Abstract
Employing an experimental design, mother-to-infant transmission of stress was examined. Mothers (N = 117) were randomized to either have a positive or conflictual discussion with their marital partners, after which infants (age = 6 months) participated in a fear and frustration task. Saliva samples were collected to assess maternal cortisol responses to the discussion, and infant cortisol responses to the challenge task. Results indicate maternal cortisol reactivity and recovery to the conflict (but not positive) discussion predicted infant cortisol reactivity to the infant challenge. Mothers’ positive affect during the discussion buffered, and intrusion during the free-play potentiated, mother-to-infant adrenocortical transmission. These findings advance our understanding of the social and contextual regulation of adrenocortical activity in early childhood.
Keywords: HPA, cortisol, inter-parental conflict, mother-infant relationships
Marital conflict is a family-system wide stressor with biobehavioral implications for all family members (Cox & Paley, 1997). For parents, conflict can become destructive, characterized by criticism, coercion, and overt anger and hostility, resulting in increased circulating cortisol levels for up to 24 hours (Robles & Kiecolt-Glaser, 2003). Though some high conflict parents are able to compartmentalize negative marital relationships (Sturge-Apple, Davies, Cicchetti, & Fittoria, 2014), parents consumed by negative marital conflict often resort to more harsh (e.g., Easterbrooks & Emde, 1988; Levendosky & Graham-Bermann, 2000) and less engaged parenting techniques (Sturge-Apple, Davies, & Cummings, 2006). Infants depend on caregivers to soothe and calm their heightened emotional and physiological arousal (Gunnar & Donzella, 2002). Specifically, low quality parenting has been related to high but flat infant cortisol responses to parental separation (Sturge-Apple, Davies, Cicchetti, & Manning, 2012), high infant cortisol levels to frustration (Blair, Raver, Granger, Mills-Koonce, & Hibel, 2011), and reduced infant recovery from a naturally occurring stressor (Albers, Riksen-Walraven, Sweep, & deWeerth, 2008). In other words, disrupted mother-child interactions may serve to upregulate infants’ stress physiology. Thus, parents’ experience of stress within the marital domain may indirectly regulate children’s stress responses during subsequent parent-child interactions, serving as the basis for the intergenerational transmission of stress reactivity (Champagne & Meaney, 2001). Employing an experimental design, we examined the effect of marital conflict on mother-infant interactions and physiology.
Mother-infant interactions consist of intricately matched exchanges in which the dyad’s behavior and physiology are transactionally influenced (e.g., Bornstein, 2009). The hypothalamic-pituitary-adrenal (HPA) axis is implicated in these transactions, with maternal cortisol reliably related to child cortisol levels (Hibel, Trumbell, & Mercado, 2014) and reactivity (Laurent, Ablow, & Measelle, 2012). Little is known about the process by which mother and child cortisol attunes, but it is thought to be a physiological manifestation of shared emotional and behavioral experiences (e.g., Feldman, 2007; Hibel et al., 2014). For example, mother-infant cortisol attunement has been found: during a diaper change in preterm infants randomized to receive skin-to-skin contact, but not in those in standard incubator care (Mörelius, Örtenstrand, Theodorsson, & Frostell, 2015); in three month old infants before, but not after, a separation period (Thompson & Trevathan, 2008); and when mothers were aware, compared to unaware, of their infant’s distress when left alone to sleep for the night (Middlemiss, Granger, Goldberg, & Nathans, 2012). Similarly, maternal behaviors thought to encourage the matching of mother-infant behavioral and emotional states (e.g., maternal sensitivity to infant cues) have also been linked to greater amounts of adrenocortical attunement (e.g., Atkinson et al., 2013). Thus, researchers often assume that adrenocortical attunement is part of the underlying process by which maternal behavior organizes the infant’s behavioral and physiological activity (e.g., Feldman, 2007).
Interestingly, mothers in violent relationships (Hibel, Granger, Blair, & Cox, 2009), or those with depressive symptoms (Laurent, Ablow, & Measelle, 2011), also have stronger attunement with their infants. These authors highlight that mother-infant attunement in the context of maternal physiological dysregulation (stemming from either contextual stress [Hibel et al., 2009] or psychopathology [Laurent et al., 2011]) may be a mechanism in the intergenerational transmission of risk. In other words, mothers under duress might also share their emotional and physiological state with their child, resulting in the attunement of negative reactivity states. Evolutionary scholars have highlighted the adaptiveness of high reactivity profiles in the context of chronic stress, and the role of harsh early experiences in calibrating high stress responsivity (Del Giudice, Ellis, & Shirtcliff, 2011). It is therefore possible that physiological attunement plays a mechanistic role in this calibrating process, thus aiding in the infants’ long-term ability to respond to contextual threats (Williams et al., 2013). Following this conceptualization, we hypothesize that marital conflict, a known physiological and emotional stressor for mothers, will facilitate mother-infant attunement of adrenocortical reactivity.
Two recent studies highlight the role that negative emotions, in particular, may play in fostering physiological attunement within a dyad. In a laboratory experiment, mothers of infants (12 – 14 months old) who were randomized into a negative evaluation task displayed higher levels of externalized (anger) affect relative to mothers in a positive evaluation or control condition (Waters, West, & Mendes, 2014). Though both evaluation conditions (positive and negative) produced sympathetic nervous system reactivity in the mothers, only reactivity in mothers exposed to the negative evaluation task predicted their infants’ physiological reactivity upon reunion (Waters et al., 2014). In a naturalistic study of diurnal rhythms, mother-adolescent dyads reported their emotions and collected cortisol at 7 time points across two days. Findings revealed mothers and adolescents who reported greater daily negative affect also displayed stronger cortisol attunement (Papp, Pendry, & Adam, 2009). Together, these studies suggest that negative affect and outward distress might serve to co-regulate dyadic physiology, though to the best of our knowledge no study has explicitly tested the role of experimentally induced maternal emotions in facilitating mother-infant attunement. Marital conflict is an ecologically valid stressor with the potential to elicit strong negative emotions (Gottman, 1979) and wives often exhibit higher negative emotionality during a marital conflict and report greater levels of distress following the interaction than their husbands (Almeida & Kessler, 1998). Thus, we expect that the negative emotions stemming from marital conflict will predict mother-infant attunement.
Further, theoretical and empirical evidence suggests that problematic parenting is an important process variable for high conflict families in influencing child developmental outcomes. Specifically, negative parenting processes in the context of marital conflict have been shown to heighten depression and anxiety levels, as well as adrenocortical reactivity (Brock & Kochanska, 2015; Hibel, Granger, Blair, & Cox, 2011). Intrusive or withdrawn parenting behaviors are thought to directly and indirectly stimulate children’s development of high behavioral and physiological reactivity by undermining their attempts at self-control (Beebe, 2006) or by failing to provide external regulation to reduce arousal in times of stress (Feldman, 2007). Although relatively fewer studies have examined poor parenting practices and mother-infant attunement, mothers who use harsh and punitive parenting techniques have been found to have greater adrenocortical attunement with their infants, compared to those who do not (Hibel et al., 2009). Therefore, we also expect negative parenting practices to play a role in facilitating mother and infant cortisol attunement.
Conceptually, attunement is thought of as a coordinated bidirectional exchange within the dyad which contributes to the dyad’s homeostasis (Butler, 2011), and accordingly, the previously mentioned studies examine mother and child adrenocortical activation concurrently. However, to date, no study has tested the potential for cortisol reactivity to be actively transmitted, as opposed to passively shared. The conceptual framework that assumes maternal stress transmits to the child proposes that heightened maternal stress physiology leads to heightened child stress physiology. In other words, transmission assumes temporal ordering and causal influence (Butler, 2011). We aim to specifically investigate transmission by examining the physiological implications of randomly assigning mothers to a stressful (marital conflict) or positive task, before engaging with their infant. The sequencing of these events creates the time-lagged design necessary for testing mother-to-infant cortisol transmission (Butler, 2011). Randomized designs also reduce the alternative explanations of stress condition effects by attempting to remove pre-existing group differences (Shadish, Cook, & Campbell, 2002). Further, we build off of Waters et al.’s informative work by examining the specific maternal emotions and parenting behaviors related to transmission, and extending their findings to cortisol.
We first hypothesize that conflict will predict negative marital behaviors, heightened maternal HPA reactivity, and negative parenting behaviors. Next, we hypothesize that maternal cortisol will transmit to the child, and that marital conflict, marital emotions, and parenting will moderate mother-to-infant cortisol transmission. Specifically, we expect marital conflict, heightened negative emotions and/or reduced positive emotions, and negative parenting to predict a stronger mother-infant cortisol relationship.
Method
Participants
Families (n = 117) were recruited from a small Midwestern city. Eligibility criteria required families to consist of a non-pregnant biological mother, residential father, and an infant between the ages of 5–8 months. We selected this age for two primary reasons. First, the transition to parenthood is marked by heightened stress on the romantic relationship and increased couple conflict (e.g., Feinberg, 2002). Thus, parents with an infant may be particularly susceptible to conflict and the subsequent spillover effects on parenting. Secondly, infancy is a time of increased dependency on parental (especially maternal) caregiving and external regulation of physiological processes. Thus, spillover effects have the potential to be particularly potent for infants.
Participating mothers were married (91%), for approximately 4.5 years (SD = 3.0). Mothers were mostly Caucasian (87%) and 51% of the infants were female. On average, infants were 5.9 months (SD = .69), mothers were 29.3 years (SD = 4.5), and fathers were 31.5 years (SD = 5.4). Just over half of the infants (55.8%) were the parents’ first child. Many parents were college graduates (mothers: 75%; fathers: 67%), and 45.8% of the sample reported an annual household income of $70,000 or less per year (see Table 1, for sample demographics).
Table 1.
Demographic characteristics split by mothers’ positive or conflict discussion
| Positive | Conflict | Overall | |
|---|---|---|---|
| Mother Caucasian N (%) | 53 (88.3) | 51 (85.0) | 104 (86.8) |
| Infant Caucasian N (%) | 36 (85.7) | 35 (83.3) | 71 (84.5) |
| Education N (%) | |||
| 9–11th grade | 2 (3.5) | 0 (0) | 2 (1.7) |
| High School/GED | 0 (0) | 5 (8.3) | 5 (4.3) |
| Some College | 6 (10.5) | 9 (15.0) | 15 (12.8) |
| Technical School | 1 (1.8) | 1 (1.7) | 2 (1.7) |
| Associate Degree | 2 (3.5) | 3 (5.0) | 5 (4.3) |
| Bachelor’s Degree | 25 (43.9) | 25 (41.7) | 50 (42.7) |
| Graduate/Professional | 21 (36.8) | 17 (28.3) | 38 (32.5) |
| Income N (%) | |||
| Less than 10,000 | 2 (4.3) | 2 (4.3) | 4 (4.3) |
| 10,000 – 29,000 | 16 (34.0) | 8 (17.0) | 24 (25.5) |
| 30,000 – 59,000 | 8 (17.0) | 17 (36.2) | 25 (26.6) |
| 60,000 – 89,000 | 10 (21.3) | 12 (25.5) | 22 (23.4) |
| 90,000 – 119,000 | 9 (19.1) | 2 (4.3) | 11 (11.7) |
| 120,000 and up | 2 (4.3) | 6 (12.8) | 8 (8.5) |
| Work Status N (%) | 34 (56.7) | 28 (46.7) | 62 (51.6) |
| First-time Parent N (%) | 33 (55.0) | 34 (56.7) | 67 (55.8) |
| Non-Smoker N (%) | 55 (91.7) | 55 (91.7) | 110 (91.7) |
Note: Positive (N = 57), Conflict (N = 60); Percentages = valid percentage; Infant Caucasian = missing data for 31 infants; Work status = mothers working part-time or fulltime; Income = A typographical error was made on the income questionnaire and one category included the range ‘50,000 – 69,000’. N = 19 mothers (Positive N =10, Conflict N = 9) indicated this income bracket. Because this range overlapped with the two adjacent ranges, these incomes were left missing.
Procedure
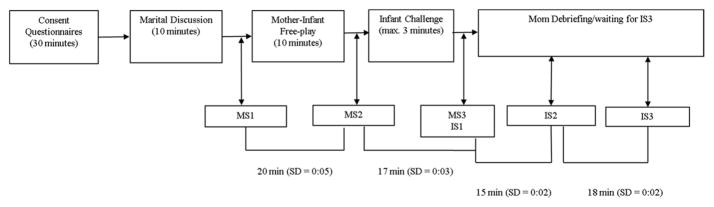
The current study was approved by the Institutional Review Board at Purdue University. Informed consent was obtained at the lab visit, which was initiated between 2 and 6pm to control for cortisol’s diurnal rhythm (see Figure 1, for a timeline of the laboratory visit). Mothers were not permitted to eat during the laboratory visit, but were given a bottle of water. Ten minutes prior to any of the saliva collections, mothers were told to refrain from drinking (Salimetrics, LLC, 2015). Families were compensated $75. Prior to the lab visit, couples were block randomized on infant gender into either a 10-minute conflict (n = 60) or positive (n = 57) interaction task (Jouriles & Farris, 1992). To reduce anticipatory anxiety, the consent informed parents they would be engaging in a discussion, but did not specify that the discussion might induce conflict. Questionnaires measuring demographic, health, and family processes were completed by both partners after consent and prior to the couple discussion tasks.
Figure 1.
Timeline of tasks and cortisol sample collections during the 2–3 hour study visit. The mask task lasted approximately 40 seconds; each mask was presented to the child for 10 seconds. The arm restraint lasted approximately 2 minutes. Transitioning between tasks, administering instructions, and saliva collection accounts for the time between tasks.
Spouses randomized to the conflict discussion each rated relationship problems on a list of commonly occurring areas of conflict. Two research assistants chose the top three corresponding problems between partners as topics of discussion (e.g., drugs and alcohol, finances, children). Couples assigned to the positive task discussed three positive relationship experiences (e.g., how they first met, what initially attracted them to each other, what drew them together as a couple). After the discussion, the mother’s first saliva sample (MS1) was collected via passive drool. Salivary cortisol is indicative of contextual influences 20 minutes prior, with measurement of salivary HPA reactivity occurring approximately 20 minutes post stressor and returning to baseline approximately 40 minutes post stressor (Dickerson & Kemeny, 2004). Thus, the saliva sample collected immediately after the discussion (MS1) reflects mothers’ HPA activation while completing their questionnaires. After completing the marital discussion, timers were set for 20 and 40 minutes, to collect maternal sample2 (MS2) and sample3 (MS3), respectively. MS2 measured discussion task cortisol reactivity; MS3 represented discussion task recovery, and reactivity to the infant challenge (discussed below). While the parents completed their questionnaires and were engaged in the discussion, sensitive and experienced babysitters interacted with the infant in a separate room with age appropriate toys. If the babysitters were unable to console the infant during the questionnaires, the infant was temporarily reunited with the mother. Further, if infants became inconsolable during the marital interaction task, the task was interrupted and parents were given an opportunity to calm the infant. The discussion task was only interrupted for a couple of families.
After the discussion, the mother was then reunited with her infant and the dyad engaged in a 10 minute semi-structured free-play interaction. The mother was instructed to play with her child as she normally would if she had a small amount of free time. Next, the infant engaged in two challenge tasks designed to elicit fear (masks) followed by frustration (arm restraint), adapted from the Laboratory Temperament Assessment Battery (LAB-TAB; Goldsmith & Rothbart, 1988). For the mask task, a research assistant presented the infant with four unusual masks while calling the child’s name. If the child reached peak behavioral reactivity (i.e., 20 seconds of hard crying), the task was discontinued and the mother was told she was free to interact with her infant; in these cases the arm restraint was not conducted (N = 3). For the arm restraint task, the experimenter stood behind the infant and gently restrained the infant’s arms for 2 minutes or until the infant reached peak reactivity. Four infants did not complete the full 2 minutes. After the task, the infant’s arms were released and the mother was told she was free to interact with her child.
Five minutes after the challenge tasks, the infant’s first saliva sample (IS1) was collected via absorbent sponge (Salimetrics Children’s Swab). IS1 represents infant adrenocortical activity prior to the free-play, and thus, infant cortisol without maternal influence. After the infant challenge, timers were set for 20 and 40 minutes, to collect infant sample2 (IS2) and sample3 (IS3), respectively. IS2 represented reactivity to the challenge tasks, IS3 represented the infant’s adrenocortical recovery. Following the five minutes of soothing, the mother was debriefed with the infant present. Mothers then engaged in a short task without the infant, while the infant was cared for by the father.
In sum, mothers’ saliva was collected to examine reactivity to and recovery from the marital discussion, while infants’ saliva was collected to examine reactivity to and recovery from the infant challenge task.
Questionnaires
The 36-item Dyadic Adjustment Scale (Spanier, 1976) assessed couples’ perceptions of, and satisfaction with, their relationship on a 6-point Likert-scale (1 = strongly agree to 6 = strongly disagree), with a few response scales formatted in terms of frequency (never to more often). The global summary scale was used in the current paper, and it demonstrated acceptable internal consistency (α = .97). The Conflict Tactics Scale (Straus, Hamby, Boney-McCoy, & Sugarman, 1996) provided measures of partner’s verbal and physical aggression using a total of 19 items rated on a 7-point Likert scale measuring event frequency (0 = never to 6 = more than 20 times). Subscales showed adequate internal consistency in the current sample (α = .80, .89). The Parenting Sense of Competence Scale (Johnston & Mash, 1989) has 17 items assessing perceptions of parenting rated on a 6-point Likert scale (1 = strongly agree to 6 = strongly disagree). Internal consistency for the two subscales used in this paper, satisfaction (n = 7 items) and efficacy (n = 7 items) was adequate (α = .72, .80). The Infant Behavior Questionnaire (Rothbart, 1981) measured infant temperament using two subscales, soothability (n = 18 items) and distress to limitations (n= 16 items) on a 6-point Likert scale of (1 = never to 7 = always). These scales displayed adequate reliability estimates in the current sample (α = .75, .76). Questionnaire data provided a check of the randomization process and potential control variables.
Task Coding and Saliva Assay
Marital discussion
The System for Coding Interactions in Dyads (Malik & Lindahl, 2004) was used to code the marital discussion task for mothers’ expressed negativity and positive affect. Negativity refers to the level of tension, frustration, and anger directed by one parent towards the other and the use of blaming and defensive statements. Positive affect refers to a happy tone of voice, affection, laughter, or the appearance of being relaxed. These codes were rated using a 5-point Likert-type scale (1 = very low to 5 = high). Coders reached a level of substantial agreement on pilot data (κ = .80) before coding participants’ data. All videos were double coded and discrepancies were resolved through conferencing.
Parenting behavior
Mother-infant free-play interactions were video recorded and later coded to assess levels of mothers’ sensitivity to non-distress, sensitivity to distress, detachment, intrusiveness, positive regard, negative regard, and stimulation of development when interacting with the child (see NICHD ECCRN, 1999). Weighted kappa coefficients were calculated for ratings made by pairs of research assistants; coders reached substantial agreement (κw > .61) on pilot data before moving on to code non-pilot videos. All videos were double coded and discrepancies were resolved through conferencing. Ratings for each code were made on a scale ranging from 1 (not at all characteristic) to 4 (highly characteristic). A factor analysis revealed two main factors that were used to examine negative maternal parenting; intrusive parenting, that consisted of intrusion and reverse coded sensitivity to non-distress, (α = .82), and emotionally disengaged parenting, that consisted of reverse coded positive regard, and flat affect (α = .86).
Infant challenge
Infant negative behavioral reactivity was determined with an adapted version of Stifter and Braungart’s (1995) coding scheme, and measured the level and duration of crying and fussing during the tasks. This scale ranged from 0 (no reactivity) to 3 (high negative reactivity). Raters coded the percentage of time spent at each reactivity mode (0 = 0%; 1 = less than 25%; 2 = roughly 25%; 3 = roughly 50%; 4 = roughly 75%; 5 = roughly 100% of the time). Coders reached acceptable inter-rater reliability on pilot data (κ = .80) before coding participants’ data, which was double coded.
The total intensity of negative reactivity during each of the tasks (masks and arm restraint) was computed by multiplying the percentage time code (0 – 5) at each intensity level by the code representing that intensity (0 – 3). For example, the total intensity score for a child that spent roughly 25% (code = 2) of the time at each intensity level during the arm restraint would be (2*0) + (2*1) + (2*2) + (2*3) = 12 for that task.
Salivary cortisol
After saliva collection, samples were placed on ice temporarily, then stored at −80 °C. Samples were assayed for cortisol via ELISA (Salimetrics, Carlsbad, CA). The assay has a test volume of 25 ul, range of sensitivity from 0.007 to 3.0 ug/dL and average intra- and inter-assay coefficients of variation of 10% and 15%, respectively. Samples were assayed in duplicate and the average was used in statistical analyses. Cortisol values were natural log transformed. All saliva samples were examined for discoloration indicative of blood contamination (Granger et al., 2007). No samples were excluded based on discoloration.
Analytical Strategy
Preliminary analyses were first conducted to check the efficacy of the randomization. To do this, differences between the positive and conflict discussion groups were assessed on a variety of demographic, marital, parenting, and child variables. Any variables found to be significantly different between the two groups were controlled for in the main analyses. This check bolsters our ability to attribute task related differences in the outcome to the experimental manipulation (i.e., the conflict task), as opposed to pre-existing differences between the groups. For the first aim, we examined the emotional, behavioral, and physiological implications of the conflict task. To do this, couples randomized into the conflict group were first examined for higher levels of negativity, and lower levels of positive affect than the positive discussion group. Second, the mother’s three cortisol samples around the discussion task were examined using a hierarchical linear model with maximum likelihood estimation, and random intercepts. The Level 1 submodel described the within mother cortisol change across the task and the Level 2 submodel described the between mother variations (Singer & Willet, 2003). Cortisol reactivity and recovery were examined using a piecewise approach. Dummy variables for the MS1 and MS3 collections (MS2 was the reference sample) estimated change in cortisol over the marital discussion task. The MS1 dummy variable estimated the difference in cortisol levels at MS1 compared to MS2; the MS3 dummy code estimated the difference in cortisol levels at MS3 compared to MS2. Interactions with the discussion task dummy (positive = 0; conflict = 1) revealed differences in cortisol reactivity and recovery by group. This same model was used to examine infant cortisol across the task.
- Level 1
- Level 2
Next, we examined conflict, marital emotions, and parenting behavior as moderators of mother-to-infant cortisol transmission. Cortisol transmission was also tested in a hierarchical piecewise model with the three maternal samples predicting the three infant samples, again utilizing maximum likelihood estimation and a random intercept. The Level 1 submodel described the within dyad cortisol change across the task; the Level 2 submodel described the between dyad variations (Singer & Willet, 2003). Collection point dummy codes, sample1 (S1) and sample3 (S3), were employed as moderators. The interaction between maternal cortisol and the S1 dummy variable, predicting infant cortisol, assessed the degree to which transmission changed from pre- to post-task. Interactions with the moderators (i.e., discussion dummy, marital emotions, maternal behavior) determined if these factors induced mother-to-infant cortisol transmission across the task. To facilitate pairwise comparisons between each of the collection points, and determine transmission at each of the collection points, S1 and S3 were also examined as the reference. Models presented in the tables employ S2 as the reference.
- Level 1
- Level 2
Model fit during the model building process was determined through the use of AIC. BIC is less tolerant of complex models, however the piecewise approach results in the addition of three parameter estimates (e.g., conflict, conflict*S1, conflict*S3) for every independent variable included. This causes a large reduction in degrees of freedom. Therefore, based on our model structure and the fact that BIC reflects a stronger penalty for over-parameterization than the AIC, AIC was judged to be a more accurate assessment than BIC. All multi-level models were run in SAS 9.4 (SAS Institute, Inc.).
Follow-up regression analyses with cortisol change scores assessed the effect of conflict on the association between mother and infant cortisol reactivity (calculated as S2 – S1) and recovery (calculated as S3 – S2). The hierarchical models with the sample collection point dummy provide a statistical test of the difference in transmission from one time point to the next. The change score regression analyses, on the other hand, determine the degree to which change in maternal cortisol predicts change in infant cortisol. In other words, by examining both the hierarchical dummy codes and the change scores, we are able to assess the strength of the mother-to-child cortisol association at each time point, how this strength changes across the task, and if the degree of mothers’ cortisol change is associated with the degree of infant cortisol change. All follow-up regression analyses were run in SPSS (IBM SPSS 24).
Results
Preliminary analyses
No differences between the discussion groups emerged on demographic, marital, parenting, and child variables. Infants with mothers in the positive discussion had marginally higher behavioral reactivity to the challenge task (M = 6.23, SD = 5.07) than infants with mothers in the conflict discussion (M = 4.68, SD = 3.79; t(98.1) = 1.81, p = .073). Infant behavioral reactivity to the challenge was controlled in all analyses. Demographics (e.g., infant gender, infant age, infant race, maternal age), health (e.g., medication usage), visit time of day, and if the infant was fed over the course of the visit, were examined in relation to cortisol level, reactivity, and transmission; however, only sample collection time-of-day proved significant. Specifically, infants’ first saliva collection time was significantly correlated with MS1 (r = −.29, p = .002), MS2 (r = −.18, p = .05), MS3 (r = −.26, p = .004), and IS2 (r = −.21, p = .027). Therefore, we controlled for the time of day IS1 was collected in all analyses with cortisol (mother’s first sample collection time was highly correlated with infant’s first sample collection time, r = .99). See Table 2 for inter-correlations of main analysis variables.
Table 2.
Bivariate correlations among main analysis variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. M S1 Cortisol | – | ||||||||
| 2. M S2 Cortisol | .47** | – | |||||||
| 3. M S3 Cortisol | .78*** | .62** | – | ||||||
| 4. I S1 Cortisol | .18 | .15 | .29 | – | |||||
| 5. I S2 Cortisol | .33*** | .35*** | .42** | .66** | – | ||||
| 6. I S3 Cortisol | .29** | .20* | .32*** | .55** | .74** | – | |||
| 7. Maternal Positivity | .02 | −.08 | −.01 | .03 | −.03 | .05 | – | ||
| 8. Maternal Intrusion | −.06 | −.01 | −.02 | −.04 | −.08 | −.08 | −.07 | ||
| 9. I S1 Time of Collection | −.29** | −.19** | −.26** | −.11 | −.21* | −.17 | .04 | .06 | – |
Note. N = 114–117. M = maternal, I = infant, S1 = sample 1, S2 = sample 2, S3 = sample 3.
p<.05;
p<.01;
p<.001.
Main analyses
1) Marital conflict will predict marital emotions, mothers’ HPA reactivity, and negative parenting behaviors
Both mothers and fathers in the conflict group exhibited more negativity (mother t(111.5) = 8.0, p < .001; father t(99.8) = 7.23, p < .001), and less positivity (mother t(77.8) = −8.1, p < .001; father t(63.11) = −9.54, p < .001) than their counterparts in the positive-discussion group. All mothers exhibited significant cortisol reactivity (b = .13, p = .05) and recovery (b = −.31, p < .001) to the discussion. There were no group differences in mother’s cortisol reactivity (b = −.07, SE = .13, p = .60) or recovery (b = .01, SE = .33, p = .98) between the conflict and positive discussion groups (see Figure 2). Lastly, conflict did not cause significantly higher levels of emotionally disengaged parenting t(114) = −0.21, ns or intrusive parenting t(114) = −0.05, ns during the mother-infant free-play.
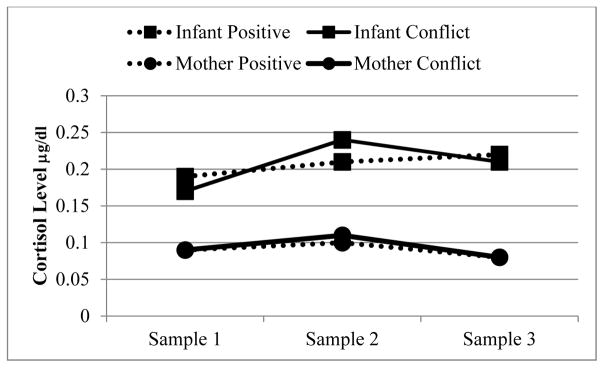
Figure 2.
Mother and infant cortisol levels across their respective tasks (marital discussion and fear/frustration challenge), split by mothers’ marital discussion group (positive and conflict). 50% of the mothers and 61.3% of the infants expressed cortisol sample 2 levels that were greater than sample 1 levels. 66.9% of the mothers and 58.5% of the infants expressed cortisol sample 3 levels that were lower than sample 2 levels. There were no significant differences by discussion group.
2) Marital conflict will predict mother-to-infant cortisol transmission
According to the unconditional model, between individual differences accounted for 67% of the variability in infant cortisol (ICC = .67), leaving 33% of the variance existing within individuals (see Table 3, for variance and model fit data). Infant cortisol significantly increased in response to the infant challenge task (b = −.17, SE = .07, p = .02), followed by a plateau (b = −.01, SE = .07, p= .85; see Figure 2). However, mothers’ marital discussion condition did not predict infant cortisol trajectories from IS1 to IS2 (b = −.08, SE = .09, p = .38) or IS2 to IS3 (b = .02, SE = .09, p = .86). Mother and infant cortisol levels before their respective stressors (S1) were not related (b = .01, SE = .08, p = .92), however post discussion task maternal cortisol (S2) predicted post infant challenge cortisol (b = .21, SE = .07, p = .002; bMCortisol; see Table 4, for model 2 estimates with S2 as the reference). Maternal cortisol explained 11.4% of the between infant variation and 8.2% of the within infant variation in cortisol.
Table 3.
Error variance and fit statistics of: the unconditional null model; 1) infant’s cortisol across the challenge task; 2) effect of mother cortisol on infant cortisol; 3) effect of conflict mother-to-child cortisol transmission; 4) effect of maternal positive affect on mother-to-child cortisol transmission; and 5) effect of intrusive parenting on mother-to-child cortisol transmission
| Null Model | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|---|
| Error Variance | ||||||
| Intercept | 0.6708*** | 0.6488*** | 0.5940*** | 0.5388*** | 0.5381*** | 0.5787*** |
| (0.1040) | (0.1025) | (0.0981) | (0.0905) | (.0900) | (0.0960) | |
| Residual | 0.3257*** | 0.3014*** | 0.2991*** | 0.2981*** | 0.2954*** | 0.2938*** |
| (0.0306) | (0.0298) | (0.0292) | (0.0292) | (.0288) | (0.0288) | |
| Model Fit | ||||||
| AIC | 830.7 | 780.7 | 776.5 | 774.6 | 774.3 | 773.1 |
| BIC | 839 | 805.1 | 807.1 | 825.3 | 823.1 | 821.1 |
Note. AIC = Akaike Information Criterion. BIC = Bayesian Information Criterion.
p<.001.
Table 4.
Multi-level models examining: 1) infant’s cortisol across the challenge task; 2) effect of mother cortisol on infant cortisol; 3) effect of conflict mother-to-child cortisol transmission; 4) effect of maternal positive affect on mother-to-child cortisol transmission; and 5) effect of intrusive parenting on mother-to-child cortisol transmission
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Effect | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE |
| Intercept | 0.61 | 0.31 | 0.25 | 0.33 | 0.32 | 0.13 | 0.09 | 0.41 | 0.29 | 0.34 |
| Time of day | −0.11 | 0.06 | −0.09 | 0.06 | −0.08 | 0.02 | −0.08 | 0.06 | −0.07 | 0.06 |
| S1 | −0.17* | 0.07 | .01 | 0.12 | −0.02 | 0.1 | 0.12 | 0.27 | 0.00 | 0.15 |
| S3 | −0.01 | 0.07 | 0.05 | 0.12 | −0.03 | 0.1 | 0.16 | 0.27 | 0.03 | 0.15 |
| M Cortisol | .21** | 0.07 | −0.01 | 0.11 | 0.88 | 0.26 | 0.02 | 0.13 | ||
| M Cortisol*S1 | −0.20* | 0.09 | −0.01 | 0.14 | −0.09 | 0.34 | 0.07 | 0.16 | ||
| M Cortisol*S3 | −0.1 | 0.08 | −0.02 | 0.15 | −0.22 | 0.29 | −0.04 | 0.16 | ||
| Conflict | −0.26 | 0.18 | −0.28+ | 0.18 | ||||||
| Conflict*S1 | 0.13 | 0.14 | 0.09 | 0.15 | ||||||
| Conflict*S3 | 0.15 | 0.14 | 0.16 | 0.16 | ||||||
| M Cortisol*Conflict | 0.36* | 0.13 | 0.35* | 0.15 | ||||||
| M Cortisol*Conflict*S1 | −0.24* | 0.21 | −0.31 | 0.21 | ||||||
| M Cortisol*Conflict*S3 | −0.03 | 0.18 | 0.00 | 0.20 | ||||||
| Moderator | 0.03 | 0.08 | −0.04 | 0.09 | ||||||
| Moderator*S1 | −0.01 | 0.07 | 0.04 | 0.08 | ||||||
| Moderator*S3 | −0.01 | 0.07 | 0.04 | 0.08 | ||||||
| M Cortisol*Moderator | −0.21** | 0.08 | −0.01 | 0.08 | ||||||
| M Cortisol*Moderator*S1 | −0.01 | 0.1 | −0.24* | 0.11 | ||||||
| M Cortisol*Moderator*S3 | 0.06 | 0.1 | 0.01 | 0.11 | ||||||
Note. N = 117. Models 3 – 5 control for the effect of infants’ behavioral reactivity to the challenge task on cortisol levels, reactivity, and recovery to the challenge task. For Model 4 ‘moderator’ refers to mothers’ positive affect during the marital discussion. For model 5 ‘moderator’ refers to mothers’ intrusion during the mother-infant free-play. M = maternal, S1 = estimates values at S1 compared to S2; S3 = estimates values at S3 compared to S2, conflict: 0 = positive, 1= conflict.
<.1;
p<.05;
p<.01.
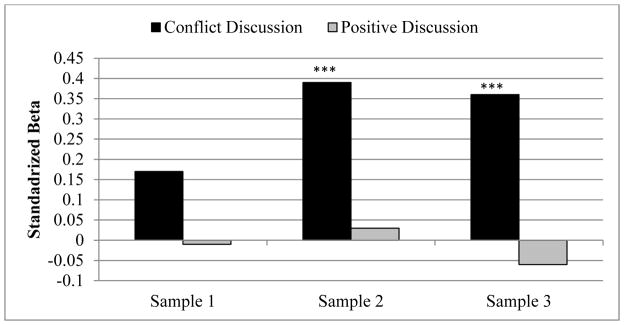
Next, we determined if conflict predicted maternal cortisol transmission by examining mothers’ discussion group as a possible moderator of the maternal-infant cortisol association (see Table 4, for model 3 estimates with S2 as the reference). Maternal cortisol did not predict infant cortisol at the S1 collection for dyads in either the positive (b = −.01, SE = .09; p = .90) or the conflict group (b = .17, SE = .14; p = .24). However, by the S2 collection, the strength of mother-infant cortisol transmission differed between the conflict and positive discussion groups (b = .36, SE = .15, p = .02; bMCortisol*conflict). Notably, only for the conflict group did higher MS2 cortisol predict higher IS2 cortisol (b = .39, SE = .08, p < .001); this was not the case for the positive discussion group (b = .03, SE = .12; p = .78). Likewise, MS3 cortisol predicted IS3 in the conflict group (b = .36, SE = .10; p < .001) but did not predict infant cortisol in the positive group (b = −.06, SE = .10; p = .56; see Figure 3). Marital conflict explained an additional 9.2% of the between infant variation and .3% of the within infant variation, beyond maternal cortisol. Likewise, regression change score analyses revealed interactions between discussion group and reactivity (b = .40, SE = .15, p = .009) as well as recovery (b = .30, SE = .11, p = .009). Specifically, maternal reactivity only predicted infant reactivity (positive: b = .01, SE = .12, p = .95; conflict: b = .59, SE = .12, p = .002) and maternal recovery only predicted infant recovery (positive: b = .18, SE = .13, p = .19; conflict: b = .30, SE = .12, p = .02) in the conflict group. In sum, at S1 there are no group differences in transmission (i.e., no transmission occurring in either group). Only after mothers engaged in the conflict task did their cortisol predict infant cortisol, with higher maternal cortisol levels, reactivity, and recovery predicting higher infant levels, reactivity, and recovery.
Figure 3.
Standardized betas representing the association between mother and infant cortisol at each sampling point, by discussion group. ***p<.001.
3) Marital emotions will predict mother-to-infant cortisol transmission
Maternal affect during the marital discussion was then examined as a predictor of transmission. Controlling for the effect of infant behavioral reactivity on infant cortisol trajectories, neither maternal negativity (b = −.03, SE = .10, p = .74) nor positive affect (b = .02, SE = .07, p = .77) were related to infant cortisol reactivity. Likewise, mothers’ negativity did not predict transmission of cortisol levels at S2 (b = .04, SE = .09, p = .62), nor induce greater transmission from S1 to S2 (b = −.11, SE = .12, p = .34). Though there was no difference in mother-infant cortisol transmission at S1 based on positive affect (b = −.07, SE = .07; p = .32), by the S2 collection, the strength of mother-infant cortisol transmission depended on mothers’ positive affect (b = −.22, SE = .09, p = .01; bMCortisol*moderator; see Table 4 model 4 for estimates with S2 as the reference). Based on a mean split, only when mothers expressed low positive affect did higher MS2 cortisol predict higher IS2 cortisol (b = .41, SE = .09, p < .001); this was not the case for high positive affect (b = .06, SE = .11; p = .59). Maternal positive affect explained an additional 0.1% of the between infant variation and 1% of the within infant variation in cortisol, beyond maternal cortisol.
Similarly, the effect of maternal positivity moderated the mother-infant cortisol reactivity association in the OLS regression utilizing change scores (b = −.31, SE = .10, p = .004), though not the recovery association (b = .10, SE = .12, p = .37). When the effect of conflict on transmission was entered into model 4, the conflict effect and the positive affect effect were both diminished to nonsignificant values (p > .05; likely due to suppression from multicollinearity, Tu, Gunnell, & Gilthorpe, 2008).
4) Negative parenting will predict mother-to-infant cortisol transmission
Parenting was then examined as a predictor of transmission, controlling for the effect of conflict on transmission. First, we examined maternal emotional disengagement followed by maternal intrusion (see Table 4, for model 5 estimates with S2 as the reference). Infants with more disengaged mothers had higher IS2 cortisol (b = .19, SE = .09, p = .02), though maternal disegagement did not predict transmission of cortisol levels at S2 (b = .06, SE = .10, p = .52), nor did disegagement induce greater transmission from S1 to S2 (b = −.06, SE = .12, p = .63). Similarly, maternal emotional disegagement did not predict transmission of cortisol reactivity using a change score (b = −.42, SE = .24, p = .09).
Although maternal intrusion was not related to infant cortisol reactivity (b = −.04, SE = .09, p = .64), it did predict transmission. Specifically, the strength of transmission changed from S1 to S2, and this change was moderated by mothers’ level of intrusion (b = −.23, SE = .11, p = .03; bMCortisol*moderator*S1). Based on a mean split of intrusion, transmission was examined across the tasks in mothers exhibiting high and low levels of intrusive behavior, separately, controlling for the effect of conflict on transmission. For mothers with intrusive behaviors above the mean, cortisol transmission was greater at S2 than at S1 (b = −.36, SE = .15, p = .02) however, for mothers with below average intrusion, transmission did not change across the task (b = .01, SE = .13, p = .96). Maternal intrusion explained an additional 2.6% of the between infant variation and 2.3% of the within infant variation in cortisol, beyond maternal cortisol and marital conflict. Again controlling for the effect of conflict on transmission, maternal intrusive parenting moderated mother-infant cortisol reactivity (b = −.48, SE = .19, p = .01), in the OLS regression utilizing change scores. In other words, for mothers with higher levels of intrusive parenting, maternal cortisol levels post-discussion predicted infant cortisol levels post-challenge task, and the degree of change from pre- to post-task was related within the dyad.
Discussion
This study utilized an experimental design to examine the association between maternal and infant cortisol reactivity. Specifically, mothers were randomized to either engage in a marital conflict or a positive marital discussion, and afterwards, infants underwent a fear and frustration inducing challenge. Findings revealed cortisol reactivity and post-task cortisol levels for mothers in the conflict group predicted their infants’ cortisol reactivity and post-task cortisol levels. No relations were found between mother and infant cortisol for the mothers in the positive discussion group. Importantly, only cortisol reactivity indicative of mothers’ and infants’ respective challenges was strongly related; pre-task levels were not. In other words, transmission appears to be induced by mothers’ engagement in the marital conflict. Further, mothers’ ability to recover, and down-regulate cortisol after the conflict, predicted the degree to which their infants were able to recover from their challenge. Similarly, mother-infant adrenocortical transmission was facilitated when mothers expressed low levels of positive affect during the marital discussion and high levels of intrusion during the mother-infant free-play. Together, this suggests that a mother’s adrenocortical activation in the context of family stress has implications for her infant’s adrenocortical regulation in times of challenge.
Early experiences play an important role in calibrating the response dynamics of stress physiology, assisting in the adaptation to analogous environments the infant may encounter in the future (Del Giudice, Ellis, & Shirtcliff, 2011). Both highly positive and highly negative experiences are hypothesized to foster the development of heightened physiological stress reactivity, increasing the individual’s adaptability and sensitivity to contextual challenges and opportunities (Del Giudice et al., 2011). Thus, mothers’ transmission, and infants’ reception, of stress physiology could function to communicate and detect immediate threats and danger. In the long term, this process might shape the developing infant’s physiological sensitivity and adaptability to future contexts. The current findings support maternal transmission of stress reactivity as a potential mechanism of calibration, with higher maternal cortisol levels and reactivity in the conflict group predicting higher infant cortisol levels and reactivity to the infant challenge. Foremost, mothers’ stress appears to be an important contributor to infant cortisol, explaining roughly 20% of the between infant variance in cortisol. However, the current study does not support a ‘duality’ of transmission (i.e., transmission in both positive and negative contexts). Specifically, transmission was only found in the conflict group, implying there may be differences in the nature of adrenocortical reactivity between a negative and positive experience, and perhaps only ‘stress’ reactivity may facilitate transmission.
The degree of mothers’ adrenocortical activation, however, did not differ between the conflict and positive groups. Mothers in both the positive and conflict groups exhibited physiological reactivity to their respective tasks. Negative and rewarding stimuli may recruit neuroendocrine activity (Koolhaas et al., 2011), and thus, reactivity by mothers in the positive discussion may reflect distinct behavioral demands compared to the conflict group. Similar reactivity profiles, but differing transmission, suggests adrenocortical activation alone is not enough to induce transmission. Differential transmission between the positive and conflict group underscores behavioral, emotional, or cognitive processes as mechanisms of transmission. It seems that there is something unique about the mother’s emotional or cognitive experience of stress, during or after engaging in a conflict with her partner, which facilitates transmission.
Emotions allow individuals to effectively communicate and convey their motives and intentions, and social animals have evolved unique sensitivities to be receptive of others’ emotions (e.g., Barsäde & Gibson, 1998). The transmission and reception of emotions across individuals therefore facilitates sociality by coordinating group behaviors and creating a mutual understanding of group needs (e.g., Butler, 2011). Our findings show that, like emotions, physiology can also be transmitted. Based on past studies (Papp et al., 2009; West et al., 2014) we expected negative emotions in particular to be responsible for this physiological transfer. Interestingly, the current findings suggest that it is not negative emotions that facilitate transmission, but positive emotions that buffer transmission. Specifically, examination of transmission across the entire sample revealed a main effect of mothers’ cortisol on infant cortisol. However, including mothers’ positive affect during the marital discussion as a moderator, revealed transmission only occurred when positive emotions were low, but not when discussions were marked with affection, laughter, and happiness. When mothers’ engaged in conversations characterized by positive affect neither her adrenocortical reactivity to the discussion, nor her recovery from the discussion, were related to the reactivity or the recovery of her infant. It is interesting that mothers’ outward negative affect during the marital discussion does not appear to facilitate transmission, yet the lack of positive affect does. It could be that the presence or absence of positive affect during a conflict is a better barometer of the quality of the interaction, compared to negativity. Though conflict is ubiquitous in close relationships, not all conflict is detrimental to the health and well-being of the couple (Greeff & Bruyne, 2000), and not all parents show disrupted parenting (Sturge-Apple et al., 2014). Interactions lacking positive emotions and affection between the partners might indicate pervasive relationship problems (Coyne, Thompson, & Palmer, 2002) and risk for stress spillover. However, it is important to note the difference in variance explained in infant cortisol between the conflict model and the model utilizing positive affect. Specifically, including the discussion grouping variable into the model explains an additional 9% of infant cortisol while positive affect only explains an additional 1%. In other words, conflict is inducing stress transfer via mechanisms other than just (the lack of) maternal positive affect. Future studies should continue to investigate the mechanisms by which stress transfers from mother to child.
In a similarly designed study to ours, maternal autonomic stress physiology was found to transfer to the infants while merely sitting on their mothers’ lap (Waters, et al., 2014), suggesting the existence of subtle channels (e.g., facial expressions, odor, posture, and/or fluctuations in vocal patterns) by which mothers’ physiology transfers to the child (LeDoux, 1996; Zhou & Chen, 2009). Our study provides two important extensions of this work. First, the Waters and colleagues experiment utilized a classic laboratory stress paradigm known to elicit a strong physiological stress response: the Trier Social Stress Task. The current analyses suggest that transmission may occur with a frequently occurring ecologically valid stressor, an episode of marital disagreement. Second, the experiment by Waters and colleagues did not include measures of maternal behavior. The authors hypothesize harsh maternal behaviors may be the proximate cause in infants’ physiological change, but do not test this. Our study empirically tests and supports the idea that mothers’ intrusive behavior serves to co-regulate mother and infant physiological reactivity.
The mother-child relationship is considered the first social relationship; however, this bond is distinct from other social bonds given the human infant is born underdeveloped and helpless. Thus, the mother-child relationship is characterized by intensive and prolonged caregiving (Flinn, Ward, & Noone, 2005). During this time of extended development, it has been hypothesized that young children concentrate their energy learning, primarily from their mothers, to regulate their emotional, behavioral, and physiological responses to social interactions (Kenrick et al., 2013). When parents respond to infant cues with body contact and interactional warmth and support, they provide external soothing and serve as effective co-regulators. However, neglectful parenting and/or punitive and controlling disciplinary practices directly and indirectly stimulate the child’s development of high behavioral and physiological reactivity during times of challenge (e.g., Blair, 2010; Repetti, Robles, & Reynolds, 2011). In support of this past work, we found poor parenting practices are linked to multiple aspects of infants’ physiological regulation to challenge.
Though not part of our main goals, we found detached maternal behaviors were related to infants’ increased adrenocortical levels following the challenge task. The infants in the current study were 6 months old and early in their development of self-regulation, and thus, had few well-developed coping resources of their own. Our findings support previous studies showing even a moderately detached mother is behaviorally and physiologically stressful for infants (Haley & Stansbury, 2003; Toda & Fogel, 1993). It could be that maternal detachment is particularly salient at this developmental stage. Interestingly, maternal detachment did not predict adrenocortical transmission. This might imply that transmission requires active behavioral engagement between the mother and infant (as opposed to the withdrawn and disengaged behaviors characterized by detachment). Our findings seem to suggest that although the lack of maternal external regulation may lead to infant over-arousal, it is not necessarily due to the mother’s over-arousal.
Further, independent of conflict, maternal intrusion facilitated adrenocortical transmission. Intrusive mothers in this study were overstimulating, did not slow down in response to infant cues, and were rough when interacting with their infant. It is possible that heightened maternal stress physiology could also induce heightened stress physiology in their infant through harsh touching. Affectionate touch is an important source of external regulation, with the power to calm, soothe, and reduce heightened physiology (Feldman, 2012). Heightened skin-to-skin contact in mother-infant dyads has been found to increase covariation of cortisol levels, and decrease infant stress reactivity as a result (e.g., Mörelius et al., 2015). These findings illuminate the dynamic relationship between parents and infants, highlighting changes in mothers’ sensitive and responsive emotional and behavioral states as down-regulators of infants’ own physiological states. It is possible that, in the same vein, a mother’s harsh and rough touch may transmit heightened physiological reactivity to the child (Waters, West, Karnilowicz, & Mendes, 2017). Interestingly, it appears that the pathways by which conflict and intrusion predict transmission are independent. Specifically, conflict continued to predict transmission, even after including intrusion in the model. This suggests that although stress may induce transmission at the group level, for some mothers (e.g., intrusive mothers) transmission may be regularly occurring in the context of harsh mother-infant interactions. That said, comparing the percent variance explained between the intrusion and conflict models, it appears that maternal conflict is a more important contributor to infant physiology, than intrusive behaviors.
The current study has several limitations that should be noted. First, the participants were predominantly married and white (reflective of the geographical location in which the study was conducted). The homogeneous nature of the participants excludes extension of these findings to other populations, yet potentially provides a baseline for the comparison of other populations. The sample was, however, fairly heterogeneous with regard to household income. Second, though the tasks involved were designed to represent ecologically valid challenges and situations, this study was carried out in a laboratory setting, and behaviors and physiology were most likely influenced by this environment. Third, infant factors such as prematurity, cosleeping, and breastfeeding status have been found to relate to cortisol levels (e.g., Grunau et al., 2007; Waynforth, 2007). These variables were not assessed and therefore may contribute to unexplained variance in the models. Fourth, it is possible that our saliva collection design affected our results. We capitalized on the 15–20 minute time lag between an external stressor and the measurement of peak salivary cortisol reactivity and collected the mother’s first saliva sample immediately after her discussion with her partner. This timing allowed our participants 40 minutes to acclimate to the lab setting, but may have resulted in a heightened sample 1 cortisol level due to some early task reactivity. Fifth, the randomization process might not have controlled for all pre-existing mother, child, and family characteristics. It is possible that these characteristics might have influenced the results. Lastly, though we felt the AIC was a better indication of model fit given our model structure, it should be noted that the BIC did not support all of the moderation models. Regardless of these limitations, the findings highlight new and important ways in which early social interactions and family dynamics influence infant physiology. In particular, the current findings underscore the ability for mothers’ heightened physiological reactivity in the context of distress to upregulate infant physiology.
Conclusion
The current investigation highlights the ability for mothers to transmit, and infants to receive, biological signals of the mother’s emotional valence. Infant biobehavioral processes appear to be particularly open to external regulation, and theorists have hypothesized that these channels of transference may serve to teach infants about the world around them (Champagne & Meaney, 2001). These signals potentially have long term implications by modifying and programing the infant’s lifelong biobehavioral responses to environmental demands. Interesting, the ability to receive another’s biobehavioral signals is not limited to infancy, and reports show these connections in salient adult relationships as well (Liu, Rovine, Cousino-Klein, & Almeida, 2013; Mercado & Hibel, 2017). Interpreting social and contextual cues, and responding appropriately is critical for a social animal like a human. Thus, these interpersonal biobehavioral connections are potentially retained to facilitate complex adult social interactions. Future investigations should continue to examine the factors that cause, potentiate, interrupt, and re-establish physiological connections across a broad range of social relationships, focusing on how the nature of these exchanges develops over the life course.
Acknowledgments
The authors gratefully acknowledge the helpful feedback Drs. Jay Belsky and Douglas Granger provided on earlier versions of this manuscript and to Dr. Wan Yeung for statistical assistance. In addition, we would like to thank Drs. German Posada, Jill Trumbell, and Umadevi Senguttavan, and the participants, without whom this project would not have been possible. Support for this research was provided by NICHD grant HD066269-01A1 (PI Hibel). The authors have no conflicts of interest to disclose.
References
- Albers EM, Riksen-Walraven MJ, Sweep FCGJ, deWeerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. Journal of Child Psychology and Psychiatry. 2008;49:97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Gonzalez A, Kashy DA, Santo Basile V, Masellis M, Pereira J, Levitan R. Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology. 2013;38(12):2943–2951. doi: 10.1016/j.psyneuen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Barsäde SG, Gibson DE. Group emotion: A view from top and bottom. Research on managing groups and teams. 1998;1(82):81–102. [Google Scholar]
- Beebe B. Co-constructing motherinfant distress in face-to-face interactions: Contributions of microanalysis. Infant Observation. 2006;9(2):151–164. doi: 10.1080/13698030600810409. [DOI] [Google Scholar]
- Blair C, Raver C. Individual development and evolution: Experiential canalization of self-regulation. Developmental Psychology. 2012;48:647–657. doi: 10.1037/a0026472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver C, Granger D, Mills-Koonce WR, Hibel LC The Family Life Project Investigators. Allostasis and Allostatic Load in the Context of Poverty in Early Childhood. Development and Psychopathology. 2011;23:845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH. Toward a model of culture↔parent↔child transactions. In: Sameroff A, editor. The transactional model of development: How children and contexts shape each other. American Psychological Association; Washington DC: 2009. pp. 139–161. [DOI] [Google Scholar]
- Brock RL, Kochanska G. Interparental conflict, children’s security with parents, and long-term risk of internalizing problems: A longitudinal study from Age 2 to 10. Development and Psychopathology. 2015:1–10. doi: 10.1017/S0954579415000279. [DOI] [PMC free article] [PubMed]
- Butler EA. Temporal Interpersonal Emotion Systems The “TIES” That Form Relationships. Personality and Social Psychology Review. 2011;15(4):367–393. doi: 10.1177/1088868311411164. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Cox MJ, Paley B. Families as systems. Annual Review of Psychology. 1997;48(1):243–267. doi: 10.1146/annurev.psych.48.1.243. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Easterbrooks MA, Emde RN. Marital and parent-child relationships: The role of affect in the family system. Relationships within families: Mutual influences. 1988:83–103. doi: 10.1177/019251398019006002. [DOI]
- Feinberg ME. Coparenting and the transition to parenthood: A framework for prevention. Clinical Child and Family Psychology Review. 2002;5(3):173–195. doi: 10.1023/A:1019695015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007;48(3–4):329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R. Bio-behavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting. 2012;12(2–3):154–164. doi: 10.1080/15295192.2012.683342. [DOI] [Google Scholar]
- Flinn MV, Ward CV, Noone R. Hormones and the human family. In: Buss D, editor. Handbook of evolutionary psychology. Wiley; New York: 2005. pp. 552–580. [Google Scholar]
- Goldsmith HH, Rothbart MK. The Laboratory Temperament Assessment Battery (Lab-TAB): Locomotor Version (No. 88-01) Oregon Center for the Study of Emotion; 1988. [Google Scholar]
- Granger DA, Cicchetti D, Rogosch F, Hibel LC, Teisl M, Flores E. Blood contamination in children’s saliva: Prevalence, stability, and impact on the measurement of salivary cortisol, testosterone, and dehydroepiandrosterone. Psychoneuroendocrinology. 2007;32:724–733. doi: 10.1016/j.psyneuen.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. Journal of Pediatrics. 2007;150:151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Doom JR, Esposito EA. Psychoneuroendocrinology of stress. Handbook of Child Psychology and Developmental Science. 2015 doi: 10.1002/9781118963418.childpsy304. [DOI]
- Hibel LC, Granger DA, Blair C, Cox MJ. Intimate partner violence moderates the association between mother–infant adrenocortical activity across an emotional challenge. Journal of Family Psychology. 2009;23(5):615. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox MJ. Maternal sensitivity buffers the adrenocortical implications of intimate partner violence exposure during early childhood. Development and Psychopathology. 2011;23(02):689–701. doi: 10.1017/S0954579411000010. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Trumbell JM, Mercado E. Work/non-workday differences in mother, child, and mother–child morning cortisol in working mothers and their children. Early Human Development. 2014;90(1):1–7. doi: 10.1016/j.earlhumdev.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Mash EJ. A measure of parenting satisfaction and efficacy. Journal of Clinical Child Psychology. 1989;18(2):167–175. doi: 10.1207/s15374424jccp1802_8. [DOI] [Google Scholar]
- Jouriles EN, Farris AM. Effects of marital conflict on subsequent parent-son interactions. Behavior Therapy. 1992;23(3):355–374. doi: 10.1016/S0005-7894(05)80163-7. [DOI] [Google Scholar]
- Kenrick DT, Neuberg SL, White AE. Relationships from an evolutionary life history perspective. The Oxford Handbook of Close Relationships. 2013:13–38. [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda BD, De Boer SF, Flügge G, Korte SM, … Richter-Levin G. Stress revisited: a critical evaluation of the stress concept. Neuroscience and Biobehavioral Reviews. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, Measelle J. Risky shifts: How the timing and course of mothers’ depressive symptoms across the perinatal period shape their own and infant’s stress response profiles. Development and Psychopathology. 2011;23(02):521–538. doi: 10.1017/S0954579411000083. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, Measelle J. Taking stress response out of the box: Stability, discontinuity, and temperament effects on HPA and SNS across social stressors in mother–infant dyads. Developmental Psychology. 2012;48(1):35. doi: 10.1037/a0025518. http://psycnet.apa.org/doi/10.1037/a0025518. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain: The Mysterious Underpinnings of Emotional Life. Simon and Schuster; New York: 1996. [Google Scholar]
- Levendosky AA, Graham-Bermann SA. Behavioral observations of parenting in battered women. Journal of Family Psychology. 2000;14(1):80. doi: 10.1037//0893-3200.14.1.80. http://psycnet.apa.org/doi/10.1037/0893-3200.14.1.80. [DOI] [PubMed] [Google Scholar]
- Malik NM, Lindahl KM. System for Coding Interactions in Dyads (SCID) In: Kerig PK, Baucom DH, editors. Couple observational coding systems. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2004. pp. 191–208. [Google Scholar]
- Middlemiss W, Granger DA, Goldberg WA, Nathans L. Asynchrony of mother–infant hypothalamic–pituitary–adrenal axis activity following extinction of infant crying responses induced during the transition to sleep. Early Human Development. 2012;88(4):227–232. doi: 10.1016/j.earlhumdev.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Mörelius E, Örtenstrand A, Theodorsson E, Frostell A. A randomised trial of continuous skin-to-skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Human Development. 2015;91(1):63–70. doi: 10.1016/j.earlhumdev.2014.12.005. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Child care and mother-child interaction in the first 3 years of life. Developmental Psychology. 1999;35:1399–1413. http://psycnet.apa.org/doi/10.1037/0012-1649.35.6.1399. [PubMed] [Google Scholar]
- Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: Within-family cortisol associations and moderators. Journal of Family Psychology. 2009;23:882–894. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti R, Robles T, Reynolds B. Allostatic processes in the family. Development and Psychopathology. 2011;23:921–938. doi: 10.1017/S095457941100040X. http://doi:10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: Pathways to health. Physiology and Behavior. 2003;79(3):409–416. doi: 10.1016/S0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981:569–578. doi: 10.2307/1129176. [DOI]
- Salimetrics LLC. Saliva Collection and Handling Advice. 3. State College, PA: Salimetrics LLC; 2015. [Google Scholar]
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference: Wadsworth Cengage learning 2002 [Google Scholar]
- Singer JD, Willet JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. A framework for investigating change over time; pp. 3–15. [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. Journal of Marriage and Family. 1976:15–28. doi: 10.2307/350547. [DOI]
- Stifter CA, Braungart JM. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31(3):448. http://psycnet.apa.org/doi/10.1037/0012-1649.31.3.448. [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scales (CTSZ) Development and Preliminary Psychometic Data Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- Sturge-Apple ML, Davies PT, Cummings EM. Hostility and withdrawal in marital conflict: effects on parental emotional unavailability and inconsistent discipline. Journal of Family Psychology. 2006;20(2):227. doi: 10.1037/0893-3200.20.2.227. http://psycnet.apa.org/doi/10.1037/0893-3200.20.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, Manning LG. Interparental violence, maternal emotional unavailability and children’s cortisol functioning in family contexts. Developmental psychology. 2012;48:237–49. doi: 10.1037/a0025419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LA, Trevathan WR. Cortisol reactivity, maternal sensitivity, and learning in 3-month-old infants. Infant Behavior and Development. 2008;31(1):92–106. doi: 10.1016/j.infbeh.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YK, Gunnell D, Gilthorpe MS. Simpson’s Paradox, Lord’s Paradox, and suppression effects are the same phenomenon – the reversal paradox. Emerging Themes in Epidemiology. 2008;5:1–9. doi: 10.1186/1742-7622-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Cash E, Daup M, Geronimi EMC, Sephton SE, Wood-Borden J. Exploring patterns in cortisol synchrony among anxious and nonanxious mother and child dyads: A preliminary study. Biological Psychology. 2013;93:287–295. doi: 10.1016/j.biopsycho.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Waters SF, West TV, Mendes WB. Stress contagion: Physiological covariation between mothers and infants. Psychological Science. 2014;25(4):934–942. doi: 10.1177/0956797613518352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waynforth D. The influence of parent-infant co-sleeping, nursing, and childcare on cortisol and SIgA immunity in a sample of British children. Developmental Psychobiology. 2007;49:640–648. doi: 10.1002/dev.20248. [DOI] [PubMed] [Google Scholar]
- Zhou W, Chen D. Fear-related chemosignals modulate recognition of fear in ambiguous facial expressions. Psychological Science. 2009;20(2):177–183. doi: 10.1111/j.1467-9280.2009.02263.x. [DOI] [PubMed] [Google Scholar]