Abstract
Introduction:
Dry Eye (DE) is a multifactorial condition with a variable clinical presentation. This highly prevalent disease has multiple symptoms and signs that often do not correlate with one another. As such, the diagnosis of DE can be challenging to make, and a systematic approach must be taken.
Areas covered:
We review the different methods commonly utilized to evaluate a patient complaining of DE symptoms. Included in this review are clinical examination techniques, point of care tests, and imaging techniques.
Expert opinion:
DE is an umbrella term that encompasses different etiologies and pathophysiological mechanisms. The current definition recognizes tear instability, high osmolarity, inflammation, and neuro-sensory dysfunction as causative entities. The approach to DE begins with a systematic assessment of symptoms and signs, evaluating for both nociceptive and neuropathic sources of symptoms. Future research is needed to develop tests that assess neurosensory status in DE and couple point of care tests with therapeutic algorithms.
Keywords: Aqueous tear deficiency, corneal imaging techniques, corneal stains, dry eye, evaporative deficiency, inflammatory biomarkers, meibomian gland dysfunction, neuropathic, nociceptive, point of care tests
1. Introduction
Dry eye (DE) is a multifactorial disease defined by “a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”1 In the Unites States more than 16 million adults have been diagnosed with this condition, with a global frequency ranging from 5 to 50% (depending on the population and disease definition).2–4 Individuals suffering from DE experience chronic unpleasant ocular sensations characterized as “dryness”, “burning”, and “aching”. Patients also complain of fluctuating or decreased vision, which can reduce work productivity and quality of life.2,5 The disease can present with a myriad of other symptoms including evoked pain to wind and light, itching, redness, ropy discharge, and tearing, which vary in frequency depending on the underlying ocular surface findings.1,6,7
Traditionally, the classification of DE was broken down into aqueous tear deficiency (ATD), evaporative deficiency, or a mixed pattern. This classification has been expanded to consider anatomical contributors to signs and symptoms, including nerve abnormalities.8 In order to appropriately manage patients, it is important to have a systematic approach to evaluate DE. A thorough understanding of the available point of care tests and imaging technologies is crucial to the diagnosis and stratification of DE.
2. The ocular surface
The ocular surface is composed of structures that work together to provide and maintain a smooth refractive surface on the cornea. It includes the corneal surface, glandular epithelium, conjunctiva, main and accessory lacrimal glands, meibomian glands, eyelashes and associated glands, and the nasolacrimal duct.9–11 Furthermore, the nervous, endocrine, vascular, and immune systems play an essential role in integrating all anatomical structures to form a functional entity.9,11,12 The structures responsible for tear secretion and the blink reflex include the cornea, conjunctiva, meibomian glands, lacrimal glands, eyelids and the nervous system which together form the lacrimal functional unit.13 Dysfunction of any part of the ocular surface system leads to instability, which can disrupt the refractive surface and lead to tear film abnormalities.
3. Tear film physiology
The health of the ocular surface is largely determined by the health of the tear film. This film was originally thought to be composed of three distinct layers, with an inner mucin layer, a middle aqueous layer, and an outer lipid layer.10,14 Recent studies suggest that there may not be a clear delineation between the mucin and aqueous phases, giving rise to a bi-layered film model.10,15 The mucins, which help stabilize the tear film and ensure it is evenly distributed, are mainly produced by goblet cells.16 The aqueous layer, which contains electrolytes, glucose, proteins, and trace elements, accounts for most of the thickness of the tear film. Produced by the main and accessory lacrimal glands and the corneal and conjunctival epithelium, it serves to provide oxygen and nutrients to the underlying epithelium. Lastly, the lipid layer forms a hydrophobic barrier that helps stabilize the tear film and protects from evaporation and contamination. It is produced primarily by meibomian glands and composed of synthesized lipids, membrane derived lipids, and bacterial degradation products.17 Many factors can influence the tear film health including co-morbid diseases, environmental exposures, and medication use.
4. Clinical examination
4.1. Dry eye questionnaires
Patients report different symptoms associated with DE. Several questionnaires have been validated to assess the burden of symptoms experienced by patients.6 The Dry Eye Questionnaire 5 (DEQ5) asks solely about symptoms by quantifying the severity and frequency of dryness, discomfort, and tearing. This questionnaire is scored on a 0 to 22 scale, with high scores indicating more severe symptoms.18 Meanwhile, the Ocular Surface Disease Index (OSDI) is used to quantify specific symptoms, such as sensitivity to light and grittiness. Additionally, symptom triggers like windy conditions, and impact of DE symptoms on quality of life are evaluated.19 The OSDI is composed of 12 questions, each graded on a scale of 0 (indicating “none of the time”) to 4 (indicating “all the time”) that are designed to be self-completed by the patient in 5 minutes, to arrive at a total score between 0 to 100. Other questionnaires, such as the Neuropathic Pain Symptom Inventory modified for the Eye (NPSI-E)20 and the Ocular Pain Assessment Survey (OPAS)21 focus specifically on pain symptoms. The use of questionnaires is an important first step in understanding the symptom profile and severity of DE symptoms. To facilitate patient flow, each practice should choose one or more questionnaires that best fit their practice and consider having patients fill out the questions prior to seeing the treating physician.
Along with validated questionnaires, individuals should also be asked to provide information about medical co-morbidities and medications. There should be a special focus on conditions that impact DE such diabetes, rheumatoid arthritis, Sjögrens, migraine, fibromyalgia, Bell’s palsy and more. In addition, alleviating and aggravating factors such as diurnal variation of symptoms and environmental exposures should be elicited and an ocular history including surgeries and contact lens use obtained.
4.2. External examination
The external examination includes a close observation of the facial and adnexal skin since dermatological conditions can underlie ocular findings. Rosacea is a common cutaneous disorder that presents with malar flushing, telangiectasias, and rhinophyma.22 This condition can affect the eye, leading to symptoms of DE. Key manifestations of ocular rosacea include eyelid telangiectasias (Figure 1), poor meibomian quality, and anterior blepharitis.
Figure 1:
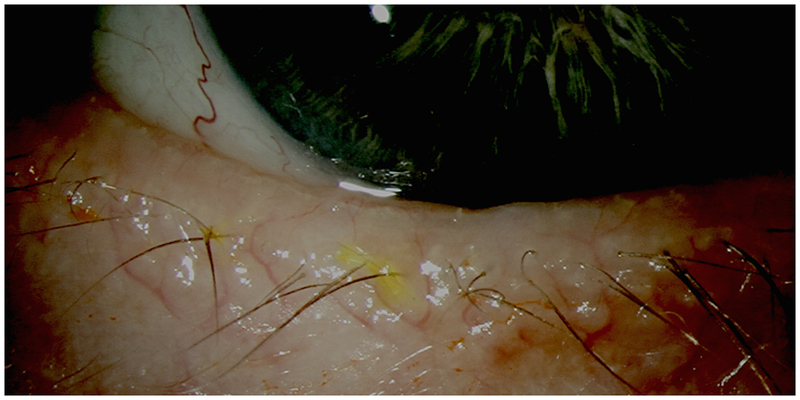
Telangiectatic Blood Vessels at the Inferior Lid Margin
Next, eyelid position and blink rate are evaluated. Blinking is an important action that serves to evenly distribute the tear film over the ocular surface. The normal blink rate ranges between approximately 13 to 15 per minute.23 A reduced blink rate can promote tear evaporation, and lead to signs of DE. For example, individuals with Parkinson’s disease have a slow blink rate with frequent disruptions in their ocular surface.24,25 Failure of the eyelids to fully close can also lead to exposure of the cornea, tear dysfunction, and ocular surface compromise.26 This may be observed in the clinic while the patient is in a seated position or may need to be elicited by leaning the patient back to look for evidence of nocturnal lagophthalmos. The physician should also note any scleral show, ectropion, or entropion when the eyes are in primary position. After noting resting abnormalities, eyelid testing proceeds by asking the patient to softly close their eyes, followed by a forceful contraction, evaluating muscle strength and symmetry, and looking for elicited eyelid abnormalities, such as spastic entropion. Eyelid laxity can be tested by pulling the eyelid inferiorly to see if they “snap back” into position (Figure 2). The skin over the eyebrow is then pulled up to see if the upper eyelids flip over, a sign of upper eyelid laxity. Eyelid abnormalities can interfere with normal tear film function and contribute to DE.
Figure 2:
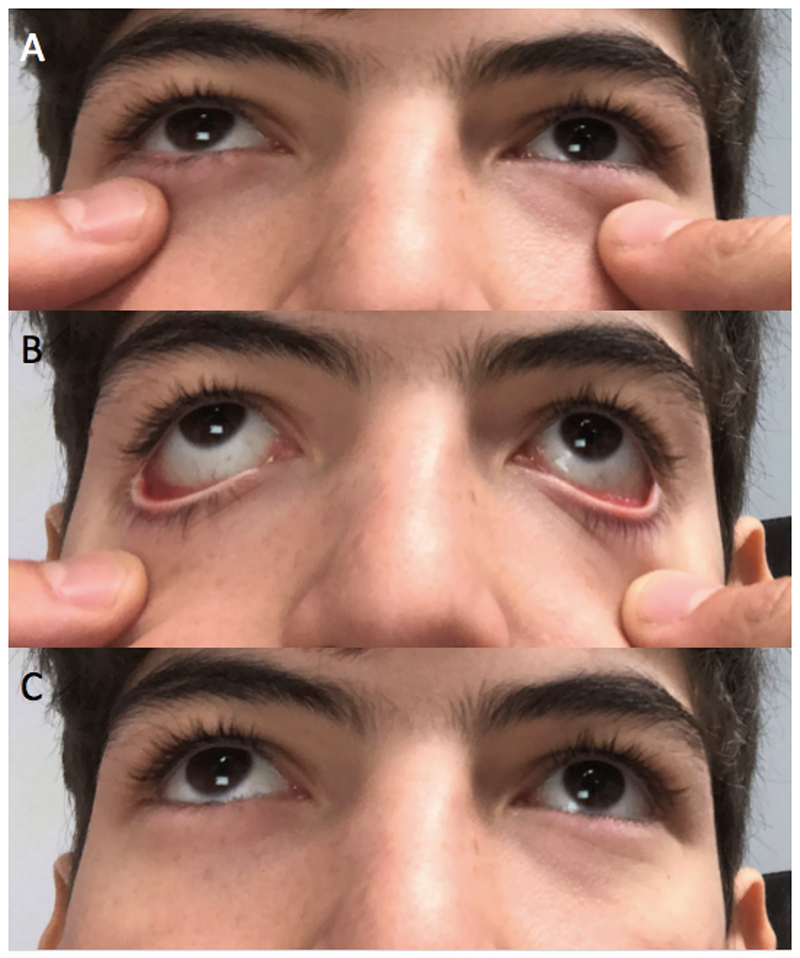
Testing for eyelid laxity by pulling the lower eyelids inferiorly and evaluating their return to anatomical position. A: Eyelids at resting position. B: Downward force on eyelids. C: “Snap back” of lower eyelids to normal position.
The order of the DE examination depends on which ancillary tests are used. Typically, non-invasive imaging is first performed, followed by tear osmolarity, Inflammatory, and then invasive tests, as pertinent.
4.3. Slit lamp examination
The slit lamp examination should be performed in a systematic fashion, starting from outside to inside the eye. Beginning with the eyelashes and lid margin, attention should be paid for anterior blepharitis, trichiasis, telangiectasias, and the condition of the meibomian glands noted. Next, the tear meniscus is examined with an expected height of ~ 0.5 mm and a concave shape. Values less than 0.2 mm are considered pathological.
The bulbar conjunctiva is then evaluated, and abnormalities noted including injection, pinguecula and pterygium. In addition, conjunctivochalasis, or redundant bulbar conjunctiva folds that can disrupt tear flow, must be identified.27 These folds can be focal, affecting the temporally or nasal portion of the eye, or they can be diffusely distributed throughout. Palpebral conjunctival changes can then be noted including hyperemia, concretions, follicles or papillae.
4.4. Ocular surface staining
Ocular surface is examined with non-toxic stains to quantify the degree and location of ocular surface disruption. Healthy epithelium does not take up dye, as it is protected by tight junctions, the plasma membrane, and surface glycocalyx. Damage to any of these components will cause the dye to diffuse into the surface cells and cause staining. This can manifest as micropunctate (small dots), macropunctate (large dots), or a coalescent patch. When performing this exam it is important to note the depth, extent, and site of staining.28 Many grading systems have been introduced to standardize the examination. For example, the National Eye Institute (NEI) scale splits the cornea into five separate sections and grades staining in that region on a scale of 0 (absent) to 3 (severe) for a maximum of 15 points.6
Fluorescein is a synthetic organic compound with a yellow-orange color that easily dissolves in water.29 With the use of a blue cobalt filter, fluorescein stains the tear film a brilliant green color. Areas of cellular degeneration or death will have increased staining intensity. Cell damage leads to disruption of plasma membranes and tight junctions that allow the fluorescein dye to penetrate into the intracellular space. When applying the stain, it is important to not overwhelm the eye. Instead, the best approach is to place a small drop in the middle or proximal end of a strip and allow for it to run down. This approach provides a concentrated amount of dye. Due to its rapid diffusion in the stroma, fluorescein is not ideal for evaluation of the conjunctiva. Instead, it is best used to diagnose corneal epithelial disruptions.30 A depressed or irregular cornea can cause pooling which must be distinguished from staining. A wisp of cotton can be used to lightly dab the area of concern. If the epithelium is intact, the pooled fluorescein will be absorbed showing that the area underneath is not stained. It is important to also note certain patterns that suggest specific pathologies. For example, interpalpebral staining is characteristic for aqueous tear deficiency, inferior limbal staining for exposure (Figure 3) and whorling of the epithelium for limbal stem cell deficiency. In addition, linear staining suggests a foreign body under the superior tarsal conjunctiva, superior bulbar conjunctival staining is characteristic of superior limbic keratoconjunctivitis, and diffuse corneal staining of a toxic etiology. Negative staining occurs when the cornea is irregular or elevated such as in anterior basement membrane dystrophy or Salzmann’s degeneration and manifests with areas of black surrounded by fluorescein. Delayed tear clearance can also be assessed after fluorescein placement. They presence of dye 10 minutes after placement is indicative of delayed tear clearance. Potential causes include punctal plug placement and nasolacrimal duct stenosis.
Figure 3:
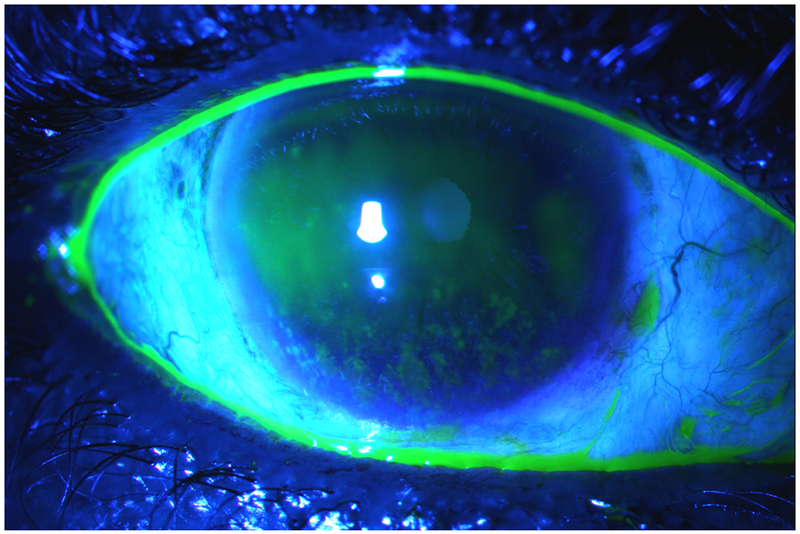
Corneal Fluorescein Staining Under Cobalt Blue Light. Note National Eye Institute grade 3 inferior epithelial disruption characteristic for exposure. Also note the concave tear meniscus highlighted with fluorescein.
Rose Bengal, a derivative of fluorescein, can stain devitalized cells and healthy cells in areas where there are breaks in the tear film integrity.31 Under a slit lamp with a white light, stained corneal and conjunctival epithelial cells will be seen as red dots. This stain is commonly used to evaluate for epithelial disruption in DE, neoplasia, and herpes.30 However, this dye can have a toxic effect on epithelial cells and causes discomfort. As such, Lissamine green is more often used as it has a similar staining profile as Rose Bengal but is less irritating. Furthermore, Lissamine green does not stain healthy cells or damage them and due to its green coloring, can better evaluate disease in patient with red eyes.
4.5. Tear film examination
A lack of stability in the tear film is seen in many DE sub-types, including both aqueous and evaporative deficiency. However, it may also occur in the setting of a poor blink rate or epithelial irregularity. The tear breakup time (TBUT) measures the time between a complete blink and the first appearance of a dry spot on the ocular surface using fluorescein.6,32 After the application of the dye, the patient is asked to blink a few times then not blink. In normal eyes, the expected TBUT is approximately 10 seconds, with values of 5 seconds or less considered abnormal.33 Due to inherent variability, 3 readings are generally measured in each eye and averaged.10 Given that the amount of instilled fluorescein can alter TBUT measurements, non-invasive measures have been developed and are described below.
The Schirmer test is used to measured tear production. This test consists of placing a standardized size strip of filter paper over the lower lid margin into the cul-de-sac (Figure 4). The patient is instructed to close their eyes for 5 minutes, after which the strip is removed and the amount of wetting is measured. Less than 5 mm of wetting at 5 minutes is often used as a cut-off for ATD.6,34 The test can be performed without (Schirmer I) or with anesthesia (Schirmer II). The Schirmer II test is thought to measure basal tear secretion, but other sensory stimuli such as eyelashes, can affect tear secretion.35 Schirmer test results can be variable. However, with more advanced ATD, the lacrimal glands become less sensitive to stimuli and Schirmer test results become more consistent. When an individual presents with ATD, systemic diseases such as Sjögren’s must be considered. Prior to and after placement of topical anesthesia, individuals should be questioned about the presence of ocular pain and/or discomfort. Persistent pain after placement of topical anesthesia suggests a central component to pain.36
Figure 4:
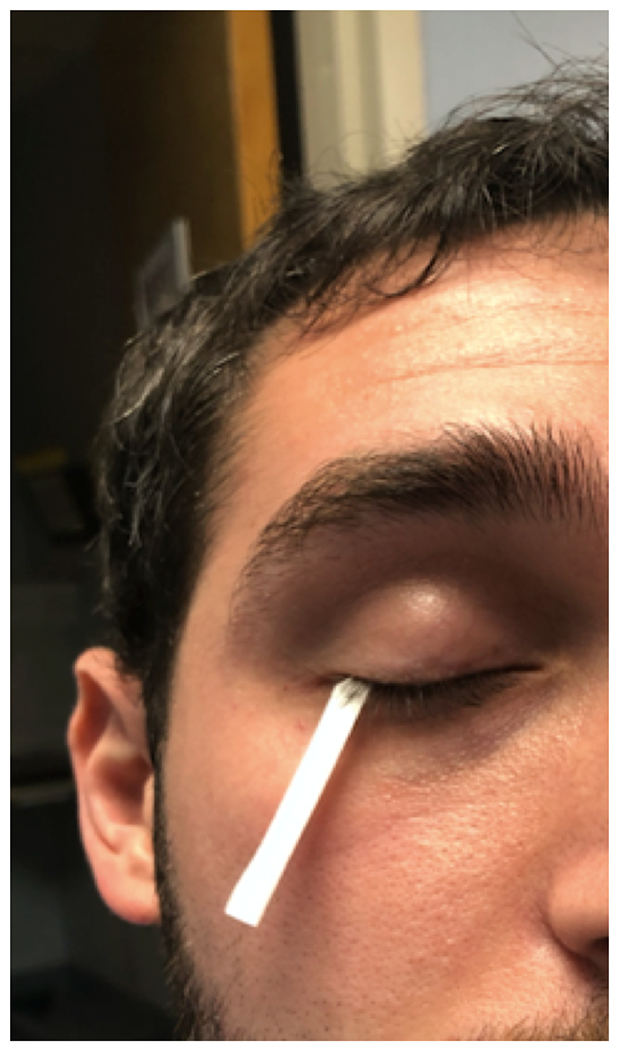
Basal Tear Production Measurement via Schirmer II Test. Filter paper strip is placed over the lower eyelid margin into the cul-de-sac and the amount of wetting is measured after 5 minutes.
The Phenol Red test can be used to assess tear volume. A cotton thread impregnated with pH sensitive phenol red is used to perform this test. When wetted by tears, the thread changes from red to yellow. The thread is inserted into the lower eyelid and the length wetted by tears is measured after 15 seconds. As the Schirmer test and the Phenol Red test measure different tear properties (production versus volume), it is not surprising that correlation between the two tests is low.37
4.6. Meibomian gland health
The lipid layer produced by the meibomian glands retards evaporation of the aqueous component of tears. Dysfunction of the meibomian glands can be due to a change in the composition or delivery of lipids.38 In fact, meibomian gland dysfunction (MGD) is a more common contributor to DE than ATD.39 The evaluation of MGD begins with the slit lamp exam. Meibomian glands should be arranged at regular intervals along the eyelid margin. Loss of this configuration can be seen in MGD, as well as orifice plugging and telangiectasias.40 The glandular structure can be observed by using a muscle light placed inside the lower lid after the application of topical anesthesia. Devices such as the Keratograph 5M (OCULUS) and LipiView interferometer devices (TearScience) can help visualize this anatomy.41 High definition dynamic imaging of the Meibomian glands may also be achieved with the LipiScan Imager (TearScience) (Figure 5). Lastly, normal meibum should be easily expressed and clear in color. Individuals with MGD display opaque, viscous meibum that is difficult to express.
Figure 5:
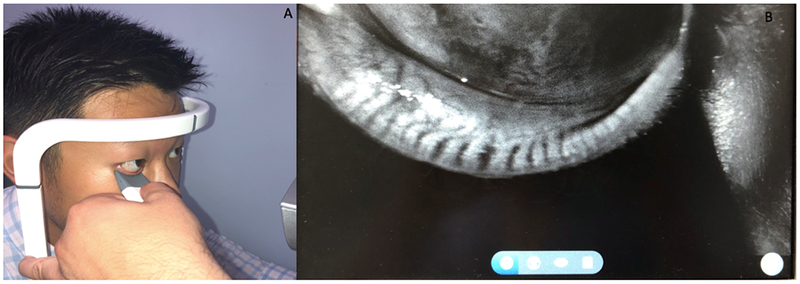
Imaging of Meibomian Glands via LipiScan (Tear Science, Morrisville). A: Performing meibography. B: Representative output depicting normal meibomian gland structure.
5. Point of care tests
5.1. Tear osmolarity
A hallmark of DE is increased tear osmolarity, and it is believed to be one of the central mechanisms of the disease.1,42 Hyperosmolarity is a global indicator of DE, regardless of etiology.1 Hyperosmolarity can occur in many situations including in the setting of decreased tear production, meibomian gland dysfunction, and exposure. As such, this test cannot distinguish between DE sub-types.42 The TearLab osmometer non-invasively measures tear osmolarity after the chip collects a 50 nL sample of tears. This test must be performed prior to administration of ocular anesthetic and other types of tests. The osmometer is a handheld device with a single-use microchip at the tip that measures the electrical impedance of the tear fluid sample. To collect the sample, the osmometer’s tip is placed on the inferior lateral meniscus of the tear film, and the microchip passively absorbs the fluid (Figure 6A). After a few seconds, the osmometer will display the osmolarity measurement (Figure 6B). This procedure should be carried out in both eyes with the higher number obtained used as the determinant value. A reading of 308 mOsms/L or greater in either eye or a difference of greater the 8 mOsms/L between eyes indicates tear osmolarity disruption.43 It is important to note that due to tear film instability, the values obtained with this test can be variable.44 As with other clinical tests, the severity of symptoms may not correlate to tear osmolarity values. Therefore, this test should be used in conjunction with the overall clinical presentation to diagnose DE.
Figure 6:
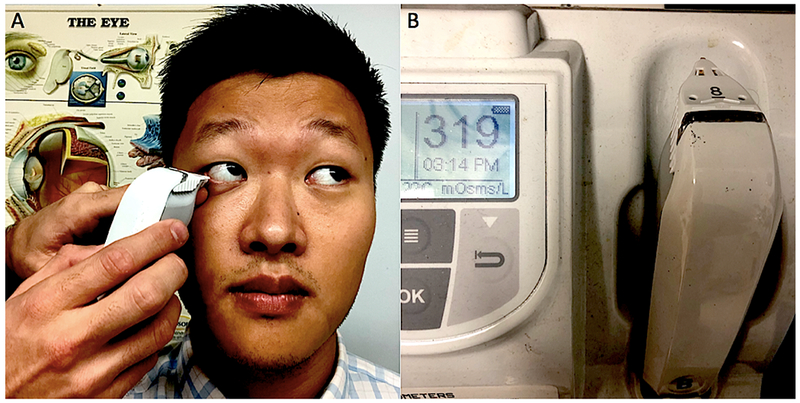
Tear Osmolarity Measurement via TearLab Osmometer (TearLab, San Diego). A: Collecting a 50nL tear fluid sample from the inferior lateral meniscus of the tear film. B: Osmolarity Reader with test pen in place and output display reading 319mOsm/L.
5.2. Inflammatory biomarkers
A key driving mechanism of DE is inflammation.45 Matrix metalloproteinase (MMP)-9 is an endopeptidase that helps remodel the extracellular matrix and plays a crucial role in DE. The point of care test Inflammadry (Quidel, San Diego) can detect this biomarker in the tear film with a lower limit of detection of 40 ng/mL.46 This test is to be carried out prior to the use of anesthetics, corneal stains, or Schirmer test. A fleece placed at the tip of a sample collector is inserted multiple times along the patient’s palpebral conjunctiva (Figure 7A). The sample collector is then placed into the test cassette and secured prior to immersing it into the buffer. Once 10 minutes have passed, the test cassette will display a blue line, representing a valid test. If the blue line does not appear, the test is considered invalid and must be repeated. A positive result is indicated by the presence of a pink and blue line (Figure 7B), thus providing a qualitative (yes or no) response. To accurately carry out this test, enough sample (5 μL) needs to be collected to avoid a false negative result. While inflammation is known to play a role in DE, it is not always present in those with symptoms. Furthermore, inflammatory biomarkers have been associated with other conditions such as contact lens use, infection, and allergy and thus, the presence of inflammation is not unique to DE.46 As with other clinical tests, the diagnosis of DE must be made by considering the entire clinical presentation and not just the presence inflammation. In those patients with a positive Inflammadry, it is not known if the levels change overtime or with treatment. As such, further studies are needed to evaluate if Inflammadry results can be used to sub-type DE and direct treatment.
Figure 7:
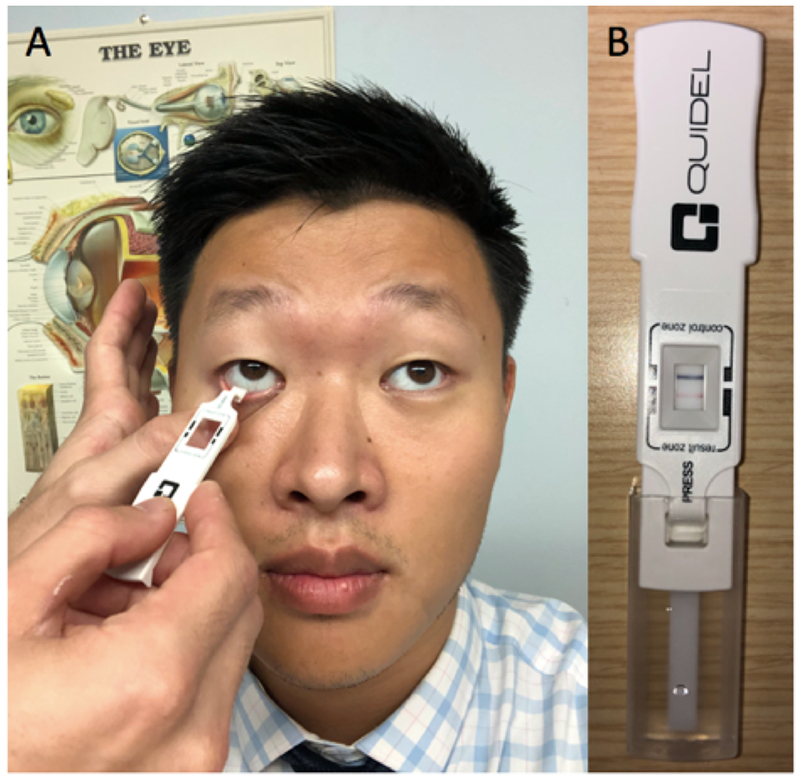
Testing for Ocular Surface Inflammation Via Inflammadry (Quidel, San Diego). A: Swabbing the right inferior palpebral conjunctiva with the sample collector. B: Test cassette with blue control line indicating validity and pink line indicating positive presence of MMP-9.
5.3. Lactoferrin
Lactoferrin is a protein that serves as an iron chelator and becomes upregulated in periods of increased oxidative stress. In DE, specifically in the ATD sub-type, levels of lactoferrin are usually increased.47 Levels of this protein can be detected with the use of the Advanced Tear Diagnostics’ TearScan system. Since lactoferrin is produced by the acinar cells of the lacrimal glands, a sample from the tear film can be used to measure levels of this protein. With the use of a micropipette, a small volume of tears is collected and transferred to a cell in a test cassette that contains a calorimetrically tagged reagent. This test cassette is then inserted into the TearScan Reader which returns a numeric result. Due to the need of additional equipment, lactoferrin levels are less commonly tested in clinic compared to tear osmolarity and MMP-9. Interestingly, over 1500 proteins have now been identified in the tear film. As the role of these proteins becomes elucidated, new targets will likely be developed for the diagnosis and treatment of DE.10
6.0. Devices used in the assessment of DE
6.1. Lipid layer thickness
The lipid layer thickness can be measured using the TearScience LipiView. This machine also captures blink rate and images meibomian gland structure.48,49 A limitation is that the lipid thickness is measured at one point and this measurement does not correlate with TBUT. Recently, a technology that provides multiple points for lipid thickness described that lipid homogeneity over the cornea is a better predictor of tear stability than central thickness. That is, a thin but homogenous lipid layer is associated with greater tear stability than a thick but heterogeneous layer.50
6.2. Vision quality
Patients with DE often complain of decreased visual acuity during certain activities such as reading and driving. Studies have shown that tear instability alone can compromise image quality.51 However, the deficiencies noted by patients can often be missed when using a test like the Snellen chart.52 While visual acuity is measured to be within normal limits, those suffering from DE can have functional visual acuity of 20/60 or less. Functional visual acuity has been measured in the research setting by using optotypes.51 In this scenario, patients are presented with optotypes and functional visual acuity is measured during 30-second blink-free intervals. Other methods utilized include speed reading, but in order to optimally evaluate functional visual acuity it is recommended that the reading assignments be of approximately 30 minutes.53
6.3. Corneal imaging
An unstable tear film and epithelial disruption can manifest as irregular astigmatism on corneal topographic imaging with both placido disk-based and Scheimpflug-based technologies (Figure 8). Optical coherence tomography (OCT) uses emission and reflection of light to image corneal structures in cross section.54 Tear film thickness over the cornea and tear meniscus volume can be quantified with some anterior segment OCT devices.
Figure 8:
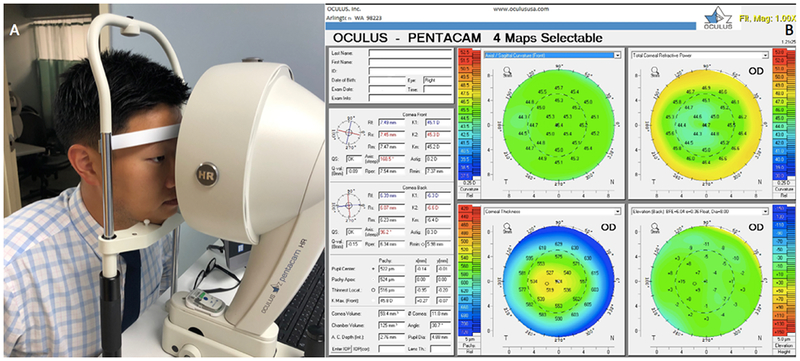
Scheimpflug-based Corneal Tomographic Imaging via Pentacam (Oculus, Arlington). A: Non-invasive measurement using the Pentacam. B: Representative output demonstrating mild irregular astigmatism.
6.4. Esthesiometry
Corneal innervation is critical for ocular surface homeostasis and decreased corneal sensation can lead to epithelial disturbances and endothelial cell loss.12,55 The nasociliary branch of the ophthalmic nerve derived from the trigeminal nerve (cranial nerve V) provides sensation to the cornea, eyelid, and conjunctiva. A feedback loop exists between this nerve and the branches of the facial nerve (cranial nerve VII) to control blinking a tear secretion. Disruption of this loop can lead to DE and anesthesia of the cornea.56 To assess corneal sensation, a wisp of a cotton-tipped swab or a piece of dental floss can be used to score sensation on a qualitative scale from none to increased. The Cochet-Bonnet esthesiometer which consists of a nylon filament of adjustable length is also used to grade sensation.57 The filament’s length is varied until the patient reports sensation. Normally, the nylon filament is sensed when fully extended at 6.0 cm. If the patient does not sense the filament at this length, the length is reduced in 0.5 cm intervals until sensation is reported. It is important to note that the central cornea is the more sensitive than the limbus.58 The Belmonte aesthesiometer (Figure 9) is used as a research instrument to more precisely quantify detection thresholds to mechanical, thermal, and chemical stimuli.59
Figure 9:
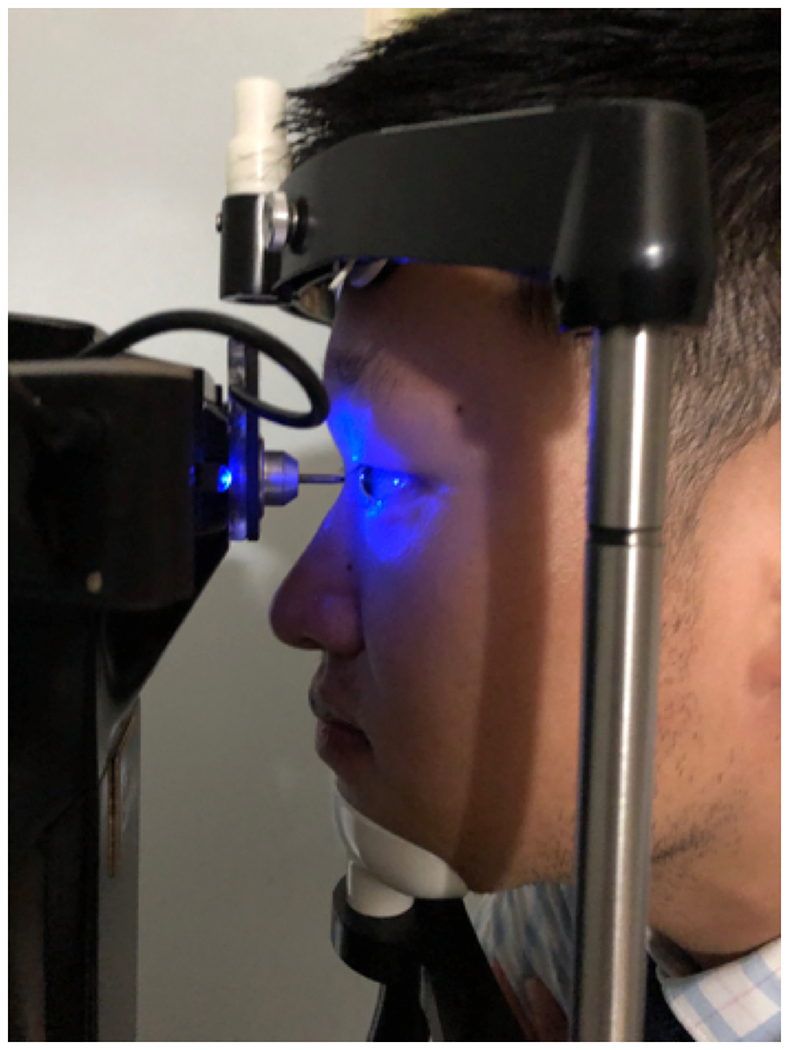
Corneal Sensitivity Measurement via Belmonte Aesthesiometer. Air flow directed at the corneal surface is incrementally increased and stimulation threshold is quantified.
6.5. Confocal microscopy
Confocal microscopy is a noninvasive technology that can be used to examine the cornea (Figure 10).60 High resolution images are able to capture sub-basal corneal nerve density and morphology and demonstrate the presence of inflammatory cells.61 Confocal microscopy requires trained operators to acquire good quality scans and lacks built in software to analyze nerves and inflammatory cells. As such, it is not as often used in the clinical setting in the diagnosis of DE.
Figure 10:
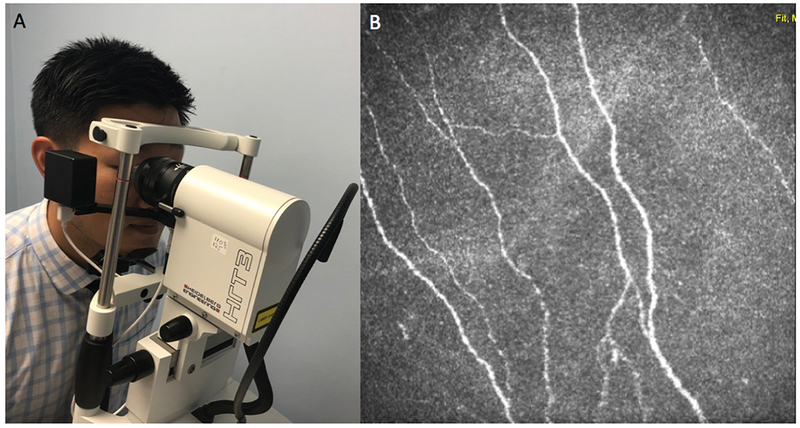
Imaging Sub-Basal Corneal Nerves with In Vivo Corneal Confocal Microscopy via Heidelberg Retina Tomograph 3 Rostock Cornea Module (Heidelberg, Germany). A: Performing in vivo corneal confocal microscopy. B: Representative output depicting the sub-basal nerve plexus.
7. Conclusion
DE is a complex disorder with multiples contributors. As such, multiple diagnostic tests are used to capture important information about patient symptoms and ocular surface status. A standardized clinical examination remains the most important aspect of the evaluation, supplemented by imaging and point of care tests, as appropriate. A standardized approach to the diagnosis of DE helps determine contributors to symptoms and allows for the development of an individual treatment approach.
8. Expert opinion
DE is a multifactorial condition that is commonly encountered in the eye clinic population. It has a variable presentation and often symptoms and signs of disease have poor correlation. As such, the diagnosis of DE can be challenging to make. The treatment of DE must be individualized to the specific underlying etiology. Therefore, a systematic approach should be utilized that takes into consideration patient symptoms along with clinical examination, point of care tests, and imaging findings.
Throughout the years, the definition of DE has expanded to represents a spectrum of disease. Parallel to the evolution of the definition, diagnostic techniques have advanced at a rapid pace. However, a paucity of data exists linking test results to therapeutic decisions. For example, it is not known if individuals with DE symptoms in the setting of ocular surface inflammation (as measured by Inflammadry) would have a more favorable response to an anti-inflammatory agent compared to individuals who are Inflammadry negative. This is an area that needs to be better studied.
Another recent development in DE is the understanding that neuro-sensory dysfunction is a causative etiology. Therefore, there is a need for objective tests that can quantify corneal nerve status. With currently available tests, it is challenging to identify which individuals have nerve dysfunction as a component of DE. In vivo confocal microscopy provides images of sub-basal corneal nerves and immune cells but does not provide information on nerve function. Furthermore, trained operators are needed to obtain quality images and it is not possible to image the same area repeatedly, limiting the ability to follow changes over time. Finally, there is no built-in software to quantify nerve parameters. These are barriers that need to be overcome. Other groups have evaluated whether tear biomarkers, such as nerve growth factor, substance P and calcitonin gene-related peptide, can provide a window to corneal nerve status. A future direction of research is the development of point-of-care tests that can assess nerve status in the clinical setting and identify which individuals with DE symptoms have underlying neuro-sensory abnormalities. Identifying the causative etiology behind each patient’s symptoms will allow for individualized treatment and, hopefully, better outcomes for this potentially debilitating disease.
Article highlights:
The definition of dry eye (DE) encompasses multiple etiologies and pathophysiologies that lead to variable clinical manifestations.
Diagnosing DE can be challenging, and a systematic approach is needed that includes a medical and ocular history, symptom severity questionnaires, and a slit lamp examination. Point of care tests and imaging techniques can be used to supplement the clinical evaluation.
The slit lamp examination starts externally by inspecting the facial skin, eyelashes, and eyelids and then moves inward to evaluate the tear film, conjunctiva and cornea.
Corneal stains assist in the evaluation of the ocular surface.
Point of care tests include tear osmolarity and inflammatory biomarkers (matrix metalloproteinase-9 and lactoferrin) and can help identify contributing mechanisms in DE.
Different imaging techniques are available to assess various aspects of the ocular surface including lipid layer thickness, meibomian gland anatomy, and corneal nerve morphology.
Acknowledgments
Funding
This paper was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (A Galor), R01EY026174 (A Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they were part of a team which developed the TearLab osmometer, however, they are no longer financially attached to the company and only possess a small amount of company stock. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- **1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 2017;15:276–83. [DOI] [PubMed] [Google Scholar]; This reference provides a thorough overview of the current definition and classification of dry eye.
- *2.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf 2017;15:334–65. [DOI] [PubMed] [Google Scholar]; This reference evaluates the prevalence, risk factors, natural history, and morbidity of dry eye.
- 3.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol 2017;182:90–8. [DOI] [PubMed] [Google Scholar]
- 4.Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol 2012;154:340–6 e2. [DOI] [PubMed] [Google Scholar]
- 5.Pouyeh B, Viteri E, Feuer W, et al. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol 2012;153:1061–66 e3. [DOI] [PubMed] [Google Scholar]
- **6.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf 2017;15:539–74. [DOI] [PubMed] [Google Scholar]; This reference reviews available dry eye tests performed in the clinical setting and research arena.
- 7.Kalangara JP, Galor A, Levitt RC, et al. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens 2017;43:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Galor A, Moein HR, Lee C, et al. Neuropathic pain and dry eye. Ocul Surf 2018;16:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference highlights the similarities between dry eye and neuropathic pain.
- *9.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017;15:438–510. [DOI] [PubMed] [Google Scholar]; This reference summarizes conclusions and recommendations from the TFOS DEWS II report.
- *10.Willcox MDP, Argueso P, Georgiev GA, et al. TFOS DEWS II Tear Film Report. Ocul Surf 2017;15:366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference summarizes biophysical and biochemical aspects of the tear film.
- 11.Gipson IK. The Ocular Surface: The Challenge to Enable and Protect Vision: The Friedenwald Lecture. Investigative Ophthalmology & Visual Science 2007;48:4391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf 2017;15:404–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res 2004;78:409–16. [DOI] [PubMed] [Google Scholar]
- 14.Holly FJ, Lemp MA. Tear Physiology and Dry Eyes . Survey of Ophthalmology 1977;22:69–87. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson W, Pugazhendhib S, Wang M. Is the main lacrimal gland indispensable? Contributions of the corneal and conjunctival epithelia. Survey of Ophthalmology 2016;61:616–27. [DOI] [PubMed] [Google Scholar]
- 16.Dratt DA, Willcox MDP. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Experimental Eye Research 2013;117:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paranjpe V, Tan J, Nguyen J, et al. Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul Surf 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye 2010;33:55–60. [DOI] [PubMed] [Google Scholar]
- 19.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118:615–21. [DOI] [PubMed] [Google Scholar]
- 20.Farhangi M, Feuer W, Galor A, et al. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology 2016;123:1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira AC, Mannis MJ. Ocular rosacea: common and commonly missed. J Am Acad Dermatol 2013;69:S36–41. [DOI] [PubMed] [Google Scholar]
- 23.Messmer EM. The Pathophysiology, Diagnosis, and Treatment of Dry Eye Disease. Dtsch Arztebl International 2015;112:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy VC, Patel SV, Hodge DO, Leavitt JA. Corneal Sensitivity, Blink Rate, and Corneal Nerve Density in Progressive Supranuclear Palsy and Parkinson Disease. 2013;32:631–5. [DOI] [PubMed] [Google Scholar]
- 25.Demirci S, Gunes A, Koyuncuoglu HR, Tok L, Tok OJNS. Evaluation of corneal parameters in patients with Parkinson’s disease. 2016;37:1247–52. [DOI] [PubMed] [Google Scholar]
- 26.Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. 2017;28:3–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhadva P, Alexander A, McClellan AL, McManus KT, Seiden B, Galor A. The impact of conjunctivochalasis on dry eye symptoms and signs. Invest Ophthalmol Vis Sci 2015;56:2867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bron AJ, Argueso P, Irkec M, Bright FV. Clinical staining of the ocular surface: mechanisms and interpretations. Prog Retin Eye Res 2015;44:36–61. [DOI] [PubMed] [Google Scholar]
- 29.Murube J Fluorescein: the most commonly used surfocular vital stain. Ocul Surf 2013;11:144–9. [DOI] [PubMed] [Google Scholar]
- 30.Kim J The use of vital dyes in corneal disease. Curr Opin Ophthalmol 2000;11:241–7. [DOI] [PubMed] [Google Scholar]
- 31.Cui J, Jin J, Hsieh Y-H, et al. Design, Synthesis and Biological Evaluation of Rose Bengal Analogues as SecA Inhibitors. 2013;8:1384–93. [DOI] [PubMed] [Google Scholar]
- 32.Sweeneya DF, Millarb TJ, Rajub SR. Tear film stability: A review. Experimental Eye Research 2013;117:28–38. [DOI] [PubMed] [Google Scholar]
- 33.Cox SM, Nichols KK, Nichols JJ. Agreement between Automated and Traditional Measures of Tear Film Breakup. 2015;92:e257–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Bijsterveld OP. Diagnostic Tests in the Sicca Syndrome. JAMA Ophthalmology 1969;82:10–4. [DOI] [PubMed] [Google Scholar]
- 35.Jordan A, Baum J. Basic tear flow. Does it exist? Ophthalmology 1980;87:920–30. [DOI] [PubMed] [Google Scholar]
- 36.Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol 2017;101:1238–43. [DOI] [PubMed] [Google Scholar]
- 37.Masmali A, Alqahtani TA, Alharbi A, El-Hiti GA. Comparative study of repeatability of phenol red thread test versus Schirmer test in normal adults in Saudi Arabia. Eye Contact Lens 2014;40:127–31. [DOI] [PubMed] [Google Scholar]
- 38.Chhadva P, Goldhardt R, Galor A. Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology 2017;124:S20–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea 2012;31:472–8. [DOI] [PubMed] [Google Scholar]
- 40.Arita R, Itoh K, Inoue K, Amano S. Noncontact Infrared Meibography to Document Age-Related Changes of the Meibomian Glands in a Normal Population. . Ophthalmology 2008;115:911–5. [DOI] [PubMed] [Google Scholar]
- 41.Zang S, Cui Y, Cui Y, Fei W. Meibomian gland dropout in Sjogren’s syndrome and non-Sjogren’s dry eye patients. Eye (Lond) 2018;32:1681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf 2007;5:75–92. [DOI] [PubMed] [Google Scholar]
- 43.Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol 2011;151:792–8 e1. [DOI] [PubMed] [Google Scholar]
- 44.Mathews PM, Karakus S, Agrawal D, Hindman HB, Ramulu PY, Akpek EK. Tear Osmolarity and Correlation With Ocular Surface Parameters in Patients With Dry Eye. Cornea 2017;36:1352–7. [DOI] [PubMed] [Google Scholar]
- 45.Lollett IV, Galor A. Dry eye syndrome: developments and lifitegrast in perspective. Clin Ophthalmol 2018;12:125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanza NL, Valenzuela F, Perez VL, Galor A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul Surf 2016;14:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Versura P, Giannaccare G, Vukatana G, Mule R, Malavolta N, Campos EC. Predictive role of tear protein expression in the early diagnosis of Sjogren’s syndrome. Ann Clin Biochem 2018;55:561–70. [DOI] [PubMed] [Google Scholar]
- 48.Jung JW, Park SY, Kim JS, Kim EK, Seo KY, Kim TI. Analysis of Factors Associated With the Tear Film Lipid Layer Thickness in Normal Eyes and Patients With Dry Eye Syndrome. Invest Ophthalmol Vis Sci 2016;57:4076–83. [DOI] [PubMed] [Google Scholar]
- 49.Kim JS, Lee H, Choi S, Kim EK, Seo KY, Kim TI. Assessment of the Tear Film Lipid Layer Thickness after Cataract Surgery. Semin Ophthalmol 2016:1–6. [DOI] [PubMed] [Google Scholar]
- 50.Segev F, Geffen N, Galor A, et al. Dynamic assessment of the tear film muco-aqueous and lipid layers using a novel tear film imager (TFI). Br J Ophthalmol 2019. [DOI] [PubMed] [Google Scholar]
- 51.Ishida R, Kojima T, Dogru M, et al. The application of a new continuous functional visual acuity measurement system in dry eye syndromes. Am J Ophthalmol 2005;139:253–8. [DOI] [PubMed] [Google Scholar]
- 52.Goto E, Ishida R, Kaido M, et al. Optical aberrations and visual disturbances associated with dry eye. Ocul Surf 2006;4:207–13. [DOI] [PubMed] [Google Scholar]
- 53.Mathews PM, Ramulu PY, Swenor BS, Utine CA, Rubin GS, Akpek EK. Functional impairment of reading in patients with dry eye. Br J Ophthalmol 2017;101:481–6. [DOI] [PubMed] [Google Scholar]
- 54.Liang Q, Pan Z, Zhou M, et al. Evaluation of Optical Coherence Tomography Meibography in Patients With Obstructive Meibomian Gland Dysfunction. Cornea 2015;34:1193–9. [DOI] [PubMed] [Google Scholar]
- 55.Kheirkhah A, Satitpitakul V, Hamrah P, Dana R. Patients With Dry Eye Disease and Low Subbasal Nerve Density Are at High Risk for Accelerated Corneal Endothelial Cell Loss. 2017;36:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labetoulle M, Baudouin C, Calonge M, et al. Role of corneal nerves in ocular surface homeostasis and disease. 2019;97:137–45. [DOI] [PubMed] [Google Scholar]
- 57.Murphy PJ, Lawrenson JG, Patel S, Marshall J. Reliability of the Non-Contact Corneal Aesthesiometer and its comparison with the Cochet–Bonnet aesthesiometer. 1998;18:532–9. [PubMed] [Google Scholar]
- 58.Beuerman RW, Tanelian DL. Corneal pain evoked by thermal stimulation. Pain 1979;7:1–14. [DOI] [PubMed] [Google Scholar]
- 59.Spierer O, Felix ER, McClellan AL, et al. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Invest Ophthalmol Vis Sci 2016;57:617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakamatsu TH, Sato EA, Matsumoto Y, et al. Conjunctival In Vivo Confocal Scanning Laser Microscopy in Patients with Sjögren Syndrome. Investigative Ophthalmology & Visual Science 2010;51:144–50. [DOI] [PubMed] [Google Scholar]
- 61.Chan TCY, Wan KH, Shih KC, Jhanji V. Advances in dry eye imaging: the present and beyond. 2018;102:295–301. [DOI] [PubMed] [Google Scholar]


