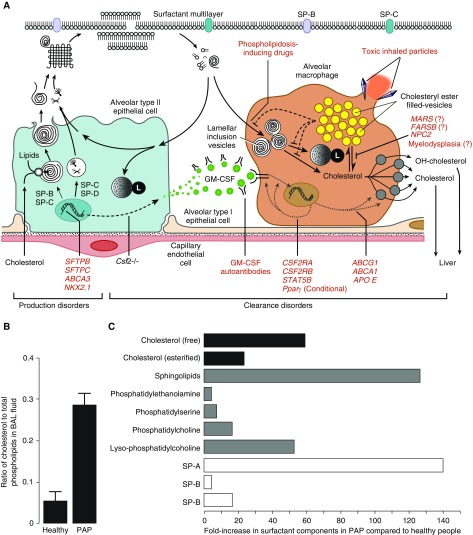
Pulmonary alveolar proteinosis (PAP) is a syndrome (not a single disease) that is characterized by the accumulation of alveolar surfactant and results in hypoxemic respiratory insufficiency/failure, which occurs in a heterogeneous group of clinically and mechanistically distinct disorders categorized (historically) as primary, secondary, or congenital PAP (1, 2). Primary PAP results from disruption of signaling by GM-CSF (granulocyte–macrophage colony–stimulating factor) and includes autoimmune PAP (caused by GM-CSF autoantibodies) and hereditary PAP (caused by mutations in genes encoding the GM-CSF receptor α and β chains [CSF2RA and CSF2RB, respectively]). Secondary PAP is caused by any one of a number of diverse underlying clinical disorders or conditions that reduce the number and/or function of alveolar macrophages, including myelodysplasia and other hematologic disorders, malignancies, immune deficiency syndromes, chronic inflammatory disorders, drugs, and toxic inhalation exposures. Congenital PAP is caused by mutations in genes required for normal surfactant production (e.g., SFTPB, SFTPC, ABCA3, and NKX2.1). The myriad of PAP-causing diseases can also be usefully considered as disorders of surfactant production or surfactant clearance (Figure 1A). Notwithstanding this etiologic diversity, autoimmune PAP accounts for approximately 90% of all patients with PAP, and all other PAP-causing diseases account for the remaining 10%.
Figure 1.
Schematic illustration of mechanisms that regulate alveolar surfactant metabolism and the disruption of these mechanisms in pulmonary alveolar proteinosis (PAP), the change in the cholesterol/phospholipid ratio in PAP, and the fold increase in various lipid species in PAP. (A) The various PAP-causing diseases classified as primary, secondary, or congenital PAP, and represented by the pathogenic mechanism (red text), can also be grouped as disorders of surfactant production or clearance (indicated) (see also Reference 2). (B) Recently, the ratio of surfactant cholesterol to phospholipids was found to be increased in PAP caused by disruption of GM-CSF (granulocyte–macrophage colony–stimulating factor) signaling in humans and mice (see References 14 and 15). (C) By carefully examining the composition of surfactant from patients with PAP and healthy control subjects collected by BAL, Griese and colleagues found that PAP caused by distinct mechanisms (including GM-CSF autoantibodies; genetic mutations in CSF2RA, MARS, FARSB, and NPC2; and myeloid leukemia) shared a similar pattern of altered surfactant composition, which is characterized by a large increase in the amount of free and esterified cholesterol and large fold increases in the normally small fraction of ceramide and other sphingolipids (16). See text for further details. SP = surfactant protein.
Pulmonary surfactant is composed of ∼80% polar lipids (primarily phosphatidylcholine and multiple less-abundant phospholipid species, sphingolipids, and others), ∼10% neutral lipids (primarily free cholesterol and small amounts of triglycerides and free fatty acids), and ∼10% surfactant proteins (A–D). Surfactant phospholipids and proteins are essential for the surface tension–lowering properties of surfactant, whereas the cholesterol content regulates its fluidity and varies inversely with environmental temperature to maintain surfactant’s biophysical properties, which are of particular importance in cold-blooded or hibernating animals (3). Surfactant lipid composition is important, and homeostasis is tightly controlled by balanced secretion by type 2 alveolar epithelial cells and clearance by these cells and alveolar macrophages in roughly equal proportions. GM-CSF is required for surfactant clearance by alveolar macrophages (4–6), and interruption of the GM-CSF signaling pathway at various locations can cause PAP (4, 7–10). Previous studies that focused on the phospholipid fraction of surfactant and demonstrated that the relative proportion of phospholipid components was preserved in patients with PAP (11) and GM-CSF–deficient mice (5) led to a widely held belief that surfactant composition was relatively unaffected (except for the accumulation of uncleared debris) and that PAP pathogenesis was caused by impaired catabolism of phospholipids in alveolar macrophages (12); however, no such mechanism has ever been identified. Recently, examinations of total surfactant lipids (i.e., both polar and neutral lipids) rather than just the polar lipid fraction identified cholesterol (free and esterified) as the predominant lipid accumulating in alveolar macrophages, and demonstrated that the ratio of cholesterol to phospholipids in BAL was markedly increased in PAP in mice and humans (Figure 1B) (13–15).
In a study reported in this issue of the Journal, Griese and coworkers (pp. 881–887) examined the alveolar “lipidome” in patients with PAP (16). Importantly, they comprehensively quantified all lipid species and included data from patients with a variety of PAP-causing diseases, including 14 with autoimmune PAP, 13 with MARS mutations, 2 with FARSB mutations, 3 with hereditary PAP caused by CSF2RA mutations, 1 with Niemann-Pick type 2, and 1 with chronic myeloid leukemia. Importantly, they demonstrated that the lipidome was similar among these mechanistically diverse PAP-causing diseases. As expected, surfactant proteins A, B, and D were all increased in PAP and phosphatidylcholine levels were 17-fold higher than normal. Moreover, there was only a small (four- to sevenfold) increase in phosphatidylethanolamine and phosphatidylserine, respectively, suggesting that lipids derived from cellular debris make only a minor contribution to the altered lipidome of PAP. In contrast, there was a large (54-fold) increase in lysophosphatidylcholine, suggesting increased activity of phospholipase A2, the expression of which is known to be increased in alveolar macrophages of Cfs2b−/− PAP mice. Importantly, there was a 60-fold increase in free cholesterol and a 24-fold increase in esterified cholesterol in PAP compared with healthy control samples. However, the ratio of free cholesterol to phospholipids was only elevated twofold above that observed in healthy control subjects, and there was no change in the ratio of esterified cholesterol to phospholipids. Interestingly, there was a 130-fold increase in ceramide and other sphingolipids, which may play an important role in the pathogenesis of PAP by contributing to an apoptotic alveolar environment. It is important to note that fold changes in the concentration of lipid species should be interpreted with care, as the absolute concentration of certain lipids may be less important than the proportional ratio to other species and vice versa. It was previously demonstrated that small changes in the total concentration, but a relative increase of threefold in cholesterol and cholesteryl esters, were associated with macrophage dysfunction in the absence of GM-CSF signaling (14). Hence, the mechanistic implications of fold changes likely depend on the individual lipid components.
Results from this study provide insight into the alveolar lipidome in PAP, with mechanistic implications for disease pathogenesis and therefore potential therapeutic targets, and provide potential biomarkers to monitor disease progression and/or therapeutic response. An important observation of the study is that the alveolar lipidome was altered similarly across a range of mechanistically distinct PAP-causing diseases, suggesting the presence of a final common pathogenic mechanism—perhaps the disruption of cholesterol metabolism that results in the development of foamy alveolar macrophages due to esterification and storage of cholesterol in intracytoplasmic lipid droplets (a cellular protective mechanism). Griese and colleagues found that the ratio of free cholesterol and esterified cholesterol to phospholipids was 2:1 and 1:1, respectively, whereas previous studies demonstrated a threefold increase (14, 15). The small differences between these studies might be explained by differences in sample preparation; for example, Griese and colleagues used centrifugation of BAL to remove cells from the surfactant before analysis, which was not done in the prior studies. Importantly, esterified cholesterol is produced within alveolar macrophages in PAP, and thus they may have underestimated the true proportion of esterified cholesterol present in the alveolar macrophages from patients with PAP. One limitation of the study is that inhibitors of lipid oxidation were not included in the sample preparation and storage, which could have affected the concentration of oxidized lipid species. Such lipid species have specific roles in modulating macrophage functional processes, particularly oxidized derivatives of cholesterol (oxysterols), which act through the LXRa pathway (10). It is also unclear whether the lipidomic profile in individuals correlates with their clinical phenotype, including the presence or absence of fibrosis—this is an area that requires further exploration. Finally, assessing dynamic changes in the alveolar lipidome may be a novel method to monitor the therapeutic response to inhaled GM-CSF or emerging cholesterol-targeted strategies.
Supplementary Material
Footnotes
Supported by NIH grant R01 HL085453 (B.C.T.) and the Translational Pulmonary Science Center, Cincinnati Children’s Hospital Medical Center.
Originally Published in Press as DOI: 10.1164/rccm.201905-1009ED on June 4, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 2.Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, Hamilton J, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers. 2019;5:16. doi: 10.1038/s41572-019-0066-3. [DOI] [PubMed] [Google Scholar]
- 3.Daniels CB, Barr HA, Power JH, Nicholas TE. Body temperature alters the lipid composition of pulmonary surfactant in the lizard Ctenophorus nuchalis. Exp Lung Res. 1990;16:435–449. doi: 10.3109/01902149009068819. [DOI] [PubMed] [Google Scholar]
- 4.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 5.Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, et al. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol. 1996;270:L650–L658. doi: 10.1152/ajplung.1996.270.4.L650. [DOI] [PubMed] [Google Scholar]
- 6.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robb L, Drinkwater CC, Metcalf D, Li R, Köntgen F, Nicola NA, et al. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci USA. 1995;92:9565–9569. doi: 10.1073/pnas.92.21.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malur A, Mccoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, et al. Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol. 2009;182:5816–5822. doi: 10.4049/jimmunol.0803504. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy MA, Barrera GC, Nakamura K, Baldán A, Tarr P, Fishbein MC, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. 1999;13:1455–1476. doi: 10.1183/09031936.99.13614779. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida M, Ikegami M, Reed JA, Chroneos ZC, Whitsett JA. GM-CSF regulates protein and lipid catabolism by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;280:L379–L386. doi: 10.1152/ajplung.2001.280.3.L379. [DOI] [PubMed] [Google Scholar]
- 13.Doyle IR, Davidson KG, Barr HA, Nicholas TE, Payne K, Pfitzner J. Quantity and structure of surfactant proteins vary among patients with alveolar proteinosis. Am J Respir Crit Care Med. 1998;157:658–664. doi: 10.1164/ajrccm.157.2.9701090. [DOI] [PubMed] [Google Scholar]
- 14.Sallese A, Suzuki T, McCarthy C, Bridges J, Filuta A, Arumugam P, et al. Targeting cholesterol homeostasis in lung diseases. Sci Rep. 2017;7:10211. doi: 10.1038/s41598-017-10879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy C, Lee E, Bridges JP, Sallese A, Suzuki T, Woods JC, et al. Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis. Nat Commun. 2018;9:3127. doi: 10.1038/s41467-018-05491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griese M, Bonella F, Costabel U, de Blic J, Tran N-B, Liebisch G.Quantitative lipidomics in pulmonary alveolar proteinosis Am J Respir Crit Care Med 2019200881–887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.