Abstract
The traditional requirement for clean rooms and specialized skills has inhibited many biologists from pursuing new microfluidic innovations. Makerspaces provide a growing alternative to clean rooms: they provide low-cost access to fabrication equipment such as laser cutters, plotter cutters, and 3D printers; use commercially available materials; and attract a diverse community of product designers. This opinion discusses the materials, tools, and building methodologies particularly suited for developing novel microfluidic devices in these spaces, with insight into biological applications and leveraging the maker community. The lower barrier to access of makerspaces ameliorates the otherwise poor accessibility and scalability of microfluidic prototyping.
Keywords: Microfluidics, Makerspaces, Laser Cutting, Plotter Cutting, Soft Lithography, Clean Room
Microfluidics and the market
Over the past few decades, thousands of novel microfluidic point-of-care (POC, see Glossary) diagnostic platforms and applications have been published in peer-reviewed journals; however, a low percentage have reached market [1]. Even with large investments from government and industry in both Europe and North America, surprisingly few lab-on-a-chip (LOC) microfluidic diagnostic tests have translated to commercial products [2]. This discrepancy somewhat constrains the potential market for these devices, which is expected to grow from $1.6 billion in 2013 to $3.6 – $5.7 billion by 2018; the key driver of this growth is the need for early detection and personalized treatment of lifestyle diseases, which have become more prominent within a growing geriatric population [3,4].
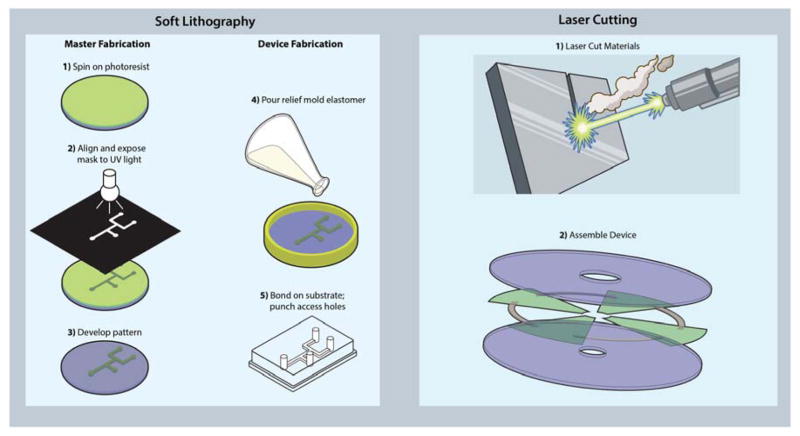
Thus far, the field of POC microfluidic diagnostics has been predominantly addressed in academia with polydimethylsiloxane (PDMS) devices manufactured using soft lithography techniques, originally popularized by the Whitesides group [5,6]. A brief review of soft lithography microfluidics is presented in Box 1 [6–15]. The focus of this Opinion will be the discussion of microfluidic prototyping that takes place in makerspaces rather than clean rooms. Alternative rapid prototyping methods that take advantage of these materials for microfluidics have been reviewed previously [16]. For example, laser cutting can be used to cut microfluidic channels in double-sided pressure sensitive adhesive (PSA) [17], to directly ablate microfluidic channels in polymer materials [18], and even to create molds for PDMS from laser cut adhesive [19]. Plotter cutting, also known as xurography, uses a drag knife printer to cut microfluidic designs from laminate and masking films [20–22]. Xurography has even been employed to directly cut microfluidic channels in PDMS and cyclic olefin copolymer films [23,24]. 3D printing technologies have also begun to show promise for microfluidic device fabrication [25–27]. While these methods do not provide the superior resolution of photolithographic methods, the use of plastic, paper, and laminate substrates are more translatable to scalable manufacturing methods—such as die cutting, hot embossing and injecting molding—to translate a finished prototype into a commercial product. An example of a rapid prototyping method amenable to scaled-up manufacturing is laser cutting. Figure 1 shows a comparison device prototyping using of soft lithography methods versus laser cutting of plastics, laminates, and paper.
Box 1. Soft Lithography Microfluidics – Pros and Cons.
Soft lithography methods for microfluidics create ‘master’ molds from photolithography techniques followed by curing of a pre-polymer (PDMS) on top of the mold master, where after curing, a PDMS negative stamp of the mold is created and bonded irreversibly to glass (Figure 1). Soft lithography techniques have proven useful in microfluidics under a wide range of applications from channel fabrication to pattern generation [7]. The key benefit of soft lithography methods is the ability to rapidly prototype [8]. The technique is ideal for biological applications because the feature resolution can match the micrometer and even nanometer feature sizes often found in biology. The PDMS polymer provides an ideal candidate for microfluidic devices as it is nontoxic, widely available, transparent, hydrophobic, gas-permeable, and elastomeric [6,9]. Oxidized PDMS surfaces can be irreversibly bonded together by a spontaneous dehydration of SiOH groups and PDMS can be passivated and functionalized through various chemistries for high efficiency molecular assays. The flexibility of the PDMS polymer enables a wide variety of geometries, layering, and unit operations applicable to a plethora of unique microfluidic manipulations [6].
On the other hand, the photo- and soft lithography methods used to create these devices suffer from the nature of artisanal and resource-consuming processes (pour, cure, cut, punch, and bond) as opposed to the traditional industry-standard injection molding process, where a mold is filled, the polymer is rapidly cured, and the part is ejected. Contract manufacturers, such as FlowJEM (Ontario, Canada) and SIMTech Microfluidics Foundry (Singapore), alsp perform soft lithography prototyping and can provide custom molds for a fee ($100 – $200 for a single layer SU-8 mold depending on the design); however, the design process is slowed down waiting for molds to be manufactured and shipped. While PDMS devices may be well-suited for the research setting, the lack of scalability in soft lithography and the high cost of PDMS (relative to cost-efficient thermoplastics) has limited commercial potential [10]. A technology map developed by Chin et al. shows how virtually none of the major players in the microfluidic in vitro diagnostics market use PDMS in their products, instead leaning towards plastic, glass, or paper materials, which can be more easily mass-manufactured through processes such as injection molding, casting, and die cutting respectively [11]. These common manufacturing materials and methods offer additional benefits such as standardization of fabrication, improving quality control, and better integration with other parts made of similar material [11,12]. A wide variety of advances in microfluidics manufacturing, materials, functions, and operations have yielded a powerful toolkit to enable plastic microfluidic development for a plethora of applications [13–15].
Figure 1.
Rapid prototyping using soft lithography vs. laser cutting. (Left) The multi-step process of soft lithography, wherein first a ‘master mold’ is developed followed by curing a pre-polymer substrate above, peeling off, bonding to a substrate, and punching access holes. (Right) The more straightforward process of laser-cutting all device parts followed by lamination or thermal bonding to assemble a device.
Makerspaces, DIY biology, and integrated thinking
The investigation of these ‘alternative’ materials is well-suited for exploration in the emerging ecosystem of community ‘makerspaces’ [28]. In the broadest sense, makerspaces are physical spaces, usually accessible to the public, where communities are able to access tools—spanning additive and subtractive techniques—for fabricating “almost anything” [29]. Such spaces can be formalized as part of an organization like the Fab Lab network (www.fabfoundation.org), or more informally organized. With over one thousand active spaces around the world, makerspaces have lowered the barrier to accessing fabrication technologies, enabling the exploration of microfluidic rapid prototyping techniques reviewed in this work.
In the past several years, there has also been a growing movement of “do-it-yourself” (DIY) biology and similar emergence of “bio-makerspaces” [30], which typically feature tools and basic infrastructure for conducting molecular biology and microbiology projects. As the majority of applications for microfluidics have involved biological systems, we believe the reviewed techniques will also be of interest, and accessible, to DIY biology communities as well.
A key factor in the shift of microfluidic manufacturing from traditional photolithographic methods to ‘maker manufacturing’ is the push for fully integrated microfluidic systems that can be readily translated to industry. A major roadblock for lab-on-a-chip devices is plugging and sealing the device to all the interfaces needed (e.g., detection, electric manipulation, and inlets/outlets) [31]. For example, Lafluer et al. used 3D-printed and paper substrates to develop an entirely integrated sample-to-result nucleic acid amplification test [32]. Kinahan et al. used laser-cut acrylic and double-sided pressure sensitive adhesive (PSA) to develop an integrated bi-plex liver assay [33]. These technologies show off the power of ‘simple’ devices that anyone can make and rapidly scale to bulk manufacturing. To enable others to take part in this type of product design and development, we review the materials and tools used by current researchers to develop these platforms.
Maker microfluidics manufacturing
This section reviews the development of microfluidic platforms using simple materials and manufacturing equipment often found in makerspaces. While microfluidics can be made from of a wide variety of materials and methods, this Opinion focuses on plastics, adhesives, and paper substrates with a brief discussion of the promise of 3D-printed microfluidics.
Materials
Plastics are a popular material choice for microfluidics as they collectively offer a wide variety of desirable properties including optical clarity, solvent resistance, and scalable manufacturing methods, which have been reviewed previously [34]. Studies have shown promise for polymeric materials with regard to biocompatibility [35], surface modification and integration of functional materials [36], and material autofluorescence [37,38]. Acrylic is one of the simplest and most useful plastics for the makerspace because of its low cost, high optical clarity, wide availability, and compatibility with a wide variety of manufacturing tools such as laser cutters. Similar plastics, such as polycarbonate, may be desired for even greater optical clarity and standardization in large-scale manufacturing; however, polycarbonate cannot be cut on a conventional laser cutter, and specialty contract manufacturers, such as Axxicon (http://axxicon.com), often require large bulk orders to make a profit. For spaces without a laser cutter, materials can be shipped pre-cut by laser cutting services such as Ponoko (www.ponoko.com) at a low cost with no minimum order. For example, a custom pre-laser cut sheet of 800 mm × 400 mm clear acrylic (1.5 mm thickness) costs approximately $50 plus shipping.
Cut double-sided adhesive tapes are ideal materials for bonding microfluidic architecture to substrates. Laser-cut microchannels in adhesive tape can be sandwiched between two pieces of plastic with access ports to serve as liquid reservoirs for a wide variety of biological applications such as a cell sorter for stem cells, a mammalian cell chemostat, a cytotoxicity assay system and even in vitro organ models [39]. Selecting a tape adhesive can be a daunting task considering the expansive selection from companies such as 3M (www.3m.com) and Adhesives Research (www.adhesivesresearch.com). The key considerations for selecting a tape are 1) fabrication considerations, 2) tape thickness, and 3) cost/availability. For fabricating a plastic device held together by double-sided thin-film adhesive, cutting microfluidic channels into the adhesive can be challenging if the product is not ‘double lined’, meaning both sides of the adhesive have a removable liner. While tape converter companies such as Converters Inc. (www.converters.com) offer to add a second liner, large minimum orders can be cost prohibitive. Converters can be avoided by purchasing tapes that already come with liner on both sides. Another consideration for adhesive selection is choosing between a transfer tape and a double-sided tape. Transfer tapes are entirely composed of adhesive material whereas traditional double-sided adhesive have a carrier layer coated on both sides with adhesive. Thus, transfer tapes are typically better suited for thinner applications (<50 μm) such as cellular and biochemical assays where reagent may be expensive, whereas double-sided adhesives are suited for thicker applications (50 – 200 μm) such as cell culture where media may need to be slowly perfused over cells with low shear. A final consideration is the cost and availability of the desired adhesive as the minimum order direct from 3M or Adhesives Research are typically on the range of 1500 foot rolls and can cost upwards of $10,000. Oftentimes, free samples of certain products are available or their products can be purchased in smaller amounts from distributors such as Grainger (www.grainger.com) and Amazon.com (www.amazon.com) depending on availability. Table S1 contains a list of adhesives appropriate for microfluidics.
Paper substrates gained renewed popularity in 2004 when the World Health Organization (WHO) declared specific performance criteria for developing POC, ultra-low cost diagnostics in low resource settings [40]. Which paper substrate is most appropriate depends on the context for its use in applications, which include nucleic acid and protein separation, immunoassays, and even cell culture [41–44]. GE Healthcare Life Sciences’ Whatman line (www.gelifesciences.com) offers a wide variety of paper substrates with thicknesses appropriate for integration into plastic/tape microfluidics and stand-alone devices. Table S2 contains a list of all of the paper substrates used by the authors along with comments to best help guide paper selection.
Tools
Laser and plotter cutting are two simple methods for cutting microfluidic channels in plastic, paper, and tape. Both of these methods are similar in workflow, feeding in a substrate to be cut by either a laser or knife. Laser cutters have the benefit of non-contact cutting and higher resolution. These benefits come at the expense of higher capital equipment costs, the requirement for a vacuum pump to clear out debris and fumes, and potential burn residue created during the cutting [45]. While material leaching of plastics and adhesives may pose problems for some sensitive biological assays, oftentimes burn products from laser cutting of particular materials have been shown to inhibit reactions such as PCR [46]. Plotter cutters (also commonly referred to as vinyl cutters or cutting plotters) are significantly cheaper, require no pumping system, and leave no burn residues. With the growing popularity of makerspaces in both academia and industry, many facilities now have these capabilities already available in a shared space. While other works directly compare results from these two cutting tools for microfluidics [45], Table S3 highlights the key differences between laser and plotter cutting.
Bridging applications
Before moving ahead to device design and prototyping, the first job is to identify which materials and tools best suit the biological application. We recommend keeping device design as simple as possible to avoid unnecessary sources of error that may invalidate an assay (e.g., too many individual steps). The simplest material for developing a device is often paper as its ability to be cut and directly wax-printed enable a variety of fluidic manipulations and assays to be performed. Paper has particular strengths for POC diagnostic immunoassays (i.e. lateral flow assays), PCR (i.e. isothermal PCR, sample preparation), and even for synthetic biology applications such as in-vitro transcription/translation. A primary weakness of paper is its opacity, which can occlude a weak signal from a fluorescent/colorimetric reporter and limit visual analyses. On the other hand, devices made from double-sided PSA with cut microfluidic channels sandwiched between plastic provide superior signal-to-nose ratios for fluorescent or colorimetric readouts as well as potential for high multiplexing through droplet microfluidic devices. Plastic and tape devices provide a viable platform for cellular assays, such as counting CD4+ T cells for HIV diagnostics from whole blood, immunophenotyping invasive cell types in vitreous fluid for ocular diagnostics, and isolating stem cells from a patient. Finally, for devices that may still require the beneficial properties of PDMS, such as gas-controlled cell chemostats, molds created from laser cut plastic or even 3D-printed materials can be used in place of traditional photolithography molds, although with the cost of reduced resolution.
Methodology
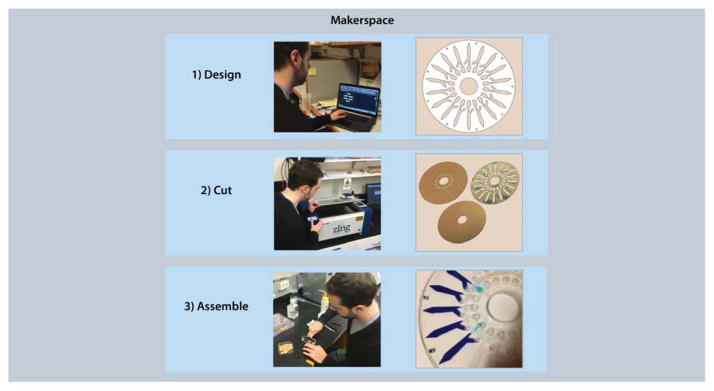
A simple and enabling methodology for maker microfluidics is design-cut-assemble, shown schematically in Figure 2. This method streamlines rapid prototyping of microfluidic devices using plastics, paper, and adhesive substrates and can be appropriately edited to incorporate different materials and technologies [47]. While more traditional material combinations such as a plastic-adhesive device may seem like an easy first step, more creative solutions may also be more efficient, such as a paper-adhesive microfluidic origami device [48]. Once the materials are chosen, a computer-aided design (CAD) file must be designed to guide the cutting process. Next, the substrates need to be cut using methods such as laser and plotter cutting. While this opinion focuses on laser and plotter cutting, wax printing and CNC-micromilling are viable alternatives. Briefly, wax printing methods are a popular and simple way to create hydrophobic patches on paper substrates to create microfluidic architecture [49]. Devices can be made as simply as using a wax-based ink printer and a hot plate to set the wax into the paper. CNC-micromilling can also be used to directly drill channels directly in plastic or drill a mold for PDMS casting [50]. Finally, once all of the parts are cut, assembly is typically completed by a manual process such as lamination, thermal bonding, or folding. A set of considerations for each step of this process is shown in Box 2.
Figure 2.
Design-cut-assemble methodology: designing device parts in CAD, cutting them out using a laser or plotter cutter, and assembling with lamination.
Box 2. Design-Cut-Assemble Considerations.
| Design Considerations | |
| Gas Permeability | While some plastic and adhesive materials such as polymethylpentene are gas permeable, most materials are not and may require venting ports |
| Inputs/Outputs | Connecting tubing to plastic microfluidics can prove challenging; consider a 3D printed connector, using ring magnets as gravity fed wells, or a PDMS block on top |
| Channel Volume | Designing microfluidic channels based on volume enables simpler protocols |
| Fiducial Marks | The addition of fiducial or registration marks play a vital role downstream in alignment for device assembly, imaging, and automation. Consideration should be made as to locations, accessibility, and orientation of fiducial markings at an early stage. |
| Fluidic Considerations | Consider the path of fluids through your device, for example sharp corners and rapid expansions can often hinder fluidic movement and lead to bubbles; also, gas permeable devices may lose fluid due to evaporation |
| Cut Considerations | |
| CAD Software Selection | Most CAD software can produce acceptable file formats for cutters (*.dxf, *.dwg), oftentimes cutters are directly compatible to select CAD software |
| Cutting Lines | Ensure that no lines are repeated in the drawing to prevent redundant cuts |
| Cutting Resolution | Best resolution can be achieved by keeping the material as flat as possible when cutting; use painter’s tape on edges of thin substrates to prevent blowing away on laser cutters or an adhesive backing to prevent unwanted skewing and bowing on plotter cutters |
| Cutting Force | Trial-and-error of laser power/speed and plotter knife force/speed/cut-style is important to get the best cut; an ideal cut for double-sided adhesive would only cut through the first liner and adhesive layer while keeping the bottom liner intact (which will prevent feature ‘droop’ during the assembly process) |
| Design vs. Cutting | While a design may look perfect on CAD, the order of cuts may cause a feature to blow away or skew during cutting; consider redundant or incomplete cuts that can be manually completed afterwards to overcome these issues |
| Assemble Considerations | |
| Cleanliness | Dust removal is important for microfluidics, a simple cleaning protocol is using a mild detergent and a sonic toothbrush to directly clean plastic surfaces, followed by a wash and dry with pressurized gas or a microfiber cloth; be wary of harsh organics, which may damage substrates |
| Feature Removal | Use tweezers to remove all unwanted features cut out from adhesive before assembly; it is best to only remove the top liner and adhesive to prevent feature ‘droop’ during assembly |
| Peeling Off First Liner | Peeling off the top liner from cut adhesive is best done in one continuous motion if possible; tweezers are useful in complicated areas |
| Alignment | Using a simple alignment rig (such as a dowel for disc devices) is recommended for aligning adhesive on substrates |
| Lamination | A laminator or even a smooth laminating roller (McMaster-Carr #7533A12) to apply heavy pressure is important to activate most adhesives to set devices together |
| Adhesive-Paper Integration | When a paper substrate is integrated into a thin-film adhesive layer, apply additional lamination pressure at the boundary between adhesive and paper to best seal the device |
3D printing
While design-cut-assemble is a powerful process for maker microfluidics, makerspaces offer other enabling technologies for microfluidic manufacturing. One of the most ubiquitous technologies in makerspaces is 3D printing, which has been referred to as the start of a ‘revolution’ in microfluidics [27]. While many devices have been developed, there are still inherent challenges faced by makerspace-available systems such as low optical clarity and material leaching [51]. These challenges are being rapidly overcome by new 3D-printing technologies such as Dolomite’s Fluidic Factory, which can produce leak-proof devices within 20 minutes made from clear, biocompatible cyclic olefin copolymer instead of traditional resins. While these printing technologies further develop to produce fully integrated microfluidic platforms, current technologies can be used to fabricate complementary microfluidic components, such as 3D-printed spinners for centrifugal devices, alignment rigs for multilayered device building, and even common laboratory equipment [52]. These tools are just as important as the microfluidics themselves to produce a complete system that replaces expensive engineering equipment, such as syringe pumps and custom fluidic locking connectors. Additionally, the design files for such complementary hardware can be easily shared via repositories such as Thingiverse (www.thingiverse.com) and specifically for microfluidics, Metafluidics (www.metafluidics.org), which is accessible to both technical experts and amateur makers alike.
Makerspace community
While general users will be enabled to make microfluidic devices with laser/plotter cutters and 3D printers, adding biological context such as POC testing, sample preparation, and post-analysis quantification may turn the user back to the biological lab for further development. The key benefit of the makerspace over having maker-manufacturing capabilities in the biology lab is the varied expertise present in the makerspace community. Rather than training biologists in machining, CAD software, and general design rules, these skills can be ‘borrowed’ through interaction with the makerspace community, which brings a wide array of human capital in forms such as mechanical, material, and electrical engineers, as well as product designers and entrepreneurs. The combined breadth of capabilities and knowledge provided by makerspaces will enable greater potential application solutions than a standalone laser or plotter cutter in a biology lab. Bridging a biological application to the materials and tools provided by a makerspace can also be facilitated through this community resource.
Accessibility and scalability of microfluidics
Along with enabling integrated microfluidic system development, maker microfluidics addresses another key limitation in microfluidics: accessibility. The use of simple materials and tools to fabricate microfluidic devices obviates the need for clean room facilities and specialized training in photo- and soft lithography methods. Additionally, the application of makerspace principles further allows non-experts in microfluidics to participate. Lesson plans have been developed for students as young as 12 years old to engage in microfluidics, which can be expanded through further makerspace involvement [53,54]. In contrast to clean room facilities, makerspaces grant low-cost access to capital-intensive manufacturing tools, span a diverse community of individuals from varying backgrounds spanning technical and even non-technical fields, and promote product development through collaboration and innovation [28]. In addition, the cost of makerspace memberships are comparable to monthly gym memberships at $40 – $75 per month, while monthly clean room memberships can cost an academic around $1500 – $3500 and a non-academic almost $10000 per month. Material costs are also considerably different, as soft lithography methods use silicon wafer masters ($6–20 ea., University Wafer), UV masks ($84 mylar mask, Fine Line Imaging), and polymer ($92/kg PDMS kit, Krayden); whereas makerspaces use low-cost plastics ($5/sqft [or $13/kg] cast 1/16″ acrylic, McMaster-Carr) and adhesives ($2/sqft Double Lintered Adhesive Tape, Amazon.com). The drastic difference in accessibility is underscored in Figure 3, which shows a technician at work in a clean room in contrast to a high school group learning in a makerspace.
Figure 3.
Contrasting clean rooms and makerspaces (a) A technician working in the George J. Kostas Nanoscale Technology and Manufacturing Research Center at Northeastern University, photo is taken outside the clean room where an orange glass window prevents particular light wavelengths from polymerizing materials inside (Reprinted with permission courtesy of Matthew Modoono and Northeastern University, Boston, Massachusetts). (b) The Technology Office Innovation Laboratory (TOIL) at MIT-Lincoln Laboratory, as an instructor teaches a group of high schoolers how to 3D-print prosthetic hands (Reprinted with permission courtesy of MIT Lincoln Laboratory, Lexington, Massachusetts).
Another key limitation addressed by maker microfluidics is the poor scalability of research-developed platforms to develop into commercial products. In addition to the greater compatibility of makerspace materials with large-scale manufacturing methods, makerspaces allow more seamless device integration with upstream and downstream processing. For example, on-chip sample preparation, sample analysis, and optical detection methods can be designed synonymously in the same space for a potentially instrument-free sample-to-result microfluidic system. These advantages come with the loss of the superior feature resolution granted by photolithography methods used in clean rooms (hundreds of nanometers) compared to laser and plotter cutters (tens to hundreds of micrometers). However, innovative new microfluidic methods, such as inertial and centrifugal microfluidics, have allowed some users to bypass the need for small features, which may be typically required in applications such as cell separations [55,56]. These methods leverage various inherent physical properties of fluids and particles such as density and size to perform a wide variety of microscale fluid manipulations and processing typically not possible in classic convective flow.
Beyond the scalable prototyping technologies makerspaces offer, one of the most enabling aspects offered by makerspaces is their community-driven nature, which often rivals professional consultants [57]. The creative diversity present in makerspaces allows technical experts in other fields (e.g., computer or electrical engineering) to more easily lend their expertise to microfluidic making in more innovative ways [58]. Maker communities can also help vet community designs (and share with others) for greater focus on reproducibility and efficacy. The big-picture view of makerspace projects will help transition the all-too-often microfluidic “one-offs” that are only used by the instrument builder to a more standardized and vetted format available to much larger communities.
Concluding remarks
The benefits afforded by makerspaces, specifically increased participation and the use of low-cost materials and prototyping methods, overcome major barriers to microfluidic device commercialization–accessibility and scalability. And while clean room manufacturing may still provide powerful research-scale solutions to massively multiplexed testing and screening (e.g., drug screening, sepsis diagnostics, and ultra-rare cell types), new innovations in microfluidics have obviated some of the need for the ultra-fine resolution of photolithographic techniques for many clinical applications (see Outstanding Questions). Makerspace prototyping promises to increase the success of microfluidics broadly by providing a thriving innovation space for a diverse population to create simple and robust POC microfluidic solutions for current clinical problems.
OUTSTANDING QUESTIONS BOX.
Can high resolution features be fabricated in makerspaces in a high-throughput manner?
Can the clean room be moved into makerspaces—similar to the SoftLithoBox by BlackHoleLab?
Will pipelines be produced to enable microfluidic product development in makerspaces for inventors to rapidly reach the market?
Will manufacturing standards be developed to easily translate devices between different spaces?
How will the advancement of 3D printing materials and techniques influence the development of microfluidic devices?
What novel materials, such as TPX ‘breathable’ plastic, can be applied to ‘maker’ microfluidics?
As makerspaces further penetrate into academic instructions, can ‘maker’ microfluidic training become a standard for future bioengineers?
World-to-chip interfaces: how rapidly will the integration of standard parts (e.g. connectors) occur with the simpler fabrication techniques described herein?
Supplementary Material
TRENDS BOX.
The use of simple tools and materials to manufacture microfluidic devices provides an opportunity for makerspaces to serve as a hotbed for microfluidic device development.
Materials such as plastic, adhesive, and paper, along with tools such as plotter/laser cutters and 3D printers, enable building integrated microfluidic systems that are more easily translated to large-scale manufacturing.
Makerspaces provide low-cost access to prototyping tools and access to technically diverse human capital, and they enable those without advanced skills to participate in microfluidic device development.
Acknowledgments
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This material is based upon work supported by the National Science Foundation Graduate Research Fellowship awarded to D.I.W. under grant NSF/DGE-0946746. Research reported in this publication was also supported by the National Cancer Institute of the National Institutes of Health under award number R01CA173712, the National Institute of General Medical Sciences and the National Institutes of Health under grant number P50 GM098792 and the National Science Foundation under grant 1521759. DISTRIBUTION STATEMENT A. Approved for public release: distribution unlimited. This material is based upon work supported by the MIT under Air Force Contract No. FA8721-05-C-0002 and/or FA8702-15-D-0001. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the MIT.
Glossary
- Contract manufacturer
A company that is used to outsource a manufacturing task. In microfluidics, contract manufacturers are commonly used to build device molds for labs without a clean room or provide device components made from tools such as CNC mills or laser cutters
- Do-it-yourself (DIY) biology
A user-based community of individuals or small organizations that study biology and life science outside the traditional academic setting, typically for education, hobbyist, or entrepreneurship applications
- Double-sided tape
a material that is composed of a carrier layer such as a film or tissue where adhesive material has been coated on both sides. These tapes are typically thicker than transfer tapes
- Droplet microfluidics
Devices that create highly monodisperse droplets from two-phase flow, such as droplets of water in oil. For example, individual cells from a sample can be trapped and sorted in droplets based on a wide variety of characteristics
- Isothermal PCR
A variation of traditional nucleic acid amplification (polymerase chain reaction, PCR) that requires no heating cycles for amplification, reducing the need for bulky and expensive equipment
- Lab on a chip (LOC)
A device that miniaturizes one or multiple functions of a laboratory, enabling automated, high-throughput characterization of biological samples, which could potentially replace traditional lab testing
- Lateral flow assay
A simple paper-based device where a sample is “flowed” along a paper strip and over a “detection” region where a colorimetric or fluorescent signal is given with intensity scaling with target analyte concentration. The best-known lateral flow assay application is the home pregnancy test
- Plotter cutter
Also known as a vinyl cutter, this computer-controlled machine controls the movement of a sharp blade over a thin material to cut out shapes. In microfluidics, plotter cutters can be used to cut channel architecture out of paper or thin-film adhesive for device manufacturing
- Point of care (POC)
In reference to microfluidics, point-of-care means deployable at the site where a patient is treated. This paradigm is in contrast to lab tests where biological samples are collected at the treatment site and sent to a dedicated lab for testing. The key benefit of POC microfluidics is the ability for more rapid diagnosis or treatment
- Polydimethylsiloxane (PDMS)
A silicon-based organic polymer that is popular for rapid prototyping of microfluidic devices due to its beneficial properties of flexibility, device bonding, optical clarity, and biocompatibility
- Soft lithography
A technique where an elastomeric material (such as PDMS) is poured over a structure-patterned mold to produce a product such as a lab on a chip
- Tape converter
A company that modifies a commercially produced adhesive with processes such as die cutting or adding a second liner to a single lined tape. Typically these companies require a large minimum order for their services
- Transfer tape
Materials that are entirely composed of pressure sensitive adhesive material (without a carrier). These are typically thinner than double-sided tape
- Xurography
The use of a programmable drag knife to cut structures out of a material to produce patterns or channels. In contract to laser cutting, xurography is a style of contact cutting that can cause material shearing but will typically avoid burn products from material ablation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sackmann EK, et al. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–9. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 2.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–73. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 3.Yole Development. Microfluidic applications in the pharmaceutical, life sciences, in-vitro diagnostic and medical device markets report 2013 [Google Scholar]
- 4.Markets and Markets. Microfluidics market by materials (polymers, silicon, glass), pharmaceuticals (microreactors, toxicity screening, lab on a chip, proteomic & genomic analysis), drug delivery devices (microneedles, micropumps), IVD (POC) - global trends & forecast to 2018 2013 [Google Scholar]
- 5.Zhao X-M, et al. Soft lithographic methods for nano-fabrication. J Mater Chem. 1997;7:1069–1074. [Google Scholar]
- 6.Whitesides GM, et al. Soft Lithography in Biology. Annu Rev Biomed Eng. 2001;3:335–73. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 7.Kim P, et al. Soft Lithography for Microfluidics: a Review. Biochip J. 2008;2:1–11. [Google Scholar]
- 8.Mcdonald JC, et al. Review - Fabrication of microfluidic systems in poly (dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Duffy DC, et al. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane) Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 10.Plouffe BD, Murthy SK. Perspective on microfluidic cell separation: A solved problem? Anal Chem. 2014;86:11481–11488. doi: 10.1021/ac5013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin C, et al. Commercialization of microfluidic point-of-care devices. Lab Chip. 2012;12:2118–2134. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- 12.Volpatti LR, Yetisen AK. Commercialization of microfluidic devices. Trends Biotechnol. 2014;32:347–350. doi: 10.1016/j.tibtech.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Fiorini GS, Chiu DT. Disposable microfluidic devices: Fabrication, function, and application. Biotechniques. 2005;38:429–446. doi: 10.2144/05383RV02. [DOI] [PubMed] [Google Scholar]
- 14.Nge PN, et al. Advances in microfluidic materials, functions, integration, and applications. Chemical Reviews. 2013;113:2550–2583. doi: 10.1021/cr300337x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren K, et al. New materials for microfluidics in biology. Curr Opin Biotechnol. 2014;25:78–85. doi: 10.1016/j.copbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Pataky K, Brugger J. Rapid prototyping techniques for the fabrication of biosensors. Woodhead Publishing Limited; 2014. [Google Scholar]
- 17.Patko D, et al. Microfluidic channels laser-cut in thin double-sided tapes: Cost-effective biocompatible fluidics in minutes from design to final integration with optical biochips. Sensors Actuators, B Chem. 2014;196:352–356. [Google Scholar]
- 18.Klank H, et al. CO(2)-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab Chip. 2002;2:242–246. doi: 10.1039/b206409j. [DOI] [PubMed] [Google Scholar]
- 19.Samuel R, et al. Simple and cost-effective fabrication of microvalve arrays in PDMS using laser cut molds with application to C. elegans manipulation in microfluidics. J Micromechanics Microengineering. 2014;24:105007. [Google Scholar]
- 20.Pessoa de Santana P, et al. Fabrication of glass microchannels by xurography for electrophoresis applications. Analyst. 2013;138:1660–4. doi: 10.1039/c3an36540a. [DOI] [PubMed] [Google Scholar]
- 21.Pješčić I, et al. Glass-composite prototyping for flow PCR with in situ DNA analysis. Biomed Microdevices. 2010;12:333–343. doi: 10.1007/s10544-009-9389-2. [DOI] [PubMed] [Google Scholar]
- 22.Bartholomeusz DA, et al. Xurography: Rapid prototyping of microstructures using a cutting plotter. J Microelectromechanical Syst. 2005;14:1364–1374. [Google Scholar]
- 23.Azouz A Ben, et al. Fast Fabrication Process of Microfluidic Devices Based on Cyclic Olefin Copolymer. Mater Manuf Process. 2014;29:93–99. [Google Scholar]
- 24.Cosson S, et al. Ultra-rapid prototyping of flexible, multi-layered microfluidic devices via razor writing. Lab Chip. 2014;15:72–6. doi: 10.1039/c4lc00848k. [DOI] [PubMed] [Google Scholar]
- 25.Ho CMB, et al. 3D printed microfluidics for biological applications. Lab Chip. 2015;15:3627–3637. doi: 10.1039/c5lc00685f. [DOI] [PubMed] [Google Scholar]
- 26.Au AK, et al. 3D-Printed Microfluidics. Angewandte Chemie - International Edition. 2016;55:3862–3881. doi: 10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharjee N, et al. The upcoming 3D-printing revolution in microfluidics. Lab Chip. 2016;16:1720–1742. doi: 10.1039/c6lc00163g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Holm EJ. What are Makerspaces, Hackerspaces, and Fab Labs? SSRN Electron J. 2012 doi: 10.2139/ssrn.2548211. [DOI] [Google Scholar]
- 29.Gershenfeld N. How to Make Almost Anything: The Digital Fabrication Revolution. Foreign Aff. 2012;91:43–57. [Google Scholar]
- 30.Scroggins M, et al. BioCoder. O’Rielly; 2013. [Google Scholar]
- 31.Temiz Y, et al. Lab-on-a-chip devices: How to close and plug the lab? Microelectron Eng. 2015;132:156–175. [Google Scholar]
- 32.Lafleur L, et al. A rapid, instrument-free, sample-to-result nucleic acid amplification test. Lab Chip. 2016 doi: 10.1039/C6LC00677A. [DOI] [PubMed] [Google Scholar]
- 33.Kinahan DJ, et al. Xurography actuated valving for centrifugal flow control. Lab Chip. 2016 doi: 10.1039/C6LC00568C. [DOI] [PubMed] [Google Scholar]
- 34.Becker H, Locascio LE. Polymer microfluidic devices. Talanta. 2002;56:267–287. doi: 10.1016/s0039-9140(01)00594-x. [DOI] [PubMed] [Google Scholar]
- 35.Van Midwoud PM, et al. Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal Chem. 2012;84:3938–3944. doi: 10.1021/ac300771z. [DOI] [PubMed] [Google Scholar]
- 36.Kitsara M, Ducrée J. Integration of functional materials and surface modification for polymeric microfluidic systems. J Micromechanics Microengineering. 2013;23:33001. [Google Scholar]
- 37.Hawkins KR, Yager P. Nonlinear decrease of background fluorescence in polymer thin-films - a survey of materials and how they can complicate fluorescence detection in microTAS. Lab Chip. 2003;3:248–252. doi: 10.1039/b307772c. [DOI] [PubMed] [Google Scholar]
- 38.Piruska A, et al. The autofluorescence of plastic materials and chips measured under laser irradiation. Lab Chip. 2005;5:1348–1354. doi: 10.1039/b508288a. [DOI] [PubMed] [Google Scholar]
- 39.Solanki S, Pandey CM. Microfluidics for Biologists. 1. Chapter 8. Springer; 2016. [Google Scholar]
- 40.Kettler H, et al. Mapping the landscape of diagnostics for sexually transmitted infections: Key findings and recommandations. 2004 at < http://www.who.int/tdr/publications/tdr-research-publications/mapping-landscape-sti/en/index.html>.
- 41.Rodriguez NM, et al. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip. 2016;16:753–763. doi: 10.1039/c5lc01392e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong B, et al. A concentration gradient generator on a paper-based microfluidic chip coupled with cell culture microarray for high-throughput drug screening. Biomed Microdevices. 2016;18:1–8. doi: 10.1007/s10544-016-0054-2. [DOI] [PubMed] [Google Scholar]
- 43.Fridley GE, et al. Highly sensitive immunoassay based on controlled rehydration of patterned reagents in a 2-dimensional paper network. Anal Chem. 2014;86:6447–6453. doi: 10.1021/ac500872j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed S, et al. Paper-based chemical and biological sensors: Engineering aspects. Biosens Bioelectron. 2016;77:249–263. doi: 10.1016/j.bios.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 45.Nath P, et al. Rapid prototyping of robust and versatile microfluidic components using adhesive transfer tapes. Lab Chip. 2010;10:2286–2291. doi: 10.1039/c002457k. [DOI] [PubMed] [Google Scholar]
- 46.Nath P, et al. Polymerase chain reaction compatibility of adhesive transfer tape based microfluidic platforms. Microsyst Technol. 2014;20:1187–1193. [Google Scholar]
- 47.Thompson BL, et al. Inexpensive, rapid prototyping of microfluidic devices using overhead transparencies and a laser print, cut and laminate fabrication method. Nat Protoc. 2015;10:875–86. doi: 10.1038/nprot.2015.051. [DOI] [PubMed] [Google Scholar]
- 48.Govindarajan aV, et al. A low cost point-of-care viscous sample preparation device for molecular diagnosis in the developing world; an example of microfluidic origami. Lab Chip. 2012;12:174–181. doi: 10.1039/c1lc20622b. [DOI] [PubMed] [Google Scholar]
- 49.Carrilho E, et al. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. 2009;81:7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 50.Guckenberger DJ, et al. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip. 2015;15:2364–2378. doi: 10.1039/c5lc00234f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh ME, et al. 3D-Printable Materials for Microbial Liquid Culture. 2016;3:113–118. [Google Scholar]
- 52.Baden T, et al. Open Labware: 3-D Printing Your Own Lab Equipment. PLoS Biol. 2015;13:1–12. doi: 10.1371/journal.pbio.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bridle H, et al. Design of problem-based learning activities in the field of microfluidics for 12- to 13-year-old participants—Small Plumbing!: empowering the next generation of microfluidic engineers. Microfluid Nanofluidics. 2016;20:1–11. [Google Scholar]
- 54.Ravgiala RR, et al. Using paper-based diagnostics with high school students to model forensic investigation and colorimetric analysis. J Chem Educ. 2014;91:107–111. [Google Scholar]
- 55.Di Carlo D. Inertial microfluidics. Lab Chip. 2009;9:3038–3046. doi: 10.1039/b912547g. [DOI] [PubMed] [Google Scholar]
- 56.Strohmeier O, et al. Centrifugal microfluidic platforms: advanced unit operations and applications. Chem Soc Rev. 2015;44:6187–6229. doi: 10.1039/c4cs00371c. [DOI] [PubMed] [Google Scholar]
- 57.Jeppesen LB, Lakhani KR. Marginality and Problem Solving Effectiveness in Broadcast Search. Organ Sci. 2010;21:1016–1033. [Google Scholar]
- 58.Poetz MK, Schreier M. The value of crowdsourcing: Can users really compete with professionals in generating new product ideas? J Prod Innov Manag. 2012;29:245–256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.