et al. RESTORE Study Investigators; Pediatric Acute Lung Injury and Sepsis Investigators Network. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313:379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson RS, Asaro LA, Hertzog JH, Sorce LR, Kachmar AG, Dervan LA, et al. RESTORE Study Investigators; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Long-term outcomes after protocolized sedation versus usual care in ventilated pediatric patients. Am J Respir Crit Care Med. 2018;197:1457–1467. doi: 10.1164/rccm.201708-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 14.Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–460. doi: 10.1007/s11136-006-9134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 16.SOI tax stats—individual income tax statistics—ZIP code data (SOI) 2011 https://www.irs.gov/uac/soi-tax-stats-individual-income-tax-statistics-zip-code-data-soi [accessed 2017 Mar 1]. Available from.
- 17.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss SL, Asaro LA, Flori HR, Allen GL, Wypij D, Curley MA Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) Study Investigators. Multiple organ dysfunction in children mechanically ventilated for acute respiratory failure. Pediatr Crit Care Med. 2017;18:319–329. doi: 10.1097/PCC.0000000000001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein B, Giroir B, Randolph A International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 21.HealthActCHQ. ITQOL-97 US norms. Boston, MA: HealthActCHQ; 2017. [Google Scholar]
- 22.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KC, Rappaport LA, et al. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr. 2003;143:67–73. doi: 10.1016/S0022-3476(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 25.Anand KJ. Effects of perinatal pain and stress. Prog Brain Res. 2000;122:117–129. doi: 10.1016/s0079-6123(08)62134-2. [DOI] [PubMed] [Google Scholar]
- 26.Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 2014;4:57–67. doi: 10.2217/pmt.13.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Als LC, Picouto MD, Hau SM, Nadel S, Cooper M, Pierce CM, et al. Mental and physical well-being following admission to pediatric intensive care. Pediatr Crit Care Med. 2015;16:e141–e149. doi: 10.1097/PCC.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 28.McGiffin JN, Galatzer-Levy IR, Bonanno GA. Is the intensive care unit traumatic? What we know and don’t know about the intensive care unit and posttraumatic stress responses. Rehabil Psychol. 2016;61:120–131. doi: 10.1037/rep0000073. [DOI] [PubMed] [Google Scholar]
- 29.Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing post intensive care syndrome in children-the PICS-p framework. Pediatr Crit Care Med. 2018;19:298–300. doi: 10.1097/PCC.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 30.Watson RS, Choong K, Colville G, Crow S, Dervan LA, Hopkins RO, et al. Life after critical illness in children—toward an understanding of pediatric post-intensive care syndrome. J Pediatr. 2018;198:16–24. doi: 10.1016/j.jpeds.2017.12.084. [DOI] [PubMed] [Google Scholar]
- 31.Herrup EA, Wieczorek B, Kudchadkar SR. Characteristics of postintensive care syndrome in survivors of pediatric critical illness: a systematic review. World J Crit Care Med. 2017;6:124–134. doi: 10.5492/wjccm.v6.i2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.