SUMMARY
While all-trans retinoic acid (ATRA) treatment in acute promyelocytic leukemia (APL) has been the paradigm of targeted therapy for oncogenic transcription factors, the underlying mechanisms remain largely unknown, and a significant number of patients still relapse and become ATRA resistant. We identified the histone demethylase PHF8 as a coactivator that is specifically recruited by RARα fusions to activate expression of their downstream targets upon ATRA treatment. Forced expression of PHF8 resensitizes ATRA-resistant APL cells, whereas its downregulation confers resistance. ATRA sensitivity depends on the enzymatic activity and phosphorylation status of PHF8, which can be pharmacologically manipulated to resurrect ATRA sensitivity to resistant cells. These findings provide important molecular insights into ATRA response and a promising avenue for overcoming ATRA resistance.
INTRODUCTION
Transcriptional deregulation plays a key role in a large array of human malignancies, in particular, in acute leukemia, which is mostly initiated by chimeric transcription factors (CTFs) that induce oncogenic transcriptional programs resulting in cellular transformation (Cheung and So, 2011). The successful application of all-trans retinoic acid (ATRA) treatment to acute promyelocytic leukemia (APL) induced by RARα fusion proteins represents a major breakthrough and is the paradigm for targeted therapy of oncogenic transcription factors (Wang and Chen, 2008). In spite of its success in achieving a complete remission (CR), APL patients receiving ATRA treatment alone do not achieve definite cure of the disease. Although refined APL treatment regimens in combination with anthracycline-based chemotherapy or arsenic trioxide (ATO) result in 90% of initial CR, a significant proportion of patients still relapse and are resistant to the treatment with 3 years overall survival in second remission of only around 50% (Sanz and Lo-Coco, 2011). Also, while the success of ATRA treatment in APL sets the stage for targeted therapy of oncogenic transcription factors, retinoic acid (RA) treatment is ineffective to other malignant diseases. Therefore, understanding the molecular mechanisms of APL pathogenesis and ATRA response are of major interest, because it may help to design better therapeutic strategies to overcome ATRA resistance and potentially extend its application to other malignancies.
Over the years, we and others have shown that RARα fusions form high-order homo-oligomers (Kwok et al., 2006; Lin and Evans, 2000; Minucci et al., 2000; Sternsdorf et al., 2006) that aberrantly recruit DNA binding cofactor RXRα (Zeisig et al., 2007; Zhu et al., 2007) and epigenetic modifying enzymes such as histone deacetylases (HDACs) (Grignani et al., 1998; Lin et al., 1998) and polycomb-repressive complexes (PRCs) (Boukarabila et al., 2009; Smith et al., 2011; Villa et al., 2007) for transcriptional suppression of their downstream targets (e.g., RARB) and oncogenic transformation. A pharmacological level of ATRA induces conformational changes of RARα fusions, which result in dissociation of corepressor complexes and recruitment of coactivators, leading to activation of downstream targets and subsequent degradation of the fusion proteins (de Thé and Chen, 2010; Wang and Chen, 2008). In spite of its critical functions in mediating ATRA response, the identity of the coactivator complex responsible for gene activation upon ATRA treatment remains unknown, and this becomes a major hurdle that significantly hinders the progress in understanding underlying mechanisms of ATRA response and designing more effective therapeutic strategies for overcoming resistance (Martens et al., 2010; Mikesch et al., 2010).
Emerging evidence indicates that dynamic histone modifications by lysine methyltransferases (KMTs) and demethylases (KDMs) play a key role in regulation of gene expression (Cheung and So, 2011; Kouzarides, 2007). Members of Jumonji-C domain (JmjC) KDMs involved in diverse biological processes including embryonic development, stem cell self-renewal, and differentiation are known to work closely together with specific KMTs by removing opposite epigenetic marks to govern gene expression (Cheung and So, 2011; Kooistra and Helin, 2012). PHF8 (also called KDM7B) is a member of the plant homeodomain finger (PHF) family KDM harboring an N-terminal plant homeodomain (PHD) that mediates binding to nucleosomes at active gene promoters as well as an active JmjC domain that is able to catalyze the demethylation of mono- or dimethyl-lysines (Feng et al., 2010; Fortschegger et al., 2010; Kleine-Kohlbrecher et al., 2010; Liu et al., 2010; Loenarz et al., 2010; Qi et al., 2010). PHF8 preferentially acts on H3K9me2 and H3K9me1 (however, some results also suggest that it can demethylate H4K20me1) and associates with transcriptional activation and retinoic acid signaling pathway in neuronal differentiation (Qiu et al., 2010). Mutations in the PHF8 gene are found in patients with X-linked mental retardation (XLMR), and knockdown of PHF8 homolog leads to brain defects in zebrafish (Abidi et al., 2007; Koivisto et al., 2007; Laumonnier et al., 2005), revealing its potential involvement in human disease.
RESULTS
PHF8 Interacts with PML-RARα and Functions as a Transcriptional Coactivator in Response to ATRA Treatment
Given the critical functions of JmjC-KDMs in transcriptional regulation, we performed a systematic biochemical screening by immunoprecipitation assay in human 293T cells for interactions between PML-RARα and seven different JmjC-KDMs from each of the subfamily (KDM2–7) with known enzymatic activity upon ATRA treatment. As a result, we identified PHF8 as the only KDM that exhibited a highly specific interaction with PML-RARα, and this interaction increased significantly in the presence of ATRA (Figure 1A; data not shown). To validate this finding in APL, we showed that endogenous PHF8 binds to PML-RARα in human NB4 cells (Figure S1A available online), which express the PML-RARα fusion.
Figure 1. Specific Interaction of PHF8 with PML-RARα Results in Alternation of Histone Marks of Transcriptional Targets in Response to ATRA Treatment.
(A–F) Representative coimmunoprecipitation (coIP) analysis in 293T cells coexpressing PML-RARα and Flag-tagged Jumonji family members cultured in the presence or absence of ATRA (A). Deleted or point mutants of PML-RARα were coexpressed with PHF8 (B), or deleted or point mutants of PHF8 were coexpressed with His-PML-RARα (C); and all samples were processed in presence of ATRA. Black arrowheads indicate mutants that cannot interact. PML-RARα or/and wild-type RARα were expressed with Flag-tagged PHF8 using the indicated amounts of expression vectors, and cells were treated with ATRA as indicated; samples were processed under mild washing conditions (D) or stringent washing conditions (E and F). Asterisk indicates unspecific band.
(G) Immunoblotting of purified histone extracts from NB4 and NB4-PHF8 cells.
(H) Histone demethylase activity of YFP-tagged PHF8 protein in 293T cells was assessed by immunostaining using confocal microscopy. White arrowheads indicate cells transfected with YFP-PHF8. Scale bar, 10 μm. Anti-H3K9me2 and anti-H3K9me3 antibodies were used.
(I and J) ChIP analysis of the binding of the endogenous PHF8 (I) and histone H3 modifications (J) on typical RARE RARB promoter region in human NB4 cells after 24 hr with or without 10−8 M ATRA.
Data representative of at least three independent experiments are shown (±SD, *p < 0.05, **p < 0.01). See also Figure S1.
To further characterize this interaction, a series of PML-RARα and PHF8 mutants were constructed for structure/function analyses to define the respective interaction domains. In contrast to the regions D (hinge region), E (ligand binding domain, LDB), and F (unknown function) of the PML-RARα that were dispensable for PHF8 interaction, the region C that partly overlaps with the DNA binding domain in the PML-RARα fusion was absolutely required for the recruitment of PHF8 (Figure 1B). On the other hand, the JmjC domain but not the catalytic activity of PHF8 was essential for the interaction with PML-RARα (Figure 1C). In contrast, the PHD domain and the C-terminal domain of PHF8 were not part of the PML-RARα-interacting motif (Figure 1C). Consistent with the recent findings of its involvement in retinoic acid signaling (Qiu et al., 2010), mapping of the PML-RARα interaction domain to the region C suggests a potential interaction between PHF8 and wild-type RARα. To gain further insights into this issue, we revealed that PML-RARα had a much higher ability than wild-type RARα to form complex with PHF8 in the presence of ATRA (Figure 1D). Moreover, the interaction between wild-type RARα and PHF8 rapidly dissociated under the stringent washing condition, while the one between PML-RARα and PHF8 remained stable (Figure 1E). Consistently, only the PML-RARα/PHF8 interaction could be detected upon ATRA treatment even when wild-type RARα was coexpressed at the almost identical level as PML-RARα in the same cells with endogenous or ectopic expression of PHF8 (Figure 1F). Together these results indicate that PML-RARα is by far the dominant PHF8 binding partner compared to wild-type RARα in response to the ATRA treatment.
To assess the effect of PHF8 expression on histone modification, we ectopically expressed PHF8 in NB4 and 293T cells. As shown in Figures 1G, 1H, and S1B, PHF8 expression resulted in a significant reduction of H3K9me2 levels in NB4 and 293T cells, consistent with its role in promoting transcriptional activation (Feng et al., 2010; Fortschegger et al., 2010; Kleine-Kohlbrecher et al., 2010; Liu et al., 2010; Loenarz et al., 2010; Qi et al., 2010). To further demonstrate that PHF8 is indeed recruited by PML-RARα to activate expression of downstream targets upon ATRA treatment in APL cells, chromatin immunoprecipitation (ChIP) assays revealed specific binding of PHF8 to the promoter region of RARB, a RARα fusion target, but not to GAPDH control, upon ATRA treatment in NB4 cells (Figure 1I). Recruitment of PHF8 was also associated with a reduction of the H3K9me2 repressive mark, increase in H3K4me3 and H3K9Ac activation marks (Figure 1J), and an increased in RARB mRNA level (Figure S1C). These results demonstrate that PHF8 binds to and modifies the promoter regions of PML-RARα targets for active gene expression in APL cells upon ATRA treatment.
PHF8 Sensitizes APL Cells to Physiological Levels of ATRA
To investigate the functional significance of PML-RARα/PHF8 interaction in mediating cellular response to ATRA treatment, we performed both gain-of-function and loss-of-function studies using human NB4 cells and mouse primary hematopoietic cells transformed by APL fusion proteins (Kwok et al., 2006; Zeisig et al., 2007). NB4 cells are highly sensitive to pharmacological concentrations (10−6 M) but only have a mild response to physiological level (10−8 M) of ATRA as assessed by activation of a RARα fusion target, RARB, and inhibition of transformation (Figures S2A and S2B). Strikingly, forced expression of PHF8 in NB4 cells (Figures 2A, 2B, S2C, and S2D) or primary hematopoietic cells transformed by different RARα fusions, including PLZF-RARα-transformed cells that are usually more resistant to ATRA treatment in the presence of the reciprocal RARα-PLZF fusion (Guidez et al., 2007), significantly sensitized their response to ATRA (Figures 2C, 2D, S2E, and S2F). This function of PHF8 highly depended on the following: (1) its enzymatic activity as a single-point mutation F279S on the catalytic domain identified in X-linked mental retardation patients (Kleine-Kohlbrecher et al., 2010; Koivisto et al., 2007) completely abolished the enhanced ATRA sensitivity including colony suppression (Figures 2A–2D and S2D), enhanced RARB expression (Figures 2E and S2G), and differentiation of APL cells (Figure S2H); and(2) the presence of RARα fusions as expression of PHF8 had no effect on the ATRA response in the control human K562 leukemic cells or E2A-PBX1-transformed primary cells (Figures 2A–2E, S2G, and S2I), which expressed the same or even higher levels of RARα protein compared with those in NB4 cells and PML-RARα-transformed primary cells, respectively (Figure S2J). Consistently, the levels of PML-RARα were much higher than those of wild-type RARα in both NB4 and PML-RARα-transformed cells (Figure S2J). Together with the competitive/dilution assays in Figures 1D–1F, these results strongly suggest that PML-RARα is the major and dominant mediator for the PHF8/ATRA response.
Figure 2. PHF8 Governs ATRA Sensitivity of APL Cells.
(A–D) Typical p-iodonitrotetrazolium-violet (INT)-stained colony pictures and bar charts representing normalized colony number of human NB4 and K562 cells (A and B) or murine primary bone marrow cells transformed by the indicated RARα fusion constructs (C and D) treated with and without indicated concentration of ATRA. Black arrowheads indicate the lowest optimal ATRA concentration employed in most of the subsequent studies. Representative data of three experiments are shown (±SEM, **p < 0.01).
(E) Quantitative RT-PCR (qRT-PCR) analysis for RARB expression in the indicated cells.
(F–J) qRT-PCR (F and I) and western blot analysis (G and J) for PHF8 expression in cell transduced with specific human (F and G) or mouse (I and J) PHF8 shRNA. Error bars indicate SD of at least three independent experiments. (H) Human NB4 cells or (K) murine primary bone marrow cells transformed by PLZF-RARα were transduced with either shRNAs for specific PHF8 knockdown (KD) or scramble control before they were plated into methylcellulose medium in the absence or presence of ATRA for colony formation assay.
Data representative of three experiments are shown (±SEM, *p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S2.
To demonstrate a critical function of endogenous PHF8 in governing ATRA response, we further confirmed the expression of endogenous PHF8 in NB4, which was indeed much higher than that in the ATRA-resistant variant, NB4-MR2 cells (Figure S2K), suggesting an association of ATRA resistance with a reduced level of PHF8. To further validate this hypothesis, endogenous PHF8 expression was downmodulated by small hairpin RNAs (shRNAs) in human NB4 cells (Figures 2F–2H) or RARα-fusion-transformed primary cells (Figures 2I–2K). As a result, suppression of PHF8 conferred ATRA resistance in both NB4 cells (Figure 2H) and RARα-fusion-transformed primary cells (Figure 2K), although the effect was more pronounced in the latter with a more defined genetic background. Together, these results indicate that PHF8 may act as a sensor to mediate ATRA response in APL and its level may govern ATRA sensitivity.
PHF8 Resensitizes ATRA-Resistant APL Cells In Vitro and In Vivo
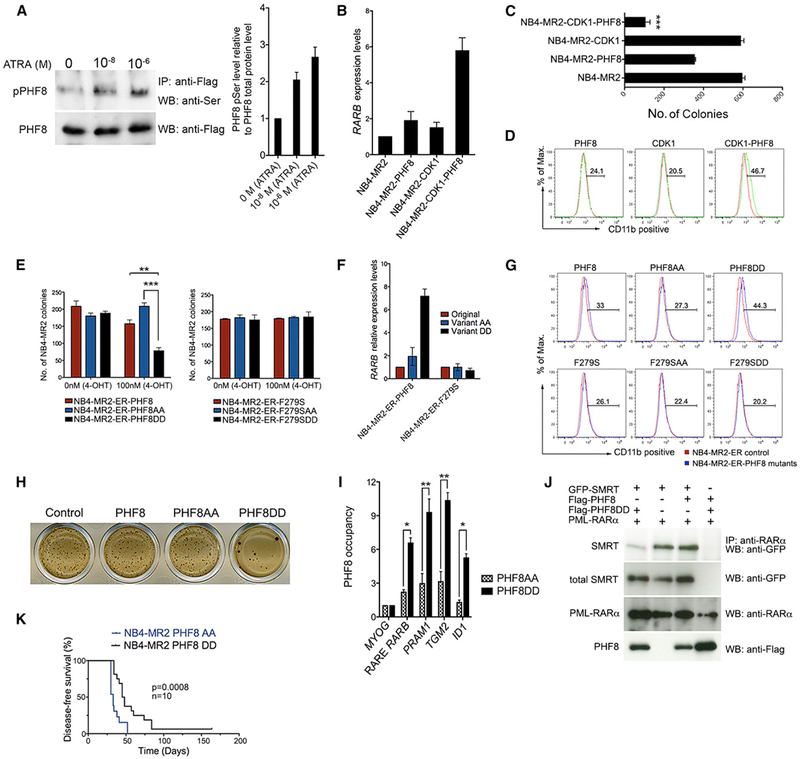
To investigate if PHF8 can indeed sensitize ATRA-resistant cells to the treatment, we induced expression of wild-type PHF8 and the catalytically dead mutant PHF8-F279S in the ATRA-resistant NB4-MR2 cells (Figures S3A and S3B). NB4-MR2 cells have an increased level of topoisomerase 2β (TOP2β) that could decrease ATRA-mediated gene expression and granulocytic differentiation by enhancing the association of repressor complexes with PML-RARα downstream target genes including RARB (McNamara et al., 2008). NB4-MR2-PHF8 cells showed a significant reduction in colony number, induction of the RARB expression, and increased differentiation of the APL cells after ATRA treatment (Figures 3A–3C and S3C–S3E). On the contrary, expression of PHF8-F279S failed to sensitize NB4-MR2 cells to the treatment, indicating an important enzymatic-activity-dependent function of PHF8, in governing ATRA response even in resistant cells (Figures 3A–3C and S3A–S3E). To further assess if PHF8 is also able to sensitize ATRA-resistant cells to treatment in vivo, we transplanted NB4-MR2, NB4-MR2-PHF8, and NB4-MR2-F279S cells into sublethally irradiated NOD-SCID-Gamma (NSG) mice for in vivo leukemogenic assay (Figures 3D and 3E). As expected, mice transplanted with NB4-MR2 cells succumbed to leukemia regardless of ATRA treatment. Strikingly, mice transplanted with NB4-MR2-PHF8 cells responded very well to the ATRA treatment, and most of them remained healthy (Figure 3D). This was in stark contrast to the untreated NB4-MR2-PHF8 control group that rapidly succumbed to leukemia. Consistent with the in vitro results (Figures 3A–3C), mice transplanted with NB4-MR2-F279S failed to respond to the ATRA treatment in vivo and developed leukemia with a similar latency as the untreated controls, confirming the importance of its enzymatic activity in ATRA response (Figure 3E). These results strongly indicate that PHF8 plays a critical role in mediating ATRA response, and its activation by an overexpression approach reverses ATRA resistance in APL cells.
Figure 3. PHF8 Sensitizes ATRA-Resistant APL Cells to Physiological Concentrations of ATRA.
(A–H) Human APL cells transduced with vector control or PHF8 wild-type or its catalytically inactive mutant F279S were treated with and without ATRA at the indicated concentrations. Typical INT-stained colony pictures of NB4-MR2 (A) and NB4-LR2 (F) cell lines. The bar charts represent NB4-MR2 normalized numbers of colonies (B). Error bars are representative of four independent experiments. (±SEM, ***p < 0.001). qRT-PCR analysis for human RARB expression in NB4-MR2 (C) or NB4-LR2 (G) cell lines. Error bars indicate SD of three independent experiments. Disease-free survival of NSG mice injected with NB4-MR2, NB4-MR2-PHF8 cells (D), NB4-MR2-F279S cells (E), or NB4-LR2, NB4-LR2-PHF8 cells (H), with and without ATRA treatment.
(I and J) M4 cells from PML-RARα LBD transgenic mouse model transduced with vector control (control M4) or PHF8 wild-type (PHF8 M4). Disease-free survival of FVB mice injected with control M4 or PHF8 M4 cells with and without ATRA treatment. Black arrowheads indicate the end of ATRA treatment (I). FACS analysis of bone marrow cells stained with Gr-1, Mac-1, and c-Kit markers for differentiation status of murine myeloid cells (J).
See also Figure S3.
Another major mechanism for ATRA resistance in human APL is the mutation of ligand binding domain (LBD) of PML-RARα (Côté et al., 2000). To further extend the role of PHF8 in ATRA-resistant APL, we employed two different APL models, namely, human NB4-LR2 cells (Roussel and Lanotte, 2001) and primary leukemic cells from a PML-RARαm4 transgenic mouse (designated “M4” herein) (Kogan et al., 2000), each carrying a different PML-RARα LBD mutation identified in human APL patients. As expected, NB4-LR2 cells were resistant to ATRA (Figures 3F, 3G, and S3F–S3H). Conversely, expression of PHF8 resensitized their response to ATRA 10−8 M (Figures 3F, 3G, and S3F–S3H). However, this effect disappeared when the mutation abolishing the catalytic activity was introduced to PHF8, consistently indicating the critical function of PHF8 enzymatic activity in mediating the ATRA response even in the PML-RARα LBD mutant (Figures 3F and 3G, S3A, and S3F–S3H). Finally, transplantation of NB4-LR2 cells into NGS mice induced ATRA-resistant leukemia, which could, however, be sensitized to ATRA treatment again when PHF8 was expressed (Figure 3H). To further validate these results in a well-defined genetic background, we performed similar in vivo experiments using the well-characterized ATRA-resistant M4 primary cell model (Kogan et al., 2000). M4 cells induced leukemia in mice regardless ATRA treatment. M4 cells expressing PHF8 also induced leukemia in mice. However, expression of active PHF8 in combination with ATRA treatment significantly extended the survival of mice and induced differentiation of M4 cells (Figures 3I and 3J). Together, these results strongly indicate that activation of PHF8 can resurrect ATRA sensitivity in a wide range of clinically relevant ATRA-resistant APL cells.
PHF8 Switches Promoter Occupancy after ATRA Treatment
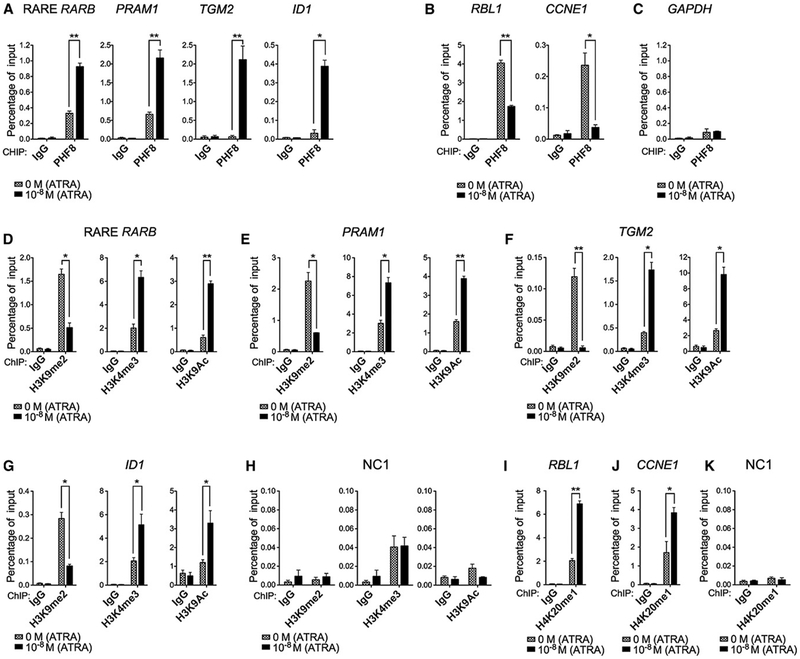
It has been shown that the binding of PHF8 to its targets is regulated by its phosphorylation status (Liu et al., 2010). PHF8 binds to promoter regions of genes involved in cell cycle progression such as RBL1 to remove H4K20me1 mark and dissociates from these promoters upon phosphorylation of S33/S84 residues by CDK1 (Liu et al., 2010). To gain further insights into the molecular regulation of PHF8 upon ATRA treatment in APL, we investigated the dynamics of PHF8 promoter occupancy by ChIP analysis in NB4-MR2 cells. Upon ATRA treatment, the binding of PHF8 to the promoter regions of multiple RARα fusion targets, including RARB, PRAM1, TGM2, and ID1 (Martens et al., 2010; Wang et al., 2010), was increased (Figure 4A), while its binding to naive PHF8 targets such as RBL1 and CCNE1 promoters that are occupied by PHF8 in the absence of ATRA was reduced (Figure 4B). ATRA treatment had no effect on the binding of PHF8 at the control GAPDH promoter (Figure 4C). This switch in promoter occupancy was also accompanied by changes in corresponding histone marks. A significant reduction in PHF8-specific repression marks (H3K9me2) and an increase in activation marks (H3K4me3 and H3K9Ac) were detected in the same promoter regions of RARB (Figure 4D), PRAM1 (Figure 4E), TGM2 (Figure 4F), and ID1 (Figure 4G), whereas H4K20me1 histone mark was significantly increased at the RBL1 (Figure 4I) and CCNE1 (Figure 4J) promoters. None of these changes could be detected in the noncoding control region (NC1) of NB4-MR2 cells (Figures 4H and 4K) or the same RARB regions in K562 cells that do not carry RARα fusions (Figures S4A and S4B). Thus, these findings suggest that PHF8 may switch promoter occupancy for driving the expression of PML-RARα targets in human APL cells upon ATRA treatment.
Figure 4. Changes of PHF8 Promoter Occupancy and Associated Histone Modifications after ATRA Induction.
ChIP analysis of PHF8 (A–C) or various histone marks including H3K9me2, H3K4me3, H3K9Ac, H4K20me1 (D–K) on both RARα-fusion-targeted promoters (e.g., RARB, PRAM1, TGM2, and ID1) and naive PHF8-targeted promoters (e.g., RBL1, CCNE1) before and after 24 hr of ATRA treatment at 0 M or 10−8 M in NB4-MR2-PHF8 cells. ChIP signals are presented as percentage of input. Error bars indicate SD of three independent experiments (±SD, *p < 0.05, **p < 0.01). GAPDH was used as negative control for PHF8 occupancy, and NC1 was used as negative control for histone marks. See also Figure S4.
PHF8-Mediated ATRA Response Is Regulated by Serine Phosphorylation
Interestingly, ATRA is known to relocate cyclin A to the nuclear compartment in leukemic cells, resulting in activation of CDK1 in AML cells (Ekberg et al., 2004). Thus, we speculated that the promoter occupancy of PHF8 might be regulated by ATRA in part by CDK1-mediated phosphorylation. Indeed, we detected an increase in PHF8 phosphorylation with increasing levels of ATRA in NB4-MR2-PHF8 cells (Figure 5A). Next, we investigated if CDK1-mediated PHF8 phosphorylation would be able to mimic ATRA response in PHF8-transduced NB4 or NB4-MR2 cells (Figures 5B–5D, S5A, and S5B). In the absence of ATRA treatment, cells transduced with PHF8 (NB4-MR2-PHF8) or CDK1 (NB4-MR2-CDK1) exhibited a mild increase of RARB gene expression as compared with vector-control (NB4-MR2)-transduced cells (Figure 5B). In contrast, cells cotransduced with CDK1 and PHF8 (NB4-MR2-CDK1-PHF8) significantly activated RARB expression even in the absence of ATRA (Figure 5B). These transcriptional activities also directly correlated with the biological readouts, in which coexpression of PHF8 with CDK1 significantly suppressed transformation and enhanced differentiation of NB4-MR2 cells even in the absence of ATRA treatment (Figures 5C and 5D). Similar results could also be obtained for NB4 cells (Figure S5B). These results suggest a critical function of CDK1 in mediating PHF8 functions, although it is known that CDK1 can have many different targets and functions, and likewise other kinases may also be able to modulate PHF8 activity. To confirm the critical role of PHF8 phosphorylation in mediating ATRA response, we generated two additional PHF8 variants at those two serine phosphorylation sites by replacing S33 and S84 with either alanines to generate a phosphorylation defective mutant (PHF8AA) or aspartic acids to mimic a constitutively phosphorylated form (PHF8DD) (Liu et al., 2010). To further demonstrate their specific activity in mediating ATRA response, these three PHF8 variants were fused to the ligand binding domain of the estrogen receptor (ER) to allow an inducible activation of the proteins by 4-hydroxy-tamoxifen (4-OHT) treatment. As expected, activation of PHF8 in the absence of ATRA had little impact on NB4-MR2 cells, and similar results were obtained for PHF8AA mutant (Figures 5E–5G, S5C, and S5D). However, expression of PHF8DD mutant significantly suppressed colony formation (Figure 5E) as well as enhanced expression of RARB (Figure 5F) and differentiation (Figure 5G) of APL cells without a significant impact on apoptosis (Figure S5D) even in the absence of ATRA. These results from ER-inducible mutants could also be directly reproduced using corresponding constitutive mutants (Figure 5H), in which the PHF8AA mutant as expected failed to collaborate with CDK1 for suppression of NB4-MR2 colony formation in the absence of ATRA (Figure S5E). Moreover, PHF8DD as compared with PHF8AA exhibited a much stronger binding to target promoters of PML-RARα (e.g., RARB, PRAM1, TGM2, and ID1) but not the MYOG control, consistent with a critical function of phosphorylation status in determining the promoter occupancy (Figure 5I). To further investigate if the inhibitory effects by PHF8DD mutant also require enzymatic activity of PHF8, the F279S mutation was introduced to these three PHF8 variants. The F279S mutation completely abolished both the transformation suppressive function and the transactivation activity of PHF8DD (Figures 5E–5G), strongly suggesting that ATRA response mediated by PHF8 is regulated by both serine phosphorylation and enzymatic activity of PHF8. Consistently, expression of PHF8DD mutant but not wild-type PHF8 could displace the transcriptional corepressor SMRT from PML-RARα in the absence of ATRA (Figure 5J). To further demonstrate that phosphorylation of PHF8 plays a key role in mediating ATRA response in APL in vivo, NB4-MR2 cells expressing either PHF8AA or PHF8DD mutant were transplanted into NSG mice for in vivo leukemogenic assay. PHF8DD significantly extended the disease latency even in the absence of ATRA treatment, confirming a key role of PHF8 phosphorylation status for APL leukemogenesis (Figure 5K).
Figure 5. PHF8-Mediated ATRA Response Is Regulated by Serine Phosphorylation.
(A) PHF8 was immunoprecipitated from total cell lysate of NB4-MR2-PHF8 cells treated with the indicated concentrations of ATRA and immunoblotted for phospho-Ser (upper panel); the membrane was stripped and then immunoblotted for total PHF8 protein (lower panel). The bar chart at the right represents quantification of serine-phosphorylated PHF8 relative to the total PHF8 protein. Error bars represent SD of three independent experiments.
(B–D) NB4-MR2 cells were transduced with empty vector control, wild-type PHF8, wild-type CDK1, or PHF8 and CDK1 together. The RARB mRNA level (B, measured by qRT-PCR, error bars indicate SD of three independent experiments), the number of colony formed (C, error bars represent SEM of three independent experiments, ***p < 0.001), and expression of the differentiation marker for myeloid cells CD11b (D, FACS analysis) of these cells were determined.
(E) The number of colonies of NB4-MR2 cells transduced with ER-fused enzymatic active (left) or dead (right) PHF8, PHF8AA, or PHF8DD in the absence or presence of 100 nM 4-OHT. Error bars represent three independent experiments (±SEM, **p < 0.01, ***p < 0.001).
(F) RARB mRNA level in cells transduced with indicated PHF8 constructs after induction with 100 nM 4-OHT shown in (E). Error bars indicate SD of three independent experiments.
(G) FACS analysis for CD11b expression of NB4-MR2-ER cells expressing PHF8 or different PHF8 mutants.
(H) Typical INT-stained colony pictures of NB4-MR2 cells transduced with empty vector control, PHF8, PHF8AA, or PHF8DD.
(I) ChIP analysis comparing the PHF8 occupancy at the indicated promoter regions of NB4-MR2 cells transduced with PHF8AA and PHF8DD variants. ChIP signals are presented as fold enrichment over MYOG (±SD, *p < 0.05, **p < 0.01).
(J) Representative coimmunoprecipitation (coIP) analysis in 293T cells. PML-RARα was coexpressed in the presence of GFP-SMRT, Flag-PHF8, or Flag-PHF8DD.
(K) Disease-free survival curves of NSG mice injected with NB4-MR2 cells expressing PHF8AA or PHF8DD.
See also Figure S5.
Inhibition of PHF8 Dephosphorylation by Okadaic Acid Sensitizes ATRA-Resistant Human APL Cells to the Treatment
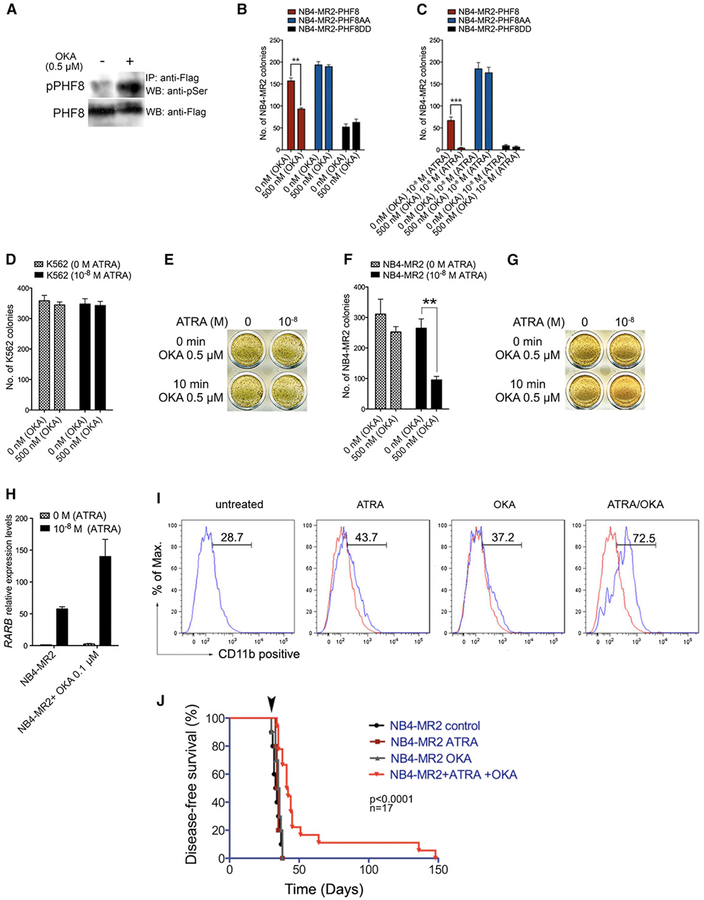
Considering the critical function of PHF8 in mediating ATRA response, we asked if pharmacological inhibition of PHF8 dephosphorylation could sensitize resistant APL cells to the ATRA treatment. To this end, we tested the effect of two common phosphatase inhibitors, calyculin A and okadaic acid (OKA), on NB4-MR2-PHF8 cells for their ability to suppress dephosphorylation of PHF8. OKA showed good efficacy in inhibiting PHF8 dephosphorylation as revealed by a significant increase of PHF8 serine phosphorylation in NB4-MR2-PHF8 cells upon OKA treatment (Figure 6A; data not shown). Consistent with this result, colony formation assays revealed that OKA alone could suppress colony formation by NB4-MR2-PHF8 cells, while it had no effect on NB4-MR2 cells expressing phosphorylation mutants NB4-MR2-PHF8AA and NB4-MR2-PHF8DD (Figure 6B). In addition, combined OKA/ATRA treatment had further enhanced tumor suppression effect on NB4-MR2-PHF8 cells but not on NB4-MR2 cells expressing PHF8AA or PHF8DD mutants (Figure 6C), indicating a critical function of these two PHF8 phosphorylation sites in mediating OKA response. To further assess if OKA can be used to sensitize ATRA-resistant APL to the treatment without genetic manipulation of PHF8, NB4-MR2 cells and K562 control cells were treated with OKA, ATRA, or their combination and subjected to colony formation assay (Figures 6D–6I). As expected, K562 cells did not respond to any of the treatments with ATRA, OKA, or their combination (Figures 6D and 6E). NB4-MR2 cells were also refractory to ATRA treatment and exhibited a very mild response to OKA treatment alone (Figures 6F and 6G). However, the combined treatment of ATRA and OKA could significantly reduce the colony formation ability of NB4-MR2 cells (Figures 6F and 6G), which was also accompanied by increased expression of RARB (Figure 6H) and expression of differentiation marker CD11b (Figure 6I). Consistently, very similar effects were also obtained using NB4 cells and NB4-LR2 cells carrying wild-type and LBD mutant of PML-RARα (Figures S6A and S6B), indicating a more general effect of OKA on different ATRA-resistant APL cells. Finally, in order to assess the in vivo efficacy of OKA/ATRA treatment on ATRA-resistant APL, NB4-MR2 cells were transplanted into NSG mice and subjected to the treatments. Mice receiving ATRA or OKA treatment alone died at almost the identical time points as the untreated control (Figure 6J). In contrast, combined OKA/ATRA treatment significantly prolonged the survival of mice even after ceasing treatment (Figure 6J). Together, these results reveal PHF8 as a critical sensor in mediating ATRA response, and pharmacological manipulation of its activity represents a potential avenue to sensitize resistant APL cells to the ATRA treatment.
Figure 6. Okadaic Acid Specifically Inhibits PHF8 Dephosphorylation and Sensitizes NB4-MR2 Cells to ATRA Treatment.
(A) Western blot analysis of PHF8 phosphorylation in NB4-MR2-PHF8 cells upon 10 min treatment with 0.5 μM OKA. Flag-tagged PHF8 was immunoprecipitated from the total lysate using anti-Flag antibody and blotted for phospho-Ser detection (upper panel) or for total PHF8 protein on the stripped membrane (lower panel).
(B and C) The effect of OKA treatment on NB4-MR2 cells transduced with PHF8, PHF8AA, or PHF8DD. Cells were plated in methylcellulose after 10 min of OKA pretreatment at the indicated concentrations without (B) or with (C) ATRA treatment. Data are representative of three independent experiments (±SEM, **p < 0.01, ***p < 0.001).
(D–G) K562 and NB4-MR2 cells were pretreated with OKA as described above and plated in methylcellulose with or without 10−8 M ATRA. The bar charts show the number of colonies after indicated treatment for K562 (D) and NB4-MR2 (F), while INT-stained represent typical results for K562 (E) and NB4-MR2 (G) colony formation assay. Data are representative of three independent experiments (±SEM, **p < 0.01).
(H and I) NB4-MR2 cells were treated with OKA and ATRA as indicated and then analyzed for the expression of RARB by qRT-PCR (H, error bars indicate SD of three independent experiments) or CD11b by FACS (I).
(J) Disease-free survival curves of NSG mice transplanted with NB4-MR2 cells and then treated as indicated. Arrow indicates the end point of the treatment.
See also Figure S6.
DISCUSSION
Transcriptional deregulation plays a key role in a large array of human cancer, in particular, in acute leukemia, which is mostly initiated by mutations affecting master transcription factors (Cheung and So, 2011). While development of small molecule inhibitors targeting transcriptional machinery has been proved extremely difficult, the discovery of epigenetic modifying enzymes such as EZH2 and DNMT3 with rigid catalytic domains that are mutated or aberrantly recruited by oncogenic transcription factors for their functions have fueled the enthusiasm for targeting these classically intractable factors (Zeisig et al., 2012). This has also led to a recent burst of international efforts in developing specific inhibitors toward these enzymes (Arrow-smith et al., 2012). In addition to their emerging role in disease development, here we reveal a critical function of KDM in regulation of treatment response, in which PHF8 governs the ATRA sensitivity in APL.
Although significant progress has been made in recent years in characterizing the corepressor complexes and the resultant epigenetic landscapes in APL cells (Martens et al., 2010; Wang et al., 2010), the molecular basis and regulation of the resultant transcriptional reactivation upon ATRA treatment are still largely unknown (Mikesch et al., 2010). In this study, we provide several lines of evidence that PHF8 functions as a critical coactivator and molecular sensor in regulating transcriptional and cellular responses to ATRA treatment in APL. In contrast to HDAC or PRC2, PHF8 is differentially recruited by the RARα fusions to remove repressive histone marks and favors active gene transcription in response to ATRA treatment, which is in agreement with PHF8 as a class of transcriptional coactivators recruited by RARα fusions to create a more permissive chromatin environment at promoters in response to ATRA treatment (Figure 7). Activation of PHF8 activity by increasing its level of either expression or phosphorylation at S33/S84 residues can sensitize ATRA-responsive or ATRA-resistant APL. Under these conditions, the respective demethylated lysine residues can be targets for acetylation and additional interactions with RNA polymerases and other proteins, which may further stimulate transcription (Fortschegger et al., 2010). Consistently, inhibition of LSD1 resulting in an increase of H3K4me2 has recently been shown to be able to reactivate ATRA differentiation pathway in AML (Schenk et al., 2012), whereas histone acetylation has been one of the highly regulated histone marks in RARE binding sites with a strong correlation with RNA polymerase II occupancy near PML-RARα binding sites upon ATRA treatment (Martens et al., 2010; Mikesch et al., 2010). Interestingly, inhibition of TOP2b that overcame ATRA resistance in NB4-MR2 also caused hyperaceylation of H3K9 (McNamara et al., 2008), which is consistent with the observed transcriptional function of PHF8. Thus, identification of PHF8 as a coactivator for RARα fusions may provide a molecular explanation for epigenetic changes associated with ATRA response and opens up promising avenues to sensitize cancer cells to ATRA treatment.
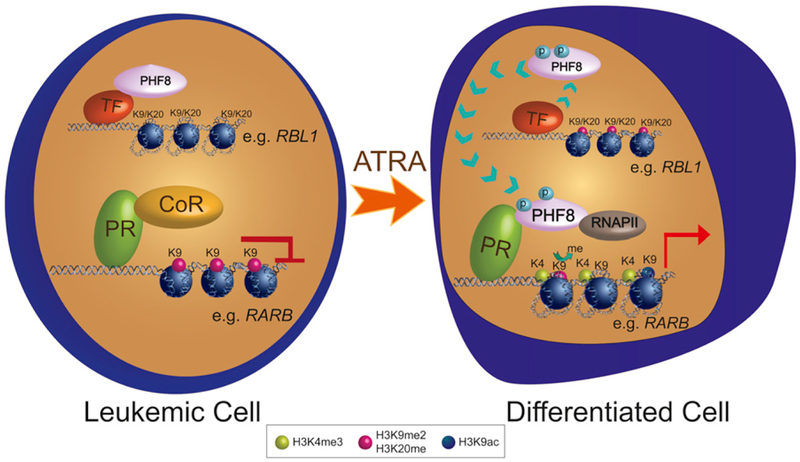
Figure 7. Schematic Diagram Illustrates the Molecular Regulation of PHF8 in Mediating ATRA Response in APL.
In leukemia, PML-RARα (PR) recruits corepressor complexes (CoR) to suppress expression of downstream targets. Upon ATRA treatment, PHF8 is phosphorylated and detaches from the original binding sites (naive PHF8 targets, e.g., RBL1 promoter) to bind to PR. PHF8 removes H3K9me2 marks and recruits additional histone modification enzymes and RNA polymerase II (RNAPII) to drive the expression of PR downstream targets (e.g., RARB) for differentiation.
RA signaling and subsequent activation of target genes for induction of differentiation, cell cycle arrest, and apoptosis have been a focus for development of differentiation-based cancer therapy (Gronemeyer et al., 2004). ATRA and 13-cis RA have been used in the clinic to treat cutaneous T cell lymphoma (CTCL) and neuroblastoma (NB) by targeting endogenous RAR to induce cell proliferation arrest and morphological differentiation in these tumors. While RA treatment after completion of chemoradiotherapy significantly improves event-free survival in high-risk NB patients (Matthay et al., 2009), more than 40% of the patients will relapse, and virtually all become resistant to the treatment. Interestingly, PHF8 can also interact with endogenous RAR in the absence of RARα fusions, and its knockdown suppresses RA-induced neuronal differentiation from mouse embryonic P19 cells (Qiu et al., 2010), suggesting its potential function in regulating RA response in the neuronal lineage. This property is reminiscent of ZNF423, which is activated by tumor suppressor NF1 (Hölzel et al., 2010) and is critically required for RA-induced differentiation of NB cells (Huang et al., 2009). However, ZNF423 constitutively associates with RAR regardless of ATRA treatment; thus, additional RA modulators (RAMs) that are differentially recruited to RAR/RXR complexes are likely required to mediate the RA response in NB. While PRAME has been reported to differentially bind to RAR upon RA treatment, it acts as a transcriptional repressor to prevent ligand-induced activation (Epping et al., 2005), and the equivalent positive RAM is still missing. In analogy to PRAME, PHF8 is differentially recruited to RAR in response to ATRA. Instead of complexing with EZH2 for making transcriptional repressive marks (Epping et al., 2005), PHF8 possesses enzymatic activity that can actively remove repressive marks and recruits other epigenetic modifying enzymes and basal transcriptional machinery for active gene expression. Discovery of the critical functions of PHF8 in ATRA-mediated transcriptional and in vivo cellular responses in APL may also suggest PHF8 as the missing positive RAM. While the activity of RAM is likely tissue specific and requires other cofactors to mediate its full response, it will be of interest to determine if PHF8 may fulfill the role of positive RAM in mediating RA response in other cell types as well. As a proof-of-principle study, we were able to show that combined treatment of ATRA and OKA, a phosphatase inhibitor suppressing PHF8 dephosphorylation, could be used successfully to treat ATRA-resistant human APL cells in vivo. Together, these results describe an important molecular regulation of PHF8 in mediating ATRA response and raise hope to develop effective therapeutic strategies combining ATRA treatment with drugs inducing specific epigenetic modifications to target RA-resistant cancer cells.
EXPERIMENTAL PROCEDURES
Plasmids and antibodies are described in Supplemental Experimental Procedures.
Immunoprecipitation and Western Blot Analysis
For generic immunoprecipitation, transfected cells were lysed in 0.5% NP-40 lysis buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 5 mM EDTA, 0.2 μM DTT, 10% glycerol, protease inhibitor, 0.5% NP-40 detergent) for mild conditions or RIPA lysis buffer (50 mM Tris-HCL [pH 8], 150 mM NaCl, 1% NP-40,0.5% sodium deoxycholate, 0.1% SDS, protease inhibitors) for stringent condition during 1 hr at 4°C. They were then incubated with the respective antibody overnight, precipitated with protein A/G Dynal beads (Invitrogen) at 4°C for 1 hr, and then washed with mild 0.5% NP-40 lysis buffer or stringent RIPA buffer. The indicated amount of ATRA was present throughout the processes. Eluted proteins were resolved by SDS-PAGE. Membranes were probed with described antibodies.
Histone Purification
Nuclei were extracted in acidic conditions to selectively remove histones, which were used subsequently for immunoblotting analysis. Details are in Supplemental Experimental Procedures.
Immunofluorescence Staining
Cytospins of a total of 5 × 104 cells were performed onto glass microscope slides and then fixed with 4% formaldehyde in phosphate-buffered saline (PBS) (pH 7.4) for 30 min on ice. Cells were washed in PBS, permeabilized, and blocked using 10% fetal calf serum (FCS)/1% BSA/0.2% TX-100/PBS for 15 min. Anti-H3K4 was used at 1:50 dilution in 10% FCS/1% BSA/PBS and incubated overnight at 4°C. Slides were washed three times with PBS and subsequently incubated with 1:100 donkey anti-mouse fluorescein isothiocyanate for 30 min at room temperature. Slides were washed five times with PBS and mounted with Vectashield (Vector Laboratories, Peterborough, UK).
Chromatin Immunoprecipitation
NB4, NB4-MR2, and K562 cells were cultured in R10 medium at 37°C and, when indicated, treated for 24 hr with 10−8 M ATRA and crosslinked with 1% formaldehyde (Sigma). Details are described in Supplemental Experimental Procedures.
Retroviral/Lentiviral Transduction and Transformation Assays
Retroviral/lentiviral transduction and transformation assays (RTTAs) were performed on primary murine or human hematopoietic cells as previously described (Zeisig and So, 2009). In brief, c-kit+ cells were isolated from murine bone marrow and cultured overnight in R10 medium (RPMI 1640, 10% FCS, 2 mM L-glutamine) supplemented with 20 ng/ml SCF, 10 ng/ml IL-3, and IL-6. Spinoculation was carried out by centrifugation at 800 × g in the presence of 5 μg/ml polybrene (Sigma-Aldrich, UK) at 32°C for 2 hr. Cells were plated in M3231 methylcellulose medium (Stem Cell Technologies, Canada) supplemented with recombinant murine 20 ng/ml SCF, 10 ng/ml IL-3, IL-6, and GM-CSF (PeproTech EC, UK), and antibiotic on the following day. Colonies were scored and replated every 7 days.
For human cell studies, cells were transduced as described before and kept in R10 with appropriate antibiotics until cells were plated into methylcellulose medium. Colonies were scored after 8 days of culture.
Flow Cytometric Analysis
Immunophenotypic analysis was performed by flow cytometric analysis using fluorochrome-conjugated monoclonal antibody to human CD11b (PE/Cy5 anti-human CD11b Clone ICRF44 Biolegend). Protocols and reagents used for murine cell analysis were as previously described (Yeung and So, 2009) and detailed in Supplemental Experimental Procedures.
In Vitro Drug Studies
Drug studies were carried out by pretreating cells at 3 × 103 cells/ml in R10 with different concentrations of Okadaic Acid (Sigma-Aldrich), 0, 100, 250, and 500 nM at different time points 0, 5, 10, or 20 min. After washing, cells were plated in 1 ml of methylcellulose in the absence or presence of 10−8 M ATRA (Sigma-Aldrich). Colony formation was examined after 8 days of incubation at 37°C in 5% CO2.
Animals and Drug Treatment Studies
All experimental procedures were approved by King’s College London and conform to the UK Home Office regulations. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (also termed NSG) (Shultz et al., 2005) or FVB mice were used for transplantation experiments. Mice were given 2.5 Gy total body gamma-irradiation and injected intravenously with up to 1 × 105 test cells. For drug studies, all treatments commenced on the next day after injection of cells. Mice were given intraperitoneal injection of daily 1 μg ATRA/g of body weight (He et al., 1998) or/and 50 ng OKA/g of body weight every other day.
Statistical Analysis
Two-tailed Student’s test was used to determine statistical significance for all bar charts. The log-rank test and Gehan-Breslow-Wilcoxon test were used to compare survival curves as previously described (Yeung et al., 2010). The p values less than 0.05 were considered statistically significant.
Supplementary Material
Significance.
Identification of the molecular functions of oncogenic transcription factors and their associated epigenetic-modifying enzymes in drug response has been an important goal for development of targeted therapy. In this study, we discover the histone demethylase PHF8 as a critical molecular sensor for mediating retinoic acid (RA) treatment response in RARα-fusion-induced leukemia. RA sensitivity is governed by both the enzymatic activity and phosphorylation status of PHF8, which mediate transactivation of downstream targets. Molecular or pharmacological manipulation of PHF8 activity can enhance or even resurrect RA response to resistant leukemic cells. These results reveal a critical function of histone demethylase in mediating drug response and open up an attractive avenue for modulation of RA sensitivity for cancer therapeutics.
ACKNOWLEDGMENTS
We would like to thank Drs. Bernd Zeisig for technical advice and inputs, Hinrich Gronemeyer for RARα antibody and insightful discussion, Hugues de Thé for PML-RARα mutant constructs, David Grimwade and Marc Timmers for critical comments on the manuscript, Miles Houslay, David Adams, and Grazia Saturno for helpful advice on the enzymology and structural basis of PHF8/PML-RARα/ATRA interaction, members of So’s lab for constructive discussion, and Pui Yi Tse for professional graphic assistance. The Leukaemia and Lymphoma Research (LLR) in the United Kingdom supported the majority of the work. The work carried out in the Helin lab was supported by the Danish Cancer Society, the Novo Nordisk Foundation, the Danish National Research Foundation, and the Excellence Program of the University of Copenhagen. M.F.A. performed all the experiments with assistance from J.-H.M. and J.Q. J.C. and K.H. supplied the PHF8 antibody. S.C.K. provided the M4 transgenic cells. C.W.E.S., M.F.A., J.-H.M., and S.D. analyzed the data. C.W.E.S. and M.F.A. wrote the manuscript with inputs from J.-H.M., S.D., and K.H. C.W.E.S. designed the overall experimental direction.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.ccr.2013.02.014.
REFERENCES
- Abidi FE, Miano MG, Murray JC, and Schwartz CE (2007). A novel mutation in the PHF8 gene is associated with X-linked mental retardation with cleft lip/cleft palate. Clin. Genet 72, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith CH, Bountra C, Fish PV, Lee K, and Schapira M (2012). Epigenetic protein families: a new frontier for drug discovery. Nat. Rev. Drug Discov 11, 384–400. [DOI] [PubMed] [Google Scholar]
- Boukarabila H, Saurin AJ, Batsché E, Mossadegh N, van Lohuizen M, Otte AP, Pradel J, Muchardt C, Sieweke M, and Duprez E (2009). The PRC1 Polycomb group complex interacts with PLZF/RARA to mediate leukemic transformation. Genes Dev. 23, 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, and So CW (2011). Transcriptional and epigenetic networks in haematological malignancy. FEBS Lett. 585, 2100–2111. [DOI] [PubMed] [Google Scholar]
- Côté S, Zhou D, Bianchini A, Nervi C, Gallagher RE, and Miller WH Jr. (2000). Altered ligand binding and transcriptional regulation by mutations in the PML/RARalpha ligand-binding domain arising in retinoic acid-resistant patients with acute promyelocytic leukemia. Blood 96, 3200–3208. [PubMed] [Google Scholar]
- de Thé H, and Chen Z (2010). Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat. Rev. Cancer 10, 775–783. [DOI] [PubMed] [Google Scholar]
- Ekberg J, Landberg G, Holm C, Richter J, Wolgemuth DJ, and Persson JL (2004). Regulation of the cyclin A1 protein is associated with its differential subcellular localization in hematopoietic and leukemic cells. Oncogene 23, 9082–9089. [DOI] [PubMed] [Google Scholar]
- Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, and Bernards R (2005). The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 122, 835–847. [DOI] [PubMed] [Google Scholar]
- Feng W, Yonezawa M, Ye J, Jenuwein T, and Grummt I (2010). PHF8 activates transcription of rRNA genes through H3K4me3 binding and H3K9me1/2 demethylation. Nat. Struct. Mol. Biol 17, 445–450. [DOI] [PubMed] [Google Scholar]
- Fortschegger K, de Graaf P, Outchkourov NS, van Schaik FM, Timmers HT, and Shiekhattar R (2010). PHF8 targets histone methylation and RNA polymerase II to activate transcription. Mol. Cell. Biol 30, 3286–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, FerRARα FF, Zamir I, et al. (1998). Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391, 815–818. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Gustafsson JA, and Laudet V (2004). Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov 3, 950–964. [DOI] [PubMed] [Google Scholar]
- Guidez F, Parks S, Wong H, Jovanovic JV, Mays A, Gilkes AF, Mills KI, Guillemin MC, Hobbs RM, Pandolfi PP, et al. (2007). RARalpha-PLZF overcomes PLZF-mediated repression of CRABPI, contributing to retinoid resistance in t(11;17) acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 104, 18694–18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LZ, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, and Pandolfi PP (1998). Distinct interactions of PML-RARalpha and PLZFRARalpha with co-repressors determine differential responses to RA in APL. Nat. Genet 18, 126–135. [DOI] [PubMed] [Google Scholar]
- Hölzel M, Huang S, Koster J, Ora I, Lakeman A, Caron H, Nijkamp W, Xie J, Callens T, Asgharzadeh S, et al. (2010). NF1 is a tumor suppressor in neuroblastoma that determines retinoic acid response and disease outcome. Cell 142, 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Laoukili J, Epping MT, Koster J, Hölzel M, Westerman BA, Nijkamp W, Hata A, Asgharzadeh S, Seeger RC, et al. (2009). ZNF423 is critically required for retinoic acid-induced differentiation and is a marker of neuroblastoma outcome. Cancer Cell 15, 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Kohlbrecher D, Christensen J, Vandamme J, Abarrategui I, Bak M, Tommerup N, Shi X, Gozani O, Rappsilber J, Salcini AE, and Helin K (2010). A functional link between the histone demethylase PHF8 and the transcription factor ZNF711 in X-linked mental retardation. Mol. Cell 38, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan SC, Hong SH, Shultz DB, Privalsky ML, and Bishop JM (2000). Leukemia initiated by PMLRARalpha: the PML domain plays a critical role while retinoic acid-mediated transactivation is dispensable. Blood 95, 1541–1550. [PubMed] [Google Scholar]
- Koivisto AM, Ala-Mello S, Lemmelä S, Komu HA, Rautio J, and Järvelä I (2007). Screening of mutations in the PHF8 gene and identification of a novel mutation in a Finnish family with XLMR and cleft lip/cleft palate. Clin. Genet 72, 145–149. [DOI] [PubMed] [Google Scholar]
- Kooistra SM, and Helin K (2012). Molecular mechanisms and potential functions of histone demethylases. Nat. Rev. Mol. Cell Biol 13, 297–311. [DOI] [PubMed] [Google Scholar]
- Kouzarides T (2007). Chromatin modifications and their function. Cell 128, 693–705. [DOI] [PubMed] [Google Scholar]
- Kwok C, Zeisig BB, Dong S, and So CW (2006). Forced homo-oligomerization of RARalpha leads to transformation of primary hematopoietic cells. Cancer Cell 9, 95–108. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Holbert S, Ronce N, Faravelli F, Lenzner S, Schwartz CE, Lespinasse J, Van Esch H, Lacombe D, Goizet C, et al. (2005). Mutations in PHF8 are associated with X linked mental retardation and cleft lip/cleft palate. J. Med. Genet 42, 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, and Evans RM (2000). Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol. Cell 5, 821–830. [DOI] [PubMed] [Google Scholar]
- Lin RJ, Nagy L, Inoue S, Shao W, Miller WH Jr., and Evans RM (1998). Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391, 811–814. [DOI] [PubMed] [Google Scholar]
- Liu W, Tanasa B, Tyurina OV, Zhou TY, Gassmann R, Liu WT, Ohgi KA, Benner C, Garcia-Bassets I, Aggarwal AK, et al. (2010). PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature 466, 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenarz C, Ge W, Coleman ML, Rose NR, Cooper CD, Klose RJ, Ratcliffe PJ, and Schofield CJ (2010). PHF8, a gene associated with cleft lip/palate and mental retardation, encodes for an Nepsilon-dimethyl lysine demethylase. Hum. Mol. Genet 19, 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, Brinkman AB, Simmer F, Francoijs KJ, Nebbioso A, FerRARα F, Altucci L, and Stunnenberg HG (2010). PML-RARalpha/RXR Alters the Epigenetic Landscape in Acute Promyelocytic Leukemia. Cancer Cell 17, 173–185. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB, and Villablanca JG (2009). Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J. Clin. Oncol 27, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara S, Wang H, Hanna N, and Miller WH Jr. (2008). Topoisomerase IIbeta negatively modulates retinoic acid receptor alpha function: a novel mechanism of retinoic acid resistance. Mol. Cell. Biol 28, 2066–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesch JH, Gronemeyer H, and So CW (2010). Discovery of novel transcriptional and epigenetic targets in APL by global ChIP analyses: Emerging opportunity and challenge. Cancer Cell 17, 112–114. [DOI] [PubMed] [Google Scholar]
- Minucci S, Maccarana M, Cioce M, De Luca P, Gelmetti V, Segalla S, Di Croce L, Giavara S, Matteucci C, Gobbi A, et al. (2000). Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol. Cell 5, 811–820. [DOI] [PubMed] [Google Scholar]
- Qi HH, Sarkissian M, Hu GQ, Wang Z, Bhattacharjee A, Gordon DB, Gonzales M, Lan F, Ongusaha PP, Huarte M, et al. (2010). Histone H4K20/H3K9 demethylase PHF8 regulates zebrafish brain and craniofacial development. Nature 466, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Shi G, Jia Y, Li J, Wu M, Li J, Dong S, and Wong J (2010). The X-linked mental retardation gene PHF8 is a histone demethylase involved in neuronal differentiation. Cell Res. 20, 908–918. [DOI] [PubMed] [Google Scholar]
- Roussel MJ, and Lanotte M (2001). Maturation sensitive and resistant t(15;17) NB4 cell lines as tools for APL physiopathology: nomenclature of cells and repertory of their known genetic alterations and phenotypes. Oncogene 20, 7287–7291. [DOI] [PubMed] [Google Scholar]
- Sanz MA, and Lo-Coco F (2011). Modern approaches to treating acute promyelocytic leukemia. J. Clin. Oncol 29, 495–503. [DOI] [PubMed] [Google Scholar]
- Schenk T, Chen WC, Göllner S, Howell L, Jin L, Hebestreit K, Klein HU, Popescu AC, Burnett A, Mills K, et al. (2012). Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat. Med 18, 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. (2005). Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol 174, 6477–6489. [DOI] [PubMed] [Google Scholar]
- Smith LL, Yeung J, Zeisig BB, Popov N, Huijbers I, Barnes J, Wilson AJ, Taskesen E, Delwel R, Gil J, et al. (2011). Functional crosstalk between Bmi1 and MLL/Hoxa9 axis in establishment of normal hematopoietic and leukemic stem cells. Cell Stem Cell 8, 649–662. [DOI] [PubMed] [Google Scholar]
- Sternsdorf T, Phan VT, Maunakea ML, Ocampo CB, Sohal J, Silletto A, Galimi F, Le Beau MM, Evans RM, and Kogan SC (2006). Forced retinoic acid receptor alpha homodimers prime mice for APL-like leukemia. Cancer Cell 9, 81–94. [DOI] [PubMed] [Google Scholar]
- Villa R, Pasini D, Gutierrez A, Morey L, Occhionorelli M, Viré E, Nomdedeu JF, Jenuwein T, Pelicci PG, Minucci S, et al. (2007). Role of the polycomb repressive complex 2 in acute promyelocytic leukemia. Cancer Cell 11, 513–525. [DOI] [PubMed] [Google Scholar]
- Wang ZY, and Chen Z (2008). Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111, 2505–2515. [DOI] [PubMed] [Google Scholar]
- Wang K, Wang P, Shi J, Zhu X, He M, Jia X, Yang X, Qiu F, Jin W, Qian M, et al. (2010). PML/RARalpha targets promoter regions containing PU.1 consensus and RARE half sites in acute promyelocytic leukemia. Cancer Cell 17, 186–197. [DOI] [PubMed] [Google Scholar]
- Yeung J, and So CW (2009). Identification and characterization of hematopoietic stem and progenitor cell populations in mouse bone marrow by flow cytometry. Methods Mol. Biol 538, 301–315. [DOI] [PubMed] [Google Scholar]
- Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, and So CW (2010). β-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 18, 606–618. [DOI] [PubMed] [Google Scholar]
- Zeisig BB, and So CW (2009). Retroviral/Lentiviral transduction and transformation assay. Methods Mol. Biol 538, 207–229. [DOI] [PubMed] [Google Scholar]
- Zeisig BB, Kwok C, Zelent A, Shankaranarayanan P, Gronemeyer H, Dong S, and So CW (2007). Recruitment of RXR by homotetrameric RARalpha fusion proteins is essential for transformation. Cancer Cell 12, 36–51. [DOI] [PubMed] [Google Scholar]
- Zeisig BB, KulasekaRARαj AG, Mufti GJ, and So CW (2012). Acute Myeloid Leukemia: Snapshot. Cancer Cell 22, 698. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nasr R, Pérès L, Riaucoux-Lormière F, Honoré N, Berthier C, Kamashev D, Zhou J, Vitoux D, Lavau C, and de Thé H (2007). RXR is an essential component of the oncogenic PML/RARA complex in vivo. Cancer Cell 12, 23–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.