To the Editor:
Asthma can affect sleep duration and sleep quality in children. Children with asthma are more likely to have shorter or disrupted sleep due to nocturnal symptoms (1), and children with frequent asthma symptoms report more daytime tiredness/sleepiness than those with less frequent or no asthma symptoms (2).
The American Academy of Sleep Medicine and the Sleep Research Society recommend that adults should have a regular sleep duration of 7 or more hours per night. Although short sleep duration has been linked to asthma in children and adolescents (3), only a few studies have examined sleep duration and asthma (4, 5) or fractional exhaled nitric oxide (FeNO) (6) in adults. We examined whether short sleep duration is associated with asthma and reduced lung function in a population-based sample of U.S. adults.
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional nationwide survey of the noninstitutionalized U.S. civilian population. Study participants are selected using a stratified multistage probability design and are representative of the U.S. population. As part of its study design, NHANES oversamples persons 60 years and older and ethnic minorities (African Americans and Hispanics) to increase statistical power for data analysis in those subgroups. Subjects 20–79 years of age who had nonmissing values for current asthma and sleep duration were included in the current analysis of the 2007–2012 NHANES dataset. Sleep duration was assessed by household interview using the Computer-Assisted Personal Interviewing system. Subjects were asked the question, “How much sleep (in hours) do you usually get at night on weekdays or workdays?”
Spirometry was performed according to American Thoracic Society recommendations (7). The best FEV1 and FVC values were selected for data analysis. The analysis of lung function measures was performed using percent predicted values calculated based on the Global Lung Function Initiative (8). FeNO was measured using the Aerocrine NIOX MINO (Aerocrine AB). In a subset of participants whose baseline spirometry showed an FEV1/FVC ratio below the lower limit of normal and/or below 70%, spirometry was repeated 15 minutes after inhalation of short-acting β2 agonists. Bronchodilator response (BDR) was expressed as a percentage, calculated as
Current asthma was defined by positive answers to the following questions: 1) “Has a doctor or other health professional ever told you that you have asthma?” and 2) “Do you still have asthma?” Subjects who answered “no” to both questions were selected as control subjects. We categorized weekday sleep duration as ≤5 hours, 6–8 hours (reference group), and ≥9 hours per night.
Logistic or linear regression was used for the analysis of sleep duration and current asthma, percent predicted lung function measures, BDR, and FeNO. All multivariable models were adjusted for annual household income, body mass index, family history of asthma, marital status, serum cotinine, smoking status, use of oral or inhaled steroids in the past 2 days, and depression severity. Models for current asthma, FeNO, and BDR were additionally adjusted for age, sex, and race/ethnicity.
Table 1 shows the main characteristics of the study participants and the results of the analysis of the relation between sleep duration and current asthma. Compared with control subjects, subjects with current asthma were more likely to sleep ≤5 hours or ≥9 hours per night and to be female, non-Hispanic white, and current smokers. They were also more likely to have an annual household income of <$20,000; higher body mass index and serum cotinine; a family history of asthma; used oral or inhaled steroids in the past 2 days; shorter sleep duration; greater depression severity; lower FEV1, FVC, and FEV1/FVC; and higher FeNO and BDR. In a multivariable analysis adjusting for depression severity and other covariates, a sleep duration of no more than 5 hours per night was significantly associated with 1.7 times increased odds of current asthma, but there was no significant association between sleeping at least 9 hours per night and current asthma. We found no significant interaction between sleep duration and other covariates on current asthma. Similar results were obtained in a secondary analysis of sleep duration and lifetime asthma (defined as a positive answer to physician-diagnosed asthma but a negative answer to still having asthma [data not shown]).
Table 1.
Main Characteristics of the Study Participants (N = 15,442) and Results of the Analysis of the Relation between Sleep Duration and Asthma (National Health and Nutrition Examination Survey 2007–2012)
| Odds Ratio (95% Confidence Interval) |
||||
|---|---|---|---|---|
| Characteristics | Control Subjects (n = 14,125)* | Current Asthma* (n = 1,317) | Unadjusted Analysis | Adjusted Analysis |
| Sleep duration/night, h | 6.9 ± 0.02 | 6.6 ± 0.06† | ||
| 6–8 h | 11,061 (81.2) | 867 (69.9)† | 1.0 | 1.0 |
| ≤5 h | 2,133 (12.7) | 348 (23.0) | 2.10 (1.78–2.48)† | 1.73 (1.32–2.27)† |
| ≥9 h | 931 (6.1) | 102 (7.2) | 1.37 (1.07–1.76)‡ | 1.02 (0.74–1.41) |
| Age, yr | 45.7 ± 0.4 | 46.0 ± 0.6 | — | 0.99 (0.98–0.996)† |
| Male sex | 7,107 (49.9) | 476 (36.0)† | — | 0.61 (0.48–0.77)† |
| Race/ethnicity | ||||
| Non-Hispanic white | 5,785 (66.6) | 627 (70.3)† | — | 1.0 |
| Mexican American | 2,447 (9.1) | 92 (3.8) | — | 0.57 (0.41–0.79)† |
| Other Hispanic | 1,538 (5.6) | 149 (5.6) | — | 0.79 (0.57–1.09) |
| Non-Hispanic black | 3,053 (11.3) | 351 (14.6) | — | 0.93 (0.73–1.19) |
| Other | 1,302 (7.3) | 98 (5.7) | — | 0.99 (0.68–1.46) |
| Marital status | ||||
| Married/living together | 8,592 (64.9) | 671 (56.2)† | — | 1.0 |
| Divorced/separated | 2,031 (12.7) | 252 (16.1) | — | 0.99 (0.71–1.37) |
| Widowed/single | 3,490 (22.4) | 394 (27.6) | — | 1.15 (0.92–1.44) |
| Annual household income ≥ $20,000 | 10,511 (85.6) | 822 (75.5)† | — | 1.41 (1.09–1.82)‡ |
| Smoking status | ||||
| Never/former smokers | 11,046 (78.9) | 932 (72.9)† | — | 1.0 |
| Current smokers | 3,067 (21.1) | 385 (27.1) | — | 0.93 (0.67–1.29) |
| Body mass index, kg/m2 | 28.6 ± 0.1 | 30.9 ± 0.3† | — | 1.03 (1.02–1.04)† |
| Serum cotinine level, ng/ml | 57.9 ± 2.8 | 72.3 ± 6.0‡ | — | 1.00 (0.999–1.001) |
| Family history of asthma | 2,294 (16.4) | 577 (43.6)† | — | 2.89 (2.39–3.51)† |
| Use of oral or inhaled steroid in past 2 d | 210 (1.9) | 373 (37.2)† | — | 28.68 (21.04–39.10)† |
| Depression severity (PHQ-9 score) | 2.9 ± 0.17 | 4.9 ± 0.21† | — | 1.04 (1.01–1.07)† |
| FEV1% predicted | 98.0 ± 0.3 | 88.7 ± 0.8† | — | — |
| FVC% predicted | 101.6 ± 0.2 | 97.2 ± 0.8† | — | — |
| FEV1% predicted/FVC | 96.1 ± 0.2 | 90.6 ± 0.5† | — | — |
| FeNO, ppb | 16.8 ± 0.2 | 22.8 ± 0.9† | — | — |
| Bronchodilator response | 5.6 ± 0.2 | 10.1 ± 0.9† | — | — |
Definition of abbreviations: FeNO = fractional exhaled nitric oxide; PHQ-9 = Patient Health Questionnaire-9.
Values are presented as number (%) or mean ± SD. Numbers may vary owing to missing data.
P < 0.01.
P < 0.05.
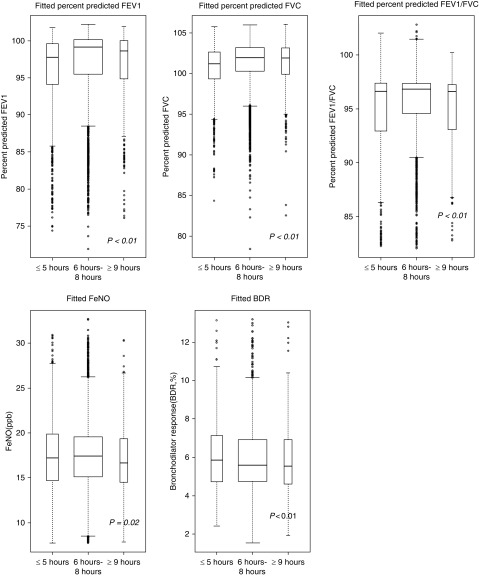
Figure 1 shows the results of the multivariable analysis of sleep duration and lung function, BDR, and FeNO. In this analysis, both short and long sleep durations were associated with lower percent predicted lung function measures (FEV1, FVC, and FEV1/FVC [P < 0.01 in all instances]) and FeNO (P = 0.02), and short sleep duration was associated with increased BDR (P < 0.01).
Figure 1.
Boxplot of model-fitted values of lung function measures against sleep duration categories (i.e., ≤5 h, 6–8 h, and ≥9 h). Linear regression was fitted for the analysis of lung function measures (FEV1% predicted, FVC% predicted, and % predicted FEV1/FVC), which was adjusted for annual household income, body mass index, current asthma status, smoking status, and use of oral or inhaled steroids in the past 2 days. The models for bronchodilator response (BDR) and fractional exhaled nitric oxide (FeNO) were additionally adjusted for age, sex, and race/ethnicity. P value is for the one-way ANOVA between the three groups. The widths of the boxes are proportional to the sample size in every group.
A population-based study of young Korean adults and a hospital-based study of U.S. adults previously reported a U-shaped association between sleep duration and asthma (4, 5). Our study differs from prior studies of asthma (3, 5, 9) with regard to geographic location (5), age range of the study participants (3, 5, 9), mode of ascertainment of asthma diagnosis (4, 5), and ability to adjust for potential confounders such as depression severity and use of inhaled steroids (4). Moreover, we found no significant evidence of modification of the estimated effect of sleep duration on current asthma by sex or overweight/obesity.
A prior study in NHANES showed a U-shaped relationship between sleep duration and FeNO but did not adjust for depression severity (6). Our novel finding of a U-shaped association between sleep duration and lung function measures or BDR may be explained by increased airway inflammation in subjects reporting sleep deprivation or excessive sleep.
We acknowledge several study limitations. First, we cannot determine temporal relationships in a cross-sectional study. Second, self-reported sleep duration is not as accurate as objective gold standards. However, our findings were essentially unchanged in a sensitivity analysis excluding 921 subjects who reported less than 4 hours of sleep (data not shown). Third, using weekday sleep duration may have led to underestimation of the true effect of insufficient sleep on asthma or lung function. Fourth, we lacked data on potential confounders of the relation between sleep duration and asthma, including sleep apnea, sleep hygiene, and insomnia (10). Lastly, misclassification of chronic obstructive pulmonary disease as asthma is possible in a study of adults.
In summary, we found that short and long sleep durations were significantly associated with reduced lung function measures and FeNO in a sample of U.S. adults, in whom short sleep duration was also associated with current asthma and increased BDR. Our findings support conducting prospective studies of sleep duration, asthma, and lung function in adults.
Supplementary Material
Footnotes
Supported by the Third Xiangya Hospital, Central South University (G.Y.), and NIH grants HD052892 (E.F.) and HL117191, HL119952, and MD011764 (J.C.C.).
Author Contributions: G.Y., W.C., and J.C.C. participated in study design, data analysis, manuscript writing, and interpretation of the study results. T.S., Y.-Y.H., L.L., F.R., E.F., and S.R.P. participated in data analysis. All authors reviewed and approved the final version of the submitted manuscript. J.C.C. is the guarantor of this work and takes responsibility for the integrity of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201905-1004LE on June 21, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Jensen ME, Gibson PG, Collins CE, Hilton JM, Latham-Smith F, Wood LG. Increased sleep latency and reduced sleep duration in children with asthma. Sleep Breath. 2013;17:281–287. doi: 10.1007/s11325-012-0687-1. [DOI] [PubMed] [Google Scholar]
- 2.van Maanen A, Wijga AH, Gehring U, Postma DS, Smit HA, Oort FJ, et al. Sleep in children with asthma: results of the PIAMA study. Eur Respir J. 2013;41:832–837. doi: 10.1183/09031936.00019412. [DOI] [PubMed] [Google Scholar]
- 3.Bakour C, O’Rourke K, Schwartz S, Wang W, Sappenfield W, Couluris M. Sleep duration, obesity, and asthma, in Florida adolescents: analysis of data from the Florida Youth Risk Behavior Survey (2009-2013) Sleep Breath. 2017;21:1039–1045. doi: 10.1007/s11325-017-1460-2. [DOI] [PubMed] [Google Scholar]
- 4.Dashti HS, Redline S, Saxena R. Polygenic risk score identifies associations between sleep duration and diseases determined from an electronic medical record biobank. Sleep (Basel) 2018;42:zsy247. doi: 10.1093/sleep/zsy247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JH, Nam GE, Kim DH, Lee JY, Han KD, Cho JH. Association between sleep duration and the prevalence of atopic dermatitis and asthma in young adults. Asian Pac J Allergy Immunol. 2017;35:150–155. doi: 10.12932/AP0772. [DOI] [PubMed] [Google Scholar]
- 6.Hyde JH, Qayyum R. The effect of sleep duration on exhaled nitric oxide levels in US adults. Sleep Breath. 2017;21:809–813. doi: 10.1007/s11325-017-1520-7. [DOI] [PubMed] [Google Scholar]
- 7.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 8.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han YY, Forno E, Celedón JC. Health risk behaviors, violence exposure, and current asthma among adolescents in the United States. Pediatr Pulmonol. 2019;54:237–244. doi: 10.1002/ppul.24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luyster FS, Strollo PJ, Jr, Holguin F, Castro M, Dunican EM, Fahy J, et al. Association between insomnia and asthma burden in the Severe Asthma Research Program (SARP) III. Chest. 2016;150:1242–1250. doi: 10.1016/j.chest.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.