Abstract
This paper evaluated the feasibility of using residual sugar cane bagasse ash with a high carbon content (as-received SCBA) as raw material to produce a pozzolan after controlled recalcination and grinding. Initially, the as-received SCBA was re-burned using rotary (continuous) and muffle (batch) kilns, both at 600 °C for 1 h. Next, the resulting ash was ground in a closed-circuit ball mill with an air classifier system to obtain a product with 50% passing particle size of approximately 10 μm (SCBA600). SCBA600 was then characterized in terms of oxide composition, loss on ignition, density, specific surface area, and pozzolanic activity. A hydration study was carried out using isothermal calorimetry, thermogravimetric analysis and mercury intrusion porosimetry. Additionally, the performance of SCBA600 in mortars was evaluated by axial compression tests. The combination of recalcination at 600 °C, low-energy ultrafine grinding of the material and classification resulted in pozzolanic SCBA. The results also showed that including SCBA600 in cement mortars reduced total accumulated heat and portlandite content in cement-based pastes, in addition to refining pore structure and significantly increasing compressive strength after 3 days of curing.
Keywords: Civil engineering, Materials science, Concrete technology, Construction engineering, Materials characterization, Sugar cane bagasse ash, Controlled burning, Ultrafine grinding, Hydration
1. Introduction
Calcining sugar cane bagasse generates sugar cane bagasse ash (SCBA), an abundantly available material rich in silica. SCBA is one of the main by-products generated worldwide and its production is estimated in 5.5 × 106 tons, considering that SCBA is equivalent to around 0.3% of the total mass of processed sugar cane [1]. To date, SCBA has largely been disposed in landfills. Several studies have shown that using SCBA as a supplementary cementitious material would solve the environmental problems related to its disposal [2, 3, 4, 5]. Partial replacement of Portland cement with SCBA positively affects concrete by improving its mechanical properties [2, 3, 4, 6], durability [2, 3, 7], rheology [3], and heat released during hydration [3, 7, 8].
It is well known that ashes with high pozzolanic properties requires burning under controlled conditions. However, SCBA is mainly generated in uncontrolled calcining processes (e.g. boilers). Calcining procedures essentially define the quality of SCBA through interaction between amorphous or partially crystalline active phases (silica and alumina) and carbon content [5]. It has been demonstrated that appropriate combinations of time, heating rate, and burning temperature of the bagasse can yield SCBA with high amorphous silica content and low loss on ignition [5, 9, 10]. Some studies have shown that SCBA recalcination can be used to adjust its chemical composition, particularly by reducing its carbon content [9, 11].
In addition to burning, grinding is also required, given its ability to homogenize and control the particle size distribution of SCBA. Thus, several grinding strategies have been studied to improve the pozzolanic activity of SCBA by increasing its specific surface area [1, 8, 12]. In a recent investigation, a selective grinding method consisting of a ball mill and two classification stages was used to reduce the quartz and cristobalite content in SCBA, increasing its amorphous content [13]. Another study demonstrated that ultrafine grinding could improve the pozzolanic activity of high quartz content SCBA [1]. In this case, the SCBA sample varied from inert (similar to quartz) to highly reactive (similar to rice husk ash) as particle size declined.
In the present study, combined recalcination and continuous controlled grinding were performed to adjust the physical and chemical characteristics of a high-carbon SCBA for use as a supplementary cementitious material. This investigation was prompted by the large amount of SCBA with high loss on ignition generated in sugar cane plants in Brazil, the world's largest sugar cane producer, with a production of 750 × 106 tons in 2017 [14]. The ash produced was characterized and applied in cementitious systems to study the hydration of cement-based pastes and mechanical performance of mortars.
2. Materials
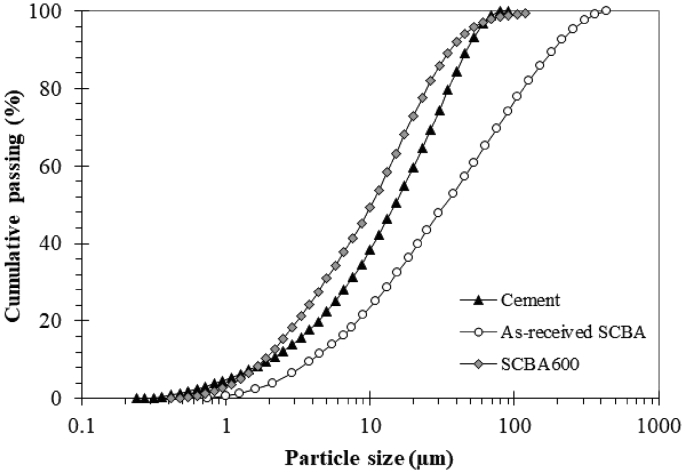
The as-received SCBA was collected from a sugar mill in Rio de Janeiro state, Brazil. The ash resulted from bagasse burned in boilers at around 800 °C and was collected directly from the particulate collector. Its chemical composition in terms of major oxides is given in Table 1, revealing the presence of carbonaceous (from high loss on ignition) and silicate-dominated particles. The sample exhibited dark coloration, indicative of the strong presence of carbonaceous particles in the ash. Laser diffraction particle size distribution of the as-received SCBA (Fig. 1) showed a D80 of 116 μm and D50 of 33 μm, suggesting ultrafine grinding was needed to reduce and control SCBA granulometry.
Table 1.
Oxide composition and loss on ignition values (% of mass) of cement, as-received SCBA, and SCBA600.
| Constituent | Cement | As-received SCBA | SCBA600 |
|---|---|---|---|
| SiO2 | 16.4 | 13.0 | 63.3 |
| Al2O3 | 3.4 | 0.6 | 8.1 |
| CaO | 69.1 | 0.2 | 4.6 |
| K2O | 0.5 | 0.4 | 3.8 |
| Fe2O3 | 5.7 | 0.2 | 3.6 |
| SO3 | 3.7 | 0.4 | 2.6 |
| P2O5 | - | 0.4 | 3.1 |
| TiO2 | - | - | 0.4 |
| MnO | - | - | 0.3 |
| MgO | - | - | 3.8 |
| Loss on ignition | 1.1 | 84.8 | 3.2 |
Fig. 1.
Particle size distribution of cement, as-received SCBA, and SCBA600.
A Brazilian Class G cement [15] from a single batch with density of 3.17 g/cm3 was used to prepare pastes and mortars. Class G is an ordinary cement used in oil well cementing operations. It is important to underscore that ordinary Portland cement is not commercially available in Brazil. Cement oxide composition and loss on ignition is presented in Table 1. Modified carboxylic ether superplasticizer (28.9% oven-dried residue and 1.21 g/cm3 density) and deionized water were used to prepare all the mixes. Fine aggregate comprised of siliceous river sand (3.56 fineness modulus and 2.56 g/cm3 density) was used in the mortars.
3. Methods
3.1. Production and characterization of SCBA
The as-received SCBA was burned in two stages. The first was carried out in a continuous electric rotary kiln to reduce the SCBA loss on ignition (carbon content). The equipment operated at a frequency of 1.72 rpm, 3% slope and variable temperature range (400–600 °C). The residence time of the sample inside the oven was approximately 1 h. The second stage was performed in a batch muffle furnace at 600 °C for 1 h, with a heating rate of 10 °C/min to reduce loss on ignition below 6% and comply with Brazilian standards [16].
After recalcination, the SCBA was ground in a rotary ball mill with 20 kg of steel grinding media with diameters varying from 24 to 30 mm. Rotation speed was 51 rpm (50% of critical) and power in operation was estimated at 68 W. The mill was fed using a Vibra Screw Incorporated (Totowa-USA) vibrating feeder, with a nominal fresh feed rate of 1.74 kg/h, resulting in a specific energy consumption of 39 kWh/t. The ground ash was classified continuously in a Raymond air classifier to obtain 50% passing particle size (D50) of about 10 μm, which is a particle size adequate for pozzolanic SCBA [1, 11]. The coarse product from the classifier was returned to the mill feed, while the resulting ash (fine product) was the SCBA used as pozzolan (named SCBA600).
From the specific energy in grinding in closed circuit, the operational Bond work index (in kWh/t) was calculated using the Bond formula (Eq. (1)) [17].
| (1) |
Where Wi,op is the operational Bond work index, W is the estimated specific energy consumption, P80 is the mill product 80% passing size (in μm) and F80 is the mill feed 80% passing size (in μm).
The oxide composition of SCBA600 was determined by energy dispersive X-ray fluorescence spectrometry (Shimadzu EDX 720, Kyoto-Japan). Particle size distribution of the ash was measured by laser scattering (Malvern Mastersizer 2000, Malvern-UK) in liquid mode (deionized water for ash and ethanol for cement), with 15 min agitation and ultrasound in the last minute. The BET specific surface area was determined in a nitrogen adsorption apparatus (Micromeritics ASAP 2020, Norcross-USA) at 200 °C for 6 h, under a 0.1 mm Hg vacuum, and density by the pycnometry test [18]. X-ray diffraction (XRD) was performed using a Miniflex 600 diffractometer (Rigaku, Tokyo-Japan) with Cu-Kα operation at 40 kV and 15 mA, angular range from 8 to 70°, 0.02° step size and 5°/min angular speed.
The soluble fraction test using sodium hydroxide [1] was performed to estimate the amorphous content of the sample. NaOH concentration was 10% and 5 g of SCBA was added to 100 mL of solution. The mix was kept in a thermal bath at 90 °C for 6 h. Next, the solution was filtered (quantitative filter GE 203, Marlborough-USA) with 800 mL of deionized water and then calcined in a muffle kiln at 800 °C for 2 h. The soluble fraction was expressed as the percentage of material dissolved in the solution. The test was performed in triplicate.
The pozzolanic activity of SCBA600 was evaluated using the modified Chapelle method based on the Brazilian standard ABNT NBR 15895:2010 [19]. The test consisted of adding 1 g of the material to a solution of 2 g of CaO and 250 g of water, kept at 90 °C for 16 h. The amount in milligrams of fixed or reacted CaO per gram of ash represented the reactivity of the material.
3.2. Production and characterization of cement-based pastes
A reference paste (denominated P-Ref) was prepared using deionized water, at a water to cement ratio of 0.35 and fixed consistency of 120 ± 10 mm, in a mini-slump test [20]. The pastes with cement replacement levels of 10 and 20% SCBA600 (denominated P-SCBA600-10% and P-SCBA600-20%, respectively) had a water to cementitious material (or water to binder, w/b) ratio of 0.35. The same consistency as P-Ref was obtained by adjusting the superplasticizer content, which was added as a percentage of the mass of cementitious material. The superplasticizer contents (expressed as percentage of superplasticizer solid mass in relation to binder mass) used were 0.05% (1.43 mL/kg of cement) for P-Ref, 0.09% (2.57 mL/kg of binder) for P-SCBA600-10%, and 0.12% (3.43 mL/kg of binder) for P-SCBA600-20%, which demonstrated higher demands for this rheology-controlling additive with greater cement replacement by SCBA600.
The effect of SCBA600 on the hydration of cement-based mixes was analyzed by isothermal calorimetry, thermogravimetric analysis (TGA), and mercury intrusion porosimetry. Each paste was mixed by adding solids to mixing water and superplasticizer in a 100-mL plastic beaker, and stirring by hand with a spatula for 30 s. Next, the pastes were mixed in an electric hand-held mixer for an additional 2 min. Isothermal calorimetry analyses were performed in a Calmetrix I-Cal 2000 calorimeter (Arlington-USA). Duplicate samples containing approximately 50 g were monitored for 72 h at 25 °C.
TGA was performed in a TA Instruments SDT Q600 system (New Castle-USA) after 1, 3, 7, and 28 days of hydration. Around 10 mg of hand-ground sample was used for each test, with a maximum N2 flow rate of 100 mL/min. Samples were heated to 45 °C at 10 °C/min, maintained for 15 min. This isotherm was required to remove free water. The samples were then submitted to a second temperature ramp up to 950 °C, with a heating rate of 10 °C/min. Raw materials (cement and ash) were tested using a single ramp up to 1000 °C. Dehydroxilization of the portlandite, chemically bound water and calcium carbonate were quantified in accordance with Rocha et al. [21].
Mercury intrusion porosimetry was performed in the pastes at 28 days using a Micrometrics AutoPore IV 9500 Series porosimeter (Norcross-USA), considering a mercury surface tension of 0.484 N/m at 25 °C and maximum pressure of 414 MPa. Paste fragments were placed in isopropanol alcohol solution to interrupt the hydration process and kept in a vacuum desiccator for 24 h before testing [22].
3.3. Production and compressive strength of mortar
Three mortars (M-Ref, M-SCBA600-10%, and M-SCBA600-20%) were proportioned using w/b and sand to cementitious material ratios of 0.35 and 2.75, respectively. Mortar consistency was measured by the flow table test [23] and maintained constant at 230 ± 10 mm for all mixtures by adding specific superplasticizer contents. These conditions were adequate to produce moldable mixes without bleeding. The mix proportions of the mortars are presented in Table 2. The control mortar (M-Ref) and those containing 10 and 20% SCBA600 (M-SCBA600-10% and M-SCBA600-20%, respectively), in mass, were prepared in a 5-L planetary mortar mixer for 3 min. Cylindrical specimens (2.5 cm diameter and 5 cm high) were cast and compacted on a vibration table. After casting, the specimens were maintained in a 100% relative humidity container for 24 h to prevent moisture evaporation. The specimens were then demolded and transferred to a lime-saturated water bath at 23 °C until testing at the established ages. Compressive strength tests were carried out at 3, 7, and 28 days of curing on a hydraulic servo-controlled machine (Emic PC200C, São José dos Pinhais-Brazil), with a displacement rate of 0.5 kN/s. Before testing, the specimen bases were ground using a wet diamond wheel. Compressive strength was considered as the average of five specimens and standard deviation values were also presented. The results were submitted to statistical analysis (analysis of variance) to assess significant differences (p ≤ 0.05) between mortars.
Table 2.
Mix proportions of mortars in kg/m³ (mass proportion values are indicated between parentheses).
| Materials | M-Ref | M-SCBA600-10% | M-SCBA600-20% |
|---|---|---|---|
| Cement | 647.3 (1) | 578.8 (0.9) | 511.2 (0.8) |
| SCBA600 | - | 64.3 (0.1) | 127.8 (0.2) |
| Sand | 1456.4 (2.25) | 1446.9 (2.25) | 1437.9 (2.25) |
| Deionized water | 225.3 (0.35) | 221.6 (0.35) | 218.7 (0.35) |
| Superplasticizer | 1.8 (0.010) | 4.9 (0.026) | 6.6 (0.035) |
Note: Volume of superplasticizer of 1.43 L/m3 for M-Ref, 2.57 L/m3 for M-SCBA600-10%, and 3.43 L/m3 for M-SCBA600-20%.
4. Results
4.1. Characterization of SCBA
Fig. 1 shows the cumulative particle size distribution of SCBA600 in relation to the as-received SCBA and cement used in this study. SCBA600 exhibited a D50 equal of 10.2 μm and grinding-classification proved efficient, since particle size distribution was significantly smaller than that of as-received SCBA (D50 of 115.7 μm). Particle size distribution for SCBA-600 and cement (Fig. 1) presented similar shape, differing only at both ends of the size spectrum (fine and coarse material). Pozzolanic SCBA samples with similar D50 were obtained in previous studies [1, 4, 12]. The finer the SCBA, the more pozzolanic it is expected to be [1, 24].
The Bond work index estimated using the Bond's law and grinding specific energy consumption (39 kWh/t) was 22.4 kWh/t. This value is close to that obtained from a standard Bond-work index test for a SCBA with low quartz and carbon SCBA, which was 22.0 kWh/t [12]. The difference, in this case, was only 1.8% for both calculated and experimental work indices, indicating that the grinding procedure was useful for estimating the specific energy associated to this operation. The results showed that SCBA600 required similar energy consumption to that estimated to produce pozzolanic SCBA in an industrial ball mill operating dry and in closed circuit with classifier (42 kWh/t) [12]. Moreover, the specific energy consumption for SCBA600 production was significantly lower than the 110 kWh/t consumed in the cement making process [25], which demonstrates yet another benefit of using this cement replacement.
The oxide compositions of both as-received SCBA and SCBA600 are shown in Table 1. As-received SCBA displayed a high residual carbon content, confirmed by its high loss on ignition (85%). The sample presented low silica content in relation to pozzolanic SCBA samples [1, 26, 27]. After burning in two kilns, low loss on ignition, high silica content and significant presence of alumina were observed in SCBA600. Its silica and alumina percentages were 63.3 and 8.1%, respectively, values comparable to the results observed for other SCBA pozzolanic samples [2, 28].
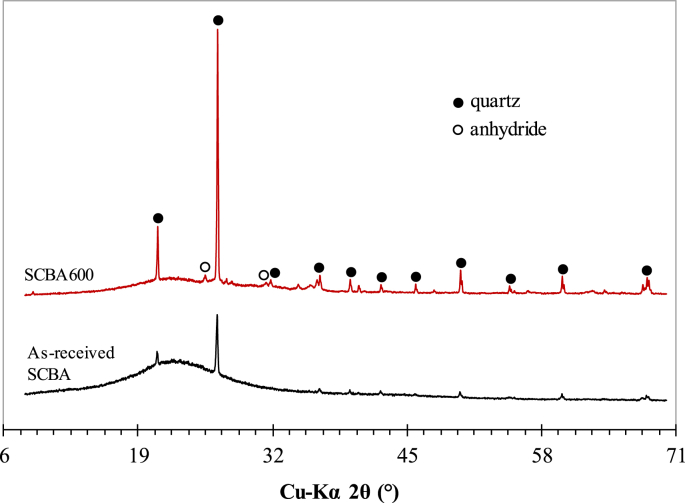
Quartz was the main crystalline phase in both as-received SCBA and SCBA600, identified by X-ray diffraction (Fig. 2). This mineral is typical for bio-ashes, such as sugar cane straw and bagasse, rice husk and elephant grass ashes, due to soil contamination during plant collection [12]. A comparison of the SCBA600 and as-received SCBA X-ray diffraction spectra indicated more intense quartz peaks after calcination. This was expected, given the decline in loss on ignition associated with the amorphous carbon in the sample, increasing the concentration of crystalline phases in the reburned ash [11]. Baseline deviation in the 2θ range between 15 and 30° was representative of amorphous silica in SCBA600. The largest deviation observed for as-received SCBA was due to the influence of carbonaceous compounds.
Fig. 2.
X-ray diffraction patterns of as-received SCBA and SCBA600 (peak intensities in arbitrary unit).
BET specific surface area and density are presented in Table 3. SCBA600 exhibited values typical of pozzolanic biomass [2, 5, 8], with specific surface area greater than 20 × 103 m2/kg. This value in association with SCBA density helped to explain the higher superplasticizer contents in pastes and mortars than those used in reference mixes. The soluble fraction (Table 3) indicated that around 32% of SCBA600 is predominantly amorphous. This result is related to other physical characteristics, whereby a high specific surface value and low D50 provided higher solubility [1]. It is interesting to note that the soluble fraction of SCBA600 was approximately 50% higher when compared to those observed in a previous study on SCBA [1]. As presented in Table 3, the modified Chapelle method indicated that SCBA600 showed good pozzolanic activity (706 mg/g), approximately 70% higher than the value obtained by Cordeiro et al. [11] for a SCBA after recalcination.
Table 3.
D50, density, BET specific surface area, soluble fraction (standard deviation values are indicated between parentheses), and modified Chapelle method values for SCBA600.
| Characteristic | Value |
|---|---|
| D50 | 10.2 μm |
| Density | 2.45 g/cm³ |
| BET specific surface area | 20,960 m2/kg |
| Soluble fraction | 31.6% (±1.6%) |
| Modified Chapelle method | 706 mg/g |
4.2. Hydration of cement-based pastes with SCBA600
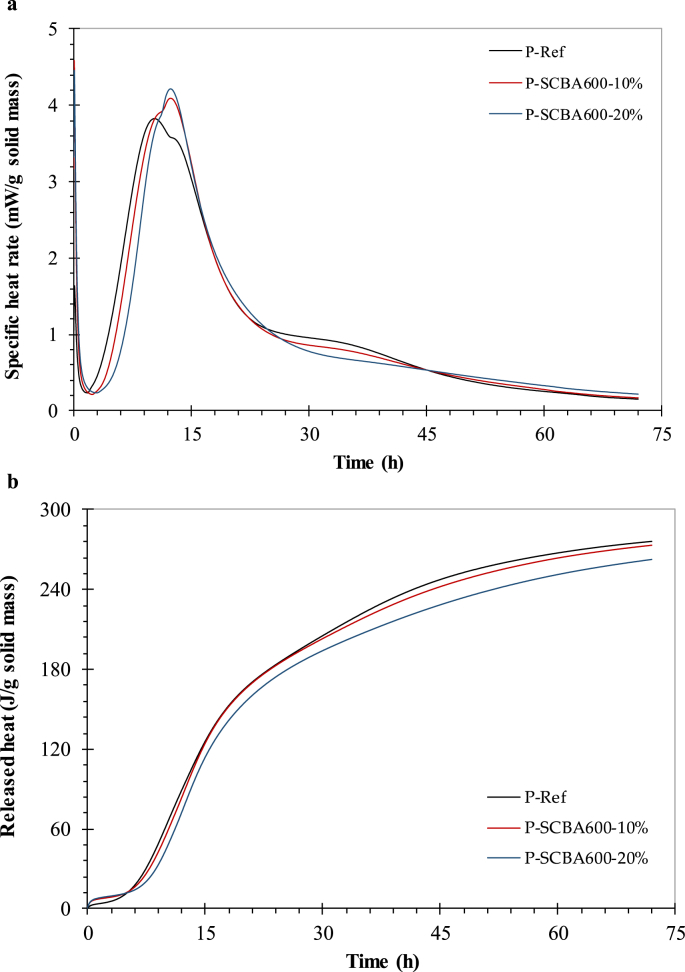
Fig. 3a shows the specific heat flow versus hydration time curves up to 72 h for P-Ref and mixes containing SCBA600. Overall, all the mixes displayed typical curves with the phases of the cement hydration reactions, as described in several studies [29, 30]. The initial reaction (first peak after a few minutes of testing) was not considered here because the mixes were produced outside the calorimeter. The results showed that replacing cement with SCBA600 provided a clear shift in the heat flow versus time curves to the right of P-Ref. This indicated that the presence of SCBA600 delayed hydration, which was more pronounced with greater cement replacement, whereby the higher the SCBA600 content, the greater the induction period. Dormancy offset for pastes containing 10 and 20% SCBA600 remained for 50 min and 1.5 h longer than P-Ref, respectively. The presence of carbon and contaminants such as K2O and SO3 in SCBA600 (3.2%) may have influenced the longer dormancy period for pastes containing ash [11, 26]. It is important to underscore that the pastes containing SCBA exhibited considerable higher superplasticizer doses when compared to P-Ref at the same consistency. A high superplasticizer content contributed to the retarding effect. Closer examination of the data revealed a significant increase in heat flow associated with the monosulfate formation reaction (third peak) provided by SCBA600, possibly due to the Al2O3 content in the ash. Fig. 3b shows that the maximum heat release values of SCBA600 pastes were lower than that of P-Ref. After 72 h hydration, the cumulative heat values for P-SCBA600-20%, P-SCBA600-10% and P-Ref were 262, 273 and 277 J/g, respectively.
Fig. 3.
Specific heat rate (a) and cumulative heat (b) curves (by mass of total solids) for different pastes.
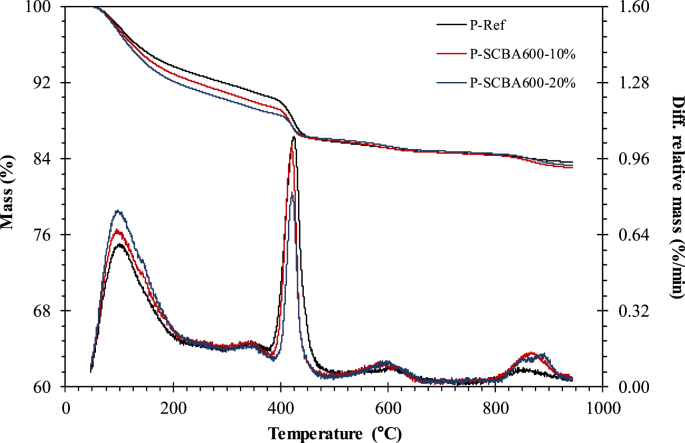
Fig. 4 shows thermogravimetric and differential thermogravimetric curves at 28 days of hydration of P-Ref and pastes containing SCBA600 indicated as a function of solid mass. In general, the same behavior was observed for all samples at the assessment ages (between 1 and 28 days of hydration). A peak was observed between 90 and 200 °C attributed to ettringite and C-S-H dehydration and phases associated with aluminate hydration. There was a significant mass loss between 350 and 500 °C due to the dehydroxilization of portlandite. At temperatures above 550 °C, calcium carbonate (CaCO3) decarbonation occurred in all samples [31].
Fig. 4.
Thermogravimetry (above) and differential thermogravimetry (below) curves of control and SCBA600 pastes at 28 days.
Portlandite, chemically bound water and calcium carbonate contents are shown in Table 4. The results of raw material analyses were vital in correcting quantification. After 1 day, portlandite content for the control paste, P-SCBA600-10% and P-SCBA600-20% was 10.8, 7.6 and 5.9%, respectively. This corroborated the calorimetry results, which confirmed the hydration delaying effect of SCBA600. As expected, portlandite content increased throughout the curing time for all pastes. However, the rate of increase was higher in P-Ref than SCBA600 pastes, with the former exhibiting higher portlandite content after 28 days of hydration. In the pastes containing SCBA600, portlandite content declined as cement replacement increased (Table 4). This was due to the dilution effect resulting from the low cement content in these mixes and the pozzolanic reaction provided by the ash. For instance, P-SCBA600-20% contained approximately 43% less portlandite than P-Ref at 28 days. It is important to note that calcium carbonate content was below 5% for all the pastes.
Table 4.
Portlandite, chemically bound water, and calcium carbonate contents in control paste and pastes with 10 and 20% of SCBA600, based on the thermogravimetric analysis.
| Time (day) | P-Ref | P-SCBA600-10% | P-SCBA600-20% | |
|---|---|---|---|---|
| Portlandite (%) | 1 | 10.8 | 7.6 | 5.9 |
| 3 | 12.9 | 8.7 | 7.8 | |
| 7 | 13.9 | 9.6 | 8.2 | |
| 28 | 15.1 | 12.2 | 8.6 | |
| Chemically bound water (%) | 1 | 10.9 | 7.7 | 8.7 |
| 3 | 14.4 | 13.7 | 13.2 | |
| 7 | 15.3 | 16.3 | 15.1 | |
| 28 | 17.0 | 16.0 | 15.6 | |
| Calcite (%) | 1 | 2.2 | 1.0 | 2.1 |
| 3 | 4.6 | 4.0 | 3.3 | |
| 7 | 3.2 | 3.2 | 3.8 | |
| 28 | 1.2 | 3.1 | 3.2 |
Chemically bound water content displayed the same behavior observed for portlandite concentrations (Table 4), with values of 17.0, 16.0 and 15.6% in P-Ref, P-SCBA600-10% and P-SCBA600-20% at 28 days, respectively. Interestingly, the drop in chemically bound water levels was less pronounced than that of portlandite content for both SCBA600 pastes. This behavior confirmed the pozzolanicity of SCBA600, as demonstrated in a previous study using a similar ash [1].
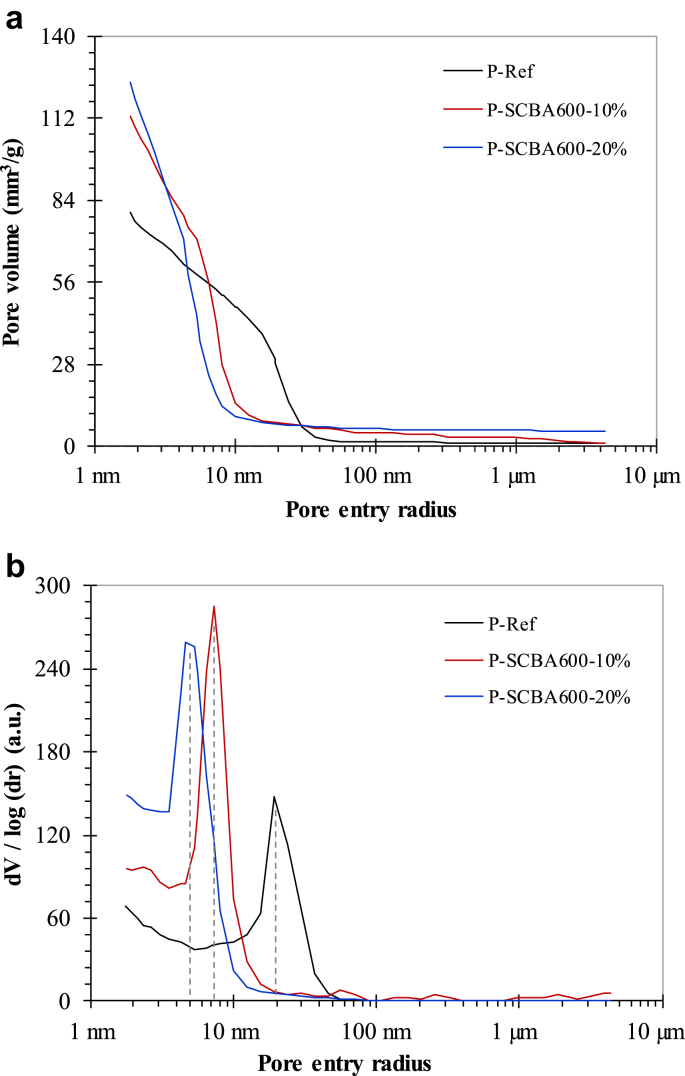
Fig. 5a shows the mercury intrusion porosimetry results for all the pastes after 28 days of hydration, indicating a difference between the mixes. The total intruded pore volume of the SCBA600 pastes was significantly higher than that obtained for P-Ref. No meaningful differences in pore volume were observed between P-SCBA-10% and P-SCBA-20%. By contrast, the threshold radius (at which the cumulative pore volume rose sharply) decreased considerably with a rise in SCBA600 replacement content, indicating finer pore structure in pastes containing SCBA600. The volume of pores with radii smaller than 10 nm increased with cement replacement by SCBA600. Fig. 5b shows that the critical pore entry radius (related to the peak indicated by dashed lines in the figure) decreased with the use of ash. This pore structure refinement is typical in cement-based systems containing pozzolans. Indeed, Andreão et al. [26] reported that SCBA caused visible pore structure refinement in cement-based pastes.
Fig. 5.
Cumulative pore volumes (a) and first derivatives of the cumulative curves (b) of pastes incorporated by 10 and 20% of SCBA600 and control paste at 28 days.
4.3. Compressive strength of mortars containing SCBA
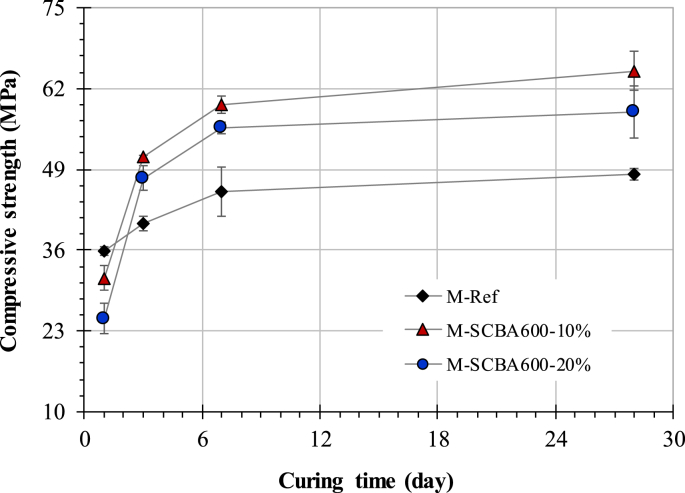
The evolution of compressive strength over curing time for all mortars is presented in Fig. 6. Overall, replacing cement with SCBA600 had a positive effect on the compressive strength of mortars, except for short curing times. At 1 day, the SCBA mixes showed significantly lower compressive strength values than those observed for M-Ref. This may be due to the delayed hydration effect of SCBA600, as discussed in 4.2. For example, M-SCBA600-20% exhibited approximately 70% of the compressive strength recorded in M-Ref. A decline in early strength of cement-based systems containing SCBA has been reported in several studies [6, 32]. As hydration progressed, more pronounced strength increases were observed for SCBA600 mixes when compared to the reference mortar. At 3 days, both SCBA600 mortars displayed higher strength values than those of M-Ref, particularly M-SCBA600-20%, which showed a 22 MPa increase in relation to its 1-day compressive strength. There were no significant compressive strength differences between mortars containing 10 and 20% SCBA600 at this age. Similar findings were observed at 7 days of hydration. At 28 days of curing, the difference in compressive strength between the SCBA600 mixes and reference increased, corroborating the hydration results. In this case, the moderate pozzolanicity of SCBA promoted portlandite consumption and pore refinement.
Fig. 6.
Compressive strength versus curing time for all mortars.
5. Conclusions
This study investigated the properties of SCBA produced in combined recalcination and controlled grinding procedures. Based on the results, the main conclusions can be summarized as follows:
-
⁃
The production of SCBA600 by combined burning and low-energy grinding-classification generated an ash with properties typical of a pozzolan;
-
⁃
Isothermal calorimetry tests indicated differences in hydration kinetics and heat release in pastes containing SCBA600. The ash caused a significant delay in the induction period due to the presence of contaminants;
-
⁃
The TGA results indicated lower portlandite contents in pastes containing SCBA600 due to the dilution effect and pozzolanic reaction of the ash;
-
⁃
SCBA600 significantly improved paste properties, with clear evidence of a pozzolanic effect and pore structure refinement;
-
⁃
SCBA600 in mortars reduced compressive strength at 1 day of curing due to the retarding effect of the ash. After 3 days, significant gains in compressive strength were observed in mortars containing SCBA600. The best mechanical performance of mortars containing ash was observed at 28 days, demonstrating the pozzolanic nature of SCBA600.
Declarations
Author contribution statement
Guilherme Chagas Cordeiro & Pryscila Vinco Andreão: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Luís Marcelo Tavares: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. This work was also supported by the Brazilian Agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). Pryscila Vinco Andreão was supported by the ELAP scholarship made available by Global Affairs Canada through the Department of Foreign Affairs, Trade and Development (DFATD).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Guilherme Chagas Cordeiro, Email: gcc@uenf.br.
Luís Marcelo Tavares, Email: tavares@metalmat.ufrj.br.
References
- 1.Cordeiro G.C., Kurtis K.E. Effect of mechanical processing on sugar cane bagasse ash pozzolanicity. Cement Concr. Res. 2017;97:41–49. [Google Scholar]
- 2.Ganesan K., Rajagopal K., Thangavel K. Evaluation of bagasse ash as supplementary cementitious material. Cement Concr. Compos. 2007;29(6):515–524. [Google Scholar]
- 3.Cordeiro G.C., Toledo Filho R.D., Fairbairn E.M.R. Use of ultra-fine sugar cane bagasse ash as mineral admixture for concrete. ACI Mater. J. 2008;105(5):487–493. [Google Scholar]
- 4.Chusilp N., Jaturapitakkul C., Kiattikomol K. Utilization of bagasse ash as a pozzolanic material in concrete. Constr. Build. Mater. 2009;23(11):3352–3358. [Google Scholar]
- 5.Cordeiro G.C., Toledo Filho R.D., Fairbairn E.M.R. Effect of calcinations temperature on the pozzolanic activity of sugar cane bagasse ash. Constr. Build. Mater. 2009;23(10):3301–3303. [Google Scholar]
- 6.Cordeiro G.C., Paiva O.A., Toledo Filho R.D., Fairbairn E.M.R., Tavares L.M. Long-term compressive behavior of concretes with sugar cane bagasse ash as a supplementary cementitious material. J. Test. Eval. 2018;46(2):564–573. [Google Scholar]
- 7.Cordeiro G.C., Toledo Filho R.D., Tavares L.M., Fairbairn E.M.R. Experimental characterization of binary and ternary blended-cement concretes containing ultrafine residual rice husk and sugar cane bagasse ashes. Constr. Build. Mater. 2012;29:641–646. [Google Scholar]
- 8.Bahurudeen A., Kanraj D., Dev V.G., Santhanam M. Performance evaluation of sugarcane bagasse ash blended cement in concrete. Cement Concr. Compos. 2015;59:77–88. [Google Scholar]
- 9.De Souza L.M.S., Fairbairn E.M.R., Toledo Filho R.D., Cordeiro G.C. Influence of initial CaO/SiO2 ratio on the hydration of rice husk ash-Ca(OH)2 and sugar cane bagasse ash-Ca(OH)2 pastes. Quim. Nova. 2014;37(10):1600–1605. [Google Scholar]
- 10.Rahmat N., Sabali M.A., Sandu A.V., Sahiron N., Sandu I.G. Study of calcination temperature and concentration of NaOH effect on crystallinity of silica from sugarcane bagasse ash (SCBA) Rev. Chim.-Bucharest. 2016;67(9):1872–1875. [Google Scholar]
- 11.Cordeiro G.C., Barroso T.R., Toledo Filho R.D. Enhancement the properties of sugar cane bagasse ash with high carbon content by a controlled re-calcination process. KSCE J. Civ. Eng. 2018;22(4):1250–1257. [Google Scholar]
- 12.Cordeiro G.C., Toledo Filho R.D., Tavares L.M., Fairbairn E.M.R. Ultrafine grinding of sugar cane bagasse ash for application as pozzolanic admixture in high-performance concrete. Cement Concr. Res. 2009;39(2):110–115. [Google Scholar]
- 13.Cordeiro G.C., Tavares L.M., Toledo Filho R.D. Improved pozzolanic activity of sugar cane bagasse ash by selective grinding and classification. Cement Concr. Res. 2016;89:269–275. [Google Scholar]
- 14.UNdata (Food and Agriculture Organization) 2019. http://data.un.org/
- 15.ABNT (Technical Standards Brazilian Association) 2006. Portland Cement for Cementing of Oil Wells – Requirements and Testing Methods. NBR 9831, Rio de Janeiro, Brazil. [Google Scholar]
- 16.ABNT (Technical Standards Brazilian Association) 2015. Pozzolanic Materials. NBR 12653, Rio de Janeiro, Brazil. [Google Scholar]
- 17.Bond F.C. The third theory of comminution. Trans. AIME. 1952;193:484–494. [Google Scholar]
- 18.ABNT (Technical Standards Brazilian Association) 1984. Soil Grains Passing through Sieve of 4.8 mm - Determination of Density. NBR 6508, Rio de Janeiro, Brazil. [Google Scholar]
- 19.ABNT (Technical Standards Brazilian Association) 2010. Pozzolanic Materials – Determination of Calcium Hydroxide Fixed – Modified Chapelle’s Method. NBR 15895, Rio de Janeiro, Brazil. [Google Scholar]
- 20.Kantro D.L. Influence of water reducing admixtures on properties of cement paste a miniature slump test. Cem. Concr. Aggregates. 1980;2(2):95–102. [Google Scholar]
- 21.Rocha C.A.A., Cordeiro G.C., Toledo Filho R.D. Use of thermal analysis to determine the hydration products of oil well cement pastes containing NaCl and KCl. J. Therm. Anal. Calorim. 2015;122(3):1279–1288. [Google Scholar]
- 22.Nehdi M.L., Suleiman A.R., Soliman A.M. Investigation of concrete exposed to dual sulfate attack. Cement Concr. Res. 2014;64:42–53. [Google Scholar]
- 23.ABNT (Technical Standards Brazilian Association) 2019. Portland Cement - Determination of Compressive Strength of Cylindrical Test Specimens. NBR 7215, Rio de Janeiro, Brazil. [Google Scholar]
- 24.Cordeiro G.C., Toledo Filho R.D., Tavares L.M., Fairbairn E.M.R. Pozzolanic activity and filler effect of sugar cane bagasse ash in Portland cement and lime mortars. Cement Concr. Compos. 2008;30(5):410–418. [Google Scholar]
- 25.Jankovic A., Valery W., Davis E. Cement grinding optimization. Miner. Eng. 2004;17:1075–1081. [Google Scholar]
- 26.Andreão P.V., Suleiman A.R., Cordeiro G.C., Nehdi M.L. Sustainable use of sugarcane bagasse ash in cement-based materials. Green Mater. 2019;7(2):61–70. [Google Scholar]
- 27.Sales A., Lima S.A. Use of Brazilian sugarcane bagasse ash in concrete as sand replacement. Waste Manag. 2010;30(6):1114–1122. doi: 10.1016/j.wasman.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Singh N.B., Singh V.D., Rai S. Hydration of bagasse ash-blended Portland cement. Cement Concr. Res. 2000;30(9):1485–1488. [Google Scholar]
- 29.Taylor H.F.M. second ed. Academic Press; London: 1997. Cement Chemistry. [Google Scholar]
- 30.Mostafa N.Y., Brown P.W. Heat of hydration of high reactive pozzolans in blended cements: isothermal conduction calorimetry. Thermochim. Acta. 2005;435(2):162–167. [Google Scholar]
- 31.Ramachandran R.V.S. first ed. Chemical Publishing Company; New York: 1969. Applications of Differential Thermal Analysis in Cement Chemistry. [Google Scholar]
- 32.Somna R., Jaturapitakkul C., Rattanachu P., Chalee W. Effect of ground bagasse ash on mechanical and durability properties. Mater. Des. 2012;36:597–603. [Google Scholar]