Abstract
Actinopathy is a group of clinically and pathologically heterogeneous myopathies due to mutations in the skeletal muscle sarcomeric α-actin 1-encoding gene (ACTA1). Disease-onset spans from prenatal life to adulthood and weakness can preferentially affect proximal or distal muscles. Myopathological findings include a spectrum of structural abnormalities with nemaline rods being the most common. We report a daughter and father with prominent finger flexors and/or quadriceps involvement. Muscle biopsies revealed rimmed vacuoles in both patients, associated with type 1 fiber atrophy in the daughter, and nemaline rods in the father. Next generation sequencing identified a novel dominant ACTA1 variant, c.149G > A (p.Gly50Asp) in both individuals and no abnormal variants in vacuolar myopathy-associated genes. Our findings expand the clinico-pathological spectrum of actinopathy.
Keywords: ACTA1, Congenital fiber type disproportion, Finger flexor weakness, Inclusion body myositis, IBM, Nemaline myopathy, Rimmed vacuoles
1. Introduction
Mutations in the skeletal muscle sarcomeric α-actin 1-encoding gene (ACTA1) cause autosomal dominant or less commonly recessive congenital myopathies with a wide range of clinical phenotypes and pathological findings [1]. Weakness generally occurs prior to teenage years, but adult-onset weakness has been reported [1]. The pattern of weakness varies from limb-girdle, scapuloperoneal [2] to the recently described distal phenotype [3]. Muscle biopsy may show classic nemaline rods or less commonly actin filament aggregates, caps, cores, fiber type disproportion, myofibrillar pathology, or zebra bodies [3].
Herein, we report a daughter and her father with a childhood-onset of the weakness and prominent finger flexor and/or quadriceps involvement due to a novel dominant ACTA1 variant. Muscle biopsies revealed no inflammatory exudate but rimmed vacuoles in both patients. This family expands the clinical and pathological spectrum of actinopathy.
2. Case reports
The proband is a 39-year-old woman with congenital joint hypermobility, congenital cyanotic heart defect requiring surgical repair and delayed motor milestones. She was a slow runner since childhood and had difficulty keeping up with her peers. At age 33, she noted inability to do sit-ups due to abdominal muscle weakness and, three years later, difficulty climbing stairs and getting up from the floor, dyspnea and orthopnea. She denied myalgia, pigmenturia or complications from exposure to anesthetics. Creatine kinase (CK) was mildly elevated at 222 U/L (normal < 173 U/L). The patient’s father carried the diagnosis of sIBM despite the onset of weakness around age 11. His weakness started worsening after age 40. The proband’s 36-year-old sister is asymptomatic. Table 1 summarizes the key clinical features of the proband and affected father. Weakness of finger flexors was present in the proband and in the father. In addition, the father had also quadriceps weakness and atrophy. The proband’s two daughters, age 3 and 6 years, respectively, had axial hypotonia and joint hypermobility at birth; the oldest had delayed motor development and now has difficulty rising up from the floor while the youngest is unable to fully close her eyelids while sleeping.
Table 1.
Clinical findings in proband and father.
| Characteristics | Proband | Father |
|---|---|---|
| Age at presentation | 39 | 69 |
| Motor milestones | Delayed | Normal |
| Age at onset (years) | Early childhood | 11 |
| Symptoms at onset | Slow runner | Slow runner and frequent falls |
| Progression | Abdominal wall weakness (at age 33); proximal leg weakness, dyspnea at rest and orthopnea (at age 36) | Gait difficulty and dyspnea on exertion (at age 40) |
| Facial weakness | ++ | + |
| Neck flexor weakness | + | + |
| Upper extremity weakness | ||
| − Proximal | +/− triceps; + others | + shoulder external rotators, elbow flexors, and elbow |
| − Distal | ++ left thumb flexors and + right thumb flexors; + finger | extensors; +/− shoulder abductors |
| extensors; | + finger flexors; +/− finger extensors and intrinsic hand | |
| +/− other muscles | muscles | |
| Abdominal wall muscle weakness |
++ | Not tested |
| Characteristics | Proband | Father |
| Lower extremity weakness | ||
| − Proximal | Nl knee extensors; ++ hip flexors; + others | ++ left knee extensors and + right knee extensors; ++ left |
| − Distal | ++ toe extensors; − plantar flexors and foot | hip flexors and + right hip flexors; + l eft knee flexors; − |
| invertors; + others | others ++ right foot evertors and + left foot evertors; + ankle dorsiflexors; − ankle dorsiflexors |
|
| Other features | High arched palate; left scapular winging; scoliosis; distal joint hyperlaxity | No scapular winging or joint hyperlaxity |
Nl, normal strength (MRC grade 5); +/−, very mild weakness (MRC grade 4 +); +, mild weakness (MRC grade 4); ++, moderate weakness (MRC grade 3); +++, severe weakness (MRC grade 1–2); ++++, very severe weakness (MRC grade 0).
Nerve conduction studies and 2 Hz repetitive stimulation of spinal accessory and peroneal nerves were normal. Needle EMG revealed short duration, low amplitude motor unit potentials with early recruitment in proximal and distal muscles, and rare fibrillation potentials in proximal lower limb muscles. EKG, echocardiogram, and overnight oximetry were normal. Blood acid alpha-glucosidase level and genetic studies for myotonic dystrophies type 1 and 2, and facioscapulohumeral dystrophy type 1, performed prior to our evaluation, were normal. Right triceps biopsy showed findings consistent with congenital fiber type disproportion but also rimmed vacuoles (Fig. 1 (A)–(C)), one of which contained congophilic material. Inflammatory changes, cytochrome c oxidase-negative fibers, core formations and nemaline rods were absent. No ectopic accumulation of α-actinin, myotilin and other Z-disk associated proteins was detected by immunohistochemical studies. In light of the patient’s clinical history and observed myopathological findings not suggestive of an immune-mediated myopathy, MHC-I immunostaining was not performed.
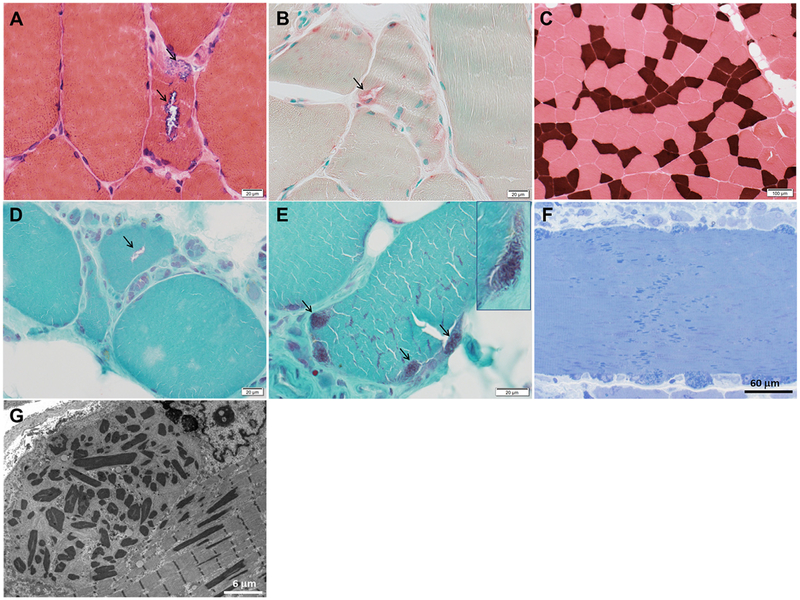
Fig. 1.
Muscle biopsies of proband and her father. Proband’s biopsy photographs (A–C) depicting fibers with rimmed vacuoles (arrows) overreactive for acid phosphatase (A, hematoxylin-eosin; B, acid phosphatase) and type 1 fiber atrophy (darkest fibers; C, ATPase pH 4.2). Proband’s father’s muscle biopsy photographs (D–G) showing a fiber with rimmed vacuole (arrow, D) in the midst of numerous pyknotic nuclear clusters and fatty replacement, and nemaline rods (arrows, E) as seen in Gomori trichrome. In the epon 1 μm sections (F, toluidine blue; G, electron microscopy), the nemaline rods are identified in subsarcolemmal groups and amidst sarcomeres across the entire diameter of some muscle fibers.
The proband’s father (now age 69) had undergone quadriceps biopsy at age 54 and diagnosed with sporadic inclusion body myositis (sIBM) elsewhere, mainly on the basis of his pattern of weakness, despite the early onset of the weakness. His muscle biopsy slides showed nearly end-stage muscle, groups of atrophic fibers, numerous pyknotic nuclear clusters, fiber splitting, increase in internalized nuclei, and rare fibers with rimmed vacuoles or nemaline rods (Fig. 1 (D) and (E)). Inflammatory changes and cytochrome c oxidase-negative fibers were absent. MHC-I immunostaining was not performed. Subsequent epon sections for light and electron microscopy studies confirmed the presence of nemaline rods (Fig. 1 (F) and (G)) and did not identify tubulofilamentous inclusions.
Next generation sequencing (NGS) of 74 myopathy genes (Supplementary material 1A) showed 2 novel heterozygous variants, one in exon 3 of ACTA1 (NM_001100.3), c.149G > A (p.Gly50Asp), and one in exon 2 of the GAA (NM_000152.3), c.109C > G (p.Leu37Val). In silico analysis predicted that the ACTA1 variant is deleterious and the GAA variant tolerated. The proband’s father carries the same ACTA1 variant. Additional NGS in the proband revealed no abnormal variants in genes causative of vacuolar myopathies and multisystem proteinopathy (Supplementary material 1B).
3. Discussion
Although both proband and father developed symptoms early in life, they did not seek medical attention until their mid-30s or early 40s, when the weakness became progressive and started impacting their activities of daily living. Thumb or finger flexors were weaker than finger extensors in proband and father; knee extensors and hip flexors were equally weak in the father. This pattern of weakness resembles the classic features of sIBM [4], and, indeed, in combination with the rimmed vacuoles on biopsy, had led to the misdiagnosis of sIBM in the father, despite his young age of disease onset arguing against sIBM [5]. The early disease-onset and family history of autosomal dominant trait were the main elements that prompted the search of an alternative diagnosis also in the father. In light of the presence of rimmed vacuoles in the muscle biopsy of both proband and father, the possibility of other hereditary muscle diseases featuring rimmed vacuoles was considered, but not supported by the molecular analysis. No pathogenic variants were found in known genes causative of myopathies with rimmed vacuoles. Recently, predominant finger flexor weakness was reported in patients with GNE-myopathy and in two subjects carrying a heterozygous VCP variant, one meeting clinical and pathological criteria of sIBM and one with possible sIBM [6,7]. Prominent finger flexor involvement can also occur in other hereditary muscle diseases, including myotonic dystrophy type 1 and 2 [8], autosomal dominant filamin C-distal myopathy [9], glycogen storage disease type 2 [10], and now also in ACTA1-myopathy.
ACTA1 is a highly conserved protein and any amino acid change is likely to be pathogenic [11]. The novel ACTA1 variant, p.Gly50Asp, affects a highly conserved residue and is predicted to be deleterious. It also co-segregates with the vacuolar myopathy and prominent finger flexor weakness in this family with autosomal dominant myopathy. The detection of nemaline rods in the father’s biopsy, despite being nearly end-stage, and the type 1 muscle fiber atrophy in the proband’s biopsy also support the pathogenicity of the p.Gly50Asp variant, in addition to underscoring the intrafamilial pathological variability of ACTA1-myopathy. A different missense mutation at the same codon, p.Gly50Cys, was reported in the online Leiden Open Variation Database (https://databases.lovd.nl/shared/genes/ACTA1), but its pathogenicity was not confirmed.
ACTA1-myopathy can present with various patterns of weakness. Distal weakness was not considered a prominent feature of ACTA1-myopathy until recently, when we reported a family of autosomal dominant distal myopathy with early finger extensor involvement due to a novel p.Gly253Arg mutation [3]. To our knowledge, the present family is the first ACTA1-myopathy with predominant finger flexor weakness. Nemaline rods are the classic pathological findings of ACTA1-myopathy, but other pathological findings do occur as mentioned above [3]. We have now shown in two patients that also rimmed vacuoles can accompany ACTA1-myopathy. Rimmed vacuoles in ACTA1-myopathy had been previously described in a single patient with zebra body myopathy [12]. That patient’s biopsy showed also myofibers containing nemaline rods and sarcoplasmic hyaline materials on trichrome stained sections, resembling myofibrillar pathology.
The present family broadens the phenotypic spectrum of ACTA1-myopathy to include the prominent finger flexor weakness and vacuolar pathology.
Supplementary Material
Funding
Study funded through a generous gift of a Mayo Clinic benefactor to MM and ZN.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.nmd.2019.02.012.
References
- [1].Nowak KJ, Ravenscroft G, Laing NG. Skeletal muscle alpha-actin diseases (actinopathies): pathology and mechanisms. Acta Neuropathol 2013;125:19–32. [DOI] [PubMed] [Google Scholar]
- [2].Zukosky K, Meilleur K, Traynor BJ, et al. Association of a novel ACTA1 mutation with a dominant progressive scapuloperoneal myopathy in an extended family. JAMA Neurol 2015;72:689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liewluck T, Sorenson EJ, Walkiewicz MA, Rumilla KM, Milone M. Autosomal dominant distal myopathy due to a novel ACTA1 mutation. Neuromuscul Disord 2017;27:742–6. [DOI] [PubMed] [Google Scholar]
- [4].Milone M. Diagnosis and management of immune-mediated myopathies. Mayo Clin Proc 2017;92:826–37. [DOI] [PubMed] [Google Scholar]
- [5].Rose MR Group EIW. 188th ENMC International workshop: inclusion body myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord 2013;23:1044–55. [DOI] [PubMed] [Google Scholar]
- [6].Weihl CC, Baloh RH, Lee Y, et al. Targeted sequencing and identification of genetic variants in sporadic inclusion body myositis. Neuromuscul Disord 2015;25:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Dios JK, Shrader JA, Joe GO, et al. Atypical presentation of GNE myopathy with asymmetric hand weakness. Neuromuscul Disord 2014;24:1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Day JW, Ricker K, Jacobsen JF, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology 2003; 60:657–64. [DOI] [PubMed] [Google Scholar]
- [9].Guergueltcheva V, Peeters K, Baets J, et al. Distal myopathy with upper limb predominance caused by filamin C haploinsufficiency. Neurology 2011;77:2105–14. [DOI] [PubMed] [Google Scholar]
- [10].Bandyopadhyay S, Wicklund M, Specht CS. Novel presentation of Pompe disease: inclusion-body myositis-like clinical phenotype. Muscle Nerve 2015;52:466–7. [DOI] [PubMed] [Google Scholar]
- [11].Laing NG, Dye DE, Wallgren-Pettersson C, et al. Mutations and polymorphisms of the skeletal muscle alpha-actin gene (ACTA1). Hum Mutat 2009;30:1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sewry CA, Holton JL, Dick DJ, Muntoni F, Hanna MG. Zebra body myopathy is caused by a mutation in the skeletal muscle actin gene (ACTA1). Neuromuscul Disord 2015;25:388–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.