Pulmonary arterial hypertension (PAH) is a devastating disease characterized by progressive vasoconstriction and obliterative vascular remodeling leading to right-sided heart failure and premature death (1). Despite recent strides in understanding the pathogenesis of the disease, no effective therapy is available to reverse vascular remodeling and inhibit right-sided heart failure. Current therapies mainly target the abnormalities in vasoconstriction in the prostacyclin, nitric oxide, and endothelin signaling pathways, and hence result in only modest improvements in PAH morbidity and mortality. Thus, novel therapeutic agents are urgently needed for patients with PAH. Recently, the aberrant expression of transcription factors and epigenetic and metabolic regulators has been implicated in the pathogenesis of PAH (2). Some drugs that target these pathways have been evaluated in clinical trials (3), and others provide great opportunities for future development (4–6).
BET (bromodomain and extraterminal motif)-containing proteins, which function as epigenetic readers, have two conserved bromodomains with distinct specificity for Lys acetylation on histones and other nonhistone targets (7). BET proteins, comprised of four members (BRD2, BRD3, BRD4, and BRDT), bind to chromatin loci such as enhancers, resulting in the aberrant expression of genes that regulate cell proliferation, survival, and inflammation (7). BRD4 is the most extensively studied BET protein and is involved in multiple diseases, including cancer, cardiovascular disease, and PAH (5, 8). BRD4 levels are markedly increased in smooth muscle cells (SMCs) in pulmonary vascular lesions and hypertrophic right ventricular (RV) tissue of patients with PAH and Sugen5416/hypoxia (SuHx)-exposed rats (5, 9). Nebulization of the pan-BET inhibitor JQ1 inhibits PAH and RV hypertrophy in SuHx rats (5).
In this issue of the Journal, Van der Feen and colleagues (pp. 910–920) report a study in which three independent groups evaluated the efficacy of a clinically available BET inhibitor, RVX208, in experimental PAH models (10). Consistent with a previous study (5), BRD4 was found to be upregulated in pulmonary endothelial cells (ECs) and SMCs isolated from patients with PAH, and to mediate the expression of FoxM1 and its transcriptional target PLK1 in PAH-SMCs. The authors provide ample evidence from a series of in vitro studies that RVX208 treatment normalized PAH pulmonary vascular SMCs and ECs, as evidenced by inhibition of cell proliferation and inflammation, induced apoptosis, and balanced TGF-β/BMP signaling. Furthermore, the authors used three rat PAH models (SuHx, monocrotaline/aortocaval shunt, and pulmonary artery banding [PAB]) to assess the therapeutic benefit and safety of RVX208. RVX208 at a clinically relevant dose improved hemodynamics and reversed pulmonary vascular remodeling in both SuHx and monocrotaline/aortocaval shunt rats. Intriguingly, RVX208 treatment of PAB rats increased RV hypertrophy and improved cardiac function. As stated by the authors, it is for the first time that methodological rigor has been implemented to assess the therapeutic potential of BET inhibitor by multiple groups’ replication and use of multiple complementary animal models of PAH.
Accumulating evidence suggests that BRD4 is implicated in the pathogenesis of PAH, and targeting BETs and BRD4 represents a novel therapeutic approach for treatment of PAH (5, 10). However, there are some concerns and questions that need to be addressed. Although inhibition of BETs via RVX208 showed a reduction or reversal of vascular remodeling and improvement of hemodynamics, no attenuation of RV hypertrophy or mortality was observed in animals with PAH (10). However, a previous study showed that the pan-BET inhibitor JQ1 reduced RV hypertrophy in SuHx rats (5), suggesting that RVX208 is less effective than JQ1, which may be attributed to different selective binding affinities of these inhibitors for BETs. JQ1 binds to both bromodomains with higher affinity on the second bromodomain, whereas RVX208 only binds the second bromodomain (11, 12). A transcriptional analysis demonstrated that JQ1 strongly affected gene transcription, with a 10-fold difference compared with RVX208 in liver carcinoma cells (12). Another possibility is that administration of JQ1 via nebulization may provide a more effective dose in the pulmonary vasculature than RVX208 by oral administration. The possible contribution of BET family members other than BRD4 should also be considered, as JQ1 exhibits broader inhibitory activity than RVX208. Additionally, although RVX208 can enhance RV function in the PAB model, it remains unclear whether RVX208 treatment can inhibit RV decompensation and right-sided heart failure in advanced stages of PAH. The survival benefit of RVX208 is not explored. Given that a variety of BET inhibitors have been evaluated in clinical trials for various diseases (7), further studies are warranted to compare the efficacy of different BET inhibitors in PAH models.
Van der Feen and colleagues show that BRD4 controls the hyperproliferative and apoptosis-resistant and proinflammatory phenotype in SMCs and ECs in vitro. However, the cell-specific role of BRD4 in the pathogenesis of PAH remains unclear. Endothelial injury is a common feature of PAH and often initiates the development of the disease (13). The authors also show that RVX208 affects the paracrine effect of ECs on SMCs, suggesting that endothelial BRD4 may play an important role in the pathogenesis of PAH. It is possible that BRD4 coordinates with endothelial transcription factors such as HIF-2α (hypoxia-inducible factor-2α) and/or SOX17 to transcriptionally activate the expression of PAH-causing genes such as PDGFB and CXCL12 in ECs (4, 6, 14). Other investigators and we have shown the important role of the Forkhead transcription factor FoxM1 in mediating pulmonary vascular SMC proliferation in response to EC-derived PDGF-B and CXCL12 and pulmonary vascular remodeling in vivo (4). Van der Feen and colleagues demonstrate that the FOXM1–PLK1 axis is likely responsible for RVX208 normalization of the hyperproliferative, apoptosis-resistant, and proinflammatory phenotype of PAH vascular cells. Thus, it is possible that smooth-muscle BRD4 may function downstream of receptor signaling to mediate FoxM1 expression in SMCs, or that endothelial BRD4 works synergistically with HIF-2α to regulate the expression of these PAH-causing growth factors in ECs (Figure 1).
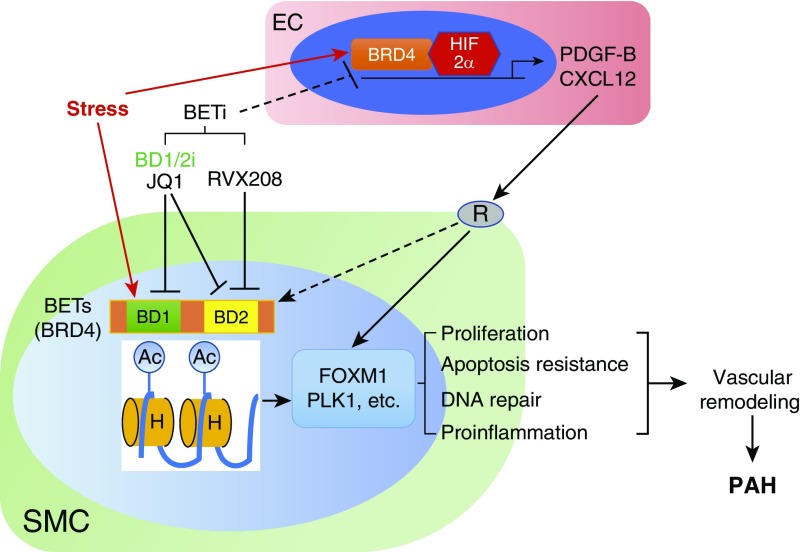
Figure 1.
Role of BETs (bromodomain and extraterminal motifs) in pulmonary arterial hypertension. Numerous stresses, including disturbed blood flow, BMPR2 mutations, drugs, toxins, and inflammation, induce the recruitment of BETs (e.g., BRD4) to the nuclei of smooth muscle cells (SMCs) to activate the expression of FoxM1 and other genes, which causes SMC hyperproliferation, apoptosis resistance, and inflammation, and thus induces pulmonary vascular remodeling and pulmonary arterial hypertension (PAH). These stresses may also induce BET recruitment to the nuclei of other cells, such as endothelial cells (ECs). BETs may coordinate with other transcriptional factors (e.g., HIF-2α [hypoxia-inducible factor-2α]) to induce expression of PAH-causing genes (PDGFB and CXCL12) in ECs. Through receptor (R) signaling, these growth factors may directly activate FoxM1 expression in SMCs or promote BET recruitment to SMC nuclei, which in turn activates FoxM1 expression in SMCs and thereby induces pulmonary vascular remodeling and development of PAH. Thus, targeting BETs is a mechanistic approach to inhibit pulmonary vascular remodeling. Efforts should be made to develop druggable BET inhibitors (BETi), preferably with dual binding affinity for both bromodomains of BRD4 (BD1/2i), for effective treatment of PAH. Ac = acetylation; H = histones.
As an epigenetic reader, BRD4 can be recruited to both enhancer and promoter loci. It has been shown that BRD4 binds to the promoter/enhancer of FOXM1 in multiple cancer cell lines. It is unclear whether other transcriptional factors and genes involved in hyperproliferation, apoptosis resistance, and/or inflammation are also controlled by BRD4 in PAH vascular cells and other cells such as fibroblasts, macrophages, and RV cardiomyocytes. It is hypothesized that the high expression of genes that are responsible for persistently activated PAH vascular cells is stable due to the open chromatin structure, which is maintained by the occupation of epigenetic regulator and transcriptional factors. Chromatin immunoprecipitation sequencing and proteomics profiling should be useful to determine the specific enhancers/promoters and genes that are directly regulated by BRD4 in various PAH cells.
In summary, RVX208 is so far the only BET inhibitor to reach phase 3 clinical trials. Patients who take RVX208 have reductions in major adverse cardiac events, including heart attack, stroke, and cardiac-related death (15). The current study provides new evidence that RVX208 might also benefit patients with PAH by reducing pulmonary arterial pressure and vascular remodeling. Thus, it is worthwhile to explore the use of BET inhibitor(s), especially those with dual binding affinity for both bromodomains of BRD4, for effective treatment and improved survival of patients with PAH.
Supplementary Material
Footnotes
Supported in part by NIH grants R01HL123957, R01HL125350, R01HL133951, R01HL140409, and P01HL077806 (Project 3) (Y.-Y.Z.); NIH grant K99HL138278; and an ATS Foundation and Pulmonary Hypertension Association Research Fellowship (Z.D.).
Originally Published in Press as DOI: 10.1164/rccm.201904-0877ED on May 21, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Hu CJ, Zhang H, Laux A, Pullamsetti SS, Stenmark KR. Mechanisms contributing to persistently activated cell phenotypes in pulmonary hypertension. J Physiol. 2019;597:1103–1119. doi: 10.1113/JP275857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sitbon O, Gomberg-Maitland M, Granton J, Lewis MI, Mathai SC, Rainisio M, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019;53:1801908. doi: 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai Z, Zhu MM, Peng Y, Jin H, Machireddy N, Qian Z, et al. Endothelial and smooth muscle cell interaction via FoxM1 signaling mediates vascular remodeling and pulmonary hypertension. Am J Respir Crit Care Med. 2018;198:788–802. doi: 10.1164/rccm.201709-1835OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, et al. Bromodomain-containing Protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 6.Dai Z, Zhu MM, Peng Y, Machireddy N, Evans CE, Machado R, et al. Therapeutic targeting of vascular remodeling and right heart failure in pulmonary arterial hypertension with a HIF-2α inhibitor. Am J Respir Crit Care Med. 2018;198:1423–1434. doi: 10.1164/rccm.201710-2079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa T, Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat Rev Mol Cell Biol. 2017;18:246–262. doi: 10.1038/nrm.2016.143. [DOI] [PubMed] [Google Scholar]
- 8.Wang CY, Filippakopoulos P. Beating the odds: BETs in disease. Trends Biochem Sci. 2015;40:468–479. doi: 10.1016/j.tibs.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Stratton MS, McKinsey TA. Acetyl-lysine erasers and readers in the control of pulmonary hypertension and right ventricular hypertrophy. Biochem Cell Biol. 2015;93:149–157. doi: 10.1139/bcb-2014-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Feen DE, Kurakula K, Tremblay E, Boucherat O, Bossers GP, Szulcek R, et al. Multicenter preclinical validation of BET inhibition for the treatment of pulmonary arterial hypertension Am J Respir Crit Care Med 2019200910–920 [DOI] [PubMed] [Google Scholar]
- 11.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci USA. 2013;110:19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2α. Circulation. 2016;133:2447–2458. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls SJ, Ray KK, Johansson JO, Gordon A, Sweeney M, Halliday C, et al. Selective BET protein inhibition with apabetalone and cardiovascular events: a pooled analysis of trials in patients with coronary artery disease. Am J Cardiovasc Drugs. 2018;18:109–115. doi: 10.1007/s40256-017-0250-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.