Abstract
Rationale: Neutrophil extracellular traps (NETs) are important in the host defense against infection, but they also promote intravascular coagulation and multiorgan failure in animal models. Their clinical significance remains unclear, and available assays for patient care lack specificity and reliability.
Objectives: To establish a novel assay and test its clinical significance.
Methods: A prospective cohort of 341 consecutive adult ICU patients was recruited. The NET-forming capacity of ICU admission blood samples was semiquantified by directly incubating patient plasma with isolated neutrophils ex vivo. The association of NET-forming capacity with Sequential Organ Failure Assessment scores, disseminated intravascular coagulation, and 28-day mortality was analyzed and compared with available NET assays.
Measurements and Main Results: Using the novel assay, we could stratify ICU patients into four groups with absent (22.0%), mild (49.9%), moderate (14.4%), and strong (13.8%) NET formation, respectively. Strong NET formation was predominantly found in sepsis (P < 0.0001). Adjusted by Acute Physiology and Chronic Health Evaluation II score, multivariate regression showed that the degree of NET formation could independently predict disseminated intravascular coagulation and mortality, whereas other NET assays (e.g., cell-free DNA, myeloperoxidase, and myeloperoxidase–DNA complexes) could not. IL-8 concentrations were found to be strongly associated with NET formation, and inhibiting IL-8 significantly attenuated NETosis. Mitogen-activated protein kinase activation by IL-8 has been identified as a major pathway of NET formation in patients.
Conclusions: This assay directly measures the NET-forming capacity in patient plasma. This could guide clinical management and enable identification of NET-inducing factors in individual patients for targeted treatment and personalized ICU medicine.
Keywords: neutrophil extracellular traps, critical illness, disseminated intravascular coagulation, multiple organ failure, sepsis
At a Glance Commentary
Scientific Knowledge on the Subject
Neutrophils are the first line of defense against bacterial infection, and formation of neutrophil extracellular traps (NETs) is an important protective mechanism. However, NETs can also cause harm by exposing cytotoxic histones and promoting intravascular coagulation. Although NETs are increasingly considered as important therapeutic targets, there is currently no robust measure of NET formation to inform clinical care and enable precision medicine in patients in the ICU.
What This Study Adds to the Field
We have established a novel assay by incubating patient plasma with neutrophils to directly induce and measure NET formation. This is different from currently available assays, which primarily detect NET breakdown products. Using this assay in a prospective cohort of 341 ICU patients, we found that the degree of NET formation is significantly associated with disease severity and independently predicted development of disseminated intravascular coagulation and mortality. This assay also enabled identification of IL-8 as a major factor that drives NETosis through mitogen-activated protein kinase pathway activation. Inhibiting IL-8 or mitogen-activated protein kinase significantly reduced NET formation. Therefore, this assay can provide information on the in vivo capacity for NET formation and its inducing factors to enable improved therapeutic targeting strategies for ICU patients.
Morbidity and mortality rates in critically ill patients remain high despite significant advances in ICU management. Sepsis is a major driver of poor outcome, and because sepsis definitions have shifted toward infection-triggered organ dysfunction (1), the pathophysiology that underlies progressive organ failure requires further elucidation (2). The microcirculation plays a key role in the development of organ dysfunction and is particularly vulnerable to the interactions between inflammation, coagulation, and innate immune activation (3). Aberration of this process can cause “immunothrombosis” (4) and promote development of disseminated intravascular coagulation (DIC) to impair microcirculation.
The role of neutrophils in immunothrombosis is increasingly recognized (4). Activated neutrophils can expel nuclear chromatin to form neutrophil extracellular traps (NETs) (5, 6) in response to different pathogens (7–12), bacterial toxins (12, 13), cytokines (12, 14–17), histones (18), and activated platelets (19, 20). Mechanistically, NETs are formed through reactive oxygen species generation via the mitogen-activated protein kinase (MAPK) pathway that specifically includes mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling (21) to trigger myeloperoxidase (MPO)-mediated activation of neutrophil elastase (NE) and protein-arginine deiminase type 4 (PAD4) activation. The resultant histone citrullination leads to chromatin decondensation and the expulsion of extracellular DNA decorated with antimicrobial enzymes (NE and MPO) and histones (22). NETs can trap and kill bacteria to form a first line of defense against infection. However, excessive NET formation facilitates immunothrombosis and even DIC (23–28) to damage microcirculation and contribute to organ failure (19, 29, 30). NETs have been recognized as therapeutic targets, particularly in critical illnesses (19, 31, 32), and monitoring the degrees of NET formation in real time may benefit these patients in clinical practice.
Although NETs can be induced and monitored in vitro and in animal models (12, 33), this has been difficult to quantify in clinical settings. Currently, assays to monitor NET formation are limited to invasive organ biopsy observations or through indirect measures, such as circulating cell-free DNA (cfDNA), nucleosomes, citrullinated histone, MPO, and citrullinated histone H3–DNA or MPO–DNA complexes (34–36). The clinical potential of these surrogate markers of NET formation have been highlighted for critical illness (26, 32, 37) but do not correlate with disease severity (34–36). Furthermore, their circulating concentrations are unstable and subject to enzymatic degradation (18, 23, 38, 39). Therefore, a more reliable assay is urgently required. In this study, we have developed an assay to directly determine the NET-forming capacity of patient plasma, and its clinical use has also been evaluated in a prospective cohort of ICU patients.
Methods
Study Design and Participants
A prospective cohort of adult patients admitted to a general adult ICU at the Royal Liverpool University Hospital in the United Kingdom between June 2009 and June 2013 was assessed. Patients were enrolled in accordance with the protocol approved by the National Research Ethics Service Committee North West – Greater Manchester West and Liverpool Central (reference nos. 07/H1009/64 and 13/NW/0089). Written informed consent was obtained for all participants, and daily serial blood samples were collected over the first 96 hours of ICU admission (study duration). Exclusion criteria were transfers from other ICUs, ICU readmissions within 30 days, preexisting causes of neutropenia (including hematological malignancy), intravenous heparin treatment (23), or insufficient plasma preserved to effectively perform functional analysis (Figure E1 in the online supplement). ICU admission diagnoses were verified by two independent experienced clinicians. Admission Acute Physiology and Chronic Health Evaluation (APACHE) II scores, daily Sequential Organ Failure Assessment (SOFA) scores, and modified SOFA scores (platelet component removed to avoid bias from thrombocytopenia) were recorded together with outcome measures, including respiratory/cardiovascular support days, length of ICU stay, and 28-day mortality (from ICU admission). Sepsis was defined using the American College of Chest Physicians/Society of Critical Care Medicine 2001 international sepsis definition (1).
DIC scoring was performed daily for the first 96 hours of ICU stay using criteria defined by the International Society for Thrombosis and Haemostasis (40). DIC was diagnosed when a cumulative score greater than or equal to 5 was reached from platelet (≥100 × 103/μl = 0; <100 × 103/μl = 1; <50 × 103/μl = 2), fibrinogen (≥1.0 g/L = 0; <1 g/L = 1), D-dimers (no increase = 0; moderate increase = 2; strong increase = 3), and prolongation of prothrombin time (3 s = 0; >3 but <6 s = 1; >6 s = 2) (40).
Ex Vivo Assay of NET-Forming Capacity
Assay development was performed using a cohort of 54 patients with sepsis (NHS REC ethical approval 13/WA/0353) admitted to the ICU at Aintree University Hospital and the Royal Liverpool University Hospital. The capacity of patient platelet-poor plasma to form NETs was tested by incubating patient or healthy control plasma (or serum, when indicated) (100 μl) with heterologous neutrophils (2 × 105) from healthy volunteers (see eMethods section in the online supplement) or patient-specific neutrophils, when indicated, for 4 hours in glass chamber slides (BD Biosciences) at 37°C in 5% CO2. After fixation (2% paraformaldehyde; Sigma-Aldrich), extracellular DNA was stained with 10 μg/ml propidium iodide (Sigma-Aldrich) and visualized by immunofluorescence microscopy (×20 magnification unless otherwise specified). Quantification was performed by double-blinded assessment of extracellular DNA release by three experienced clinical scientists, and the average percentage was used for analysis. Degree of NETs formed was categorized into four groups: absence of NETs = 0% neutrophils forming NETs per microscopic field, mild NETs = 1–25%, moderate NETs = 26–50%, and strong NETs greater than or equal to 50%, including amalgam of webs. For validation, plasma-induced NETs were stained with antihuman NE (Santa Cruz Biotechnology) and antihuman MPO (Abcam) together with fluorescein isothiocyanate and Alexa Fluor 700 (Thermo Fisher Scientific) secondary antibodies. Mechanistic studies were performed using specific inhibitors of either PAD4 (Cl-amidine; Cambridge Biolabs), IL-8 (IL-8 monoclonal antibody [mAb], R&D Systems; CXCR1/2 [reparixin], Dempé; or AZD5069, AstraZeneca) or MAPK signaling (U0126; Sigma-Aldrich).
Clinical Samples
After ICU admission, surplus blood samples were collected daily from all patients for the first 96 hours, in accordance with ethically approved protocols. Measurements included whole-blood cell counts, coagulation parameters, NET-related markers, and cytokines (see eMethods section in the online supplement).
Statistical Analysis
Distributions of continuous variables were assessed by Q–Q plots, histograms, and Shapiro-Wilk tests. Clinical parameters were nonparametric in nature and are presented as medians and interquartile ranges (first and third quartiles). NET-forming capacity was analyzed in two ways: 1) as continuous variables (percentage of NETs per microscopic field) and 2) as categorical groups based on the degree of NETs. Differences in medians between two (Mann-Whitney U test) or more groups (Kruskal-Wallis test) were assessed. For cytokine analysis and comparator NET assays, the NET categories were also compared with healthy control subjects. The χ2 test was used for categorical variables (sex, ethnicity, presence/absence of ICU admission diagnosis, DIC, and 28-d mortality) between either two or more groups. Correlation analysis used Spearman rank’s correlation test. To test whether our NET assay and other NET-related markers were independent predictors of DIC and mortality, multivariable analysis of crude and adjusted odds ratios were performed (with patients adjusted for APACHE II scores). Multivariate model construction is detailed in the eMethods section in the online supplement (Table E1). Receiver operating characteristic curves assessed the performance of the different parameters (using continuous variables on ICU admission) for predicting DIC and mortality. Comparisons of receiver operating characteristic curves were performed using the DeLong test with MedCalc software. All other analyses were performed using IBM SPSS Statistics version 22 statistical software (IBM). A two-tailed P value less than 0.05 was considered statistically significant.
Results
NETs Can Be Directly Induced by Incubating Neutrophils with Plasma or Sera from Patients with Sepsis
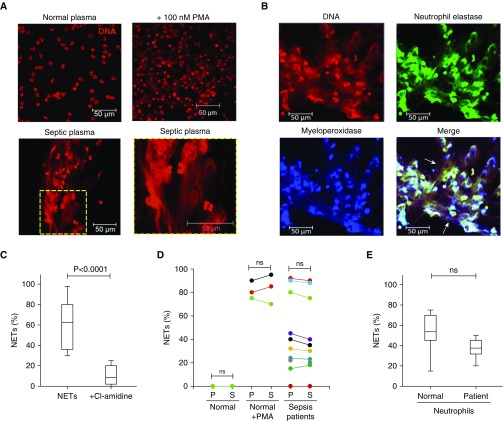
We observed that NETs were directly induced by heterologous healthy neutrophils incubated with platelet-poor plasma taken from a cohort of 54 patients with sepsis from two ICUs (Figure 1A). Typical NET structures were observed in the wells containing certain septic plasma or serum. By contrast, NETs did not form in the wells containing plasma or serum from healthy donors (n = 20) (Figure 1A), unless coincubated with 100 nM phorbol myristate acetate, a known positive control for NET formation. For further validation of patient plasma-induced NETs, antihuman NE and antihuman MPO and corresponding fluorescein isothiocyanate and Alexa Fluor 700–conjugated secondary antibodies were used, and we confirmed that the typical features of NETs existed (Figure 1B). Cl-amidine, an inhibitor of PAD4 and NETosis, was able to block the plasma- or serum-induced NETs (Figure 1C). We compared the plasma and serum isolated from blood samples taken from the same patients at the same time and found that either plasma or serum could induce similar amount of NETs (Figure 1D). Moreover, experiments were also performed using patient-specific (n = 10) neutrophils incubated with patients’ own plasma to compare the degree of NETs generated by normal donor neutrophils (n = 10), and we found no obvious difference in NET formation (Figure 1E). The degree of NET formation from patient plasma (n = 10) was repeatable with neutrophils isolated from different healthy donors (n = 10) (data not shown). Similarly, there was no exception among plasma from different healthy volunteers (n = 20), with none of them inducing NETs (data not shown). Differential degrees of NET formation between patients with sepsis (n = 54) (Figure 2A) were quantified as a percentage (NETs per microscopic field; see Methods) and categorized into four groups: absent (no neutrophils forming NETs per microscopic field) (n = 21), mild (1–25%) (n = 15), moderate (26–50%) (n = 10), and strong (≥50%) (n = 8) (Figure 2A). The strong NETs induced by patient plasma were equivalent to phorbol myristate acetate–induced NETs in healthy samples, whereas patient samples with absent NETs were indistinguishable from those of healthy control subjects.
Figure 1.
Neutrophil extracellular traps (NETs) can be directly induced by plasma or serum from patients with sepsis. (A) Normal healthy human neutrophils were incubated with either normal plasma with or without 100 nM phorbol myristate acetate (PMA) (n = 20) or critically ill patient plasma (n = 54) for 4 hours, and extracellular DNA was stained with propidium iodide. Typical images are presented. (B) NET formation was induced by incubating normal healthy human neutrophils with critically ill patient plasma, and extracellular DNA was stained with propidium iodide together with human neutrophil elastase (fluorescein isothiocyanate; green) and human myeloperoxidase (Alexa Fluor 700; blue) using specific antibodies. NET formation (arrows) was visualized using confocal microscopy. (C) Preincubation of normal neutrophils with Cl-amidine (protein-arginine deiminase type 4 inhibitor) before treatment with plasma of patients with sepsis blocked NET formation (n = 10) (ANOVA; P < 0.05). (D) NET formation was comparable when induced by either plasma (P) or serum (S). Matched normal plasma (n = 20) and serum (n = 20) did not induce NET formation when incubated with normal healthy neutrophils, unless incubated with 100 nM PMA (n = 3). There were no significant differences between plasma (n = 10) and serum (n = 10) of patients with sepsis in inducing NET formation (ANOVA; P > 0.05). (E) Incubating either normal neutrophils or neutrophils of patients with sepsis with matched plasma of patients with sepsis induced comparable NETs (n = 10) (ANOVA; P < 0.05). ns = not significant.
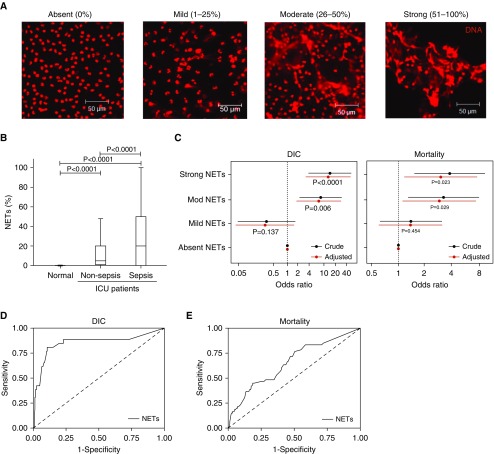
Figure 2.
The degree of neutrophil extracellular trap (NET) formation is associated with sepsis and poor clinical outcomes. (A) NET formation was categorized into four groups based on the percentage of neutrophils forming NETs per microscopic field, which were visualized using fluorescence microscopy and propidium iodide staining. Typical images are shown. (B) NET formation was associated with an admission diagnosis of sepsis. Plasma from normal healthy donors did not induce NET formation when incubated with normal neutrophils (n = 20). When patients (n = 341) were stratified on the basis of admission diagnosis into those without (n = 198) and those with sepsis (n = 143), NET formation was significantly elevated in those patients with sepsis (ANOVA; P < 0.05). (C) Multivariable analysis of crude and adjusted odds ratios (with patients adjusted for Acute Physiology and Chronic Health Evaluation II scores) demonstrated that NETs were an independent predictor of disseminated intravascular coagulation (DIC) development (left panel) and 28-day mortality (right panel) (n = 341). (C and D) Receiver operating characteristic curves for measuring the NET-forming capacity of patient plasma on ICU admission are presented for predicting DIC development (D) and for mortality (E) (n = 341).
These data indicate that the assay is robust and reliable for quantification. On the basis of this extensive assay validation work, we progressed to examine plasma taken from a large cohort of ICU patients (n = 341) to examine the clinical relevance of NETosis.
Sepsis Is the Predominant ICU Condition Associated with NET Formation
In total, 341 patients meeting the inclusion criteria were recruited (Figure E1). The clinical characteristics of patients are described in Table 1. We found that in 266 of 341 (78%) patients, their plasma could induce NET formation. In the remaining 75 of 341 (22%) patients, no NET formation was observed (Table 1). The degree of NET formation differed between patients: in 170 of 341 (49.9%), it was mild; in 49 of 341 (14.4%), moderate; and in 47 of 341 (13.8%), strong. The degrees of NET formation were not associated with age, sex, or ethnicity (Table 1) (P > 0.05), but they were strongly associated with primary diseases, in particular a diagnosis of sepsis (Table 1 and Figure 2B). Two-thirds of moderate and strong NET formation was induced by plasma from patients with sepsis, whereas more than 70% of mild NET formation was induced by plasma from patients without sepsis. There was no significant correlation between NET formation and white blood cell (R = −0.336; P = 0.062) and neutrophil counts (R = −0.309; P = 0.114) (Table 2) or with other NET-related markers such as cfDNA (R = −0.134; P = 0.864), MPO (R = 0.327; P = 0.204), and DNA–MPO complexes (R = 0.158; P = 0.982) (Table E2).
Table 1.
Characteristics of Absent, Mild, Moderate, and Strong Neutrophil Extracellular Trap Formation in ICU Patients
| Total Patients | Correlation (R Value) | Absent NET Formation | Mild NET Formation | Moderate NET Formation | Strong NET Formation | P Value* | |
|---|---|---|---|---|---|---|---|
| Total number | 341 | — | 75 | 170 | 49 | 47 | |
| NET percentage, median (IQR) | 8.0 (0.0–25.8) | — | 0.0 (0.0–0.0) | 8.0 (4.0–19.0)† | 45.0 (37.3–50.0)†‡ | 85.5 (67.0–95.0)†‡§ | <0.0001 |
| Age, yr, median (IQR) | 62.0 (48.0–72.0) | 0.276 | 60.0 (47.0–69.0) | 62.0 (49.0–73.0) | 62.0 (44.0–76.0) | 61.0 (52.0–71.0) | 0.730 |
| Male sex, n (%) | 167 (49.0%) | — | 37 (49.3%) | 85 (50.0%) | 22 (44.9%) | 23 (48.9%) | 0.940 |
| White ethnicity, n (%) | 298 (87.4%) | — | 62 (82.7%) | 150 (88.2%) | 45 (91.8%) | 41 (87.2%) | 0.474 |
| APACHE II score, median (IQR) | 19.0 (14.0–25.0) | 0.442 | 19.0 (13.0–23.0) | 19.0 (14.0–24.0) | 20.0 (15.0, 27.0) | 23.0 (17.0–29.0)†‡ | 0.013 |
| Admission diagnosis, n (%) | |||||||
| Sepsis | 143 (41.9%) | — | 31 (41.3%) | 48 (28.2%)† | 31 (63.3%)†‡ | 33 (70.2%)†‡ | <0.0001 |
| Respiratory sepsis | 48 (14.1%) | — | 10 (13.3%) | 16 (9.4%) | 16 (32.7%)†‡ | 6 (12.8%)§ | 0.0004 |
| Abdominal sepsis | 50 (14.7%) | — | 12 (16.0%) | 21 (12.4%) | 4 (8.2%) | 13 (27.7%)‡§ | 0.032 |
| Urological sepsis | 18 (5.3%) | — | 6 (8.0%) | 2 (1.2%)† | 5 (10.2%)‡ | 5 (10.6%)‡ | 0.008 |
| Other septic location | 25 (7.3%) | — | 3 (4.0%) | 9 (5.3%) | 6 (12.2%) | 7 (14.9%)†‡ | 0.047 |
| Trauma | 61 (17.9%) | — | 13 (17.3%) | 36 (21.2%) | 6 (12.2%) | 6 (12.8%) | 0.366 |
| Cardiovascular | 33 (9.7%) | — | 8 (10.7%) | 21 (12.4%) | 1 (2.0%) | 3 (6.4%) | 0.149 |
| Respiratory | 48 (14.1%) | — | 10 (13.3%) | 29 (17.1%) | 8 (16.3%) | 1 (2.1%)†‡ | 0.071 |
| Gastrointestinal | 35 (10.3%) | — | 9 (12.0%) | 22 (12.9%) | 2 (4.1%) | 2 (4.3%) | 0.142 |
| Renal | 5 (14.7%) | — | 1 (1.3%) | 2 (1.2%) | 1 (2.0%) | 1 (2.1%) | |
| Central nervous system | 16 (4.7%) | — | 3 (4.0%) | 12 (7.1%) | 0 (0.0%) | 1 (2.1%) | |
| SOFA score, median (IQR) | |||||||
| Admission | 7.0 (4.0–9.0) | 0.521 | 6.0 (3.8–9.0) | 6.0 (3.0–8.0) | 7.0 (4.0–11.0)‡ | 9.0 (7.0–12.0)†‡§ | <0.0001 |
| 24 h after admission | 7.0 (4.0–10.0) | 0.567 | 7.0 (4.0–8.0) | 6.0 (4.0–9.0) | 8.0 (5.0–12.0)†‡ | 10.0 (7.0–13.0)†‡ | <0.0001 |
| 48 h after admission | 7.0 (4.0–10.0) | 0.597 | 6.0 (4.0–8.0) | 6.0 (3.0–8.0) | 9.0 (4.0–11.0)†‡ | 11.0 (7.0–13.0)†‡§ | <0.0001 |
| 72 h after admission | 6.0 (3.0–10.0) | 0.605 | 6.0 (3.0–9.0) | 5.0 (3.0–7.0) | 9.0 (4.0–12.0)†‡ | 10.5 (8.0–14.0)†‡ | <0.0001 |
| Modified SOFA score, median (IQR) | |||||||
| Admission | 6.0 (3.0–8.0) | 0.465 | 6.0 (3.0–8.0) | 6.0 (3.0–8.0) | 6.0 (3.0–10.0) | 8.0 (5.0–11.0)†‡ | 0.002 |
| 24 h after admission | 7.0 (4.0–9.0) | 0.483 | 7.0 (4.0–8.0) | 6.0 (4.0–8.3) | 7.0 (4.0–11.0) | 8.0 (6.0–11.0)†‡ | 0.001 |
| 48 h after admission | 6.0 (4.0–9.0) | 0.506 | 5.0 (4.0–8.0) | 5.5 (3.0–7.3) | 7.0 (3.0–10.0) | 8.5 (6.0–11.0)†‡§ | <0.0001 |
| 72 h after admission | 6.0 (3.0–8.0) | 0.522 | 6.0 (3.0–8.0) | 5.0 (3.0–7.0) | 7.0 (3.3–9.0)‡ | 8.5 (6.0–11.0)†‡ | <0.0001 |
| Organ support, d, median (IQR) | |||||||
| Mechanical ventilation | 6.0 (0.0–10.5) | 0.322 | 6.0 (0.0–10.5) | 4.0 (1.0–10.0) | 2.0 (0.0–16.0) | 8.0 (3.0–14.0)‡ | 0.194 |
| Cardiovascular support | 8.0 (5.0–11.0) | 0.356 | 8.0 (5.0–11.0) | 7.0 (3.0–14.0) | 8.0 (4.0–19.5) | 10.0 (7.0–17.0)†‡ | 0.050 |
| Length of stay, d, median (IQR) | 9.0 (5.0–17.0) | 0.321 | 8.0 (4.0–15.0) | 9.0 (5.0–16.3) | 7.0 (4.0–20.5) | 11.0 (7.0–19.0)‡ | 0.144 |
| Mortality, n (%) | 67 (19.6%) | — | 9 (12.0%) | 27 (15.9%) | 15 (30.6%)†‡ | 16 (34.0%)†‡ | 0.003 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; IQR = interquartile range; NET = neutrophil extracellular trap; SOFA = Sequential Organ Failure Assessment.
P value for comparison of patients with absent versus mild versus moderate versus severe NET formation collectively, calculated using Kruskal-Wallis test for continuous variables and χ2 test for categorical variables.
Significant versus patients with absent NETs.
Significant versus patients with mild NETs.
Significant versus patients with moderate NETs.
Table 2.
Peripheral Blood Measurements of Absent, Mild, Moderate, and Strong Neutrophil Extracellular Trap Formation in ICU Patients
| Total | Correlation (R value) | Absent NET Formation | Mild NET Formation | Moderate NET Formation | Strong NET Formation | P Value* | |
|---|---|---|---|---|---|---|---|
| Total number | 341 | 75 | 170 | 49 | 47 | ||
| Peripheral blood cell counts | |||||||
| White blood cells, ×109/L, median (IQR) | |||||||
| Admission | 12.0 (7.8–18.0) | −0.336 | 12.1 (6.8–17.5) | 12.4 (8.5–18.1) | 12.3 (7.0–18.9) | 9.6 (5.0–18.4)† | 0.145 |
| 24 h after admission | 11.9 (8.0–17.4) | −0.289 | 11.6 (7.9–17.7) | 12.1 (8.7–17.2) | 11.2 (7.7–17.4) | 10.5 (5.0–18.0) | 0.835 |
| 48 h after admission | 12.0 (8.5–16.4) | −0.276 | 12.7 (8.8–15.0) | 12.0 (9.2–16.4) | 10.8 (7.9–18.3) | 12.0 (6.0–17.0) | 0.523 |
| 72 h after admission | 11.5 (8.0–16.0) | −0.114 | 11.4 (8.0–15.0) | 11.4 (9.0, 16.2) | 10.4 (8.0–15.3) | 12.0 (6.9–20.1) | 0.862 |
| Neutrophils, ×109/L, median (IQR) | |||||||
| Admission | 10.1 (6.6–15.4) | −0.309 | 9.8 (6.4–15.5) | 10.4 (7.3–15.4) | 10.2 (6.3–14.4) | 8.3 (3.9–17.7) | 0.469 |
| 24 h after admission | 9.5 (6.8–15.0) | −0.311 | 9.5 (6.5–15.1) | 10.5 (7.4–15.2) | 8.5 (6.3–16.2) | 8.2 (4.9–13.9) | 0.399 |
| 48 h after admission | 9.6 (6.4–13.9) | −0.348 | 10.2 (7.0–14.0) | 9.7 (7.1–14.0) | 9.0 (6.1–13.8) | 8.3 (4.1–14.0) | 0.430 |
| 72 h after admission | 9.5 (6.7–13.1) | −0.329 | 10.1 (7.2–12.8) | 9.4 (7.0–13.6) | 8.7 (5.9–14.9) | 7.6 (4.3–14.4) | 0.484 |
| Platelets, ×109/L, median (IQR) | |||||||
| Admission | 203.0 (136.5–299.0) | −0.648 | 241.0 (173.0–320.0) | 217.5 (175.5–331.0) | 135.0 (72.0–222.0)†‡ | 106.0 (61.0–166.0)†‡ | <0.0001 |
| 24 h after admission | 203.5 (120.0–277.8) | −0.643 | 218.5 (173.3–303.3) | 223.0 (163.0–306.8) | 136.0 (64.0–233.3)†‡ | 91.5 (53.0–150.8)†‡ | <0.0001 |
| 48 h after admission | 193.0 (102.5–267.0) | −0.677 | 236.0 (170.0–287.0) | 214.0 (163.0–301.0) | 113.5 (48.8–203.3)†‡ | 69.0 (39.5–131.0)†‡ | <0.0001 |
| 72 h after admission | 193.0 (102.0–285.5) | −0.639 | 222.0 (165.5–303.8) | 225.0 (166.3–309.5) | 99.0 (42.0–205.8)†‡ | 82.0 (42.0–118.0)†‡ | <0.0001 |
| Coagulation parameters | |||||||
| PT, s, median (IQR) | |||||||
| Admission | 15.0 (13.2–18.1) | 0.435 | 14.8 (13.6–16.8) | 14.6 (12.9–16.6) | 15.4 (13.3–19.2) | 17.7 (13.3–21.3)†‡ | 0.014 |
| 24 h after admission | 14.6 (13.0–17.2) | 0.399 | 14.5 (13.2–16.8) | 14.4 (12.9–16.5) | 14.7 (13.3–18.5) | 16.4 (12.8–20.4) | 0.332 |
| 48 h after admission | 13.9 (12.5–16.0) | 0.292 | 14.0 (12.8–15.7) | 13.8 (12.3–15.7) | 13.9 (12.1–18.1) | 14.7 (12.1–17.1) | 0.706 |
| 72 h after admission | 13.7 (12.3–15.2) | −0.126 | 13.9 (12.8–16.2) | 13.6 (12.2–14.9) | 13.9 (11.7–16.9) | 13.7 (12.5–15.4) | 0.446 |
| aPTT, s, median (IQR) | |||||||
| Admission | 32.3 (28.6–38.4) | 0.621 | 30.3 (27.3–35.5) | 31.4 (28.0–37.0) | 35.8 (30.2–44.2)†‡ | 40.2 (32.8–51.4)†‡ | <0.0001 |
| 24 h after admission | 33.3 (29.0–39.6) | 0.553 | 31.9 (28.2–35.9) | 32.8 (28.9–38.6) | 35.7 (30.6–43.0)‡ | 39.4 (31.8–45.5)†‡ | <0.0001 |
| 48 h after admission | 32.1 (28.6–38.3) | 0.581 | 30.6 (28.4–35.5) | 31.5 (28.4–37.3) | 34.5 (30.5–42.6)†‡ | 37.5 (30.4–43.9)†‡ | <0.0001 |
| 72 h after admission | 31.5 (28.4–36.5) | 0.550 | 30.5 (28.3–34.0) | 31.0 (28.3–35.8) | 34.4 (29.8–41.6)†‡ | 34.7 (29.0–39.5)†‡ | 0.004 |
| Fibrinogen, g/L, median (IQR) | |||||||
| Admission | 3.8 (2.5–5.0) | −0.565 | 4.5 (3.0–5.4) | 4.0 (3.0–5.1) | 3.4 (2.1–4.9)‡ | 2.3 (1.5–3.5)†‡§ | <0.0001 |
| 24 h after admission | 4.1 (2.9–5.2) | −0.568 | 4.5 (3.2–5.5) | 4.3 (3.3–5.4) | 3.7 (2.6–5.2)†‡ | 2.7 (1.8–3.8)†‡§ | <0.0001 |
| 48 h after admission | 4.4 (3.4–5.4) | −0.560 | 4.6 (4.0–5.8) | 4.6 (3.5–5.7) | 4.1 (2.1–5.1)†‡ | 3.1 (2.3–4.4)†‡ | <0.0001 |
| 72 h after admission | 4.6 (3.5–5.6) | −0.531 | 4.8 (3.9–6.0) | 4.8 (3.8–5.9) | 3.6 (2.2–5.2)†‡ | 3.7 (2.5–5.0)†‡ | <0.0001 |
| D-dimer, ng/ml, median (IQR) | |||||||
| Admission | 4,073.5 (2,054.8–7,759.4) | 0.377 | 3,788.0 (1,925.3–6,335.0) | 3,756.2 (1,865.0–6,284.8) | 5,549.3 (1,877.6–12,276.0) | 6,261.0 (2,464.4–15,527.6)†‡ | 0.143 |
| 24 h after admission | 4,485.0 (2,147.0–8,737.0) | 0.214 | 4,687.5 (2,941.0–10,879.3) | 4,975.8 (1,227.0–7,251.2) | 5,064.0 (2,015.0–7,022.0) | 5,791.0 (3,809.0–15,167.0) | 0.494 |
| 48 h after admission | 5,044.1 (2,175.9–7,394.8) | 0.333 | 4,145.0 (2,783.0–10,341.0) | 5,150.1 (1,803.3–7,252.7) | 4,504.5 (2,252.3–9,001.2) | 4,729.0 (2,784.0–14,336.9) | 0.888 |
| 72 h after admission | 4,931.0 (2,386.5–7,762.7) | 0.319 | 4,407.0 (2,211.0, 9,089.0) | 4,743.0 (2,952.0–6,919.0) | 5,275.9 (2,733.2–14,048.1) | 5,437.0 (1,563.5–19,292.0) | 0.983 |
| Total DIC, n (%) | 58 (17.0%) | — | 8 (10.7%) | 7 (6.7%) | 16 (32.7%)†‡ | 27 (57.4%)†‡§ | <0.0001 |
| Time to develop DIC, n (%) | |||||||
| Admission | 28 (8.2%) | — | 5 (6.7%) | 5 (2.9%) | 4 (8.2%) | 14 (30.0%)†‡§ | <0.0001 |
| 24 h after admission | 13 (3.8%) | — | 0 (0.0%) | 1 (0.6%) | 5 (10.2%)†‡ | 7 (14.9%)†‡ | <0.0001 |
| 48 h after admission | 10 (2.9%) | — | 3 (4.0%) | 1 (0.6%) | 2 (4.1%) | 4 (8.5%)† | 0.001 |
| 72 h after admission | 7 (2.1%) | — | 0 (0.0%) | 0 (0.0%) | 5 (10.2%)‡ | 2 (4.3%) | <0.0001 |
| Developed DIC ≥24 h after admission, n (%) | 30 (8.8%) | — | 3 (4.2%) | 2 (1.2%) | 12 (26.6%)†‡ | 13 (39.4%)†‡ | <0.0001 |
Definition of abbreviations: aPTT = activated partial thromboplastin time; DIC = disseminated intravascular coagulation; IQR = interquartile range; NET = neutrophil extracellular trap; PT = prothrombin time.
R correlation with percentage NET formation was performed using Spearman’s rank correlation.
P value for comparison of patients with absent versus mild versus moderate versus strong NET formation collectively, calculated using Kruskal-Wallis test for continuous variables and χ2 test for categorical variables.
Significant versus patients with mild NET formation.
Significant versus patients with absent NET formation.
Significant versus patients with moderate NET formation.
Degrees of NET Formation in Patient Plasma Strongly Predict DIC Development
High degrees of NET formation were strongly associated with thrombocytopenia (platelets, <150 × 109/L; P < 0.0001). Over 60% of patients whose plasma induced moderate and strong NET formation had thrombocytopenia compared with 15.9% in the absent and mild groups (χ2 test; P < 0.001). Abnormality in prothrombin time, activated partial thromboplastin time, and fibrinogen, as well as D-dimer, were also significantly associated with moderate or strong NET formation (P < 0.05 compared with mild or absent groups) (Table 2). These parameters are collectively indicative of DIC (40), and indeed, DIC development was significantly higher in patients with strong (39.4%) and moderate (26.6%) NET formation than in the mild (1.2%) and absent (4.2%) groups (χ2 test; P < 0.0001). NET formation was significantly higher in patients with DIC (median, 50.0%; interquartile range, 25.0–88.0%) than in those without DIC (5.0%; 0.0–20.0%) (P < 0.0001). To address whether NET formation on ICU admission could predict DIC development after admission, we excluded patients with existing DIC on ICU admission (n = 28) (Table 2). Univariate analysis using the continuous percentages of NET formation demonstrated an odds ratio for DIC of 1.06 (95% confidence interval [CI], 1.04–1.08; P < 0.0001). Using categorical data, similar results were obtained (odds ratio, 14.52; 95% CI, 3.76–56.06; P < 0.0001) for strong (8.12; 95% CI, 2.14–30.77; P = 0.002) and for moderate NET formation groups (Table 3 and Figure 2C, left panel).
Table 3.
Neutrophil Extracellular Trap Formation Is an Independent Predictor of Disseminated Intravascular Coagulation and Mortality in Critically Ill Patients
| Crude Odds Ratio | P Value* | Adjusted Odds Ratio (APACHE II) | P Value† | AUC | P Value‡ | |
|---|---|---|---|---|---|---|
| DIC | ||||||
| Absent NET formation | Reference | — | Reference | — | — | — |
| Mild NET formation | 0.274 (0.045–1.677) | 0.161 | 0.248 (0.039–1.560) | 0.137 | — | — |
| Moderate NET formation | 8.121 (2.143–30.770) | 0.002 | 7.176 (1.765–29.177) | 0.006 | — | — |
| Strong NET formation | 14.517 (3.759–56.057) | <0.0001 | 13.035 (3.157–53.829) | <0.0001 | — | — |
| NETs (%) | 1.059 (1.041–1.078) | <0.0001 | 1.058 (1.039–1.078) | <0.0001 | 0.851 | <0.0001 |
| cfDNA | 1.001 (1.000–1.001) | 0.060 | 1.001 (1.000–1.001) | 0.118 | 0.607 | 0.324 |
| MPO | 1.001 (0.997–1.004) | 0.664 | 1.001 (0.997–1.005) | 0.689 | 0.609 | 0.236 |
| DNA–MPO complex | 17.428 (1.976–153.679) | 0.010 | 9.780 (0.972–98.424) | 0.053 | 0.713 | 0.013 |
| IL-1β | 0.993 (0.923–1.068) | 0.845 | 0.999 (0.920–1.084) | 0.973 | 0.588 | 0.272 |
| IL-6 | 1.000 (1.000–1.000) | 0.113 | 1.000 (1.000–1.000) | 0.139 | 0.546 | 0.499 |
| TNF-α | 0.999 (0.994–1.003) | 0.501 | 0.999 (0.995–1.003) | 0.593 | 0.658 | 0.044 |
| IL-8 | 1.000 (1.000–1.000) | 0.049 | 1.000 (1.000–1.001) | 0.083 | 0.666 | 0.002 |
| APACHE II score | 1.144 (1.084–1.208) | <0.0001 | — | — | 0.753 | <0.0001 |
| SOFA score | 1.435 (1.274–1.616) | <0.0001 | — | — | 0.837 | <0.0001 |
| Mortality | ||||||
| Absent NET formation | Reference | — | Reference | — | — | — |
| Mild NET formation | 1.385 (0.617–3.109) | 0.430 | 1.370 (0.601–3.125) | 0.454 | — | — |
| Moderate NET formation | 3.235 (1.284–8.152) | 0.013 | 2.889 (1.114–7.494) | 0.029 | — | — |
| Strong NET formation | 3.785 (1.506–9.511) | 0.005 | 2.995 (1.162–7.720) | 0.023 | — | — |
| NETs (%) | 1.020 (1.010–1.030) | <0.0001 | 1.016 (1.006–1.026) | 0.002 | 0.851 | <0.0001 |
| cfDNA | 1.000 (1.000–1.000) | 0.232 | 1.000 (1.000–1.000) | 0.532 | 0.607 | 0.324 |
| MPO | 0.998 (0.0994–1.002) | 0.353 | 0.998 (0.993–1.002) | 0.261 | 0.609 | 0.236 |
| DNA–MPO complex | 2.005 (0.430–9.359) | 0.376 | 1.432 (0.286–7.161) | 0.662 | 0.713 | 0.013 |
| IL-1β | 1.008 (0.0981–1.035) | 0.570 | 1.003 (1.011–1.119) | 0.804 | 0.501 | 0.984 |
| IL-6 | 1.000 (1.000–1.000) | 0.907 | 1.000 (1.000–1.000) | 0.904 | 0.596 | 0.064 |
| TNF-α | 0.999 (0.997–1.002) | 0.671 | 0.999 (0.996–1.002) | 0.598 | 0.511 | 0.846 |
| IL-8 | 1.000 (1.000–1.000) | 0.380 | 1.000 (1.000–1.000) | 0.563 | 0.574 | 0.141 |
| APACHE II score | 1.087 (1.047–1.128) | <0.0001 | — | — | 0.683 | <0.0001 |
| SOFA score | 1.087 (1.017–1.162) | 0.014 | — | — | 0.604 | 0.009 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; AUC = area under the curve; cfDNA = cell-free DNA; DIC = disseminated intravascular coagulation; MPO = myeloperoxidase; NET = neutrophil extracellular trap; SOFA = Sequential Organ Failure Assessment; TNF-α = tumor necrosis factor-α.
Bold indicates P < 0.05.
P value for crude odds ratio to predict DIC and mortality.
P value for adjusted odds ratio to predict DIC and mortality in a multivariable analysis (with patients adjusted for APACHE II scores).
P value for receiver operating characteristic analysis to predict DIC and mortality.
Degrees of NET Formation Are Associated with Multisystem Organ Failure and Mortality
Because DIC is associated with development of organ dysfunction and poor outcome, we examined the relationship between NETs and multiorgan failure (MOF). Assessment with both SOFA and modified SOFA (platelet count removed) scores showed that the degree of NET formation was associated with organ injury throughout the study (Table 1). Patients in the moderate and strong NET categories had higher admission SOFA scores (median, 7 [4, 11] and 9 [7, 12], respectively) than the absent and mild groups (SOFA, 6 [4, 9] and 6 [3, 8], respectively) (P < 0.001). SOFA scores in the moderate and strong NET groups remained significantly elevated throughout the study duration. Furthermore, patients in the strong NET group had higher admission modified SOFA scores (median, 8 [5, 11]) than in the absent (6 [3, 8]), mild (6 [3, 8]), and moderate groups (6 [3, 10]) (P = 0.002), which also remained significant throughout the study duration. Patients whose plasma induced strong NET formation also required more cardiovascular support days than patients with no NETs (median, 10.0 d [interquartile range, 7.0–17.0] vs. 8.0 [5.0–11.0]. The mortality rates in both moderate (30.6%) and strong (34.0%) NET formation groups were higher than in the absent (12.0%) and mild (15.9%) groups (P < 0.003). Univariate analysis demonstrated odds ratios of 3.24 for mortality (95% CI, 1.28–8.15; P = 0.013) in the moderate NET formation group and 3.79 (95% CI, 1.51–9.51; P = 0.005) in the strong NET formation group (Table 3). Using continuous percentages of NET formation data, the odds ratio was 1.02 (1.01–1.03) (P < 0.0001). As for other NET-related assays, there was no significant association between cfDNA, MPO, or DNA–MPO complexes with mortality (Table 3).
APACHE II is a commonly used scoring system for severity-of-disease classification. We used Spearman’s rank correlation with continuous percentages of NET formation data and found that NET-forming capacity was significantly associated with APACHE II scores (r = 0.442; P = 0.013) (Table 1). However, subsequent multivariate analysis demonstrated that NET-forming capacity was independently associated with both DIC and mortality after adjustment for APACHE II (Table 3 and Figure 2C). We found that NET formation was a strong predictor of DIC (area under the curve [AUC], 0.851; P < 0.001) (Figure 2D and Table 3). Although less strong in predicting mortality (AUC, 0.656; P < 0.001), NET formation was comparable to both APACHE II (AUC, 0.683; P < 0.001) (DeLong test vs. NETs; P = 0.440) and SOFA scores (AUC, 0.604; P = 0.009) (DeLong test vs. NETS; P = 0.381) (Figure 2E and Table 3).
Anti–IL-8 Partially Blocks Patient Plasma from Inducing NETs
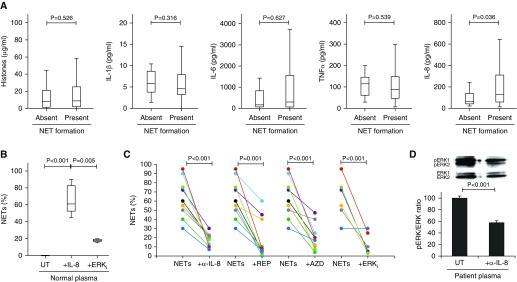
Multiple inducers of NETs have been reported, such as IL-1β, IL-6, IL-8, tumor necrosis factor-α, and extracellular histones. Using our novel assay for NET formation and cytokine profile multiplexes, we found that in our cohort, IL-8 was the only cytokine that was significantly positively associated with NET formation (Table E3 and Figure 3A), compared with a large number of negatively correlated cytokines, including IL-5, IL-9, IL-12, IL-13, IL-17, bFGF (basic fibroblast growth factor), GM-CSF (granulocyte–macrophage colony–stimulating factor), and RANTES (regulated upon activation, normal T cell expressed and secreted) (Table E3). To functionally investigate if IL-8 was the cytokine responsible for NET formation in our assay, IL-8 was added to normal plasma at relevant circulating concentration (100 pg/ml). Upon incubation with healthy neutrophils, NET formation was induced (P = 0.008) (Figure 3B). NET-forming capacities of plasma from patients with sepsis (median, 57.5; 47.5–78.8; n = 10) were significantly attenuated by a functional anti–IL-8 blocking mAb (median, 19; 10.0–22.5; P < 0.001) and the clinically trialed IL-8 receptor antagonists reparixin (median, 7.0; 3.5–41.3; P < 0.001) and AZD5069 (median, 18.5; 10.0–28.8; P < 0.001) (Figure 3C). Mechanistically, IL-8 signaling is predominately through Ras/Raf/MAPK pathways (41) (Figure E2), which are essential for NET formation (21). Specific inhibition of MAPK activation using an ERK inhibitor (U0126) significantly blocked IL-8–induced NET formation in normal plasma (P = 0.005) (Figure 3B) as well as NET-forming capacity of patient plasma (P < 0.001) (Figure 3C). Moreover, ERK phosphorylation induced by patient plasma was also significantly reduced by anti–IL-8 mAb treatment (P < 0.001) (Figure 3D). Collectively, this supports MAPK activation as the major pathway of IL-8–induced NET formation in patients.
Figure 3.
IL-8 contributes to the neutrophil extracellular trap (NET)-forming capacity of critically ill patient plasma, and this capacity is partially blocked by anti–IL-8 and anti–mitogen-activated protein kinase (anti-MAPK) treatment. (A) Quantification of circulating factors known to stimulate NET formation (histones, IL-1β, IL-6, IL-8, and tumor necrosis factor [TNF]-α) in patient plasma on ICU admission demonstrated that IL-8 was elevated in patients who were able to induce NET formation (ANOVA; P < 0.05) (n = 341). (B) Incubation of 100 pg/ml IL-8 in normal plasma with normal healthy neutrophils for 4 hours induced NET formation compared with normal plasma alone (n = 10), which was blocked by inhibiting MAPK activation with U0126 (extracellular signal-regulated kinase inhibitor [ERKi]) (n = 3) (ANOVA; P < 0.05). (C) Preincubation of normal neutrophils with either anti–IL-8 monoclonal antibody (α-IL-8) (n = 10), reparixin (REP) (n = 10), AZD5069 (AZD) (n = 10), or MAPK inhibitor U0126 (ERKi) (n = 6) before treatment with plasma of patients with sepsis partially blocked NET formation (ANOVA; P < 0.05). (D) Western blot analysis of ERK activation (pERK/ERK ratio) in normal neutrophils incubated for 15 minutes with plasma of patients with sepsis preincubated without (UT) or with anti–IL-8 monoclonal antibody (α-IL-8) (n = 3) (ANOVA; P < 0.05).
Discussion
We found that NET formation could be directly induced by patient plasma and was associated with clinically relevant information on disease severity, complications, and outcome in the ICU. The extent of NET formation on ICU admission was significantly associated with sepsis and independently predicted development of DIC and 28-day mortality. The elucidation of IL-8 as a major contributing factor to the NET-forming capacity of patient plasma could bridge important clinical utility with biological plausibility on the role of NETs in critical illness.
NETs have been increasingly recognized in disease pathogenesis since Brinkman and colleagues (12) described their ability to trap and kill bacteria in tissue samples from patients with infection. Because NETosis represents an integral component of the regulated immune response in preventing translocation and dissemination of infection (42–44), our results led us to speculate that dysregulated intravascular NETosis may promote platelet trapping and cause consumptive coagulopathy to impair end-organ perfusion and provoke MOF. In support of this theory, McDonald and colleagues (45) showed that NET-induced intravascular coagulation caused widespread microvascular occlusion and MOF in several murine models of sepsis. They also found that histones did not promote platelet adhesion to NETs or production of NETs. This could be relevant to our findings that demonstrated lack of association between histone concentrations and NET formation.
Our findings that moderate to strong NET formation is commonly observed in patients with sepsis of respiratory origin are of particular relevance to patients with pneumonia-induced acute respiratory distress syndrome (46). NET formation in these patients is associated with localized alveolar inflammation and with high IL-8 concentrations within BAL fluid (47).
We believe that a key strength of this study on NETs is the demonstration of how clinically relevant information links to mechanistic understanding and identification of therapeutic strategies. Our findings are supported by those of Yang and colleagues, who showed that plasma from patients with sepsis was more likely to induce NETs than that of patients without sepsis (48). However, their findings were limited to 62 patients with no correlation to clinical outcomes. We found that compared with other NET-related assays, cfDNA, MPO, and MPO–DNA complexes (36, 49–53) were all poorly associated with admission severity, clinical course, and outcomes. This may be due to our assay directly measuring NET-forming capacity and not being affected by NET degradation rates, factors altering the stability of NET breakdown products, contamination from neutrophil respiratory burst, or death of other types of cells (Figure E2). Therefore, our assay is clearly distinct from other NET-related assays and more accurately reflects the degrees of NET formation in patients.
A further strength of the assay is its ability to identify the driving factors for NETosis in individual patient plasma, including important signaling pathways. This could be used for determining potential targets or guiding clinical management as part of precision or personalized medicine. We identified IL-8 as an important factor promoting NETosis in some patients using this assay, and targeting IL-8 by specific inhibitors (reparixin, AZD5069, and anti–IL-8 mAb) could significantly inhibit NET formation in these patients. Using the same assay, we also identified MAPK activation as a major pathway for IL-8 in driving NETosis in patients. In multivariate analysis, IL-8 concentrations could not independently predict DIC and mortality, similar to other reported activating factors for NET formation (IL-1β, IL-6, and tumor necrosis factor-α) (Table 3). This is because IL-8 concentrations are not uniformly elevated in patients, and there are unknown factors involved that remain to be identified in future studies.
Our assay does not necessarily require isolation of individual patient neutrophils, because concordant results were obtained when patient plasma was incubated with either homologous (patient-specific) or heterologous (healthy individual) neutrophils. This allows flexibility of use in clinical practice with the choice of using patients’ or healthy donors’ neutrophils. Because fresh neutrophils are available in most large hospitals with blood banks, and because this NET assay can be easily categorized by clinical scientists (into absent, mild, moderate, or strong groups), it has clear potential to be integrated into routine clinical laboratory practice. Our assay was developed in a cohort of patients with sepsis and evaluated in a separate cohort of ICU patients. However, limitations of this study are that our results for clinical associations were obtained in a single ICU only, but our patient cohort has consistently been representative of U.K. Intensive Care National Audit and Research Centre data.
In summary, this study demonstrates how a simple, direct approach to understanding NET-forming potential in the circulation could be applied clinically to identify patients at risk of DIC and poor outcomes in the ICU. We have highlighted its potential as a stratification tool for use upon ICU admission that could enable administration of early organ support or as a companion diagnostic for novel therapies that inhibit NET formation. Because NETs and platelets interact to promote intravascular coagulation and its dissemination, there is a highly persuasive rationale for targeting NETs in sepsis and DIC. Intravenous DNase has been reported to significantly reduce end-organ damage in sepsis models (45). Our finding that IL-8 is a major inducer of NETs in many critically ill patients presents an exciting opportunity for more precise therapeutic targeting by using our novel assay system with incorporation of IL-8 inhibitors.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all of the patients, their families, and staff involved in this study. In particular, the authors accord special thanks to Colin Downey and Carol Powell for assistance with sample processing.
Footnotes
Supported by grants from the British Heart Foundation (BHF) (PG/14/19/30751, PG/16/65/32313) and the National Institute for Health Research (NIHR) (IMPALA [16/136/35]). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, BHF, NIHR, or Department of Health.
Author Contributions: S.T.A., Y.A., and M.A. performed in vitro and ex vivo experiments. S.T.A., B.M., Y.A., and S.L. analyzed the data and performed statistical analysis. S.T.A., Y.A., and I.D.W. collected the clinical data. S.T.A., B.M., G.W., and C.-H.T. edited and reviewed the manuscript and figures. G.W. and C.-H.T. supervised the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201811-2111OC on June 4, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez G, Bruhn A, Ince C. Microcirculation in sepsis: new perspectives. Curr Vasc Pharmacol. 2013;11:161–169. [PubMed] [Google Scholar]
- 4.Kimball AS, Obi AT, Diaz JA, Henke PK. The emerging role of nets in venous thrombosis and immunothrombosis. Front Immunol. 2016;7:236. doi: 10.3389/fimmu.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakazawa D, Tomaru U, Ishizu A. Possible implication of disordered neutrophil extracellular traps in the pathogenesis of MPO-ANCA-associated vasculitis. Clin Exp Nephrol. 2013;17:631–633. doi: 10.1007/s10157-012-0738-8. [DOI] [PubMed] [Google Scholar]
- 6.Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camicia G, Pozner R, de Larrañaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42:286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Toiyama Y, Inoue Y, Araki T, Mohri Y, Mizoguchi A, et al. Imaging neutrophil extracellular traps in the alveolar space and pulmonary capillaries of a murine sepsis model by multiphoton microscopy. Am J Respir Crit Care Med. 2015;191:1088–1089. doi: 10.1164/rccm.201501-0121LE. [DOI] [PubMed] [Google Scholar]
- 9.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 10.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-Kichik V, Mondragón-Flores R, Mondragón-Castelán M, Gonzalez-Pozos S, Muñiz-Hernandez S, Rojas-Espinosa O, et al. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb) 2009;89:29–37. doi: 10.1016/j.tube.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep. 2016;6:37252. doi: 10.1038/srep37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, et al. Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS One. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin Oyarzún CP, Carestia A, Lev PR, Glembotsky AC, Castro Ríos MA, Moiraghi B, et al. Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci Rep. 2016;6:38738. doi: 10.1038/srep38738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi MB, Lad A, Bharath Prasad AS, Balakrishnan A, Ramachandra L, Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587:2241–2246. doi: 10.1016/j.febslet.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, et al. Interferon-γ production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med. 2011;183:1391–1401. doi: 10.1164/rccm.201004-0592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz G, Caudrillier A, DerHovanessian A, et al. Lung Transplant Outcomes Group Investigators. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191:455–463. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 22.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould TJ, Lysov Z, Liaw PC. Extracellular DNA and histones: double-edged swords in immunothrombosis. J Thromb Haemost. 2015;13:S82–S91. doi: 10.1111/jth.12977. [DOI] [PubMed] [Google Scholar]
- 25.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 26.Delabranche X, Stiel L, Severac F, Galoisy AC, Mauvieux L, Zobairi F, et al. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. Shock. 2017;47:313–317. doi: 10.1097/SHK.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 27.McDonald B, Davis R, Jenne CN.Neutrophil extracellular traps (NETS) promote disseminated intravascular coagulation in sepsis [abstract] J Immunol 20161961 Suppl):60.8 [Google Scholar]
- 28.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015;75:2653–2662. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 31.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraaij T, Tengström FC, Kamerling SW, Pusey CD, Scherer HU, Toes RE, et al. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun Rev. 2016;15:577–584. doi: 10.1016/j.autrev.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Sha LL, Ma TT, Zhang LX, Chen M, Zhao MH. Circulating level of neutrophil extracellular traps is not a useful biomarker for assessing disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. PLoS One. 2016;11:e0148197. doi: 10.1371/journal.pone.0148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garnacho-Montero J, Huici-Moreno MJ, Gutiérrez-Pizarraya A, López I, Márquez-Vácaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18:R116. doi: 10.1186/cc13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirose T, Hamaguchi S, Matsumoto N, Irisawa T, Seki M, Tasaki O, et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS One. 2014;9:e111755. doi: 10.1371/journal.pone.0111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIlroy DJ, Jarnicki AG, Au GG, Lott N, Smith DW, Hansbro PM, et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care. 2014;29:1133, e1–e5. doi: 10.1016/j.jcrc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 38.von Köckritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qaddoori Y, Abrams ST, Mould P, Alhamdi Y, Christmas SE, Wang G, et al. Extracellular histones inhibit complement activation through interacting with complement component 4. J Immunol. 2018;200:4125–4133. doi: 10.4049/jimmunol.1700779. [DOI] [PubMed] [Google Scholar]
- 40.Toh CH, Hoots WK SSC on Disseminated Intravascular Coagulation of the ISTH. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007;5:604–606. doi: 10.1111/j.1538-7836.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- 41.Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 42.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 43.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 44.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 45.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikacenic C, Moore R, Dmyterko V, West TE, Altemeier WA, Liles WC, et al. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit Care. 2018;22:358. doi: 10.1186/s13054-018-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bendib I, de Chaisemartin L, Granger V, Schlemmer F, Maitre B, Hüe S, et al. Neutrophil extracellular traps are elevated in patients with pneumonia-related acute respiratory distress syndrome. Anesthesiology. 2019;130:581–591. doi: 10.1097/ALN.0000000000002619. [DOI] [PubMed] [Google Scholar]
- 48.Yang S, Qi H, Kan K, Chen J, Xie H, Guo X, et al. Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock. 2017;47:132–139. doi: 10.1097/SHK.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lämmle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120:1157–1164. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JE, Lee N, Gu JY, Yoo HJ, Kim HK. Circulating levels of DNA-histone complex and dsDNA are independent prognostic factors of disseminated intravascular coagulation. Thromb Res. 2015;135:1064–1069. doi: 10.1016/j.thromres.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Arai Y, Yamashita K, Mizugishi K, Watanabe T, Sakamoto S, Kitano T, et al. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1683–1689. doi: 10.1016/j.bbmt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol Lett. 2014;160:186–194. doi: 10.1016/j.imlet.2014.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.