Abstract
Rationale & Objective:
Traditional risk estimates for atherosclerotic vascular disease (ASVD) and death may not perform optimally in the setting of CKD. We sought to determine whether the addition of measures of inflammation and kidney function to traditional estimation tools improves prediction of these events in a diverse cohort of patients with CKD.
Study Design:
Observational cohort study
Setting & Participants:
2399 Chronic Renal Insufficiency Cohort (CRIC) study participants without history of cardiovascular disease at study entry.
Predictors:
Baseline plasma levels of biomarkers of inflammation (interleukin (IL)-1β, IL-1RA (IL-1 receptor antagonist), IL-6, tumor necrosis factor (TNF)-α, transforming growth factor β (TGFβ), high sensitivity C-Reactive protein (hs-CRP), fibrinogen, and serum albumin), measures of kidney function (estimated glomerular filtration rate (eGFR) and albuminuria), and the Pooled Cohort Equation Probability (PCEP) estimate.
Outcomes:
Composite of ASVD events (incident myocardial infarction (MI), peripheral arterial disease (PAD), and stroke) and death.
Analytical Approach:
Cox proportional hazard models adjusted for PCEP estimates, albuminuria, and eGFR.
Results:
During a median follow-up of 7.3 years, 86, 61, 48, and 323 participants experienced MI, PAD, stroke, or death, respectively. 1-decile greater levels of IL-6 (adjusted Hazard Ratio [aHR], 1.12; 95% CI, 1.08-1.16; p<0.001), TNF-α (aHR, 1.09; 95% CI, 1.05-1.13; p<0.001), fibrinogen (aHR, 1.07; 95% CI, 1.03-1.11; p<0.001), and serum albumin (aHR, 0.96; 95% CI, 0.93-0.99; p<0.002) were independently associated with the composite ASVD-death outcome. A composite inflammation score (CIS) incorporating these four biomarkers was associated with a graded increase in risk for the composite outcome. The incidence of ASVD-death increased across the quintiles of risk derived from PCEP, kidney function, and CIS. The addition of eGFR, albuminuria, and CIS to PCEP improved (p=0.003) the area under the receiver operating characteristic curve for the composite outcome from 0.68 (95% CI, 0.66-0.71) to 0.73 (95% CI, 0.71-0.76).
Limitation:
Data on cardiovascular death were not available.
Conclusion:
Biomarkers of inflammation and measures of kidney function are independently associated with incident ASVD events and death in CKD patients. Traditional cardiovascular risk estimates could be improved by adding markers of inflammation and measures of kidney function.
Keywords: myocardial infarction (MI), cytokines, C-reactive protein (CRP), stroke, cardiovascular disease (CVD), atherosclerosis, Pooled Cohort Equation Probability (PCEP), atherosclerotic vascular disease (ASVD), inflammatory biomarkers, risk stratification, kidney function, chronic kidney function (CKD), estimated glomerular filtration rate (eGFR), albuminuria
Introduction
Atherosclerosis is a chronic immuno-inflammatory, fibro-proliferative disease that is characterized by lipid infiltration.1 Chronic kidney disease (CKD) patients have elevated circulating levels of acute phase proteins and pro-inflammatory cytokines.2 Considerable evidence indicates that biomarkers of inflammation are associated with adverse cardiovascular outcome in the general population.3 The American College of Cardiology (ACC) and American Heart Association (AHA) Task Force on Practice Guidelines opined that that assessment of C-Reactive protein (CRP) levels is reasonable for patients at intermediate cardiovascular disease (CVD) risk category.4 In population-based cohorts with participants in early stages of CKD or studies involving small numbers of dialysis patients, measurement of a limited number of biomarkers has suggested an association between inflammation and CVD outcomes.5-7 However, these results have not been confirmed in a larger and more ethnically diverse CKD cohorts with a wide range of glomerular filtration rates (GFR) and long-term follow-up for clinical outcomes. Recently, the ACC-AHA introduced the new Pooled Cohort Equations to estimate 10-year atherosclerotic vascular disease (ASVD) risk;8 however, to our knowledge, the applicability of this risk estimate to CKD patients has not been tested.
In this study, we examine the association of incident ASVD events and death with a panel of circulating inflammatory biomarkers and kidney function measures in participants from the Chronic Renal Insufficiency Cohort (CRIC) study.9 We also evaluate whether adding measures of kidney function and biomarkers of inflammation improve upon traditional risk variables for risk-stratifying CKD patients for CVD outcomes and death. One of the purposes of this manuscript is to provide results that can inform the development of future risk prediction equations.
Methods
Study Participants
The CRIC Study includes 3,939 racially and ethnically diverse group of men and women with CKD recruited from seven clinical sites within the US, aged 21 to 74 years at entry, and with approximately half of all participants having diabetes mellitus.10 The main exclusion criteria were cirrhosis, class III or IV heart failure, HIV infection, cancer, active or recent immunosuppression, polycystic kidney disease, pregnancy. The study complies with the Declaration of Helsinki and is approved by the Institutional Review Board at each participating site. All participants provided written informed consent. For this study, we excluded participants who had any history of CVD at baseline, and those with missing medication data. The enrollment period was 2003 to 2007 and the administrative censoring date was March 2013.
CRIC Data Collection
Demographic characteristics, medical history, and medication use were recorded at baseline. Serum creatinine concentration was measured by coupled enzymatic method. Glomerular filtration rate was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation. Albuminuria was assessed by urinary albumin-creatinine ratio (UACR). Diabetes was defined as fasting glucose ≥126 mg/dL (7 mmol/L), random glucose ≥200 mg/dL (11.1 mmol/L) or use of insulin or anti-diabetic medication. Hypertension (HTN) was defined as systolic blood pressure (SBP) ≥140 mm Hg, and/or diastolic blood pressure (DBP)≥ 90 mm Hg, and/or self-reported antihypertensive medication use.
Event Adjudication
The study participants were queried twice annually during study visits about possible CVD events, death, and hospitalizations. When the ICD-9 discharge codes indicated myocardial infraction (MI), cerebrovascular accident (stroke), peripheral arterial disease (PAD) or death, medical records were reviewed using event-specific guidelines by two physicians. MI was adjudicated based on a combination of abnormalities of the electrocardiogram, elevations of cardiac injury enzymes and the presence of symptoms. PAD, as ascertained by a trained nurse abstractor, necessitated evidence of amputation or peripheral surgical or percutaneous revascularization procedures. Two neurologists adjudicated stroke based on clinical and radiographic evidence of ischemic stroke or intracranial hemorrhage.
Measurement of Biomarkers of Inflammation
Biomarkers were measured in baseline blood samples at the time of initial thawing.2 High-sensitivity ELISAs (Quantikine HS, R&D Systems, Minneapolis, MN) were used to measure plasma interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α levels. Standard ELISAs (Quantikine, R&D Systems) were used to quantify IL-1 receptor antagonist (IL-1RA) and transforming growth factor β (TGFβ) levels. All assays were performed in duplicate and mean values were used in the analysis. The coefficient of variation was <13% for all cytokines assays except for TNF-α and TGFβ, for which the estimated imprecisions were 15.2% and 21.5%, respectively. High sensitivity (hs)-CRP and fibrinogen were quantified in EDTA plasma samples using specific laser-based immunonephelometric methods on the BNII (Siemens Healthcare Diagnostics, Deerfield, IL). The imprecision for hs-CRP and fibrinogen was < 5%. Serum albumin was measured by Bromcresol Green method. These biomarkers were chosen based on their potential role in CVD in CKD.11;12
Predictors and Outcomes
The predictors were baseline measures of IL-1β, IL-1RA, IL-6, TNF-α, TGFβ, hs-CRP, fibrinogen, serum albumin, as well as measures of kidney function (eGFR and albuminuria). The primary outcome was a composite of incident ASVD (MI, PAD, or stroke) and death during the follow-up period. Secondary outcomes were individual components of the primary outcome, as well as a CVD composite (MI/stroke/PAD).
Statistical Analysis
Standard descriptive statistics were used to describe baseline characteristics for the full cohort. Univariate associations of inflammatory biomarkers with the composite outcome were examined using separate Cox proportional hazard models for each inflammatory biomarker. Covariates, including the natural log-transformed Pooled Cohort Equation probability (PCEP; ln[PCEP]), natural log-transformed UACR (lnUACR), and eGFR, were then added to these models in order to determine whether each inflammatory marker had an independent association with the composite outcome after adjusting for traditional risk factors and measures of kidney function. All Cox models were tested to ensure there was no violation of the proportional hazards assumption, and no problem with multicollinearity. In all survival analyses, cases without events were right-censored at last study visit. We tested for linearity of the relationship of markers with the incident ASVD–death composite outcome by examining the significance of the linear regression coefficient between marker quartile and the outcome.
In order to assess which of the inflammatory biomarkers had associations with the composite outcome that were independent of each other, a single Cox proportional hazard model was then tested including only the inflammatory biomarkers that had significant multivariable effects in the prior analysis. Those that remained significant in this model were used to create a composite inflammation score (CIS) by summing the deciles of these biomarkers. We also tested a weighted version, in which the weights were the parameter estimates in the above model. Also, in order for the hazard ratios (HR) to be used to compare the effect sizes between biomarkers, we first divided each marker by its standard deviation.
To test the improvement in prediction accuracy when kidney function variables and the CIS were added to traditional risk predictors, we examined the area under the receiver operating curve (AUC) for each model, calculated from the Cox models using the SurvCstd SAS macro13 and compared them using a chi-square test.14 Rather than testing at a single risk cut point, this method tests the overall improvement in discrimination across risk levels.
As a secondary analysis, we examined the independent associations of each inflammatory biomarker separately, with time to first MI, PAD, stroke, or death, and composite of MI, Stroke, or PAD individually, using Cox proportional hazards regression with biomarkers coded into deciles. We then examined interaction effects for each of the significant inflammatory biomarkers with baseline eGFR (<30 vs ≥ 30 ml/min/1.73m2), and albuminuria (UACR ≥ 30 vs < 30 mg/g).15 This was done using a marker x covariate interaction term in the Cox proportional hazards model, including PCEP and measures of kidney function as covariates.
We conducted the number of sensitivity analyses. First, we examined the association of the biomarkers with only the ASVD events, using a competing risk model16 to estimate the cumulative incidence function for biomarker quintile with the incident composite ASVD outcome, where death was considered as the competing risk. Second, we tested a traditional Cox model that included the variables used to compute the PCEP as individual predictors. These included age, race, sex, ln(total cholesterol) (lnTC), ln(high density lipoprotein) (lnHDL), smoking, diabetes, treated or untreated SBP. Blood pressure-treated was coded yes if patient had HTN and received angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), beta blocker, calcium channel blocker, or diuretic. The AUC of this model was computed, and then compared with the AUC when kidney function variables and the CIS were added as predictors. Third, compared the AUC for the PCEP-only model versus PCEP plus kidney function and inflammation measures, looking only at participants over age 40, and fourth, stratifying these Cox models by statin use.
We explored whether there was a cut-point relevant for clinical decision-making using either the model that included CIS, kidney function, and the PCEP, or the PCEP alone. To be clinically useful, at a minimum, the predicted outcome should have sensitivity and specificity both ≥ 0.70, and preferably ≥ 0.80. We used the logistic regression model including CIS, kidney function variables, and PCEP to calculate a predicted probability of reaching ASVD-death during follow-up, for each participant, based on the following formula: probability = exp(risk) / (1 + exp(risk)), where risk = intercept + x1(CIS) + x2(lnPCEP) + x3(lnUACR) + x4(eGFR), where each xn is a model parameter estimate.
All analyses were done using SAS (version 9.3 or 9.4, Cary, NC). p<0.05 was considered statistically significant.
Results
Participant Characteristics
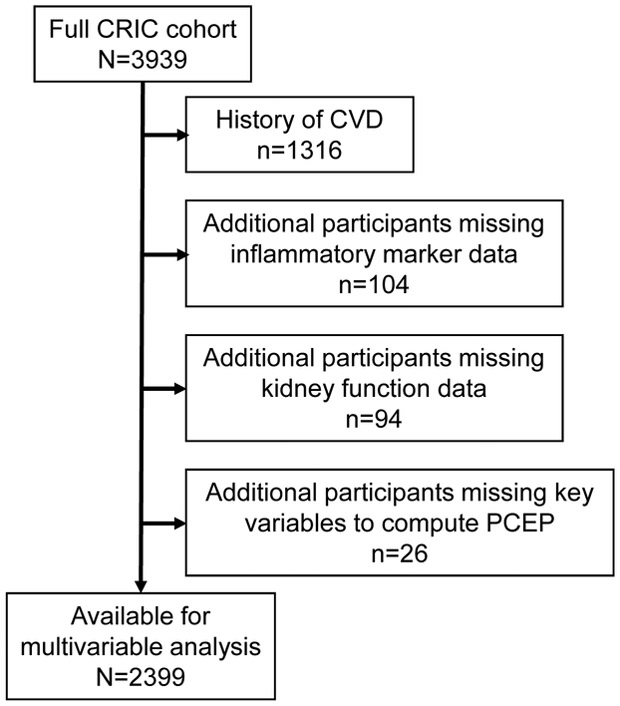
After excluding 1316 participants who had history of CVD and an additional 104 who were missing data on inflammatory markers, 94 with missing kidney function data, and 26 for whom key data needed to calculate PCEP were not available, 2399 participants were available for multivariable analysis (Figure 1). Participants were followed for a median period of 7.3 years (interquartile range [IQR], 5.4-8.4). During follow-up, 86, 61, and 48 participants developed MI, PAD, or stroke, respectively, and 323 participants died. There were 471 participants with the primary outcome, a composite of incident ASVD (MI, PAD, stroke) or death. Characteristics of study participants are shown in Table 1. The mean age of the study participants was 56 years, 48% were female, 38% were black and 41% had DM at baseline. Mean baseline eGFR was 44.1 ml/min/1.73 m2 and the median PCEP was 9.6% [IQR, 3.5%-18.8%] (ie, the 10-year risk of a first ASVD event), with 58% of the sample having baseline raw PCEP ≥ 7.5% and 46% were being treated with statins. IL-1β was highly skewed, so when this was coded into quintiles, quintiles 1 & 2 were combined.
Figure 1.
Patient flow into the study
Table 1.
Baseline characteristics of study participants
| Variable | Study cohort |
|---|---|
| Age (years) | 56.0 ± 11.6 |
| Female sex | 1144 (48%) |
| Race | |
| Black | 913 (38%) |
| White | 1192 (50%) |
| Other | 294 (12%) |
| Current smoker | 279 (12%) |
| Body mass Index (kg/m2 ) | 31.8 ± 7.8 |
| Diabetes | 995 (41%) |
| Hypertension | 1994 (83%) |
| SBP (mm Hg) | 126.7 ± 20.7 |
| DBP (mm Hg) | 72.5 ± 12.3 |
| HbA1c (%) | 6.4 ± 1.5 |
| Total Cholesterol (mmol/L) | 187.7 ± 44.0 |
| High density lipoprotein (mmol/L) | 48.7 ± 16.0 |
| eGFR (ml/min/1.73 m2) | 44.1 ± 13.9 |
| UACR (mg/g) | 39.9 [7.4 – 395.9] |
| Raw PCEP for CVD | 9.6% [3.5%-18.7%] |
| Raw PCEP ≥ 7.5% | 1393 (58%) |
| Inflammatory Biomarkers | |
| hs-CRP (mg/L) | 2.46 [1.00–6.04] |
| Fibrinogen (g/L) | 3.95 [3.31-4.66] |
| Serum albumin (g/dL) | 4.00 [3.70-4.30] |
| IL-1β (pg/mL) | 0.19 [0.06-1.27] |
| IL-1RA (pg/mL) | 694.8 [367.6-1504.2] |
| IL-6 (pg/mL) | 1.69 [1.03-2.80] |
| TNF-α (pg/mL) | 2.10 [1.40-3.20] |
| TGFβ (ng/mL) | 11.00 [6.51-17.98] |
| Medications | |
| Beta blocker | 912 (38%) |
| ACEi/ARB | 1545 (64%) |
| Calcium channel blockers | 892 (37%) |
| Diuretic | 1256 (52%) |
| Aspirin | 811 (34%) |
| Statins | 1110 (46%) |
(n=2399). Values for continuous data given as mean ± sd or median [Interquartile Range]; for categorical data as count (percentage).
ACEi/ARB= ACE inhibitors/Angiotensin receptor blockers, hs-CRP= High sensitivity C-Reactive Protein, IL= Interleukin, IL-1RA= Interleukin-1 Receptor antagonist, PCEP= Pooled Cohort Equation probability, TGF-β=Transforming growth factor β, TNF-α= Tumor necrosis factor α and UACR=Urinary albumin-creatinine ratio,
Association of Inflammatory Markers with the Composite Outcome
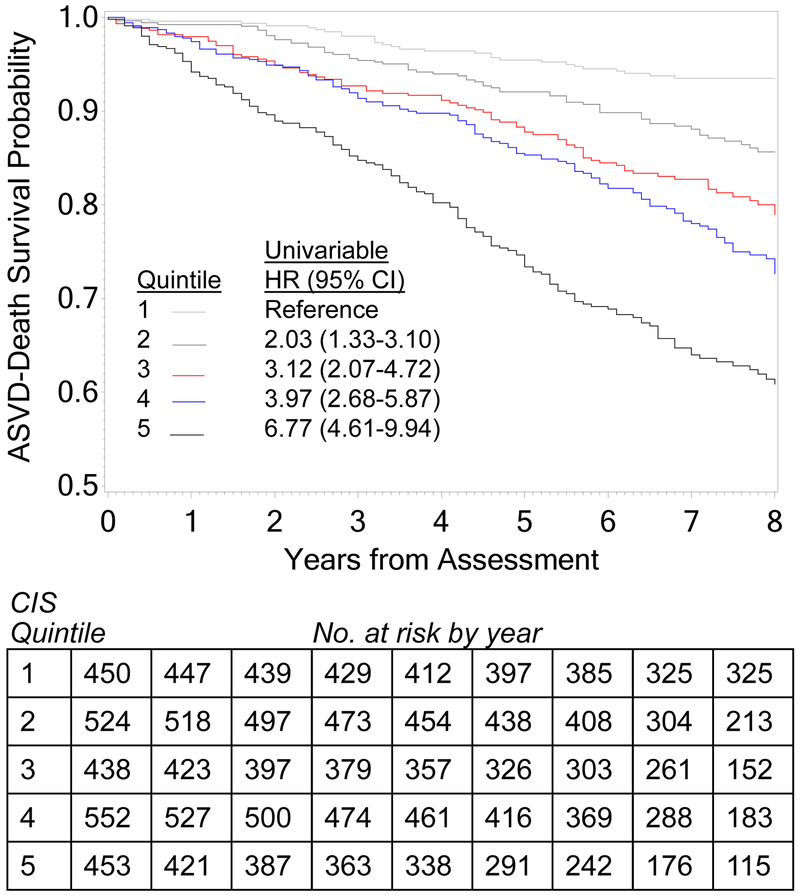
In survival analysis, all eight inflammatory markers investigated here had significant univariable associations with the composite outcome (Table 2). However, after adjusting for PCEP, eGFR, and albuminuria, only IL-6, TNF-α, hs-CRP, fibrinogen, and serum albumin remained significantly associated with the outcome. All of these markers had significant linear associations with the outcome, when tested by marker quartile (all p<0.001) (Table S1). When these five markers were combined as predictors in a single Cox proportional hazards model for the composite outcome, without other covariates, hs-CRP became non-significant, but others remained significant (Table 3). We summed the deciles for the four markers (IL-6, TNF-α, fibrinogen, and reverse-coded serum albumin) to construct the CIS. Kaplan-Meier analysis showed a significant graded positive association between quintiles of the CIS and the composite outcome (log-rank chi-square p <0.001; Figure 2). Those in the highest quintile of CIS had 3.1-fold higher hazard for the composite event, compared to those in the lowest quintile, after adjusting for baseline eGFR, proteinuria, and PCEP. Kaplan-Meier survival estimates for time to first ASVD–death event stratified by biomarker quintiles are shown in Figure S1.
Table 2.
Associations of each decile greater level of inflammatory marker with the composite outcome using separate Cox proportional hazards models for each marker.
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| Marker | HR (95% CI) | p | HR (95% CI) | p |
| IL-1β | 1.05 (1.02-1.09) | 0.002 | 1.01 (0.98-1.05) | 0.5 |
| IL-1RA | 1.04 (1.01-1.07) | 0.02 | 1.01 (0.98-1.04) | 0.5 |
| IL-6 | 1.19 (1.15-1.23) | <0.001 | 1.12 (1.08-1.16) | <0.001 |
| TGFβ | 1.04 (1.01-1.08) | 0.008 | 1.03 (0.99-1.06) | 0.1 |
| TNF-α | 1.17 (1.13-1.21) | <0.001 | 1.09 (1.05-1.13) | <0.001 |
| hs-CRP | 1.10 (1.06-1.13) | <0.001 | 1.08 (1.05-1.12) | <0.001 |
| Fibrinogen | 1.16 (1.13-1.20) | <0.001 | 1.07 (1.03-1.11) | <0.001 |
| Serum albumin | 0.90 (0.87-0.92) | <0.001 | 0.96 (0.93-0.99) | 0.02 |
The multivariable models were adjusted for Pooled Cohort Equation probability, albuminuria, and eGFR. The composite outcome is ASVD (MI, PAD, stroke) or death.
hs-CRP= High sensitivity C-Reactive Protein, IL= Interleukin, IL-1RA= Interleukin 1 Receptor antagonist, TGFβ=Transforming growth factor β, and TNF-α= Tumor necrosis factor α ; HR, hazard ratio; CI, confidence interval
Table 3.
Cox proportional hazard model for the composite outcome using significant inflammatory markers together in one model with no other covariates.
| Marker | HR (95% CI) | p | Parameter estimate (SE) |
|---|---|---|---|
| IL-6 | 1.12 (1.08-1.17) | <0.001 | 0.112 (.02) |
| TNF-α | 1.10 (1.06-1.14) | <0.001 | 0.097 (.02) |
| hs-CRP | 0.99 (0.95-1.03) | 0.5 | −0.013 (.02) |
| Fibrinogen | 1.07 (1.03-1.12) | <0.001 | 0.069 (.02) |
| Serum Albumin | 0.95 (0.92-0.99) | 0.004 | −0.050 (.02) |
HR is shown for 1-decile greater level of the inflammatory marker. The composite outcome is ASVD (MI, PAD, stroke) or death.
hs-CRP= High sensitivity C-Reactive Protein, IL= Interleukin and TNF-α= Tumor necrosis factor α; HR, hazard ratio; CI, confidence interval ; SE, standard error
Figure 2.
Kaplan Meier estimates of freedom from the ASVD-death composite outcome stratified by quintile of the composite inflammation score (CIS), which was computed by adding the deciles of IL-6, TNF-α, fibrinogen and reverse-coded serum albumin. Log-rank chi-square 162.28 (p<0.001). Adjusted hazard ratios (HR) for quintiles 2 through 5 are shown, versus quintile 1, based on a multivariable Cox proportional hazard model using CIS quintile coded as a class variable, adjusting for PCEP, eGFR, and UACR.
When the CIS was included in a Cox proportional hazard model for the composite outcome with PCEP, eGFR, and albuminuria, it remained significant, as did the other three covariates (Table 4). After standardizing the covariates so that their effect sizes could be compared using z-scores, and re-running this model, the adjusted hazard ratios (aHR) for ASVD-Death, per 1-SD higher level on the covariates, were 1.63, 1.47, 1.21, and 0.87, for PCEP, CIS, proteinuria, and eGFR, respectively. We found no evidence of violation of the proportionality assumption for Cox models that included the CIS or individual inflammatory markers with any other covariates. We also did not find evidence of significant multicollinearity (i.e., variance inflation factors ranging from 1.02 to 1.4).
Table 4.
Cox proportional hazard model for the composite incident CVD–death outcome using the CIS, adjusted for ln(PCEP), ln(UACR), and eGFR.
| Parameter | aHR (95% CI) per 1-unit increase | p | aHR per 1-SD increase |
|---|---|---|---|
| ln(PCEP) | 2.81 (2.32-3.39) | <0.001 | 1.62 (1.48-1.77) |
| ln(UACR) | 1.10 (1.05-1.15) | <0.001 | 1.22 (1.10-1.35) |
| eGFR | 0.99 (0.98-1.00) | 0.02 | 0.88 (0.79-0.98) |
| CIS | 1.05 (1.04-1.07) | <0.001 | 1.47 (1.32-1.65) |
Size of HR cannot be used to indicate relative strength of these predictors, since their variances are not equal. However, the standardized HRs can be compared.
CIS= Composite inflammation score, eGFR= Estimated glomerular filtration rate, ln(PECP)= natural logarithm of Pooled Cohort Equation probability, ln(UACR)= natural logarithm of albumin-creatinine ratio; aHR, adjusted hazard ratio; CVD, cardiovascular disease; SD, standard deviation
Multivariable Association with Death, MI, PAD and Stroke
All the inflammatory markers had significant univariable associations with death during follow-up (Table S2). Those that remained significant after adjusting for PCEP and kidney function included IL-6, TGFβ, TNF-α, hS-CRP, fibrinogen, and the CIS. Only serum albumin and the CIS were independently associated with PAD. While several inflammatory biomarkers had significant univariable associations with incident MI during follow-up, only the CIS had a significant independent association with this outcome. No inflammatory biomarker had an independent association with stroke after adjusting for covariates. hs-CRP and CIS had a significant association with the composite of MI, stroke, and PAD in the fully adjusted model .
Competing Risk Analysis
When death was considered as a competing risk and composite ASVD was the primary outcome in univariable time-to-event analysis, quintiles of hs-CRP, fibrinogen, serum albumin, IL-6, TNF-α, and the CIS remained significantly associated with time to the outcome (Figure S2). However, only the CIS retained the step-function structure in which increasing level of the marker was associated with increasing risk of reaching the outcome.
Interaction Analyses
Interaction analysis, adjusted for measures of kidney function and PCEP, showed that in general, the inflammatory biomarkers’ association with the composite outcome was not altered by either baseline eGFR or albuminuria (Figure S3A and S3B).
Discrimination for Cox Models Predicting ASVD Events and Death
The AUC for the Cox model using just the PCEP to predict time to ASVD event or death was 0.68 (95% CI, 0.66-0.71). When eGFR, albuminuria, and the CIS were added, the AUC became 0.73 (95% CI, 0.71-0.76), a significant improvement (p=0.003). We used the adjusted parameter estimates from the Cox model that included just the inflammation variables (shown in Table 3) as weights, so the weighted CIS (wCIS) was defined as wCIS = 0.112([IL-6] × 10) + 0.97([TNF-α] × 10) + 0.069([fibrinogen] × 10) - 0.05([serum albumin] × 10). When weighed CIS was used in the estimates, the results remained unchanged. Addition of kidney function variables and CIS to PCEP improved the AUC in individuals older than 40 years (0.72 [95% CI, 0.70-0.75] vs. 0.67 [95% CI 0.64-0.69], p=0.001), those using statin (0.71 [95% CI 0.68-0.74] vs. 0.66 [95% CI, 0.62-0.69], p=0.02) and those not using statin (0.75 [95% CI, 0.72-0.78] vs. 0.70 [95% CI, 0.67-0.74], p<0.05).
Clinically Relevant Risk Threshold
We examined sensitivity, specificity, positive and negative predictive values at various probability cut points for the kidney model and the PCEP (Table 5). At a 20% probability cut point, the sensitivity and specificity for the kidney model were close to being clinically useful (0.71 and 0.70, respectively). However, there was no probability cut-point where the PCEP could be used for clinical purposes. The equation for calculating the estimated event probability during follow-up, using the kidney model, was probability = exp(risk) / (1 + exp(risk)), where risk = −0.766 + 0.055(CIS) + 1.171(ln[PCEP]) + 0.09(ln[UACR]) − 0.011(eGFR).
Table 5.
Sensitivity, specificity, PPV, and NPV for the kidney model and the PCEP in predicting the ASVD-death composite, at various probability cut points.
| Probability Cut Point for Predicting an Event | ||||||
|---|---|---|---|---|---|---|
| 5% | 7.5% | 10% | 15% | 20% | 25% | |
| Kidney model | ||||||
| Sensitivity | 0.99 | 0.95 | 0.89 | 0.81 | 0.71 | 0.59 |
| Specificity | 0.10 | 0.24 | 0.36 | 0.56 | 0.70 | 0.79 |
| PPV | 0.21 | 0.23 | 0.25 | 0.31 | 0.37 | 0.41 |
| NPV | 0.98 | 0.95 | 0.93 | 0.92 | 0.91 | 0.89 |
| OR for event (95% CI) | 13.21 (4.88-35.74) | 5.73 (3.78-8.69) | 4.52 (3.34-6.12) | 5.29 (4.13-6.77) | 5.62 (4.51-7.01) | 5.40 (4.36-6.68) |
| PCEP | ||||||
| Sensitivity | 0.87 | 0.81 | 0.71 | 0.56 | 0.42 | 0.29 |
| Specificity | 0.37 | 0.48 | 0.57 | 0.72 | 0.82 | 0.89 |
| PPV | 0.25 | 0.27 | 0.29 | 0.33 | 0.37 | 0.38 |
| NPV | 0.92 | 0.91 | 0.89 | 0.87 | 0.85 | 0.84 |
| OR for event (95% CI) | 4.00 (3.01-5.33) | 3.89 (3.04-4.99) | 3.25 (2.61-4.05) | 3.29 (2.67-4.05) | 3.40 (2.74-4.23) | 3.14 (2.46-4.00) |
The kidney model probability estimate is calculated as exp(risk) / (1 + exp(risk)), where risk = −0.766 + 0.055(CIS) + 1.171(ln[PCEP]) + 0.09(ln[UACR]) − 0.011(eGFR). Cut points for calculating inflammatory marker deciles, which are needed to calculate the CIS, are provided in the Supplementary Material.
Pooled Cohort Equation probability (PCEP), PPV=Positive predictive value, NPV=Negative predictive value, OR= Odds ratio; CI, confidence interval
Cut points for calculating inflammatory marker deciles, which are needed to calculate the CIS, are provided in Table S3.
Traditional model using components of PCEP as separate variables
In the Cox model including the component variables of the Pooled Cohort Equation as separate variables, the significant independent predictors of time to ASVD-Death included ln(age) (aHR, 5.82; 95% CI, 3.41-9.93; p<0.001), ln(HDL) (aHR, 0.61; 95% CI, 0.43-0.87; p=0.005), smoking (aHR, 1.77; 95% CI, 1.38-2.29; p<0.001), diabetes (aHR, 1.86; 95% CI, 1.53-2.27; p<0.001), blood pressure-treated (aHR, 1.47; 95% CI, 1.06-2.05; p=0.02), SBP (aHR per 1-mm Hg greater, 1.01; 95% CI, 1.01-1.02; p<0.001), and female sex (aHR, 0.81; 95% CI, 0.66-0.99; p=0.04. Race (p=0.5), ln(TC) (p=0.9), ln(HDL) (p=0.2), and whether a participant was treated for HTN (p=0.1) did not have significant independent associations with the outcome.
The AUC for this model was 0.70 (95% CI, 0.68-0.72). When eGFR, proteinuria, and the CIS were added to this model, the AUC was 0.74 (95% CI, 0.72-0.77), a significant improvement (p=0.008). In the model that included the Pooled Cohort Equation variables, kidney function variables, and the CIS, the significant independent predictors of ASVD-Death included female sex (aHR, 0.81; 95% CI 0.66-0.99; p=0.04), ln(age) (aHR, 8.88; 95% CI, 5.04-15.62; p<0.001), smoking (aHR, 1.51; 95% CI, 1.17-1.95; p=0.002), diabetes (aHR, 1.46; 95% CI, 1.19-1.79; p<0.001), SBP (aHR per 1-mm Hg greater, 1.01; 95% CI, 1.00-1.01; p=0.008), ln(UACR) (aHR, 1.13; 95% CI, 1.07-1.19; p<0.001), eGFR (aHR per 1-mL/min/1.73 m2 greater, 0.99; 95% CI, 0.98-1.00; p=0.04), and the CIS (aHR per 1-point greater, 1.05; 95% CI, 1.03-1.06; p<0.001).
Weighted CIS
We used the adjusted parameter estimates from the Cox model that included just the inflammation variables (shown in Table 3) as weights, so the weighted CIS (wCIS) was defined as wCIS = 0.112([IL-6] × 10) + 0.97([TNF-α] × 10) + 0.069([fibrinogen] × 10) −0.05([serum albumin] × 10). In univariable Cox models with the ASVD-Death outcome, both the non-weighted CIS and the weighted CIS had identical AUC of 0.68 (95% CI, 0.65-0.70). When wCIS was used with the individual risk variables that were used to calculate the PCEP in a multivariable Cox model for ASVD-Death, the AUC and its confidence interval was identical to the model using CIS, and the aHRs for the other covariates were nearly identical.
Test of Cox models in participants over age 40
About 89% of participants were ≥40 years old. Among participants aged 40 or more, the AUC was 0.72 (95% CI, 0.70-0.75) using the model with covariates PCEP, CIS, and kidney function variables, versus an AUC of 0.67 (95% CI, 0.64-0.69) using PCEP as the sole covariate, a significant difference (p=0.001).
Test of Cox models stratified by statin use
Statins were used by 46% of participants. In the sample without statin use, the AUC for the Cox models examining time to first ASVD–death event were 0.75 (95% CI, 0.72-0.78) in the model using CIS, kidney function variables, and PCEP; and 0.70 (95% CI, 0.67-0.74) in the model using just PCEP. This difference remained significant (p<0.05). In the sample with statin use, the AUC for the Cox model using inflammation score, kidney function variables, and PCEP was 0.71 (95% CI, 0.68-0.74), versus 0.66 (95% CI, 0.62-0.69) in the model using just PCEP, a significant difference (p=0.02).
Discussion
In this study, we sought to identify the associations of a select panel of inflammatory biomarkers with risk for incident ASVD events and death in patients with CKD. Plasma levels of fibrinogen, IL-6 and TNF-α measured at baseline had positive independent association with a composite of incident MI, PAD, stroke, and death, whereas serum albumin had a negative association with the outcome. Study participants in the highest quintile of CIS had a 3.1-fold higher adjusted hazard for the composite event, compared to those in the lowest quintile. The proportion of subjects with incident ASVD–death showed a monotonic increase across the quintiles of the risk score computed using PCEP, kidney function, and the CIS. The discrimination for Cox models predicting the ASVD-Death outcome assessed by AUC improved significantly when kidney function and CIS were added to PCEP.
In a systematic review of 39 studies that followed a total of 1,371,990 participants, Tonelli noted that CKD is consistently associated with increased all-cause and CV mortality.17 Indeed, a 10 ml/min/1.73 m2 lower GFR is associated with a 19% increase in risk for ASVD.18 Findings from the PROSPECT study showed that CKD patients have higher atherosclerotic plaque burden, greater luminal encroachment, more necrotic core, and higher calcium content compared to those without CKD.19 Experimental studies suggest that CKD increases the potential for foam cell formation by enhancing macrophage influx into the vascular wall and inhibiting efflux of cholesterol.20;21 In our study, after excluding the 1,316 participants with history of CVD, 20% of the participants experienced incident ASVD events during follow-up or died.
In a prospective, nested, case-control study involving 244 Nurses’ Health Study participants, increased levels of hs-CRP, IL-6, and soluble TNF Receptor I and II, were found to be significantly associated with coronary events only in those with reduced kidney function.7 High CRP level was also an independent risk factor for cardiovascular death in the Modification of Diet in Renal Disease (MDRD) Study participants.22 An inverse association between serum albumin level and ASVD is reported in the general population as well as in patients with kidney disease.23;24 Zocalli et al measured CRP, IL-1β, IL-6, IL-18, and TNF-α in 217 end-stage renal disease (ESRD) patients and noted that IL-6 captured almost entirely the death prediction power of the inflammation burden in these patients.6 In our study, each 1-decile greater IL-6 level was associated with 12% increase in adjusted hazard for reaching the composite outcome. We also found that TNF-α, fibrinogen, and serum albumin also were associated with incident ASVD independently of IL-6.
Fibrinogen, an acute phase protein promotes atherogenesis and thrombogenesis.25 Experimental studies have shown that vascular endothelium and smooth muscle cells produce IL-6, which may have a procoagulant effect.26 IL-6 gene transcripts are expressed in human atherosclerotic lesions and injection of recombinant IL-6 has been reported to exacerbate atherosclerosis in mice.27 TNF-α enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular cells.28 The ACC/AHA guidelines suggested that measurement of CRP may be useful in improving risk stratification of those in an intermediate CVD risk category.4 When IL-6, TNF-α, hs-CRP, fibrinogen, and serum albumin were combined as predictors in a single Cox model for the composite outcome, only hs-CRP became non-significant. (Table 3) Also, when hs-CRP decile is added to the Cox regression model that included lnPCEP, lnUACR, eGFR, and the CIS, hs-CRP did not have a significant independent contribution (aHR, 1.03; 95% CI, 1.00-1.07; p=0.08). This could be because we adjusted for other inflammatory biomarkers and the risk relationship is different in patients with CKD.29;30
There is emerging interest in integrating multiple biomarkers to generate a single score to predict clinical outcomes.31 Cytokines act as a highly complex and coordinated network and hence it is logical to examine their combined effect on outcome measures.32 In this study, we combined the deciles of four biomarkers that exhibited significant independent association with the composite outcome to construct a composite inflammation score. The CIS was an independent predictor of the composite outcome after adjusting for the PCEP, eGFR, and albuminuria. CIS was associated with death, PAD, and MI, but not with stroke in the fully adjusted model. No significant association between inflammatory biomarkers and stroke was discernable in our study, perhaps due to the small number of events or the definition of stroke used that combines both ischemic stroke and intracranial hemorrhage.
The ) CKD-CVD model in SHARP predicted long-term cardiovascular event risk, kidney disease progression, and survival in CKD patients.33 The Pooled Cohort Equation was derived from a more diverse population when compared to Framingham Risk Score.8 In adults enrolled in the REGARDS study, Muntner noted that using the Pooled Cohort Equation, the observed and predicted 5-year atherosclerotic CVD events were similar.34 However, other studies have shown that the Pooled Cohort Equation overestimates CVD risk, and there is call for recalibration.35 In our study, a risk score incorporating PCEP, kidney function, and the CIS showed that the proportion of individuals with composite event increased across the risk score quintiles. Discrimination is the ability of a prediction model to separate those who had outcome events from those who did not have events, by assigning higher risk scores to those with events. Addition of kidney function and CIS significantly improved the AUC in our full sample, and also after stratifying by age or use of statins.
Our study is important in that we show increased levels of inflammatory biomarkers are associated with incident ASVD events and death in CKD patients, after adjusting for PCEP and measures of kidney function, and when considering death as a competing risk. Other strengths of the study include analysis of a large cohort of CKD patients with a broad range of decreased kidney function, the extent of long-term followup, the measurement of multiple biomarkers, examining the utility of an inflammation score, and a significant number of outcome events. Limitations include the measurement of biomarkers at only one time point and lack of data about CVD mortality. Inflammation markers may be associated with a higher risk for all-cause mortality other than that related to ASVD. Preliminary evidence suggest that a single baseline measure accurately reflects an individuals' inflammatory status over time36 and predicts mortality in patients with ESRD and earlier stages of CKD.37 Our findings need external validation since the deciles of inflammation biomarker in CRIC Study participants might not reflect deciles of the same biomarker in other CKD populations.
To summarize, select inflammatory biomarkers are independently associated with a composite of incident MI, PAD, stroke and death in CKD patients. A composite inflammation score based on deciles of IL-6, TNF-α, fibrinogen, and reverse-coded serum albumin showed a positive and graded association with incident ASVD events and death. A risk score that incorporates PCEP, kidney function, and the CIS was associated with the ASVD-Death outcome. We found that the inclusion of eGFR, albuminuria, and the CIS improved the model discrimination beyond that achieved by PCEP. These observations should be confirmed in an independent cohort of CKD patients.
Supplementary Material
Figure S1. Kaplan-Meier survival estimates for freedom from ASVD-death, stratified by quintiles of each inflammatory marker.
Figure S2. Cumulative incidence functions for time to first ASVD event, with death as a competing risk, stratified by biomarker quintile.
Figure S3. Interactions for inflammatory biomarkers by level of eGFR, adjusted for ln(UACR) and ln(PCEP).
Table S1. Univariable odds ratios by quartile, for 5 inflammatory markers predicting incident CVD–death during follow up.
Table S2. Associations of inflammatory markers with individual outcomes, using separate Cox proportional hazards models for each marker.
Table S3. Cut points for calculating inflammatory marker deciles.
Acknowledgments
Support: Dr Raj is supported by R01 DK073665-01A1, 1U01DK099914-01 and 1U01DK099924-01 from the National Institute of Health. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
CRIC Study Investigators: Jeffrey Fink MD, Lawrence J. Appel, James P. Lash.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R Atherosclerosis--an inflammatory disease. New Eng J Medicine 1999;340(2):115–126. [DOI] [PubMed] [Google Scholar]
- 2.Gupta J, Mitra N, Kanetsky PA et al. Association between albuminuria, kidney function, and inflammatory biomarker profile. Clin J Am Soc Nephrol 2012;71938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaptoge S, Seshasai SR, Gao P et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014;35(9):578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenland P, Alpert JS, Beller GA et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56(25):e50–103. [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Heimburger O, Paultre F et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 1999;55(5):1899–1911. [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C, Tripepi G, Mallamaci F. Dissecting inflammation in ESRD: do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 2006;17(12 Suppl 3):S169–S173. [DOI] [PubMed] [Google Scholar]
- 7.Knight EL, Rimm EB, Pai JK et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol 2004;15(7):1897–1903. [DOI] [PubMed] [Google Scholar]
- 8.Goff DC Jr., Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 9.Feldman HI, Appel LJ, Chertow GM et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol 2003;14(7 Suppl 2):S148–S153. [DOI] [PubMed] [Google Scholar]
- 10.Lash JP, Go AS, Appel LJ et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009;4(8):1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amdur RL, Feldman HI, Gupta J et al. Inflammation and Progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 2016;11(9):1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 2008;3(2):505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremers WK. Concordance for Survival Time Data Including Time-Dependent Covariates Accounting for Ties in Predictor and Time Technical Report. 2008. Mayo Clinic College of Medicine. [Google Scholar]
- 14.Gonen M. Analyzing receiver operating characteristic curves with SAS. 2007. Cary, NC, SAS Institute Inc. [Google Scholar]
- 15.Sung KC, Ryu S, Lee JY et al. Urine Albumin/Creatinine Ratio Below 30 mg/g is a Predictor of Incident Hypertension and Cardiovascular Mortality. J Am Heart Assoc 2016;5(9). e003245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94496–504. [Google Scholar]
- 17.Tonelli M, Wiebe N, Culleton B et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17(7):2034–2047. [DOI] [PubMed] [Google Scholar]
- 18.Manjunath G, Tighiouart H, Ibrahim H et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 2003;41(1):47–55. [DOI] [PubMed] [Google Scholar]
- 19.Baber U, Stone GW, Weisz G et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc Imaging 2012;5(3 Suppl):S53–S61. [DOI] [PubMed] [Google Scholar]
- 20.Ponda MP, Barash I, Feig JE, Fisher EA, Skolnik EY. Moderate kidney disease inhibits atherosclerosis regression. Atherosclerosis 2010;210(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suganuma E, Zuo Y, Ayabe N et al. Antiatherogenic effects of angiotensin receptor antagonism in mild renal dysfunction. J Am Soc Nephrol 2006;17(2):433–441. [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Greene T, Wang X et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int 2005;68(2):766–772. [DOI] [PubMed] [Google Scholar]
- 23.Beddhu S, Kaysen GA, Yan G et al. Association of serum albumin and atherosclerosis in chronic hemodialysis patients. Am J Kidney Dis 2002;40(4):721–727. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JJ, Liao D, Sharrett AR et al. Serum albumin level as a predictor of incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000;151(5):468–477. [DOI] [PubMed] [Google Scholar]
- 25.Kaptoge S, White IR, Thompson SG et al. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol 2007;166(8):867–879. [DOI] [PubMed] [Google Scholar]
- 26.Stouthard JM, Levi M, Hack CE et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost 1996;76(5):738–742. [PubMed] [Google Scholar]
- 27.Seino Y, Ikeda U, Ikeda M et al. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine 1994;6(1):87–91. [DOI] [PubMed] [Google Scholar]
- 28.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000; 102(21):2636–2642. [DOI] [PubMed] [Google Scholar]
- 29.Sarnak MJ, Levey AS, Schoolwerth AC et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108(17):2154–2169. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 31.Duncan BB, Schmidt MI, Pankow JS et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2003;52(7):1799–1805. [DOI] [PubMed] [Google Scholar]
- 32.Conen D, Ridker PM, Everett BM et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J 2010;31(14):1730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlackow I, Kent S, Herrington W et al. A policy model of cardiovascular disease in moderate-to-advanced chronic kidney disease. Heart 2017;103(23):1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muntner P, Colantonio LD, Cushman M et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA 2014;311(14):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pencina MJ, Navar-Boggan AM, D'Agostino RB Sr., et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370(15):1422–1431. [DOI] [PubMed] [Google Scholar]
- 36.Snaedal S, Heimburger O, Qureshi AR et al. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis 2009;53(6):1024–1033. [DOI] [PubMed] [Google Scholar]
- 37.Kimmel PL, Phillips TM, Simmens SJ et al. Immunologic function and survival in hemodialysis patients. Kidney International 1998;54(1):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan-Meier survival estimates for freedom from ASVD-death, stratified by quintiles of each inflammatory marker.
Figure S2. Cumulative incidence functions for time to first ASVD event, with death as a competing risk, stratified by biomarker quintile.
Figure S3. Interactions for inflammatory biomarkers by level of eGFR, adjusted for ln(UACR) and ln(PCEP).
Table S1. Univariable odds ratios by quartile, for 5 inflammatory markers predicting incident CVD–death during follow up.
Table S2. Associations of inflammatory markers with individual outcomes, using separate Cox proportional hazards models for each marker.
Table S3. Cut points for calculating inflammatory marker deciles.