Abstract
Background
Berberine (BBR), a natural alkaloid isolated from Coptis chinensis, has frequently been reported as an antidiabetic reagent, partly due to its lipid-lowering activity. Evidence suggests that BBR ameliorates palmitate-induced lipid deposition and apoptosis in renal tubular epithelial cells (TECs), which tracks in tandem with the enhancement of peroxisome proliferator-activated receptor a (PPAR-a). The study aim was to investigate the roles of BBR in renal lipotoxicity in vitro, and investigate whether PPAR-a was the underlying mechanism.
Material/Methods
Human TECs (HK-2 cells) were injured with palmitic acid (PA), and then treated with BBR, BBR+PPAR-a inhibitor (GW6471), and PA+PPAR-a agonist (fenofibrate). Endoplasmic reticulum (ER) stress was assessed by measuring the expression of prospective evaluation of radial keratotomy (PERK), C/EBP-homologous protein (CHOP), and 78 kDa glucose-regulated protein (GRP78). Lipid metabolism was assessed by determining lipid anabolism-associated genes, including fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC), and lipoprotein lipase (LPL), as well as lipid catabolism-associated gene, including carnitine palmitoyl transferase 1 (CPT1). Inflammatory response of HK-2 cells was evaluated by measuring interleukin (IL)-6 and tumor necrosis factor (TNF)-a. Cell apoptosis and protein levels of cleaved-caspase-3 were evaluated.
Results
PA downregulated PPAR-a and induced server lipotoxicity in HK-2 cells by ER stress, increasing lipid deposition, and elevating inflammatory response of HK-2 cells accompanied with inducting cell apoptosis and cleaved-caspase-3, which were obviously reversed by additional treatment of BBR or PPAR-a agonist. However, the protective effect of BBR in PA-induced lipotoxicity in HK-2 cells was significantly ameliorated by PPAR-a inhibitor.
Conclusions
BBR attenuated PA-induced lipotoxicity via the PPAR-a pathway.
MeSH Keywords: Lipid Regulating Agents, Medicine, Molecular Biology
Background
Increased storage of lipids in renal tubular epithelial cells (TECs) or glomerular mesangial cells contributes to kidney injury, kidney dysfunction, and tubulointerstitial fibrosis through the process of lipotoxicity [1]. Lipotoxicity is a metabolic syndrome that arises from the accumulation of lipid intermediates [2,3], and is believed to have a role in chronic kidney disease (CKD) [4]. Thus, a better understanding of lipid metabolism at a molecular level and exploring lipid-lowering agents might represent therapeutic strategy to block lipotoxicity and protect human renal functions.
Berberine (BBR), an alkaloid extracted from Coptis chinensis, has a significant effect of decreasing blood lipid, including cholesterol, triglycerides, and low-density lipoprotein-cholesterol [5], and has been involved in clinical therapy of abnormal lipid metabolism-associated CKDs, including diabetic nephropathy [6]. Some studies have demonstrated that BBR ameliorates renal lipid accumulation, probably through accelerating fatty acid oxidation (FAO) [7]. However, lipid-lowering mechanisms of BBR have not been completely understood.
Peroxisome proliferator-activated receptor a (PPAR-a), an FAO-regulating protein, is a central regulator in fatty acid metabolism. Deficiency in PPAR-a contributes to overloading lipid-associated tubular injury, while agonist of PPAR-a leads to the opposite [8]. Evidence suggests that BBR promotes PPAR-a, and subsequently influences FAO in palmitate-injured TECs [7]. However, whether and how PPAR-a involved in renal protective effect of BBR in TECs remained largely unknown. In the present study, palmitic acid (PA)-induced lipotoxicity in HK-2 cells were established, and then lipotoxicity was assessed by determining endoplasmic reticulum (ER) stress, lipid accumulation, the release of inflammatory cytokines, and the apoptosis of HK-2 cells. BBR, GW6471 (PPAR-a inhibitor) and fenofibrate (PPAR-a agonist) were used for treatment. Our results demonstrated a promising strategy targeting PPAR-a to treating lipotoxicity in TECs.
Material and Methods
Cell culture and treatment
Culture medium for HK-2 cells (ATCC, Manassas, VA, USA) was Dulbecco’s Modified Eagle Medium (DMEM)/F12 (SH30023.01B, Hyclone) with 10% fetal bovine serum (FBS; 16000-044, Gibco, USA) and 100 U/mL penicillin (Solarbio, Beijing, China) added. Under 5% CO2 at 37°C, HK-2 cells grew to 80% confluency, seeded in a 96-plated well (4×103 cells/well in 100 μL of cultured medium), and then continued to be cultured for 12 hour.
To study the involvement of PPAR-a in the anti-lipotoxicity effect of BBR on HK-2 cells induced by PA, HK-2 cells, stimulated with 0.1 mM of PA (P5585-10G, Sigma), were treated with BBR, BBR+5 μM of PPAR-a inhibitor GW6471 (G5045-5MG, Sigma), or 10 μM of PPAR-a agonist fenofibrate (F6020-5G, Sigma). BBR, fenofibrate, and GW6471 were added to culture medium of HK-2 cells as a solution in dimethyl sulfoxide (DMSO) with final dosage of DMSO in culture medium (v/v) of 0.1%, 0.01%, and 0.05%.
Cell proliferation analysis
To confirm the concentration of BBR used, cells were treated with BBR (B139120, Aladdin) at a dose of 0, 1, 5, 10, 50, and 100 μM, and then proliferation at 24 hours was assessed using Cell Counting Kit-8 (CCK-8) (CP002, SAB), according to the manufacturer’s instructions.
Flow cytometry analysis
After treatment, the apoptotic rate in the Control, PA, PA+BBR, PA+BBR+PPAR-a inhibitor and PA+PPAR-a agonist groups was determined, using Annexin V-FITC apoptosis detection kit (C1062, Beyotime, Shanghai, China). Briefly, cells in darkness were maintained with Annexin V-FITC (5 μL) followed by propidium iodide (PI) for 15 minutes, respectively. Flow cytometry (BD Biosciences, USA) was used for analysis, and apoptotic HK-2 cells (Annexin V+/PI−) were seen in the lower right quadrant.
Enzyme-linked immunosorbent assay (ELISA) assay
Enzyme-linked immunosorbent assay (ELISA) was conducted to assess interleukin (IL)-6 and tumor necrosis factor (TNF)-a in cultured supernatants, using 96T human IL-6 ELISA kit (Catalog Number XY-E10140) and 96T human TNF-a ELISA kit (Catalog Number XY-E10110), respectively, according to the supplier’s protocols (X-Y Biotechnology Co., Ltd., Hangzhou, China).
Both human IL-6 ELISA kit and TNF-a ELISA kit had high sensitivity (ranged 0.8 to 20 ng/L and 20 to 400 ng/L, respectively) and excellent specificity for detection of human IL6 or TNF-a with no significant cross-reactivity or interference being observed.
Western blot analysis
Bicinchoninic acid (BCA) protein assay kit (Thermo, Shanghai, China) was adopted to quantify total protein, and 50 μg of which was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoretic pure including PPAR-a, fatty acid synthase (FAS), acetyl-CoA carboxylase polyclonal (ACC), lipoprotein lipase (LPL), carnitine palmitoyl transferase 1 (CPT1), cleaved-caspase-3, prospective evaluation of radial keratotomy (PERK), C/EBP-homologous protein (CHOP), 78 kDa glucose-regulated protein (GRP78), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were transferred to nitrocellulose membranes (Millipore, USA), and then incubated with antibody against PPAR-a (ab24509, Abcam, dilution 1: 1000), anti-FAK antibody (ab128856, Abcam, dilution 1: 1000), anti-ACC antibody (PA5-17564, Invitrogen, dilution 1: 1000), anti-LPL antibody (ab21356, Abcam, dilution 1: 1000), anti-CPT1 antibody (Ab107425, Abcam, dilution 1: 100), antibody against cleaved-caspase-3 (Ab32351, Abcam, dilution 1: 5000), anti-PERK antibody (PA5-15305, Invitrogen, dilution 1: 1000), anti-GRP78 antibody (Ab22410, Abcam, dilution 1: 1000), antibody against CHOP (Ab11419, Abcam, dilution 1: 2000) and anti-GAPDH antibody (#5174, CST, dilution 1: 2000) at 4°C overnight followed by secondary antibodies (A0208 and A0216, Beyotime, dilution 1: 1000) for 1 hour at 25°C. Primary antibodies were diluted in blocking solution (5% nonfat milk) and secondary antibodies were diluted in Tris-buffered saline with Tween 20 (TBST solution, pH=8.0) prior to use. Immunoreactive bands of PPAR-a, FAS, ACC, LPL, CPT1, PERK, GRP78, CHOP, and GAPDH were qualified and quantitated by electrochemiluminescent (ECL) system (GE Healthcare/Amersham Biosciences) with ImageJ software (NIH, Bethesda, USA).
Real-time polymerase chain reaction (RT-PCR)
Total RNA within HK-2 cells in Control, PA, PA+BBR, PA+BBR+PPAR-a inhibitor and PA+PPAR-a agonist groups was acquired using TRIzol regent (Invitrogen), and reverse transcribed using cDNA synthesis kit (Fermentas). The mRNA levels of PPAR-a, FAS, ACC, LPL, CPT1, PERK, GRP78, CHOP, and GAPDH were quantitative analysis by real-time polymerase chain reaction (RT-PCR) using SYBR Green PCR Kit (Thermo) with ABI Prism 7300 SDS Software (Applied Biosystem, Foster City, CA, USA). The primer sequences are presented in Table 1.
Table 1.
Primers used in real-time polymerase chain reaction analysis.
| Name | GenBank | Primer (5′-3′) |
|---|---|---|
| PPAR-α | NM_001001928.3, at 1687–1872 position | Forward: CTGAAGCTGACAGCACTAC; |
| Reverse: TGAGATTAGCCACCTACCC; 186 bps | ||
|
| ||
| FAS | NM_000043.5, at 3138–3286 position | Forward: TTCCCTCCTGTGTTATGG; |
| Reverse: ACTTGCCCTACTTCTGTC; 149 bps | ||
|
| ||
| ACC | NM_198834.2, at 9573–9677 position | Forward: CTTCAGAGGCAGGGTGGGTTAC; |
| Reverse: GGGAGGAGGCATTACAGGGTTC; 104 bps | ||
|
| ||
| LPL | NM_000237.2, at 1178–1299 position | Forward: CGCTCCATTCATCTCTTCATC; |
| Reverse: CAGCGGTTCTTTCTACAACTC; 122 bps | ||
|
| ||
| CPT1 | NM_001031847.2, at 1166–1414 position | Forward: GCACATCGTCGTGTACCATC; |
| Reverse: GCTGCTTTCTCCACAGCATC; 249 bps | ||
|
| ||
| PERK | NM_001313915.1, at 291–535 position | Forward: TCATCCAGCCTTAGCAAAC; |
| Reverse: CCCAGAGCTGAACAGATATAC; 245 bps | ||
|
| ||
| GRP78 | NM_005347.4, at 450–584 position | Forward: GTCCTATGTCGCCTTCACTC; |
| Reverse: ACAGACGGGTCATTCCAC; 135 bps | ||
|
| ||
| CHOP | NM_001195053.1, at 640–830 position | Forward: AACCAGGAAACGGAAACAG; |
| Reverse: TCACCATTCGGTCAATCAG; 191 bps | ||
|
| ||
| GAPDH | NM_001256799.1, at 436–653 position | Forward: AATCCCATCACCATCTTC; |
| Reverse: AGGCTGTTGTCATACTTC; 218 bps | ||
RT-PCR – real-time polymerase chain reaction; PPAR – peroxisome proliferator-activated receptor a; FAS – fatty acid synthase; ACC – acetyl-CoA carboxylase; LPL – lipoprotein lipase; CPT1 – carnitine palmitoyl transferase 1; PERK – prospective evaluation of radial keratotomy; GRP78 – 78 kDa glucose-regulated protein; CHOP – C/EBP-homologous protein; GAPDH – glyceraldehyde-3-phosphate dehydrogenase.
Statistics and data analysis
Data was showed as mean ± standard error of the mean (SEM), calculated from 3 parallel results in each experiment. Comparison between Control and PA groups, PA and PA+BBR groups, PA and PA+PPAR-a agonist groups as well as PA+BBR and PA+BBR +PPAR-a inhibitor groups was conducted using one-way ANOVA with post-hoc Tukey test, and P<0.05 was considered to be of statistical significance.
Results
BBR attenuated PA-induced endoplasmic reticulum (ER) stress in HK-2 cells via upregulating PPAR-a
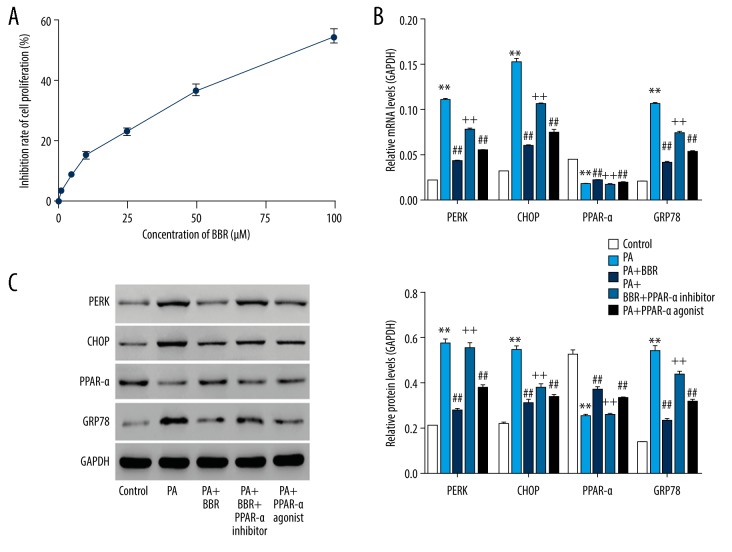
In 2011, Gao et al. investigated the protective effect of BBR on PA-induced lipotoxicity in HIT-T15 cells with the dose of 0 to 100 μM [9]. In our present study, HK-2 cells were treated with vehicle (DMSO) or BBR (1, 5, 10, 50, and 100 μM) (Figure 1A). Then 24 hours later, proliferation at 24 hours was assessed using CCK-8 method. Our data suggested that BBR dose-dependently repressed HK-2 cell proliferation, thus, we chose 100 μM of BBR for the following study. After treatment for 24 hours, expression of PPAR-a was assessed in the Control, PA, PA+BBR, PA+BBR+PPAR-a inhibitor, or PA+PPAR-a agonist groups. Figure 1B and 1C showed that expression level of PPAR-a was significantly reduced by PA, confirming the involvement of PPAR-a in PA-induced lipotoxicity in HK-2 cells. Interestingly, both BBR and PPAR-a agonist significantly enhanced PPAR-a in PA-injured HK-2 cells. However, BBR-induced enhancement of PPAR-a in PA-injured HK-2 cells was significantly reversed by additional treatment of PPAR-a inhibitor.
Figure 1.
BBR ameliorated PA-induced ER stress in HK-2 cells via PPAR-a pathway. (A) Cell proliferation was assessed after 24 hours of BBR exposure (0, 1, 5, 10, 50, and 100 μM), using method CCK-8. HK-2 cells, stimulated with PA (0.1 mM), were treated with 100 μM of BBR, BBR+10 μM of PPAR-a inhibitor (GW6471), and 5 μM of PPAR-a agonist (fenofibrate), respectively. After treatment at 24 hours: (B) mRNA and (C) protein levels of PERK, CHOP, PPAR-a, and GRP78 were assessed by RT-PCR and western blot, respectively. ** P<0.01 versus Control; ## P<0.01 versus PA; ++ P<0.01 versus PA+BBR. BBR – berberine; PA – palmitic acid; ER – endoplasmic reticulum; PPAR – peroxisome proliferator-activated receptor a; CCK-8 – Cell Counting Kit-8; PERK – prospective evaluation of radial keratotomy; CHOP – C/EBP-homologous protein; GRP78 – 78 kDa glucose-regulated protein; RT-PCR – real-time polymerase chain reaction.
Endoplasmic reticulum (ER) stress was magnified on PA-induced hepatocyte lipotoxicity [10]. Figure 1B and 1C showed that expression levels of PERK, GRP78, and CHOP (3 ER stress hallmarks) were increased by PA while suppressed following BBR or PPAR-a agonist treatment, indicating that BBR and PPAR-a agonist ameliorated PA-induced ER stress in HK-2 cells. However, BBR-induced the enhancement of ER stress in PA-injured HK-2 cells were significantly reversed by additional treatment of PPAR-a inhibitor, substantiating that upregulating PPAR-a was the underlying mechanism, whereby BBR exerted inhibitory effect on PA-induced ER stress in HK-2 cells.
BBR decreased PA-induced lipid accumulation in HK-2 cells via upregulating PPAR-a
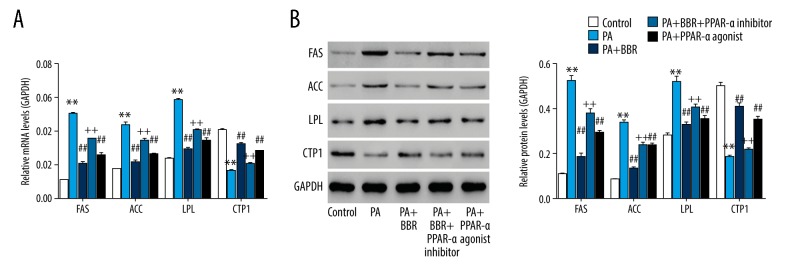
After treatment for 24 hours, expression levels of genes responsible for lipogenesis, including FAS, ACC, and LPL as well as gene related to lipolysis, including CPT1 were assessed [11]. Figure 2A and 2B showed that PA increased FAS, ACC and LPL while decreased CPT1, suggesting a severe lipid accumulation in HK-2 cells. PA-induced the changed expression of FAS, ACC, LPL, and CPT1 were significantly reversed by BBR or PPAR-a agonist. However, FAS, ACC, and LPL were significantly enhanced while CPT1 was reduced in PA+BBR+PPAR-a inhibitor group when compared with PA+BBR (all P<0.01), substantiating that upregulating PPAR-a was the underlying mechanism, whereby BBR exerted inhibitory effect on PA-induced lipid accumulation in HK-2 cells.
Figure 2.
BBR ameliorated PA-induced lipid accumulation in HK-2 cells via PPAR-a pathway. (A) mRNA and (B) protein levels of FAS, ACC, LPL, and CPT1 were assessed. ** P<0.01 versus Control; ## P<0.01 versus PA; ++ P<0.01 versus PA+BBR. BBR – berberine; PA – palmitic acid; PPAR – peroxisome proliferator-activated receptor a; FAS – fatty acid synthase; ACC – acetyl-CoA carboxylase; LPL – lipoprotein lipase; CPT1 – carnitine palmitoyl transferase 1.
BBR ameliorated PA-induced inflammation in HK-2 cells via upregulating PPAR-a
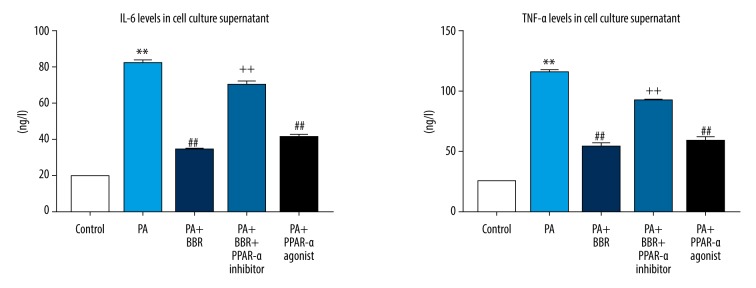
After treatment for 24 hours, concentrations of inflammatory cytokine IL-6 and TNF-a in cell cultured supernatant were assessed. Figure 3 shows that PA increased IL-6 and TNF-a, suggesting a severe inflammation. Interestingly, the release of IL-6 and TNF-a in PA-injured HK-2 cells was significantly suppressed by both BBR and PPAR-a agonist. However, BBR-induced those changes in PA-injured HK-2 cells were significantly reversed by additional treatment of PPAR-a inhibitor, substantiating that upregulating PPAR-a was the underlying mechanism, whereby BBR exerted inhibitory effect on PA-induced inflammation in HK-2 cells.
Figure 3.
BBR ameliorated PA-induced inflammation in HK-2 cells via PPAR-a pathway. After treatment at 24 hours, levels of IL-6 and TNF-a were assessed by ELISA. ** P<0.01 versus Control; ## P<0.01 versus PA; ++ P<0.01 versus PA+BBR. BBR – berberine; PA – palmitic acid; PPAR – peroxisome proliferator-activated receptor a; IL – interleukin; TNF – tumor necrosis factor; ELISA – enzyme-linked immunosorbent assay.
BBR ameliorated PA-induced apoptosis in HK-2 cells via upregulating PPAR-a
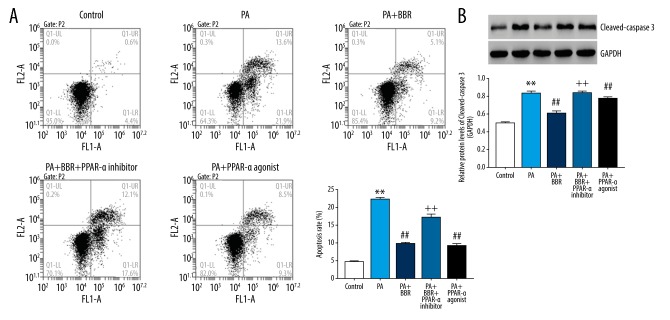
After treatment for 24 hours, cell apoptosis and expression of cleaved-caspase-3 were determined. The apoptotic rate was significant enhanced in the PA group (22.00±0.46%) when compared with the Control group (4.23±0.57%), demonstrating severe apoptosis in HK-2 cells induced by PA (Figure 4). BBR and PPAR-a agonist dropped the apoptotic rate to 9.53±0.42% and 16.7±1.15%, respectively, when compared to the PA group (22.00±0.46%). However, the promoted effect of BBR on apoptosis in the PA group were significantly reversed by additional treatment of PPAR-a inhibitor, substantiating that upregulating PPAR-a was the underlying mechanism, whereby BBR exerted inhibitory effect on PA-induced apoptosis in HK-2 cells. Furthermore, similar change treads of cleaved-caspase-3 expression was also observed in control, PA, PA+BBR, PA+BBR+PPAR-a inhibitor and PA+PPAR-a agonist groups, suggesting that BBR acted on PPAR-a to regulate the apoptosis of PA-injured HK-2 cells at a molecular level.
Figure 4.
BBR ameliorated PA-induced apoptosis in HK-2 cells via PPAR-a pathway. (A) Cell apoptosis assessed using flow cytometry; (B) protein levels of cleaved-caspase-3. ** P<0.01 versus Control; ## P<0.01 versus PA; ++ P<0.01 versus PA+BBR. BBR – berberine; PA – palmitic acid; PPAR – peroxisome proliferator-activated receptor a.
Discussion
Enhanced ER stress in TECs appears to be a consequence of lipid accumulation, and ultimately leads to cells apoptosis [12]. Furthermore, TECs display lipid accumulation frequently accompanied with the release of inflammatory cytokines, which exacerbates ER stress and accelerates tubulointerstitial fibrosis [13–15]. In this study, our data confirmed that PA significantly: 1) enhanced ER stress through increasing PERK, GRP78, and CHOP; 2) increased intracellular lipid accumulation as evidenced by obviously upregulating FAS, ACC, and LPL (lipogenesis genes) while downregulating CPTI (a lipolysis gene); 3) contributed to inflammation by promoting the release of IL-6 and TNF-a; and 4) promoted cell apoptosis and protein expression of cleaved-caspase-3, suggesting a severe lipotoxicity induced by PA in HK-2 cells.
Evidence suggests that BBR inhibits PA-induced lipid accumulation and apoptosis in TECs [7], which was confirmed in our present study (Figures 2, 4). Prolonged ER stress leads to increased apoptosis of TECs [16]. BBR suppresses ER stress, and thereby retards hypoxia/reoxygenation (H/R)-induced apoptosis of HK-2 cells [16]. Furthermore, elimination of ER stress ameliorates the production of pro-inflammatory cytokines in palmitate-induced lipotoxicity in cardiomyocytes [17]. Our data first suggested the inhibitory effect of BBR on ER stress and the release of pro-inflammatory cytokines in PA-injured HK-2 cells, implicating the possible involvement of ER stress pathway in protective effect of BBR on lipotoxicity-associated apoptosis and inflammation in TECs.
PPAR-a functioned in renal lipotoxicity, and PPAR-a agonist has become more and more popular as a potential agent for protecting renal function, likely through reducing lipid deposition, retarding inflammation, and fibrosis, as well as ameliorating renal apoptosis [18,19]. BBR has been shown to significantly enhance PPAR-a in PA-induced lipotoxicity in HK-2 cells [7], thus, we further investigated whether PPAR-a was the underlying mechanism. Similar to BBR, PPAR-a agonist (fenofibrate) increased the expression of PPAR-a, downregulated lipid accumulation, inflammation, and apoptosis, yet, exerted inhibitory effect on ER stress, substantiating the inhibitory effect of PPAR-a on lipotoxicity in HK-2 cells induced by PA. However, BBR induced the enhancement of PPAR-a expression, and the changes in the events associated with lipotoxicity were significantly reversed by additional treatment with PPAR-a inhibitor (GW6471), suggesting that the PPAR-a pathway was the mechanism by which BBR showed inhibitory effect on PA-induced lipotoxicity in HK-2 cells.
Concluisons
In summation, our data confirmed the inhibitory effect of BBR on PA-induced lipotoxicity in HK-2 cells through restricting ER stress, reducing lipid accumulation, alleviating the release of inflammatory factors, as well as attenuating apoptosis; and the mechanism was associated with the upregulation of PPAR-a. Moreover, targeting PPAR-a could provide a new possibility to ameliorate renal lipotoxicity and protect renal function in the further.
Footnotes
Source of support: The study was supported by Talent Training Plan Foundation of Shanghai Fifth People’s Hospital affiliated to Fudan University (2017WYRCJY08), Natural Science Foundation of Minhang District, Shanghai (2016MHZ13) and Key Hospital-Level Project of Shanghai Fifth People’s Hospital (2018WYZD04)
Conflict of interest
None.
References
- 1.Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens. 2010;19:393–402. doi: 10.1097/MNH.0b013e32833aa4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escasany E, Izquierdo-Lahuerta A, Medina-Gomez G. Underlying mechanisms of renal lipotoxicity in obesity. Nephron. :2019. doi: 10.1159/000494694. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Bosma M, Kersten S, Hesselink MK, Schrauwen P. Re-evaluating lipotoxic triggers in skeletal muscle: Relating intramyocellular lipid metabolism to insulin sensitivity. Prog Lipid Res. 2012;51:36–49. doi: 10.1016/j.plipres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Vaziri ND. Lipotoxicity and impaired high-density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J Ren Nutr. 2010;20:S35–43. doi: 10.1053/j.jrn.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: A systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79:437–46. doi: 10.1055/s-0032-1328321. [DOI] [PubMed] [Google Scholar]
- 6.Ni WJ, Ding HH, Tang LQ. Berberine as a promising anti-diabetic nephropathy drug: An analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–12. doi: 10.1016/j.ejphar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Chen X, Liu T, et al. Berberine protects against palmitate-induced apoptosis in tubular epithelial cells by promoting fatty acid oxidation. Med Sci Monit. 2018;24:1484–92. doi: 10.12659/MSM.908927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo N, Zheng X, Liu H, Ma X. Fenofibrate, a PPAR-alpha agonist, protect proximal tubular cells from albumin-bound fatty acids induced apoptosis via the activation of NF-kB. Int J Clin Exp Pathol. 2015;8:10653–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Gao N, Zhao TY, Li XJ. The protective effect of berberine on beta-cell lipoapoptosis. J Endocrinol Invest. 2011;34:124–30. doi: 10.1007/BF03347042. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Zhang H, Deng X, et al. Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation. Chem Biol Interact. 2017;278:189–96. doi: 10.1016/j.cbi.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Mi Y, Tan D, He Y, et al. Melatonin modulates lipid metabolism in HepG2 cells cultured in high concentrations of oleic acid: AMPK pathway activation may play an important role. Cell Biochem Biophys. 2018;76:463–70. doi: 10.1007/s12013-018-0859-0. [DOI] [PubMed] [Google Scholar]
- 12.Katsoulieris E, Malley JG, Samai M, et al. Alpha-linolenic acid protects renal cells against palmitic acid lipotoxicity via inhibition of endoplasmic reticulum stress. Eur J Pharmacol. 2009;623:107–12. doi: 10.1016/j.ejphar.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Peter A, Weigert C, Staiger H, et al. Induction of stearoyl-CoA desaturase protects human arterial endothelial cells against lipotoxicity. Am J Physiol Endocrinol Metab. 2008;295:E339–49. doi: 10.1152/ajpendo.00022.2008. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Wen Y, Lv LL, et al. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin. 2015;36:821–30. doi: 10.1038/aps.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piret SE, Olinger E, Reed AAC, et al. A mouse model for inherited renal fibrosis associated with endoplasmic reticulum stress. Dis Model Mech. 2017;10:773–86. doi: 10.1242/dmm.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu W, Sheng M, Xu R, et al. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J Transl Med. 2013;11:24. doi: 10.1186/1479-5876-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Zhang L, Ni R, et al. Disruption of calpain reduces lipotoxicity-induced cardiac injury by preventing endoplasmic reticulum stress. Biochim Biophys Acta. 2016;1862:2023–33. doi: 10.1016/j.bbadis.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balakumar P, Kadian S, Mahadevan N. Are PPAR alpha agonists a rational therapeutic strategy for preventing abnormalities of the diabetic kidney? Pharmacol Res. 2012;65:430–36. doi: 10.1016/j.phrs.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Lin S, Zhang L, Li Y. Resveratrol prevents renal lipotoxicity in high-fat diet-treated mouse model through regulating PPAR-alpha pathway. Mol Cell Biochem. 2016;411:143–50. doi: 10.1007/s11010-015-2576-y. [DOI] [PubMed] [Google Scholar]