Abstract
Sustaining virological suppression among HIV-infected adolescents is challenging. We evaluated a home-based adherence intervention and characterized self-reported adherence, virological response and drug resistance among adolescents failing atazanavir/ritonavir (ATV/r)-based 2nd line treatment.
Methods:
HIV-positive adolescents (10–18 years) on ATV/r-based 2nd line treatment with virological failure (viral load (VL) ≥1 000 copies/ml) were randomized to either standard care (SC) or SC with addition of modified directly administered antiretroviral therapy (mDAART) for 90 days. VL was measured and questionnaires were administered at study entry and at 3 months. Genotyping was done for participants with continued failure. Primary outcome was suppression to VL < 1 000 copies/ml.
Results:
Fifty adolescents aged 10–18 years on 2nd line treatment for >180 days were enrolled, 23(46%) were randomized to mDAART and 27(54%) to SC. Fifty-four percent were female; mean age was 15.8 years; mean baseline VL was 4.8(log10) copies/ml; 40% reported adherence <80% in previous 1 month at baseline; 40% suppressed (VL <1 000 copies/ml) after follow-up. mDAART resulted in significantly increased self-reported adherence (RR= 0.1; 95% CI=0.02–0.8, p=0.023); closely following dosing schedule (RR= 4.8; 95% CI=1.6–13.8, p=0.004); VL decrease (p=0.031) and modest increase in virological suppression to <1 000 copies/ml (p=0.105). Genotyping in 28/30 participants with continued virological failure demonstrated high level atazanavir resistance (I50L, N88S and I84V) in 6(21%); 3(11%) of whom also had high level resistance to lopinavir and darunavir (V32I, I50L, I54V, 147V and V82A).
Discussion:
The mDAART intervention modestly improved virological suppression among adolescents with ATV/r-based 2nd line treatment failure, significantly increased self-reported adherence and decreased viral load. High level ATV/r resistance was demonstrated.
Conclusion:
Targeting mDAART to adolescents who are virologically failing PI-based 2nd line treatment decreases viral load and increases self-reported adherence. Early drug-resistance testing could reduce morbidity and mortality.
Keywords: Adolescents, HIV, second-line treatment failure, adherence, resistance
INTRODUCTION
Global scale-up of antiretroviral therapy (ART) has significantly reduced HIV-related morbidity and mortality. However, sub-Saharan Africa (SSA) continues to bear the highest burden of HIV infection in the world, accounting for about 90% of all HIV infections (World Health Organisation, 2014). About 2.1 million adolescents (10–19 years of age) in 2012 were living with HIV globally (Lowenthal et al., 2014; WHO, UNAIDS, UNICEF, 2011). Over 10,000 HIV-infected adolescents were registered in HIV-care services in 2008 in Zimbabwe (Ferrand et al., 2010). Adolescents present important challenges to access, adherence and retention in care. Literature reports that 20 to 50% of HIV-infected adolescents on 2nd line are failing treatment (Nglazi et al., 2012; Suaysod et al., 2015). Adolescents who fail boosted protease inhibitor (PI)-based 2nd line regimens in resource-limited settings (RLS) have limited treatment options for salvage therapy, poor treatment outcomes, pose a risk of transmitting drug resistant virus and are at higher risk of subsequent treatment failure (Gupta et al., 2012; Hosseinipour et al., 2013).
Virological failure in adolescents is thought to be a result of poor adherence (Garone et al., 2014; Lessells et al., 2013; Levison et al., 2012). Paterson reported that >95% adherence is required for viral suppression on non-nucleotide reverse transcriptase inhibitors (NNRTIs) and boosted bPIs (Paterson et al., 2000). However, Kobin and Shutter later argued that for patients on boosted PIs, adherence rates of at least 80% are required for a minimum of 80% of patients to achieve viral suppression and that mean adherence required for viral suppression is 75% (Roux et al., 2011; Shuter et al., 2007; Shuter, 2008). Boosted PIs are therefore more ‘forgiving’ than NNRTIs.
Drug resistance could also cause 2nd line treatment failure. Poor adherence selects drug resistance mutations due to on-going viral replication at sub-inhibitory PI concentrations (Nachega et al., 2009). However, boosted PIs have high genetic barrier to resistance, typically requiring multiple mutations, rather than single point mutations, for clinically significant drug resistance (Rhee et al., 2015; Tang and Shafer, 2012). Many studies of boosted PIs have noted the absence PI resistance in patients failing PI-based 2nd line treatment (Garone et al., 2014; Levison et al., 2012).
The reasons why a high proportion of adolescents may fail boosted PI based 2nd line treatment include poor adherence and evolution of drug resistance. If sub-optimal adherence is the reason, intensive adherence interventions should result in viral suppression. If drug resistance is the cause of treatment failure, then HIV drug resistance testing and the use of 3rd line drugs, such as darunavir/ritonavir and raltegravir, amongst others, should be prioritised (Panel on antiretroviral guidelines for adults and adolescents and Department of Health and Human Services 2012; Panel on Antiretroviral Therapy and Medical Management of HIV Infected Children 2012). Identifying and addressing the cause of treatment failure in adolescents on boosted PI-based regimens will reduce the need for largely unavailable and expensive 3rd line treatment.
This study sought to determine and quantify the causes of virological non-suppression, and determine if a home-based adherence intervention and standard care improved virological suppression in HIV-infected adolescents who are virologically failing atazanavir/ritonavir (ATV/r)-based 2nd line treatment compared to standard care alone.
METHODS
Study design
A randomised, controlled trial (RCT) comparing modified directly administered antiretroviral therapy (mDAART) + standard care (SC) versus SC + self-administered treatment (SAT) for 90 days. Data was collected between January 2015 and May 2016. Eligible participants were included if they: were HIV positive with a documented result; were aged between 10 and 18 years; were on ATV/r-based 2nd line treatment for ≥6 complete consecutive months; had virological treatment failure (viral load ≥1 000 copies/ml); knew their HIV status; provided informed consent and assent; were registered at Harare hospital paediatric opportunistic infections clinic and stayed within Harare hospital catchment area. Adolescents were consecutively screened for eligibility using a questionnaire and viral load measurement. The screening viral load was also used as baseline for enrolled participants. Participants were excluded if they were on anti-TB treatment; did not want to be followed-up at home; had viral load <1 000 copies/ml within the previous 2 months or were on ATV/r as 1st line treatment.
Total patient sampling of eligible, assenting and consenting adolescents was considered after noting that the clinic had 267 children, adolescents and young adults on boosted protease inhibitors from 0 to 22 years of age, either as 1st or 2nd line treatment. The study was divided into 2 phases:
Phase 1:
Eligible participants were randomised to intervention (mDAART + SC) or control (SC + SAT) arms. Randomisation was done using random numbers sealed in opaque envelopes. Questionnaires were administered at baseline and after follow-up. Participants were followed for 90 days. At the end of follow-up, viral load was measured again. Self-reported adherence was measured using AIDS Clinical Trials Group (ACTG) adherence follow-up questionnaire (QLO702) and visual analogue scale (VAS) (Chesney et al., 2000; Walsh et al., 2002).
Phase 2:
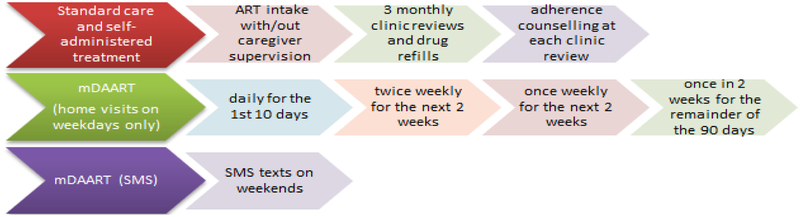
Participants with continued treatment failure (viral load ≥1 000 copies/ml) had genotypic HIV drug resistance testing. Components of each arm are summarised in Figure 1. Standard care (SC) consisted of 3 monthly hospital visits to see clinic doctors, adherence counselling by trained peer counsellors and drug refills at each hospital visit. SAT consisted of participants taking medication on their own, with or without supervision by caregivers. The intervention, mDAART, consisted of scheduled home visits during the week and short message service (SMS) on weekends by trained field workers. Home visits and SMS text messages were timed to coincide with the time participant was taking ATV/r. Home visits were scheduled during weekdays only (Mondays to Fridays) as shown in Figure 1. Trained field workers observed participants swallow medication and completed home visit charts. Participants were given a “pill chart” to complete over the 90 days.
Figure 1.
Components of study arms.
Samples for viral load and HIV drug resistance testing were collected in 2×4 ml K-EDTA tubes respectively, gently inverted 8 to 10 times to prevent clotting, transported at atmospheric temperature to the laboratory. The Roche COBAS AmpliPrep/COBAS Taqman HIV-1 Test version 2.0 was used for viral load measurement, with a linear range of 20 to 10,000000 copies/ml. HIV drug resistance mutations were generated by the Celera ViroSeq® HIV-1 genotyping system version 2.0 (Abbott Molecular Diagnostics). Sequencing was done on 3500 Genetic Analyser supplied by Thermo Fisher, Life Technologies. Mutations were identified with ViroSeq software and analysed with the Stanford database (www.HIVDB.stanford.edu) to interpret drug susceptibility.
Ethical approval
This study was approved by Harare hospital institutional review board, Joint Research Ethics Committee (JREC/51/14), Biomedical and Research Training Institute (BRTI) and Medical Research Council of Zimbabwe (MRCZ/A/1840). This clinical trial was registered with Pan African Clinical Trial Registry (PACTR201502001028169) and National Institutes of Health (NIH) Clinical Trials.gov ().
Statistical considerations
Data from questionnaires was entered into research electronic data capture (REDCap), a web-based application (Harris et al., 2009). All data was analysed in Stata version 14 (Stata Corp). Treatment failure was defined as viral load ≥1 000 copies/ml after 90 days follow-up. We used Chi-square (and Fischer’s test where appropriate) and student’s t test to determine associations between mDAART, standard of care, self-reported adherence and virological suppression (<1 000 copies/ml. P-values are 2-sided and considered statistically significant if <0.05. Primary treatment outcome was defined as viral load <1 000 copies/ml after 90 days of follow-up.
Possible confounders and factors with p<0.25 in bivariate analysis were considered in multivariate analysis to adjust for the effect of mDAART on viral load and self-reported adherence including: age, gender, level of education, orphan and caregiver status; World Health Organisation (WHO) clinical stage at ART initiation; baseline, latest and on-treatment peak CD4 cell counts; time on 1st line, 2 line and total time on ART; baseline, follow-up and change in viral load; pill burden per day; dosing frequency and body-mass index (BMI)-for-age (World Health Organisation Multicentre Growth Reference Study Group 2007). Stepwise logistic regression was used in multivariate analysis.
RESULTS
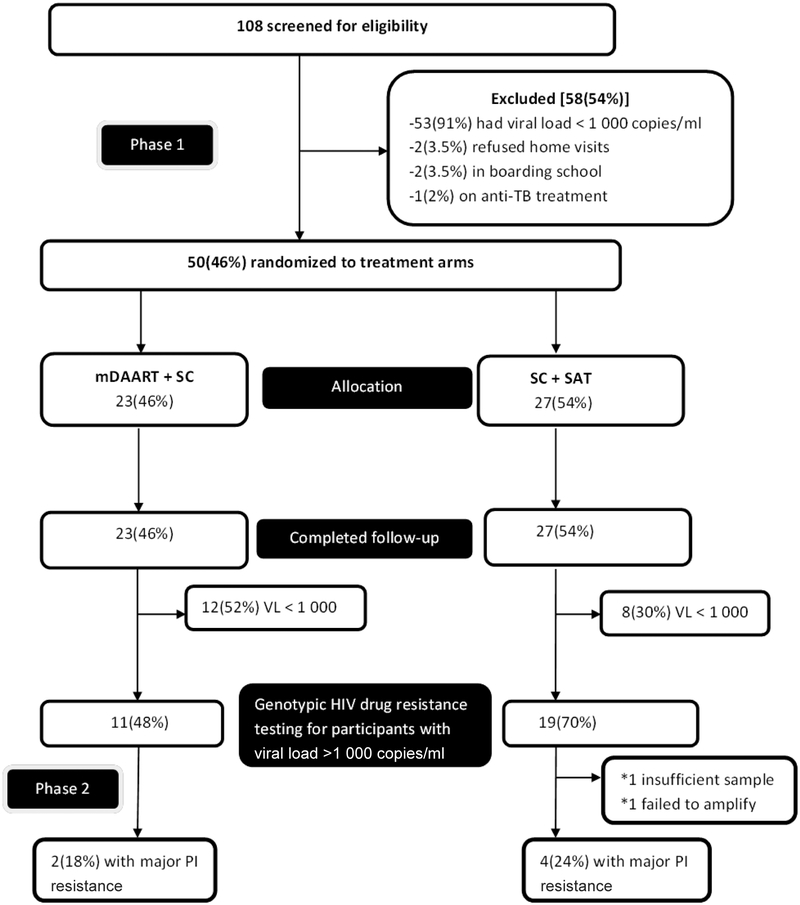
Fifty participants were recruited. Of the participants who were screened, 53/108 (49%) were virologically suppressed (viral load <1 000 copies/ml). One hundred and six (98%) participants accepted home visits. Only 2/108 (2%) participants who were eligible refused home visits citing their intrusive nature. Twenty-three (46%) and 27(54%) participants were randomised to intervention and control arms respectively (Figure 2).
Figure 2.
Consort flow chart of participants. PI, protease inhibitor.
Mean age was 15.8 years. Most participants were either in secondary or high school (form 1–6) (78%). There were more females (54%) than males. 46% were double orphans. Only 20% lived with their biological parent(s). At initiation of 1st line ART, 68% had WHO clinical stage 3 or 4 disease, and 42% had a CD4 cell count <200 cells/mm3. At enrollment into study, 52% had CD4 count <200 cells/mm3 and 30% had low BMI-for-age (thinness or severe thinness). Eighty-six percent were taking tenofovir/lamivudine (300 mg/300 mg) fixed dose combination (FDC) and ATV/r (300 mg/100 mg) FDC; 90% were taking a total of 2 to 4 ART tablets (including cotrimoxazole prophylaxis) a day; and 90% were taking ART (including cotrimoxazole prophylaxis) once a day. Mean total time on ART was 78 months (Table 1).
Table 1.
Baseline socio-demographic and treatment characteristics.
| Variable | Result (n=50) n(%) or mean(SD); 95% CI |
|---|---|
| Age (years) | 15.8 (1.8); 11 – 18 |
| Gender | |
| Female | 27(54) |
| Male | 23(46) |
| Current level of education | |
| Primary | 4(8) |
| Secondary/advanced | 39(78) |
| Other | 7(14) |
| Orphan status | |
| Non-orphan (both parents alive) | 7(14) |
| Single orphan | 20(40) |
| Double orphan | 23(46) |
| Caregiver | |
| Parent/s | 10(20) |
| Other (grandparent/s, sibling, aunt/uncle) | 40(80) |
| WHO clinical stage at ART initiation | |
| 1–2 | 16(32) |
| 3–4 | 34(68) |
| CD4 cell count at ART initiation (cells/mm3) | |
| <200 | 21(42) |
| 200–350 | 9(18) |
| >350 | 20(40) |
| CD4 cell count at enrollment (cells/mm3) | |
| <200 | 26(52) |
| 200–350 | 12(24) |
| >350 | 12(24) |
| On-treatment peak CD4 cell count (cells/mm3) | |
| <200 | 2(4) |
| 200–350 | 4(8) |
| >350 | 44(88) |
| Basis of diagnosis of 1st line treatment failure | |
| Clinical | 33(66) |
| Immunological | 47(94) |
| Virological | 28(56) |
| Time on 1st line ART (months) | 55(26); 6–107 |
| Time on 2nd line ART (months) | 22(10); 8–66 |
| Total time on ART (months) | 78(26); 24–134 |
| Current treatment | |
| Tenofovir/lamivudine/atazanavir/ritonavir | 43(86) |
| Zidovudine/lamivudine/atazanavir/ritonavir | 3(6) |
| Abacavir/lamivudine/atazanavir/ritonavir | 2(4) |
| Abacavir/didanosine/atazanavir/ritonavir | 2(4) |
| Cotrimoxazole prophylaxis | 49(98) |
| Pill burden per day | |
| 2–4 | 45(90) |
| 5–6 | 5(10) |
| Dosing frequency per day | |
| Once daily | 45(90) |
| Twice daily | 5(10) |
| BMI-for-age | |
| Underweight (severe thinness and thinness) | 14(30) |
| Normal | 25(55) |
| Overweight | 7(15) |
WHO = World Health Organization; ART= antiretroviral therapy; BMI = body mass index.
Treatment arms were well matched at baseline. Forty percent had average self-reported adherence <80% at baseline compared to 22% after follow-up, and 66% reported an increase in self-reported adherence after follow-up. Average self-reported adherence and ATV/r adherence by visual analogue scale were similar. Mean viral load change was −1.1 log10 copies/ml, 74% had overall decrease in viral load, 46% had ≥1 log10 decrease in viral load and 40% achieved virological suppression (viral load <1 000 copies/ml) (Table 2).
Table 2.
Treatment characteristics at baseline and after follow-up.
| Variable | Baseline (n=50) n(%) or mean(SD); 95% CI |
After follow-up (n=50) n(%) or mean(SD); 95% CI |
|---|---|---|
| Average self-reported adherence, VAS (%) | ||
| ≥95 | 15(30) | 25(50) |
| 80–94 | 15(30) | 14(28) |
| <80 | 20(40) | 11(22) |
| ATV/r self-reported adherence, VAS (%) | ||
| ≥95 | 16(32) | 28(56) |
| 80–94 | 15(30) | 11(22) |
| <80 | 19(38) | 11(22) |
| Change in average self-reported adherence, VAS: | ||
| No change | - | 7(14) |
| Decreased | - | 10(20) |
| Increased | - | 33(66) |
| Missed all doses in a day in past 4 days | ||
| Yes | 15(30) | 5(10) |
| No | 35(70) | 45(90) |
| Missed at least 1 dose in past 4 days | ||
| Yes | 18(36) | 18(36) |
| No | 32(64) | 32(64) |
| Closely followed dosing schedule in past 4 days | ||
| Yes | 22(44) | 29(58) |
| No | 28(56) | 21(42) |
| Missed at least 1 dose previous weekend | ||
| Yes | 12(24) | 12(24) |
| No | 38(76) | 38(76) |
| Last time a dose/s was missed | ||
| 0–4 weeks ago | 28(56) | 18(36) |
| >4 weeks ago | 22(44) | 32(64) |
| Viral load (log 10 copies/ml) | 4.8(0.8); 3–7 | 3.7(1.5); 1.3–5.9 |
| Viral load change (log10 copies/ml) | - | −1.1(1.5); −5.5–2 |
| Viral load change: | ||
| Decreased | - | 37(74) |
| Increased | - | 13(26) |
| ≥1 log10 decrease in viral load | - | 23(46) |
| <1 log10 decrease in viral load | - | 27(54) |
| Viral load, copies/ml | ||
| <1 000 | - | 20(40) |
| ≥1 000 | 30(60) |
VAS, visual analogue scale; ATV/r, atazanavir/ritonavir.
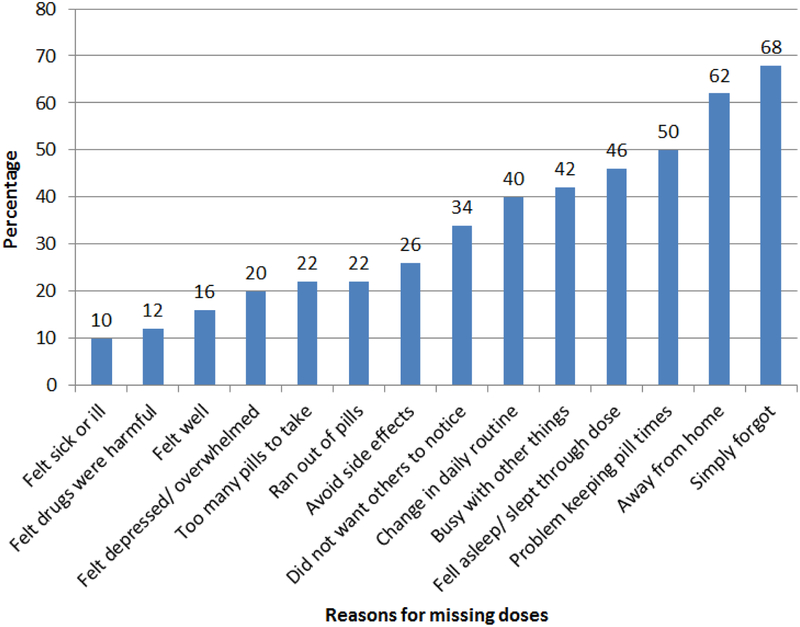
Common reasons for missing ART were simply forgetting (68%), being away from home (62%), problem with keeping time (50%) and falling asleep before taking medication or waking up late (46%) (Figure 3).
Figure 3.
Reasons for missing ART doses.
52% of the participants in mDAART achieved virological suppression compared to 30% in standard care. There was a modest increase in viral load suppression in mDAART compared to SC after stratifying by viral load <1 000 vs ≥1 000 copies/ml (p=0.105). Viral load decreased more in mDAART arm compared to standard care (p=0.03) and viral load at follow-up was lower in mDAART compared to standard care (p=0.04). Average self-reported adherence in previous 1 month measured by visual analogue scale at follow-up was higher in mDAART compared to standard care (p=0.05), and the number of participants who reported closely following their dosing schedule in the previous 4 days was higher in mDAART compared to standard care at follow-up (p=<0.001) (Table 3).
Table 3.
Comparison of participants’ treatment characteristics by treatment arms.
| Variable | mDAART (n=23) n(%) or mean(SD); 95% CI |
Standard care (n=27) n(%) or mean(SD); 95% CI |
p-value |
|---|---|---|---|
| Viral load at follow-up | |||
| <1 000 copies/ml | 12(52) | 8(30) | 0.105 |
| ≥1 000 copies/ml | 11(48) | 19(70) | |
| Viral load change | |||
| ≥1 log10 decrease | 12(52) | 11(41) | 0.399 |
| <1 log10 decrease | 11(48) | 16(59) | |
| Follow-up viral load (log10 copies/ml) | 3.3(1.5); 2.6–3.9 | 4(1.5); 3.4–4.6 | 0.048 |
| Viral load decrease (log10 copies/ml) | −1.5(1.6); −2.2– −0.9 | −0.8(1.3); −1.3– −0.3 | 0.031 |
| Average self-reported adherence, (VAS) at follow-up (%) | |||
| ≥95 | 15(65) | 10(37) | |
| 80–94 | 6(26) | 8(30) | 0.050 |
| <80 | 2(9) | 9(33) | |
| Change in average self-reported adherence (VAS) | |||
| No change | 3(13) | 4(15) | |
| Increased | 17(74) | 16(59) | 0.538 |
| Decreased | 3(13) | 7(26) | |
| Missed all doses in a day in past 4 days at follow-up | |||
| Yes | 1(4) | 4(15) | 0.357 |
| No | 22(96) | 23(85) | |
| Missed at least 1 dose in past 4 days | |||
| Yes | 2(9) | 7(26) | 0.114 |
| No | 21(91) | 20(74) | |
| Closely followed dosing schedule in past 4 days at follow-up | |||
| Yes | 19(83) | 10(37) | <0 001 |
| No | 4(17) | 17(63) | |
| Missed at least 1 dose in previous weekend at follow-up | |||
| Yes | 3(13) | 3(11) | 0.985 |
| No | 20(87) | 24(89) | |
| Last time a dose was missed at follow-up | |||
| 0–4 weeks ago | 7(30) | 11(41) | 0.449 |
| >4 weeks ago | 16(70) | 16(59) |
VAS, visual analogue scale.
There were no significant differences between suppressed and unsuppressed participants. Multivariate models were assessed comparing mDAART to SC, fitting self-reported adherence characteristics associated with virological suppression (Table 4).
Table 4.
Comparison by viral load suppression to <1 000 copies/ml after 3 months.
| Variable | Viral load <1 000 copies/ml (n=20) n(%) or mean(SD); 95% CI |
Viral load ≥1 000 copies/ml (n=30) n(%)or mean(SD); 95% CI |
p-value |
|---|---|---|---|
| Age (years) | 15(1.98); 14.4–16.3 | 16(1.66); 15.4–16.7 | 0.08 |
| Gender: | |||
| Female | 10(50) | 17(57) | 0.643 |
| Male | 10(50) | 13(43) | |
| Current level of education | |||
| Primary | 2(11) | 2(8) | |
| Secondary/advanced | 15(83) | 24(92) | 0.582 |
| Other | 1(6) | 0(0) | |
| Orphan status: | |||
| None | 2(10) | 5(17) | |
| Single orphan | 8(40) | 12(40) | 0.858 |
| Double orphan | 10(50) | 13(43) | |
| Caregiver: | |||
| Parent/s | 3(15) | 7(23) | 0.470 |
| Other (grandparent/s, sibling, aunt/uncle) | 17(85) | 23(77) | |
| WHO clinical stage at ART initiation | |||
| 1–2 | 8(40) | 8(27) | 0.322 |
| 3–4 | 12(60) | 22(73) | |
| CD4 cell count at ART initiation (cells/mm3) | |||
| <200 | 8(40) | 13(43) | |
| 200–350 | 5(25) | 4(13) | 0.563 |
| >350 | 7(35) | 13(43) | |
| CD4 cell count at enrollment (cells/mm3) | |||
| <200 | 7(35) | 19(63) | |
| 200–350 | 6(30) | 6(20) | 0.133 |
| >350 | 7(35) | 5(17) | |
| On treatment peak CD4 cell count (cells/mm3) | |||
| <200 | 0(0) | 2(7) | |
| 200–350 | 2(10) | 2(7) | 0.650 |
| >350 | 18(90) | 26(86) | |
| Time on 1st line ART (months) | 57.3(18.6); 48–62 | 52.8(30); 41–64 | 0.281 |
| Time on 2nd line ART (months) | 21.8(8.3); 17.8–25.9 | 22.5(11); 18.3–26.7 | 0.409 |
| Total time on ART (months) | 81.3(17.6); 73–90 | 75.3(30.8); 63–87 | 0.217 |
| Dosing frequency per day at follow-up | |||
| Once daily | 19(95) | 28(93) | 1.000 |
| Twice daily | 1(5) | 2(7) | |
| BMI-for-age | |||
| Normal | 12(63) | 13(48) | |
| Underweight (severe thinness and thinness) | 4(21) | 10(37) | 0.499 |
| Overweight | 3(16) | 4(15) | |
| Treatment arm | |||
| mDAART | 12(60) | 11(37) | 0.105 |
| Standard care | 8(40) | 19(63) | |
| Average self-reported adherence, (VAS) at follow-up (%) | |||
| ≥95 | 10(50) | 15(50) | |
| 80–94 | 8(40) | 6(20) | 0.143 |
| <80 | 2(10) | 9(30) | |
| Change in self-reported adherence (VAS) | |||
| No change | 5(25) | 2(7) | |
| Increased | 11(55) | 22(73) | 0.181 |
| Decreased | 4(20) | 6(20) | |
| Missed all doses in a day in past 4 days at follow-up | |||
| Yes | 1(5) | 4(13) | 0.636 |
| No | 19(95) | 26(87) | |
| Missed at least 1 dose in past 4 days | |||
| Yes | 3(15) | 6(20) | 0.652 |
| No | 17(85) | 24(80) | |
| Closely followed dosing schedule in past 4 days at | |||
| follow-up | |||
| Yes | 14(70) | 15(50) | 0.160 |
| No | 6(30) | 15(50) | |
| Missed at least 1 dose in previous weekend at follow-up | |||
| Yes | 3(15) | 3(10) | 0.672 |
| No | 17(85) | 27(90) | |
| Last time a dose was missed at follow-up | |||
| 0–4 weeks ago | 7(35) | 11(37) | 0.904 |
| >4 weeks ago | 13(65) | 19(63) |
mDAART, modified directly administered antiretroviral therapy; VAS, visual analogue scale.
Participants in mDAART were 90% less likely to report <80% adherence in the previous 1 month (p=0.023), were 4.8 times more likely to closely follow their dosing schedule in the previous 4 days (p=0.004) compared to those who were not exposed to the intervention (Table 5).
Table 5.
Multivariate logistic regression comparing mDAART referenced to standard care.
| Variable | Relative risk (95% confidence interval) | p Value |
|---|---|---|
| Average self-reported adherence, (VAS) at follow-up (%) | ||
| ≥95 | - | - |
| 80–94 | 0.4(0.1–1.5) | 0.162 |
| <80 | 0.1(0.02–0.8) | 0.023 |
| Closely followed dosing schedule in past 4 days at follow-up | ||
| No | - | - |
| Yes | 4.8(1.6–13.8) | 0.004 |
Genotypic HIV drug resistance test
Thirty (60%) participants had viral load >1 000 copies/ml at 3 months and 28/30 (93%) had a genotypic HIV drug resistance test within 1 month of follow-up viral load measurement. Three (11%) participants had wild type virus (Table 6). PI resistance was seen in 10(36%). High level atazanavir/ritonavir resistance was detected in 6(21%) of the 28 participants, 5 of whom had intermediate and/or low level ATV/r resistance mutations and 1 had a single I50L mutation. Three (11%) of the 4 participants with multiple PI resistance mutations had high level resistance to ATV/r, lopinavir/ritonavir (LPV/r) and darunavir/ritonavir (DRV/r) (V32I, I50L, I54V, I47V and V82A) and were switched to 3rd line integrase strand transfer inhibitors (InSTI)-based regimens (raltegravir). The other 3 had no resistance to LPV/r, and were switched to LPV/r, which is the available alternative 2nd line treatment (Table 6). The most frequent PI mutations were A71I/T/V (18%), V82A/M (14%), M46I (11%), L10F/V (11%) and I50L (11%) (Figure 4).
Table 6.
Resistance mutations by ARV drug class.
| Participant | Protease inhibitor mutations | NRTI mutations | NNRTI mutations |
|---|---|---|---|
| 1 | L10F, M46I, Q58E, A71I, I84V* | M41L, D67G, T69N, K70N, V75I, M184V*, T215F | A98G, V179E, Y181C*, G190A* |
| 2 | I50L* | M41L, D67G, V75I, M184V/I*, K70Q, T215F | Y188L* |
| 3 | Q58E, V82M | D67G, M184V*, T69D, K70R, K219Q | A98G, Y181C*, G190A*, K101E |
| 4 | - | D67G, K70R, T215I, T219E | G190A*, E138G |
| 5 | - | - | A98G, Y181C*, V90I |
| 6 | - | - | - |
| 7 | - | - | K101H/Q |
| 8 | - | M184V* | K103N*, E138A |
| 9 | - | ||
| 10 | - | D67G, M184V*, K70G | A98G, Y318F |
| 11 | - | T69N | Y181C*, G190A*, K101E, V90I |
| 12 | - | - | Y181C*, K103T, H221Y |
| 13 | - | V75I, M184V*, K65R*, D67N, Y115F, K219E | Y181C*, V108I |
| 14 | A71T | M41L, T69N, K70R, D67N, T215L, K219E | A98G, Y181C*, K103N*, K238T |
| 15 | - | M184V*, K70E/G/R, D67N | V90I, K103N*, Y318F |
| 16 | - | M184I/V* | G190A* |
| 17 | L90M | T69N | - |
| 18 | - | M41L, M184V*, T215C/Y | K103N*, Y318F, E138Q |
| 19 | - | M41L, V75I, M184V*, T215F/Y | E138A, H221Y, Y181I |
| 20 | A71I/T, N88S*, L10V | T69A/N, M184V*, T215F, K70R, K219E, D67S, L74V | K103N*, L100I*, M230L* |
| 21 | _ | T69D/N | Y181C*, G190A* |
| 22 | - | T69N | Y181C* |
| 23 | - | - | |
| 24 | M46I, I50L*, L10V, L33F, I47V, A71V, G73C/S, V82A# | M184V*, T215F | A98G, G190A*, K101E, E138A |
| 25 | M46I | - | Y181C*, E138G, H221Y |
| 26 | I50L*, V82M, V32I#, L24I, N83D | M41L, K70N, V75I, M184V*, T215Y | H221Y, K103S*, V106M*, F227L# |
| 27 | V82M, A71V, L24I, K43T, F53L, I54V#, T74P | M184V*, A62V, 69deIT, V75T, Q151M | Y188L* |
| 28 | - | - | K103N*, E138A |
| Without mutations, n(%) | 18(64) | 8(29) | 4(14%) |
high level resistance;
intermediate level resistance;
Italics-low level resistance; PI, protease inhibitor; NNRTI, non-nucleotide reverse transcriptase inhibitors; NRTI, nucleot/side reverse transcriptase inhibitors.
Figure 4.
Frequency of HIV drug resistance mutations by ARV drug class.
DISCUSSION
Directly observed treatment (DOT) has been successfully implemented in anti-TB treatment. However, its use in HIV treatment is controversial. In our study, a short-term mDAART intervention provided to adolescents failing 2nd line treatment was associated with a significantly greater decrease in viral load and increase in self-reported adherence compared to standard care, and it also modestly increased virological suppression. Our findings support earlier findings which found that DAART decreases viral load by an effect size between 20 and 30% and increases self-reported adherence when targeted to at-risk populations (Altice et al., 2004; Altice et al., 2007; Amico et al., 2006; Berg et al., 2011; Ford et al., 2009; Goggin et al., 2007; Lucas et al., 2006; Nachega et al., 2010; Wohl et al., 2006). At-risk groups include drug-abusers, patients with poorly controlled mental illness, homeless and marginally housed people.
We also found that 40% of adolescents had adherence <80% at baseline. Adolescent adherence to treatment is lower than that for children and adults (Kim et al., 2014; Sohn and Hazra, 2013). As children grow older, responsibility of HIV care usually shifts from caregiver to adolescent self-management (Modi et al., 2012; Taddeo et al., 2008). This transition usually coincides with complex psycho-social factors typical of this age group at a time of physical and emotional transition to adulthood (Davies et al., 2008; Lowenthal et al., 2014). Moreover, vertically infected adolescents are also likely to have been on ART for longer periods, resulting in treatment fatigue.
Forgetfulness was the most common cited reason for missing doses, and concurs with findings from earlier studies in adults (Barfod et al., 2006; Koole et al., 2016). mDAART allows direct observation of dose ingestion, reminding adolescents to take medication and providing psycho-social support. This increases adherence and decreases viral load if there is no drug resistance and drug exposure is adequate. Interestingly, among the common reasons for missing doses cited, there were no treatment related reasons. This finding is encouraging and supports earlier findings that ATV/r and tenofovir/lamivudine FDCs are tolerable due to favourable side effect profiles, once daily dosing and low pill burden (Achenbach et al., 2011; Dong et al., 2016; Wensing et al., 2010). This allows policy makers to concentrate on addressing psycho-social causes of non-adherence in adolescents.
Acceptance rate for home visits in our study was surprisingly higher than previously reported (Altice et al., 2007; Wohl et al., 2006). This finding is encouraging. Adolescents who are failing 2nd line regimens are often going to school. A community or clinic-based DOT intervention could face challenges in implementation due to busy lifestyles and stigmatisation. A home-based adherence intervention offers lesser burden to adolescents. However, the cost involved in mDAART, the intrusive nature of the intervention, breach of confidentiality of HIV status and migration of participants pose challenges to implementation. If DAART is going to be implemented, there needs to be careful consideration to confidentiality of patients’ HIV status, convenience to the patient and flexibility. Community health workers can assume this responsibility as they are familiar with communities they work in and have a portfolio full of other responsibilities (contact tracing for TB, dysentery and other communicable diseases, and health awareness). Use of technology (SMS, automated calls, camera phones and video internet) could reduce the need for many physical home visits. Family members/friends could also observe dose ingestion on days that mDAART will not be done. Once daily ART regimens also ease implementation DAART.
Time on 2nd line ART was shorter than time on 1st line ART in this study. This finding concurs with findings from previous studies, and is worrying. Risk of subsequent treatment failure increases after 1st line failure (Chawana et al., 2014). Adolescents that are failing 2nd line ART are at high risk of failing 3rd line and salvage regimens. Third line regimens are largely unavailable and where they are available, they require HIV drug resistance testing prior to switch to 3rd line (Conradie et al., 2012; Federal Ministry of Health Nigeria, 2010; Ministry of Health Botswana, 2012; National Department of Health, 2012; World Health Organisation (WHO) 2010a; World Health Organisation (WHO) 2010b; World Health Organisation HIV/AIDS Programme, 2013). However, HIV genotypic drug resistance testing is unavailable in public health care in RSL, and is expensive in private laboratories (USD$382 and USD$795), which ship their samples to South Africa. Maintaining adequate adherence in adolescents could reduce the need for expensive 3rd line treatment and HIV drug resistance testing.
Nearly one-fifth of participants demonstrated high level ATV/r resistance, and was the same as that found in adults (Boender et al., 2016). This finding contradicts previous studies which found that patients on boosted PIs who develop virological treatment failure do not have clinically significant PI mutations and they re-suppress after intensive adherence interventions (Garone et al., 2014; Levison et al,. 2012). Although ATV/r has high genetic barrier against resistance, perinatally infected adolescents often have long treatment histories, inconsistent treatment adherence and multi-drug experience resulting from numerous switches when treatment failure has occurred, all favouring evolution of drug resistance (MacDonell et al., 2013). This finding is extremely worrying due to limited supply of 3rd line regimens in RSL. Beyond 2nd line treatment, prognosis is poor. Persistence of high level NNRTI resistance in this study is also worrying because it rules out the possibility of future use of this drug class in the event that patients run out of treatment options.
Conclusion
Administering a home-based DAART intervention with direct observation of dose ingestion and SMS reminders to adolescents who were failing 2nd line treatment increased self-reported adherence and decreased viral load. High level PI resistance was also demonstrated. We recommend that HIV drug resistance testing and 3rd line antiretroviral treatment, like darunavir/ritonavir and raltegravir, be made more available in RSL in anticipation of a surge in PI resistance. We also propose that HIV drug resistance testing be done at time of diagnosis of 2nd line treatment failure. Waiting 3 to 6 months for a 2nd viral load results in disease progression and creates a window for spread of PI resistant virus.
Limitations
The mDAART intervention was based on SMS reminders and observation of dose ingestion. It is therefore difficult to separate the effect of each component. Future studies could separate these 2 components and compare their effects individually. In addition, frequency of home visits were intensive at the beginning and reduced with time, therefore their effect might have waned off as the visits reduced. The intervention was administered for 3 months, which is relatively short. There was no follow-up after the intervention was discontinued to see if the effect of mDAART would be sustained. Measurement of adherence using self-reports is known to overestimate adherence due to recall bias and social desirability. Even in the presence of adequate adherence, drug exposure may be inadequate (such as in chronic gastroenteritis and increased drug clearance in enzyme induction), resulting in treatment failure. Our sample was also small for power to be adequate.
ACKNOWLEDGEMENTS
The author would like to thank Dr Rashida Ferrand for helping with study conceptualisation; Dr Hildah Mujuru for providing guidance; Dr Monica Gandhi for critically revising and editing the protocol; Harare hospital adolescent patients and staff for participating; Child Protection Society for providing trained field workers for home visits; the research nurse, Noleen Chifamba; and Departments of Clinical Pharmacology and Paediatrics, College of Health Sciences of University of Zimbabwe for providing supervision and guidance.
Funding
This study was funded by University of Zimbabwe Staff Development Fellowship (SDF), Fogarty HIV Implementation Science Research Training Program (FHISRTP) (1D43TW009539-03) and Fogarty International Clinical, Operational and Health Services Research and Training Award (ICOHRTA) (2U2RTW007367-01). The contents of this publication are solely the responsibility of the authors, and do not represent the views of the funders.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript. Preliminary results were presented as a poster at the AIDS 2016 conference in Durban.
REFERENCES
- Achenbach CJ, Darin KM, Murphy RL, Katlama C (2011). Atazanavir/Ritonavir-Based Combination Antiretroviral Therapy for treatment of HIV-1 Infection In Adults. Future Virol. 6(2):157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH (2007). Superiority Of Directly Administered Antiretroviral Therapy Over Self-Administered Therapy Among HIV-Infected Drug Users: A Prospective, Randomized, Controlled Trial. Clin. Infect. Dis 45(6):770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, Springer SA, Friedland GH (2004). Developing A Directly Administered Antiretroviral Therapy Intervention For HIV-Infected Drug Users: Implications For Program Replication. Clin. Infect. Dis 38(S5):S376–S387. [DOI] [PubMed] [Google Scholar]
- Amico KR, Harman JJ, Johnson BT (2006). Efficacy Of Antiretroviral Therapy Adherence Interventions: A Research Synthesis Of Trials, 1996 To 2004. J. Acquir. Immune. Defic. Syndr 41(3):285–297. [DOI] [PubMed] [Google Scholar]
- Barfod TS, Sorensen HT, Nielsen H, Rodkjaer L, Obel N (2006). ‘Simply Forgot’ Is The Most Frequently Stated Reason For Missed Doses of HAART Irrespective of degree of adherence. HIV Med. 7(5):285–290. [DOI] [PubMed] [Google Scholar]
- Berg KM, Litwin A, Li X, Heo M, Arnsten JH (2011). Directly Observed Antiretroviral Therapy Improves Adherence And Viral Load In Drug Users Attending Methadone Maintenance Clinics: A Randomized Controlled Trial. Drug Alcohol Depend. 113(2–3):192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boender TS, Hamers RL, Ondoa P, Wellington M, Chimbetete C, Siwale M, Labib Maskimos EEF, Balinda SN, Kityo CM, Adeyemo TA, Akanmu AS, Mandaliya K, Botes ME, Stevens W, Rinke De Wit TF, Sigaloff KCE (2016). Protease Inhibitor Resistance In The First 3 Years Of Second-Line Antiretroviral Therapy For HIV-1 In Sub-Saharan Africa. J. Infect. Dis 214:873–883. [DOI] [PubMed] [Google Scholar]
- Chawana T, Reid A, Bwakura T, Gavi S, Nhachi C (2014). Factors Influencing Treatment Failure In HIV Positive Adult Patients on First-Line Antiretroviral Therapy. Cent. Afr. J. Med 60:5–8. [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW (2000). Self-Reported Adherence to Antiretroviral Medications among Participants in HIV Clinical Trials: The AACTG Adherence Instruments. Patient Care Committee & Adherence Working Group of The Outcomes Committee Of The Adult AIDS Clinical Trials Group (AACTG). AIDS Care 12(3):255–266. [DOI] [PubMed] [Google Scholar]
- Conradie F, Wilson D, Basson A, De Oliveira T, Hunt G, Joel G, Papathanasopoulos M, Preiser W, Klausner J, Spencer D, Stevens D, Venter F, Van Vuuren C, Levin L, Meintjes G, Orrell C, Sunpath H, Rossouw T, Van Zyl G, Southern Africa HIV Clinicians Society (2012). The 2012 Southern Africa ARV Drug Resistance Testing Guidelines. South Afr. J. HIV Med. 13(4):162–167. [Google Scholar]
- Davies MA, Boulle A, Fakir T, Nuttall J, Eley B (2008). Adherence To Antiretroviral Therapy In Young Children In Cape Town, South Africa, Measured By Medication Return And Caregiver Self-Report: A Prospective Cohort Study. BMC Pediatr. 8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong BJ, Ward DJ, Chamberlain LA, Reddy YS, Ebrahimi R, Flaherty JF, Owen WF (2016). Safety And Effectiveness Of Tenofovir/Emtricitabine Or Lamivudine Plus Ritonavir Boosted Atazanavir In Treatment Experienced HIV Infected Adults At Two Urban Private Medical Practises. J. Antivir. Antiretrovir 4:1–5. [Google Scholar]
- Federal Ministry of Health Nigeria (2010). National Guidelines for HIV and AIDS Treatment And Care In Adolescents And Adults 2010. Abuja, Nigeria. [Google Scholar]
- Ferrand R, Lowe S, Whande B, Munaiwa L, Langhaug L, Cowan F, Mugurungi O, Gibb D, Munyati S, W illiams BG, Corbett EL (2010). Survey of Children Accessing HIV Services In A High Prevalence Setting: Time For Adolescents To Count? Bull. World Health Organ 88(6):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford N, Nachega JB, Engel ME, Mills EJ (2009). Directly Observed Antiretroviral Therapy: A Systematic Review And Meta-Analysis Of Randomised Clinical Trials. Lancet 374(9707):2064–2071. [DOI] [PubMed] [Google Scholar]
- Garone D, Conradie K, Patten G, Cornell M, Goemaere E, Kunene E, Kerschberger B, Ford N, Boulle A, Van Cutsen G (2014). High Rate Of Virologic Re-Suppression Among Patients Failing Second-Line Antiretroviral Therapy Following Enhanced Adherence Support: A Model of Care In Khayelitsha, South Africa. South African J. HIV Med. 14(4):166–169. [Google Scholar]
- Goggin K, Liston RJ, Mitty JA (2007). Modified Directly Observed Therapy For Antiretroviral Therapy: A Primer From The Field. Public Health Rep. 122(4):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, Sawyer Aw, Hamers RL, Ndembi N, Pillay D, Bertagnolio S (2012). Global Trends In Antiretroviral Resistance In Treatment-Naive Individuals With HIV After Rollout Of Antiretroviral Treatment In Resource-Limited Settings: A Global Collaborative Study And Meta-Regression Analysis. Lancet 380(9849):1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Pa, Taylor R, Thielke R, Payre J, Gonzalez N, Conde JG (2009). Research Electronic Data Capture-A Metadata-Driven Methodology And W orkflow Process For Providing Translational Research Informatics Support. J. Biomed. Inform 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB (2013). Emergence of HIV Drug Resistance During First-And Second-Line Antiretroviral Therapy In Resource-Limited Settings. J. Infect. Dis 207(S2):S49–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Sh, Gerver SM, Fidler S, Ward H(2014). Adherence to Antiretroviral Therapy In Adolescents Living With HIV: Systematic Review And Meta-Analysis. AIDS 28(13):1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole O, Denison JA, Menten J, Tsui S, Wabwire-Mangen F, Kwesigabo G, Mulenga M, Auld A, Agolory S, Mukadi YD, Van Praag E, Torpey K, W illiams S, Kaplan J, Zee A, Babgsberg DR, Colebunders R (2016). Reasons for Missing Antiretroviral Therapy: Results from a Multi-Country study in Tanzania, Uganda and Zambia. PlosOne 11(1): E0147309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessells RJ, Avalos A, De Oliveira T (2013). Implementing HIV-1 Genotypic Resistance Testing In Antiretroviral Therapy Programs in Africa: Needs, Opportunities, and Challenges. AIDS Rev. 15(4):221–229. [PMC free article] [PubMed] [Google Scholar]
- Levison JH, Orrell C, Gallien S, Kuritzkes DR, Fu N, Losina E, Freedberg KA, Wood R (2012). Virologic Failure of Protease Inhibitor-Based Second-Line Antiretroviral Therapy Without Resistance In A Large HIV Treatment Program In South Africa. PlosOne 7(3):E32144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA (2014). Perinatally Acquired HIV Infection In Adolescents From Sub-Saharan Africa: A Review Of Emerging Challenges. Lancet Infect. Dis 14(7):627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, W eidle PJ, Hader S, Mccaul ME, Moore RD (2006). Directly Administered Antiretroviral Therapy In Methadone Clinics Is Associated With Improved HIV Treatment Outcomes, Compared With Outcomes Among Concurrent Comparison Groups. Clin. Infect. Dis 42(11):1628–1635. [DOI] [PubMed] [Google Scholar]
- Macdonell K, Naar-King S, Huszti H, Belzer M (2013). Barriers to Medication Adherence In Behaviorally and perinatally infected Youth Living With HIV. AIDS Behav. 17(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Botswana 2012. Botswana National HIV And AIDS Treatment Guidelines 2012. Republic Of Botswana. [Google Scholar]
- Modi AC, Pai AL, Hommel KA, Hood KK, Cortina S, Hilliard ME, Guilfoyle SM, Gray WN, Drotar D (2012). Pediatric Self-Management: A Framework for Research, Practice, and Policy. Pediatrics 129(2):E473–E485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, Cotton M, Maartens G (2009). Antiretroviral Therapy Adherence, Virologic And Immunologic Outcomes In Adolescents Compared With Adults In Southern Africa. J. Acquir. Immune. Defic. Syndr 51(1): 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Mills EJ, Schechter M (2010). Antiretroviral Therapy Adherence and Retention In Care In Middle-Income And Low-Income Countries: Current Status Of Knowledge And Research Priorities. Curr. Opin. HIVAIDS 5(1):70–77. [DOI] [PubMed] [Google Scholar]
- National Department of Health South Africa (2012). Clinical Guidelines for the Management of HIV And AIDS In Adults And Adolescents. Pretoria, South Africa. [Google Scholar]
- Nglazi MD, Kranzer K, Holele P, Kaplan R, Mark D, Jaspan H, Lawn SD, Wood R, Bekker LG (2012). Treatment Outcomes In HIV-Infected Adolescents Attending A Community-Based Antiretroviral Therapy Clinic In South Africa. BMC Infect. Dis 12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents (2012). Guidelines for the Use of Antiretroviral Agents In HIV-1 Infected Adults And Adolescents. Department of Health and Human Services (DHHS) 2012.
- Panel on Antiretroviral Therapy And Medical Management Of HIV-1 Infected Children (2012). Guidelines for The Use Of Antiretroviral Agents In Paediatric HIV Infection. Department of Health and Human Services (DHHS) 2012.
- Paterson dL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N(2000). Adherence to Protease Inhibitor Therapy And Outcomes In Patients With HIV Infection. Ann. Intern. Med 133(1):21–30. [DOI] [PubMed] [Google Scholar]
- Rhee SY, Jordan MR, Raizes E, Chua A, Parkin N, Kantor R, Van Zyl GU, Mukui I, Hosseinipour MC, Frenkel LM, Ndembi N, Hamers rL, Rinke De Wit TF, Wallis CL, Gupta RK, Fokam J, Zeh C, Schapiro JM, Carmona S, Katzenstein D, Tang M, Aghokeng AF, De Oliveira T, Wensing AM, Gallant JE, Wainberg MA, Richman DD, Fitzgibbon JE, Schito M, Bertagnolio S, Yang C, Shafer RW (2015). HIV-1 Drug Resistance Mutations: Potential Applications for Point-Of-Care Genotypic Resistance Testing. PlosOne 10(12):E0145772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Kouanfack C, Cohen J, Marcellin F, Boyer S, Delaporte E, Carrieri P, Laurent C, Spire B (2011). Adherence to Antiretroviral Treatment In HIV-Positive Patients In The Cameroon Context: Promoting The Use Of Medication Reminder Methods. J. Acquir. Immune. Defic. Syndr 57(S1):S40–S43. [DOI] [PubMed] [Google Scholar]
- Shuter J (2008). Forgiveness of Non-Adherence to HIV-1 Antiretroviral Therapy. J. Antimicrob. Chemother 61(4): 769–773. [DOI] [PubMed] [Google Scholar]
- Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS (2007). HIV-Infected Patients Receiving Lopinavir/Ritonavir-Based Antiretroviral Therapy Achieve High Rates Of Virologic Suppression Despite Adherence Rates Less Than 95%. J. Acquir. Immune. Defic. Syndr 45(1): 4–8. [DOI] [PubMed] [Google Scholar]
- Sohn AH, Hazra R (2013). The Changing Epidemiology Of The Global Paediatric HIV Epidemic: Keeping Track Of Perinatally HIV-Infected Adolescents. J. Int. AIDS Soc 16(1):18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaysod R, Ngo-Giang-Huong N, Salvadori N, Cressey TR, Kanjanavanit S, Techakunakorn P, Krikajornkitti S, Srirojana S, Laomanit L, Chalermpantmetagul S, Lallemant M, Le CS, Mcintosh K, Traisathit P, Jourdain G (2015). Treatment Failure in HIV-Infected Children On Second-Line Protease Inhibitor-Based Antiretroviral Therapy. Clin. Infect. Dis 61(1):95–101. [DOI] [PubMed] [Google Scholar]
- Taddeo D, Egedy M, Frappier JY (2008). Adherence to treatment in adolescents. Paediatr. Child Health 13(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MW, Shafer RW (2012). HIV-1 Antiretroviral Resistance: Scientific Principles and Clinical Applications. Drugs 72(9):E1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JC, Mandalia S, Gazzard BG (2002). Responses To A 1 Month Self-Report On Adherence To Antiretroviral Therapy Are Consistent With Electronic Data And Virological Treatment Outcome. AIDS 16(2):269–277. [DOI] [PubMed] [Google Scholar]
- Wensing AM, Van Maarseveen NM, Nijhuis M (2010). Fifteen Years Of HIV Protease Inhibitors: Raising The Barrier To Resistance. Antiviral Res. 85(1):59–74. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2011). Joint United Nations Programme On HIV/AIDS (UNAIDS), United Nations International Childrens’ Emergency Fund (UNICEF) (2011) Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access. 2011 Progress Report Geneva, Switzerland: World Health organization; 2011. [Google Scholar]
- Wohl AR, Garland WH, Valencia R, Squires K, W itt MD, Kovacs A, Larsen R, Hader S, Anthony MN, Weidle PJ (2006). A Randomized Trial of Directly Administered Antiretroviral Therapy And Adherence Case Management Intervention. Clin. Infect. Dis 42(11):1619–1627. [DOI] [PubMed] [Google Scholar]
- World Health organization (WHO) (2014). Adolescent HIV Testing, Counselling And Care: Implementation Guidelines For Health Providers And Planners. Geneva, Switzerland. [Google Scholar]
- World Health organization (WHO) (2010a). Antiretroviral Therapy For HIV Infection In Adults And Adolescents: Recommendations For A Public Health Approach (2010 Revision). Geneva, Switzerland. [PubMed] [Google Scholar]
- World Health organization (WHO) (2010b). Antiretroviral Therapy For HIV Infection In Infants And Children: Recommendations For A Public Health Approach (2010 Revision). Geneva, Switzerland. [PubMed] [Google Scholar]
- World Health organization (WHO) HIV/AIDS Programme (2013). Consolidated Guidelines On The Use Of Antiretroviral Drugs For Treating And Preventing HIV Infection. Recommendations For A Public Health Approach. [PubMed]
- World Health organization (WHO) Multicentre Growth Reference Study Group (2007). WHO Child Growth Standards Based On Length/Height, Weight And Age. Acta Paediatr. 95(S450):76–85. [DOI] [PubMed] [Google Scholar]