Abstract
Naturally occurring polyamines are ubiquitously distributed and play important roles in cell development, amino acid and protein synthesis, oxidative DNA damage, proliferation, and cellular differentiation. Macrophages are essential in the innate immune response, and contribute to tissue remodeling. Naïve macrophages have two major potential fates: polarization to 1) the classical pro-inflammatory M1 defense response to bacterial pathogens and tumor cells, or 2) the alternatively-activated M2 response, induced in the presence of parasites and wounding, and also implicated in development of tumor-associated macrophages. ODC, the rate-limiting enzyme in polyamine synthesis, leads to an increase in putrescine levels, which impairs M1 gene transcription. Additionally, spermidine and spermine can regulate translation of pro-inflammatory mediators in activated macrophages. In this review, we focus on polyamines in macrophage activation patterns in the context of gastrointestinal inflammation and carcinogenesis. We seek to clarify mechanisms of innate immune regulation by polyamine metabolism and potential novel therapeutic targets.
Keywords: Polyamines, Macrophages, Polarization, Gastric cancer, Colitis
Polyamine Synthesis
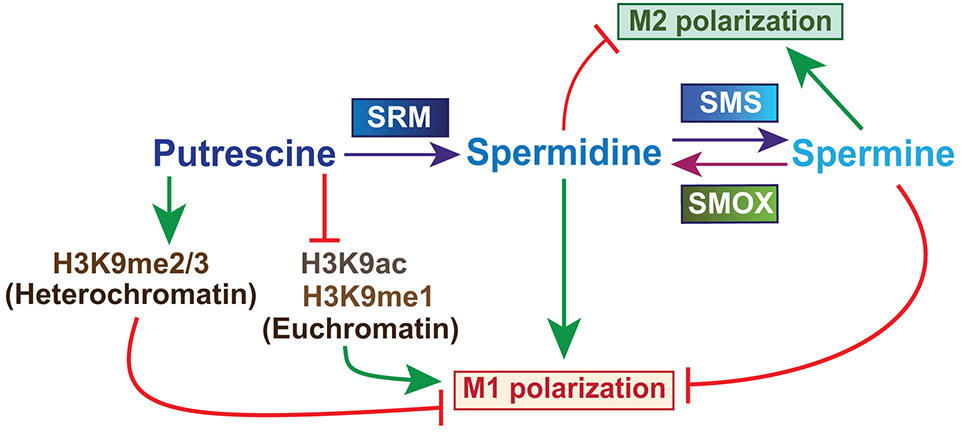
Polyamines are naturally occurring, ubiquitously distributed amino acids that are synthesized from L-ornithine by ornithine decarboxylase (ODC; also known as ODC1) (Pegg and McCann 1982; Pegg 2009). Once transported into the cell by the solute carrier family 7 member ½ (SLC7A½), L-arginine is metabolized by arginase to L-ornithine and urea (Pegg and McCann 1982; Kakuda et al. 1999; Pegg 2009). Arginase is present in the cell in two isoforms; arginase 1 (ARG1) is abundantly found in liver and is involved in the urea cycle, and arginase 2 (ARG2) is found in kidney and localizes to the mitochondria. Ornithine is then converted into putrescine by ODC in the cytosol (Pegg 2006; Asim et al. 2010). Putrescine can then be converted to spermidine by spermidine synthase (SRM) and spermidine converted to spermine by spermine synthase (SMS) (Fig. 1). The conversions to spermidine and spermine require the transfer of an aminopropyl group that is donated by the decarboxylated form of S-adenosylmethionine, referred to as dcSAM (Pegg and McCann 1982; Pegg 2009). Methionine is first metabolized to SAM, which can then be decarboxylated to dcSAM by S-adenosylmethionine decarboxylase (SAMDC), which is encoded by the gene AMD1. The activity of SAMDC is positively regulated by putrescine and inhibited by spermidine (Pegg 2009).
Fig. 1.
Overview of polyamine synthesis and the effect on macrophage polarization. Putrescine is converted to spermidine by spermidine synthase (SRM) and spermidine is converted to spermine by spermine synthase (SMS). Spermine oxidase (SMOX) back-converts spermine to spermidine. Macrophages have two main polarization states; classically activated pro-inflammatory M1 macrophages and alternatively activated, anti-inflammatory and pro-tumoral M2 macrophages. Putrescine inhibits the formation of euchromatin thus downregulating the expression of M1 genes. Spermidine favors M1 polarization while spermine favors M2
Polyamines play a role in a wide range of cellular functions including cell development, amino acid and protein synthesis, oxidative DNA damage, proliferation, and differentiation (Pegg 2009). More recently, it has been found that polyamine levels may contribute to alterations of histone modifications and chromatin structure consequently affecting DNA stability and transcription (Hobbs and Gilmour 2000; Huang et al. 2009; Brooks 2013; Pasini et al. 2014; Hardbower et al. 2017a; Singh et al. 2018). The overall rate-limiting step of polyamine synthesis, the decarboxylation of L-ornithine by ODC, has been highly studied and inhibition of ODC by difluoromethylornithine (DFMO) has entered clinical trials as a treatment to prevent relapse in patients with neuroblastoma and in patients at high risk of developing colorectal or gastric adenocarcinoma (Pegg 2006; Linsalata et al. 2014; Chaturvedi et al. 2015; Saulnier Sholler et al. 2015; Bassiri et al. 2015). Endogenous regulation of ODC is achieved by the induction of antizyme, a natural inhibitor of ODC that is translationally controlled by polyamine levels (Hayashi et al. 1996). Antizyme directly binds to ODC, triggering its rapid degradation by the proteasome.
Macrophage Polarization and Function
Macrophages are bone marrow-derived monocytes and are the first line of defense against invading pathogens by acting as a surveillance system, and are thus a key component of the innate immune response. The fate of macrophages is dependent on environmental factors that stimulate polarization to either classically activated pro-inflammatory M1 or alternatively activated M2. Specific ligands mediate these distinct changes through toll-like receptors. Pathogen-associated molecular markers such as lipopolysaccharide (LPS), damage-associated molecular markers, growth factors, and IFN-γ, a Th1 cytokine, elicit pro-inflammatory activation, while Th2 cytokines, such as IL-4 and IL-13, elicit the alternative response (Anderson and Mosser 2002; Benoit et al. 2008).
Nitric oxide (NO) production, through upregulation of inducible NO synthase (NOS2), is a key characteristic of M1 macrophages (Martinez and Gordon 2014). These classically activated M1 macrophages also produce high levels of reactive oxygen species (ROS) and pro-inflammatory cytokines, including TNFα, IL-1β, and IL-12, contributing to pathogen killing and recruitment of other pro-inflammatory cell types (Mosser and Edwards 2008). In contrast, tissue remodeling, wound healing, tumor environment regulation, allergic reactions, and responses to helminths involve M2 macrophages (Strauss-Ayali et al. 2007). These alternatively activated macrophages have enhanced arginase activity and produce IL-10. Due to the high diversity of M2 macrophage functions, the alternatively activated subset can further be subdivided into regulatory macrophages (Mregs), tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and profibrotic macrophages (M2a) (Mosser 2003; Mosser and Edwards 2008; Murray and Wynn 2011). Each of these M2 subtypes have distinct activators and effector roles, but are overall immunosuppressive. Mregs are both activated by, and secrete IL-10, express neither arginase nor NOS2, but function to suppress the classically activated M1 macrophages (Fleming and Mosser 2011; Martinez and Gordon 2014). Tumor-derived factors within the tumor environment (e.g. hypoxia), in addition to the classic M2 stimuli, promote the differentiation and polarization of TAMs (Yang and Zhang 2017). This contributes to both the initiation and progression of tumor growth by immune suppression and angiogenesis (Yang and Zhang 2017). MDSCs are thought to be the predecessors of TAMs, but have high expression of GR1, a proinflammatory marker, in mice, low expression of F4/80, and have both arginase and NOS2 activity (Gabrilovich and Nagaraj 2009). MDSCs function to suppress the innate and T-cell response in cancer. M2a macrophages express fibronectin and secrete high amounts of IL-4 and IL-13, promoting wound healing and extracellular matrix formation (Lech and Anders 2013). Once activated, macrophages retain plasticity and can switch from one functional phenotype to another based on environmental signals, but excessive activity of either polarization state can result in tissue damage, inflammatory disease, fibrosis, or tumor growth (Galli et al. 2011; Wynn et al. 2013; Lichtnekert et al. 2013; Hardbower et al. 2017b; Coburn et al. 2018; Singh et al. 2018). Thus, understanding the mechanisms of macrophage regulation is essential for disease management.
Polyamines in the context of Helicobacter pylori infection
Helicobacter pylori is a Gram-negative bacterium that selectively colonizes the human stomach and can cause a spectrum of disease from chronic gastritis to gastric cancer. Our lab has shown that H. pylori induces dysregulation of polyamine synthesis and metabolism that affects disease progression (Chaturvedi et al. 2010, 2011, 2014b, 2015). Expression of the inducible arginine transporter SLC7A2, also known at cationic amino transporter 2 (CAT2), was found to be upregulated in both mouse and human gastric tissues with H. pylori-induced gastritis (Kakuda et al. 1999; Chaturvedi et al. 2010). H. pylori infection also upregulates ARG2 and ODC expression in human gastric tissues at both the mRNA and protein levels, however, ARG1 is not induced (Gobert et al. 2002; Lewis et al. 2010). This upregulation of ARG2 comes at a cost to the host: ARG2 competes with NOS2 for the availability of L-arginine (Lewis et al. 2010). NOS2 produces NO as a defense against invading pathogens and upregulation of arginase has been shown as a survival mechanism by the intracellular bacteria Chlamydia psittaci, Chlamydia pneumoniae, Mycobacterium tuberculosis, intracellular parasites, Leishmania major and Toxoplasma gondii, and the extracellular parasite Trypanosoma brucei brucei (Gobert et al. 2000; Iniesta et al. 2001; Huang et al. 2002; Duleu et al. 2004; El Kasmi et al. 2008). In addition, during H. pylori infections, the increased activity of ARG2 directly inhibits the translation of NOS2, effectively inhibiting NO production (Lewis et al. 2010).
In conjunction with upregulation of ARG2, it has been reported that H. pylori also induces the increased expression of spermine oxidase (SMOX), an enzyme responsible for the catabolism of spermine to spermidine (Chaturvedi et al. 2012). This induction is highly dependent on the presence of the H. pylori virulence factor CagA, which is also associated with a high risk of developing gastric cancer (Chaturvedi et al. 2011). Patient gastric tissue and in vitro studies using clinical isolates showed higher levels of SMOX expression and risk of developing gastric cancer in correlation with functional CagA secretion (Chaturvedi et al. 2011, 2015). The back-conversion of spermine to spermidine is also responsible for the release of H2O2, leading to DNA damage and apoptosis. Gerbil studies showed that inhibition of either ODC or SMOX reduced the rate of adenocarcinoma development and DNA damage in cells resistant to apoptosis (Chaturvedi et al. 2015).
The role of polyamines in macrophage activation and function – Stomach
Macrophages are among the first cells recruited to the gastric lamina propria and play a significant role in the pathogenicity of H. pylori infection. To demonstrate the involvement of macrophages in gastritis, Kaparakis et al. were able to show that mice injected with dichloromethylene diphosphonate (Cl2MDP)-loaded liposomes had an overall depletion of circulating CD11b+ cells of the monocyte/macrophage lineage and substantial reduction of the recruitment of CD11b+ cells to gastric tissues, normally seen with H. pylori infection (Kaparakis et al. 2008). This reduction did not affect the colonization or survival of H. pylori, however, there was markedly less inflammation suggesting that macrophages are a key contributor to the pathogenicity of H. pylori-associated gastritis (Kaparakis et al. 2008). This supports one of the hallmarks of H. pylori infection: evasion of the elicited innate and adaptive immune response leads to the chronic inflammation responsible for the progression of infection to gastric adenocarcinoma (Wilson and Crabtree 2007; Peek Jr et al. 2010; Wroblewski et al. 2010). We propose that one mechanism underlying the ability of H. pylori to evade the immune response is through the dysregulation of polyamine metabolism and competition for L-arginine availability in macrophages, thus modulating both effector functions and signaling (Fig. 2).
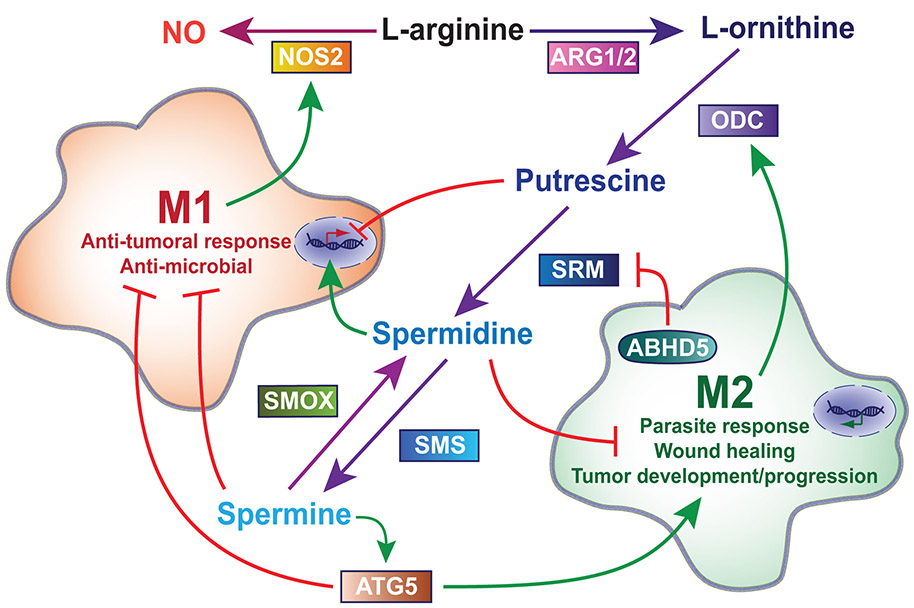
Fig. 2.
Regulation of macrophage function by polyamine synthesis. In general, once activated, M1 macrophages metabolize L-arginine by NOS2 to produce NO while M2 macrophages (Mregs, TAMs, MDSCs, M2a) upregulate the ARG1/2-ODC pathway to produce polyamines, however, both retain the ability to change activation states. Putrescine downregulates transcription of M1 genes including Nos2 and spermine inhibits the translation of NOS2, thus hindering an M1 response. Spermine also supports autophagy via ATG5, which inhibits M1 polarization while promoting M2 polarization. In contrast, spermidine upregulates transcription of Nos2 and can both directly and indirectly inhibit tumor growth. TAM survival is mediated by upregulation of ABHD5, which suppresses spermidine by inhibiting translation of spermidine synthase (SRM)
Arginase in Macrophages
Our lab has shown that the L-arginine metabolic enzymes induced in gastric tissue and epithelial cells are also upregulated in macrophages during H. pylori infection. L-arginine is selectively transported into macrophages by SLC7A2 during H. pylori infection (Yeramian et al. 2006; Chaturvedi et al. 2010). There is a significant increase of Arg2, but not Arg1, mRNA expression in H. pylori-infected murine macrophages, with the cell line RAW 264.7, and also in primary macrophages (Gobert et al. 2002; Lewis et al. 2010). Enhanced expression of ARG2 protein levels correlates with the increase in gene expression; ARG1 protein was, again, not induced (Gobert et al. 2002; Lewis et al. 2010). Past studies demonstrate that the induction or inhibition of arginase and NOS2 inversely vary due to direct competition for available L-arginine. Peritoneal macrophages isolated from Arg2 knockout mice infected with H. pylori, express increased levels of NOS2 and produce more NO compared to infected wild-type (WT) mice (Lewis et al. 2010). These findings were confirmed in vivo using H. pylori-infected mice with treatment of the arginase inhibitor S-(2-boronoethyl)-L-cysteine (BEC) or ARG2 deletion (Lewis et al. 2010).
ARG2 impairs the host response to H. pylori in addition to competing with NOS2 for the available L-arginine (Lewis et al. 2011). Arg2 knockout mice infected with H. pylori have decreased bacterial colonization and increased histologic inflammation compared to infected WT mice (Lewis et al. 2011). Additionally, these mice exhibit enhanced transcription of the genes encoding the pro-inflammatory cytokines IFN-γ, IL-12p40, and IL-17A, and downregulation of the immune regulatory cytokine IL-10 (Lewis et al. 2011). ARG2 deletion also results in an increased influx of macrophages to the gastric tissues with infection, and these recruited macrophages express higher levels of NOS2 with less evidence of macrophage apoptosis (Lewis et al. 2011). A more recent study from our laboratory has expanded on these findings and demonstrates the effects of ARG2 on macrophage polarization (Hardbower et al. 2016). Bacterial colonization and gastric inflammation do not differ between Arg2 knockout and Arg2;Nos2 double knockout mice, suggesting that the effects of ARG2 in vivo are independent of NOS2 (Hardbower et al. 2016). Arg2 knockout mice exhibit enhanced M1 macrophage activation through increased mRNA expression of Ifng, Il17a, Nos2, Il1b, and Tnfa, and increased production of the pro-inflammatory markers TNF-α, IL-1β, CCL3, CCL4, CCL5, and NO in both gastric tissues and bone marrow-derived macrophages (BMDMs) (Hardbower et al. 2016). Loss of ARG2 also modulates changes in the expression of enzymes involved in the polyamine metabolism pathways. The genes encoding ARG1, ODC, SAMDC, diamine acetyltransferase 1 (SAT1), and SMOX are all upregulated in H. pylori-infected gastric tissues and BMDMs as a compensatory mechanism for ARG2 deficiency (Hardbower et al. 2016). The decrease of putrescine and increase of spermine by ARG2 deficiency also elicited a more vigorous Th1/Th17 response, indicating that the expression of Th1/Th17 cytokines in the H. pylori-infected stomach is dependent on relative polyamine levels (Hardbower et al. 2016). Altogether, these data support the concept that metabolism of L-arginine by arginase drives macrophages away from an M1 response.
To assess the role of chronic inflammation on macrophage function, Chaturvedi et al. isolated gastric macrophages after a 4 month infection with H. pylori and re-stimulated them with H. pylori lysate (Chaturvedi et al. 2010). These cells exhibited increased expression of Slc7a2, Odc, and Nos2 (Chaturvedi et al. 2010). Nevertheless, the increase of mRNA did not result in a corresponding increase in protein levels in macrophages isolated from the infected mice. In fact, there was a decrease in NOS2 protein levels, NO production, and L-arginine uptake compared to cells from uninfected mice, suggesting that polyamine synthesis during chronic infection may favor and maintain an M2-like response, in that M1 responses are blunted (Chaturvedi et al. 2010). Moreover, in that study it was shown that spermine can impair L-arginine uptake into macrophages, providing one potential mechanism for diminished NOS2 protein expression and NO production (Chaturvedi et al. 2010) as H. pylori-stimulated NOS2 protein translation is specifically dependent on L-arginine availability in macrophages (Chaturvedi et al. 2007).
ODC and SMOX in Macrophages
Not only does H. pylori induce ARG2, but infection upregulates the expression of ODC in macrophages (Gobert et al. 2002). Similar to ARG2, the induction of ODC diverts the utilization of L-arginine towards polyamine synthesis and away from NOS2. Bussière et al. knocked down Odc expression with siRNA, demonstrating an inverse relationship with NOS2 (Bussière et al. 2005). Odc knockdown increased NOS2 protein expression and NO production, without affecting Nos2 mRNA levels, which was associated with decreased spermine concentrations within macrophages (Bussière et al. 2005; Chaturvedi et al. 2010). Inhibiting ODC and putrescine synthesis in H. pylori-infected BMDMs with the pharmacological inhibitor DFMO decreased levels of putrescine, supporting the host immune response by enhancing the M1 phenotype without altering M2 activation (Hardbower et al. 2017a). These findings are recapitulated in vivo with mice treated with DFMO and inoculated with H. pylori (Chaturvedi et al. 2010; Hardbower et al. 2017a). There is an increase of arginine uptake, Nos2 translation, and NO production in gastric macrophages. The outcome of this upregulation is decreased H. pylori colonization and decreased gastric inflammation in infected mice (Chaturvedi et al. 2010; Hardbower et al. 2017a). Supplementation of putrescine in BMDMs rescues ODC inhibition by decreasing M1 macrophage and NLR family, pyrin domain containing (NLRP) 3-driven inflammasome activation demonstrating that putrescine, generated by the induction of ODC, has a role in macrophage function (Hardbower et al. 2017a).
In partial contrast to the findings with DFMO, myeloid specific Odc knockout (OdcΔmye) mice exhibit decreased H. pylori colonization, but increased histological inflammation scores (Hardbower et al. 2017a). Using OdcΔmye mice, Hardbower et al. demonstrated that ODC and putrescine alter histone modifications and attenuate the M1 response (Hardbower et al. 2017a). H3K4 monomethylation (H3K4me1) and H3K9 acetylation (H3K9ac) are known histone modifications that enhance euchromatin formation and thus gene expression, while H3K9 di/trimethylation (H3K9me2/3) is associated with decreased gene expression (Georgopoulos 2002; Shlyueva et al. 2014). Hardbower et al. found that during H. pylori infection of OdcΔmye BMDM, there is a significant increase of H3K9me1 and H3K9ac, but a decrease of H3K9me2/3 (Hardbower et al. 2017a). Treatment with BIX 01924, a selective inhibitor of H3K9 methyltransferase, removed the inhibitory methylation of Odcfl/fl BMDMs and increased M1 markers, while having no effect on OdcΔmye BMDMs (Hardbower et al. 2017a). In contrast, treatment with anacardic acid, an inhibitor of lysine transferase 2A, reversed the increased M1 expression in OdcΔmye BMDMs while having no effect in Odcfl/fl BMDMs (Hardbower et al. 2017a). The histone modification and euchromatin formation of H. pylori-infected OdcΔmye mice leads to an increases of gene expression of macrophage-derived proinflammatory markers Il1b, Il6, Il12a, Il12b, Tnfa, and Nos2 in gastric tissue and stimulated BMDMs (Hardbower et al. 2017a). Infection of OdcΔmye mice resulted in an increase in production of proinflammatory cytokines CCL2, CCL3, CCL4, CCL5, CXCL1, CXCL2, CXCL10, IL-17, and TNF-α (Hardbower et al. 2017a). This enhanced M1 macrophage activation was also observed in human THP-1 macrophage-like cells treated with DFMO (Hardbower et al. 2017a). The exogenous addition of putrescine reversed the histone modifications and M1 marker expression observed in OdcΔmye BMDMs, highlighting the role of putrescine in macrophage polarization (Hardbower et al. 2017a) (Fig. 1).
Increased polyamine synthesis provides SMOX with available substrate. Since the catabolism of spermine to spermidine by SMOX produces H2O2, this upregulation results in increased macrophage apoptosis of H. pylori-infected RAW 246.7 cells (Chaturvedi et al. 2004). Chemical inhibition of SMOX or detoxification of H2O2 with catalase attenuated the infection-induced apoptosis (Chaturvedi et al. 2004). Our lab also found that knocking down SMOX expression with shRNA decreased arginine uptake by RAW 246.7 macrophages (Chaturvedi et al. 2014a). This resulted in a decrease of H. pylori lysate-stimulated production of NO and an increase in H. pylori survival (Chaturvedi et al. 2014a). These results were reversed with transfection of a SMOX overexpression vector with subsequent decrease of spermine levels in RAW 246.7 cells and the human moncyte cell line THP-1 (Chaturvedi et al. 2014a). Bussière et al. found that increased concentrations of spermine within the cell correlated with decreased NOS2, attributed to a direct inhibition of spermine on the translation of NOS2 (Bussière et al. 2005). In combination, these findings suggest that the macrophage effector function of pathogen killing by NO production is either downregulated by spermine or upregulated by spermidine (Fig. 2).
The role of polyamines in macrophage activation and function – Colon
Polyamine regulation of macrophage polarization is not specific to H. pylori-induced inflammation within the gastrointestinal tract. ODC and arginase are upregulated during infection with the colonic pathogen Citrobacter rodentium, increasing polyamines and decreasing NO production (Gobert et al. 2004). In contrast to H. pylori-induced gastric inflammation, ARG1 is upregulated during C. rodentium infection and other mouse models of colitis (Gobert et al. 2004). Knocking out NOS2, even with L-arginine supplementation, reduced markers of M1 activation (Gobert et al. 2004). Arginine supplementation did, however, increase the total concentration of polyamines (Gobert et al. 2004). OdcΔmye macrophages infected with C. rodentium displayed increased H3K9ac and decreased H3K9me2/3 levels indicative of euchromatin and enhanced capacity for gene transcription, and accordingly, infected OdcΔmye mice exhibit significantly increased histologic colitis and disease severity (Hardbower et al. 2017a).
Inflammatory bowel disease (IBD) is a strong risk factor for developing colitis-associated carcinogenesis (CAC) because of the constant inflammatory state (Ekbom et al. 1990; Eaden et al. 2001). Macrophage ODC augments epithelial injury-associated colitis and CAC by impairing the M1 responses that can stimulate epithelial repair, antimicrobial defense, and antitumoral immunity (Singh et al. 2018). As with H. pylori infections, myeloid cell-specific deletion of Odc results in upregulation of the M1 response during induced colonic inflammation (Singh et al. 2018). ODC-expressing macrophages are also significantly increased in the colonic tissue of patients with Crohn’s disease, active ulcerative colitis, dysplasia, and colitis-associated colon cancer compared to normal or inactive ulcerative colitis tissues (Singh et al. 2018). Singh et al. used dextran sulfate sodium (DSS) and azoxymethane (AOM)-DSS as models of colitis and CAC, respectively. OdcΔmye mice treated with DSS exhibited lower histologic injury scores (Singh et al. 2018). OdcΔmye mice treated with DSS also had a significant increase of the pro-inflammatory cytokines CSF2, TNFα, IL-1α, and IL-1β; the prototype Th1 cytokine IFNγ; and the chemokines CXCL2, CXCL9, CXCL10, CCL2, CCL3, CCL4, CCL4, and CCL5; and a significant increase in the mRNA expression of the M1 genes Nos2 and Il1b, and no change in the M2 genes Arg1 and Chil3 (Singh et al. 2018). When assessing disease progression, OdcΔmye mice treated with AOM-DSS exhibited a significant decrease in tumor number, tumor burden, and severity of dysplasia (Singh et al. 2018). There were also notable differences in the tumor versus non-tumor tissues in the AOM-DSS model (Singh et al. 2018). In tumor tissues of the OdcΔmye mice that received AOM-DSS, there was a significant increase of the M1 markers Nos2, Il1b, Il12a, Il12b, Tnfa, and the Th1 marker Ifng (Singh et al. 2018). The protein levels of NOS2, TNF-α and IFN-γ were also significantly increased (Singh et al. 2018). These findings provide evidence that inhibiting ODC may be protective against CAC due to the enhanced pro-inflammatory/anti-tumoral activity of M1 macrophages activated by lowered levels of putrescine and the resulting increase of histone modifications and euchromatin formation, which was demonstrated by increased macrophage immunofluorescent staining for H3K9ac in colon tumor macrophages of OdcΔmye mice (Singh et al. 2018).
AB-hydrolase containing 5 (ABHD5) is a co-activator of the adipose triglyceride lipase (ATGL), a key enzyme involved in lipolysis of triglycerides into diglycerides and free fatty acids (Lass et al. 2006). ABHD5 expression is shown to be increased in TAMs during colorectal cancer (CRC) (Miao et al. 2016). TAMs are rich in lipid droplets, thus ABHD5 may be a key factor in TAM utilization of the lipid deposits for energy (Miao et al. 2016). The anti-tumor response via M1 macrophages is suppressed by the enhanced TAM survival in the tumor environment of CRC. The M2 response is also sustained by ABHD5-mediated suppression of SRM and accumulation of putrescine (Miao et al. 2016). ABHD5 suppresses SRM in macrophages by inhibiting the reactive oxygen species-dependent expression of C/EBPɛ, a transcription factor of the gene encoding SRM (Miao et al. 2016). Overexpression of ABHD5 in macrophages supported tumor growth while knocking down ABHD5 suppressed tumor growth by increasing spermidine production through C/EBPε (Miao et al. 2016). Spermidine, by overexpression of SRM is also able to directly inhibit the growth of CRC cells (Miao et al. 2016). Tumor progression was rescued by knocking down C/EBPε and inhibiting Srm transcription (Miao et al. 2016). ABHD5 suppression of spermidine synthesis is another example of the how polyamines can be instrumental in the balance between M1 versus M2 macrophage activation (Fig. 2).
The role of polyamines in macrophage activation and function – Other
Tumors
In a study to characterize the effects of novel polyamine blockade treatment (PBT), Alexander et al. demonstrated that co-treatment with DFMO and a trimer polyamine transport inhibitor (PTI) decreased the M2 response and increased the anti-tumor M1 immune response (Alexander et al. 2017). The PBT inhibits polyamine synthesis as well as exogenous polyamine uptake, thus depleting polyamine concentrations (Alexander et al. 2017). The treatment promoted macrophage infiltration of various tumors in vivo marked by increased concentrations of IL-10, IFN-γ, and monocyte chemoattractant protein-1 (MCP-1) (Alexander et al. 2017). The alteration of cytokine production decreased immunosuppressive activity of MDSCs and increased cytotoxic T cell activity (Alexander et al. 2017).
Bacterial-induced Inflammation
All bacterial pathogens elicit an innate response upon detection at the site of infection. RAW 246.7 cells and BMDM treated with various inflammation-inducing stimuli including LPS, Bacillus Calmette–Guerin (BCG, TB vaccine), and carbon tetrachloride (CCl4, hepatotoxin), produce varying responses to modulations of ODC (Jiang et al. 2018). Knockdown of ODC resulted in increased TNF-α, IL-1β, and IL-6 when stimulated with LPS, and decreased IL-1β and IL-6 when stimulated with BCG and CCl4 (Jiang et al. 2018). Overexpression of Odc resulted in decreased TNF-α, IL-1β, and IL-6 when stimulated with LPS, and an opposite effect with BCG or CCl4 (Jiang et al. 2018). This suggests that macrophages respond differently to varying stimuli and highlights the need to screen the response of ODC to small molecules and the resulting effect on immune cell function. Inhibition of ODC potentiates NO production while spermine supplementation inhibits production of NO in LPS-activated murine macrophage J774 cells (Szabó et al. 1994; Baydoun and Morgan 1998). Increased concentrations of DFMO correlated with increased concentrations of NO with LPS stimulation in the first 48 hours of LPS stimulation (Baydoun and Morgan 1998). Spermine post-translationally suppresses the production of IL-12p40 in peritoneal macrophages, coinciding with decreased INF-γ (Hasko et al. 2000). Spermine also inhibits proinflammatory cytokine synthesis in human mononuclear cells, a counterregulatory mechanism that restrains the immune response (Zhang et al. 1997). Increased concentrations of spermine pre-treatment decreased TNF, MIP-1α, MIP-1β, IL-1β, IL-6, and TGF-β1 secretion of human peripheral blood mononuclear cells (PBMCs) stimulated with LPS (Zhang et al. 1997). TNF secretion was also inhibited in RAW 264.7 cells with LPS stimulation (Zhang et al. 1997). Alveolar infection by Pneumocystis organisms reduces levels of antizyme inhibitor (AZI), an endogenous inhibitor of the ODC inhibitor, antizyme, resulting in more active ODC combined with an increase in polyamines. This further increases overall polyamine levels within macrophages favoring Pneumocystis survival via an M2 response and macrophage apoptosis (Liao et al. 2009).
Liver Inflammation
Spermine pre-treatment of Kupffer cells (resident liver macrophages) isolated from mice injected with thioacetamide, a hepatotoxin that causes acute and chronic liver injury, had reduced expression of Il1b and Nos2, but increased expression of Arg1 and Mrc1 (macrophage mannose receptor 1) (Zhou et al. 2018). The spermine pre-treatment also resulted in a decrease of CCL2, CXCL-10, TNF-α and IL-6, but an increase of IL-10 in the tissues, thus suppressing the recruitment of immune cells (Zhou et al. 2018). This is supported by low levels of proinflammatory markers and increased IL-10 in the serum, and evidence of less macrophage infiltration in the tissues (Zhou et al. 2018). Knockdown of autophagy protein 5 (ATG5) mediates autophagy and increases M1 markers, while decreasing M2 markers (Zhou et al. 2018) (Fig. 2). This suggests that the Kupffer cell M1/M2 polarization is autophagy-dependent and spermine enhances autophagy via increased ATG5 expression. Overall, spermine attenuates injury and apoptosis due to thioacetamide in the liver.
Parasites
Upregulation of L-arginine transport during Leishmania donovani infection leads to increased levels of polyamines and an M2 response characterized by increased IL-10 and decreased IL-12 and TNF-α (Mandal et al. 2017). Leishmania donovani is an intercellular parasite and can reside in macrophages; this survival is L-arginine dependent and SLC7A2 is needed for arginine transport (Mandal et al. 2017). Leishmania donovani promotes arginine uptake upregulating ARG2 and ARG1 expression, however, there is no observed change in the expression of NOS2 (Mandal et al. 2017).
Allergy/Asthma
High levels of circulating polyamines have been reported in the blood of patients with severe asthma (Jain 2018). M2 macrophages were among the most prevalent immune cells within the lung of asthmatic patients due to a Th2-rich environment (Jain 2018). A characteristic of macrophages activated in response to asthma and Taenia crassiceps helminths is the expression of E-cadherin. Van den Bossche et al. found that the interaction of E-cadherin with catenins is regulated in a polyamine-dependent manner (Van den Bossche et al. 2009). M2 macrophage activation by IL-4 requires polyamines, more specifically putrescine, to have fully functional E-cadherin and acquire the ability to adhere to other cells (Van den Bossche et al. 2009). Dysregulation of polyamine synthesis by DFMO or diethylnorspermine (DENSPM), a polyamine analogue that depletes the polyamine pool, decreased Cdh1 mRNA levels, the gene that encodes cadherin 1 (E-cadherin) (Van den Bossche et al. 2009).
Polyamine depletion resulted in increased expression of LPS-induced proinflammatory genes, but not excretion (Van den Bossche et al. 2012). Polyamine synthesis may be ARG1-independent in macrophages, as Arg1-deficient macrophages did not have reduced IL-4-induced polyamine production (Van den Bossche et al. 2012). Using DFMO and DENSPM, Van den Bossche et al. were able to identify multiple markers of M2 macrophages that are dependent on polyamines for IL-4-induced expression, including Retnla, Chil3, Pdcd1lg2, Slc7a2, Rnase2a, Ccl17, Cldn11, Cdh1, and Mrc1 (Van den Bossche et al. 2012).
Conclusions
Polyamines are essential for multiple processes, many of which are not fully understood and vary between beneficial and harmful. Competition for L-arginine and the relative ratios of putrescine, spermidine, and spermine contribute to the effects of polyamine metabolism on cellular function. We propose that polyamines regulate the activation and thus the role of macrophages in the innate immune response to gastrointestinal pathogens. Our lab has shown that inhibiting the production of putrescine supports innate immunity by inducing polarization of macrophages to a pro-inflammatory response. This is accomplished by either directly inhibiting ODC with DFMO, or by alleviating the competition for L-arginine by inhibiting arginase. It has been shown that pathogens, including H. pylori and L. donovani, upregulate polyamine metabolism as a survival mechanism to impair M1 macrophage responses and produce an M2-like state and increase the rate of ROS-induced macrophage apoptosis, enhancing the risk of disease progression. These findings suggest that polyamines regulate myeloid-derived macrophage function via both biochemical pathways and epigenetic modifications providing insight into potential novel therapeutic targets. Studies, such as those ongoing in our laboratory, are needed to elucidate further the mechanisms by which polyamines alter macrophage immunometabolism, a key aspect to host cell function in the setting of infections or tumorigenesis.
Acknowledgements.
We thank the members of the Wilson Lab for their work related to the original research summarized in this article.
Funding. This work was funded by NIH Grants R01AT004821, R01CA190612, P01CA116087, and P01CA028842 (K.T.W.), Merit Review Grant I01BX001453 from the United States Department of Veterans Affairs Biomedical Laboratory R&D (BLRD) Service (K.T.W.), the Vanderbilt Digestive Disease Research Center, supported by NIH Grant P30DK058404, the Vanderbilt Ingram Cancer Center, supported by NIH Grant P30CA068485, Department of Defense Grant W81XWH-18-1-0301 (K.T.W.), the Thomas F. Frist Sr. Endowment (K.T.W.), and the Vanderbilt Center for Mucosal Inflammation and Cancer (K.T.W.).
Footnotes
Compliance with ethical standards. All studies from the Wilson Lab that are referenced here were conducted under proper ethical standards for either animal or human research.
Conflict of interest. The authors declare that no conflict of interest exists.
References:
- Alexander ET, Minton A, Peters MC, Phanstiel O 4th, Gilmour SK (2017) A novel polyamine blockade therapy activates an anti-tumor immune response. Oncotarget 8:84140–84152. 10.18632/oncotarget.20493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CF, Mosser DM (2002) A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol 72:101–106. 10.1189/jlb.72.1.101 [DOI] [PubMed] [Google Scholar]
- Asim M, Chaturvedi R, Hoge S, Lewis ND, Singh K, Barry DP, Algood HS, de Sablet T, Gobert AP, Wilson KT (2010) Helicobacter pylori induces ERK-dependent formation of a phospho-c-Fos c-Jun activator protein-1 complex that causes apoptosis in macrophages. J Biol Chem 285:20343–20357. 10.1074/jbc.M110.116988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiri H, Benavides A, Haber M, Gilmour SK, Norris MD, Hogarty MD (2015) Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Transl Pediatr 4:226–238. 10.3978/j.issn.2224-4336.2015.04.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun AR, Morgan DM (1998) Inhibition of ornithine decarboxylase potentiates nitric oxide production in LPS-activated J774 cells. Br J Pharmacol 125:1511–1516. 10.1038/sj.bjp.0702231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege J-L (2008) Macrophage polarization in bacterial infections. J Immunol 181:3733–3739. 10.4049/jimmunol.181.6.3733 [DOI] [PubMed] [Google Scholar]
- Brooks WH (2013) Increased polyamines alter chromatin and stabilize autoantigens in autoimmune diseases. Front Immunol 4:91 10.3389/fimmu.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussière FI, Chaturvedi R, Cheng Y, Gobert AP, Asim M, Blumberg DR, Xu H, Kim PY, Hacker A, Casero RA, Wilson KT (2005) Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem 280:2409–2412. 10.1074/jbc.C400498200 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Barry DP, Frye JW, Casero RA Jr, Wilson KT (2014a) Spermine oxidase is a regulator of macrophage host response to Helicobacter pylori: enhancement of antimicrobial nitric oxide generation by depletion of spermine. Amino Acids 46:531–542. 10.1007/s00726-013-1531-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP, de Sablet T, Piazuelo MB, Sarvaria AR, Cheng Y (2010) Polyamines impair immunity to Helicobacter pylori by inhibiting L-arginine uptake required for nitric oxide production. Gastroenterology 139:1686–1698. 10.1053/j.gastro.2010.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Lewis ND, Algood HMS, Cover TL, Kim PY, Wilson KT (2007) L-arginine availability regulates inducible nitric oxide synthase-dependent host defense against Helicobacter pylori. Infect Immun 75:4305–4315. 10.1128/IAI.00578-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Piazuelo MB, Yan F, Barry DP, Sierra JC, Delgado AG, Hill S, Casero RA Jr, Bravo LE, Dominguez RL, Correa P, Polk DB, Washington MK, Rose KL, Schey KL, Morgan DR, Peek RM Jr, Wilson KT (2014b) Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology 146:1739–51.e14. 10.1053/j.gastro.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Romero–Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F (2011) Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141:1696–1708. 10.1053/j.gastro.2011.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Cheng Y, Asim M, Bussière FI, Xu H, Gobert AP, Hacker A, Casero RA, Wilson KT (2004) Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem 279:40161–40173. 10.1074/jbc.M401370200 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, Sierra JC, Hardbower DM, Delgado AG, Schneider BG (2015) Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 34:3429–3440. 10.1038/onc.2014.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, de Sablet T, Peek RM, Wilson KT (2012) Spermine oxidase, a polyamine catabolic enzyme that links Helicobacter pylori CagA and gastric cancer risk. Gut Microbes 3:48–56. 10.4161/gmic.19345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn LA, Singh K, Asim M, Barry DP, Allaman MM, Al-Greene NT, Hardbower DM, Polosukhina D, Williams CS, Delgado AG (2018) Loss of solute carrier family 7 member 2 exacerbates inflammation-associated colon tumorigenesis. Oncogene 38, 1067–1079. 10.1038/s41388-018-0492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duleu S, Vincendeau P, Courtois P, Semballa S, Lagroye I, Daulouède S, Boucher J-L, Wilson KT, Veyret B, Gobert AP (2004) Mouse strain susceptibility to trypanosome infection: an arginase-dependent effect. J Immunol 172:6298–6303. 10.4049/jimmunol.172.10.6298 [DOI] [PubMed] [Google Scholar]
- Eaden JA, Abrams KR, Mayberry JF (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48:526–535. 10.1136/gut.48.4.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A, Helmick C, Zack M, Adami H-O (1990) Ulcerative colitis and colorectal cancer. N Engl J Med 323:1228–1233. 10.1056/NEJM199011013231802 [DOI] [PubMed] [Google Scholar]
- El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, König T, Schleicher U, Koo M-S (2008) Toll-like receptor–induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol 9:1399–1406. 10.1038/ni.1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming BD, Mosser DM (2011) Regulatory macrophages: setting the threshold for therapy. Eur J Immunol 41:2498–2502. 10.1002/eji.201141717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174. 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12:1035–1044. 10.1038/ni.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos K (2002) Haematopoietic cell-fate decisions, chromatin regulation and Ikaros. Nat Rev Immunol 2:162–174. 10.1038/nri747 [DOI] [PubMed] [Google Scholar]
- Gobert AP, Cheng Y, Akhtar M, Mersey BD, Blumberg DR, Cross RK, Chaturvedi R, Drachenberg CB, Boucher J-L, Hacker A, Casero RA, Wilson KT (2004) Protective role of arginase in a mouse model of colitis. J Immunol 173:2109–2117. 10.4049/jimmunol.173.3.2109 [DOI] [PubMed] [Google Scholar]
- Gobert AP, Cheng Y, Wang J-Y, Boucher J-L, Iyer RK, Cederbaum SD, Casero RA, Newton JC, Wilson KT (2002) Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol 168:4692–4700. 10.4049/jimmunol.168.9.4692 [DOI] [PubMed] [Google Scholar]
- Gobert AP, Daulouede S, Lepoivre M, Boucher JL, Bouteille B, Buguet A, Cespuglio R, Veyret B, Vincendeau P (2000) L-Arginine availability modulates local nitric oxide production and parasite killing in experimental trypanosomiasis. Infect Immun 68:4653–4657. 10.1128/IAI.68.8.4653-4657.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, Steeves MA, Cleveland JL, Schneider C, Piazuelo MB, Gobert AP, Wilson KT (2017a) Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci USA 114:E751–E760. 10.1073/pnas.1614958114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardbower DM, Asim M, Murray-Stewart T, Casero RA Jr, Verriere T, Lewis ND, Chaturvedi R, Piazuelo MB, Wilson KT (2016) Arginase 2 deletion leads to enhanced M1 macrophage activation and upregulated polyamine metabolism in response to Helicobacter pylori infection. Amino Acids 48:2375–2388. 10.1007/s00726-016-2231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardbower DM, Coburn LA, Asim M, Singh K, Sierra JC, Barry DP, Gobert AP, Piazuelo MB, Washington MK, Wilson KT (2017b) EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis. Oncogene 36:3807–3819. 10.1038/onc.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Marton A, Nemeth ZH, Deitch EA, Szabó C (2000) Spermine differentially regulates the production of interleukin-12 p40 and interleukin-10 and suppresses the release of the T helper 1 cytokine interferon-gamma. Shock 14:144–149 [DOI] [PubMed] [Google Scholar]
- Hayashi S, Murakami Y, Matsufuji S (1996) Ornithine decarboxylase antizyme: a novel type of regulatory protein. Trends Biochem Sci 21:27–30. 10.1016/S0968-0004(06)80024-1 [DOI] [PubMed] [Google Scholar]
- Hobbs CA, Gilmour SK (2000) High levels of intracellular polyamines promote histone acetyltransferase activity resulting in chromatin hyperacetylation. J Cell Biochem 77:345–360. [DOI] [PubMed] [Google Scholar]
- Huang J, DeGraves FJ, Lenz SD, Gao D, Feng P, Li D, Schlapp T, Kaltenboeck B (2002) The quantity of nitric oxide released by macrophages regulates Chlamydia-induced disease. Proc Natl Acad Sci 99:3914–3919. 10.1073/pnas.062578399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Marton LJ, Woster PM, Casero RA (2009) Polyamine analogues targeting epigenetic gene regulation. Essays Biochem 46:95–110. 10.1042/bse0460007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta V, Gómez-Nieto LC, Corraliza I (2001) The inhibition of arginase by Nω-hydroxy-L-arginine controls the growth of Leishmania inside macrophages. J Exp Med 193:777–784. 10.1084/jem.193.6.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V (2018) Role of polyamines in asthma pathophysiology. Med Sci (Basel, Switzerland) 6(1):4 10.3390/medsci6010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Gao Y, Dong C, Xiong S (2018) ODC1 inhibits the inflammatory response and ROS-induced apoptosis in macrophages. Biochem Biophys Res Commun 504:734–741. 10.1016/J.BBRC.2018.09.023 [DOI] [PubMed] [Google Scholar]
- Kakuda DK, Sweet MJ, Mac Leod CL, Hume DA, Markovich D (1999) CAT2-mediated L-arginine transport and nitric oxide production in activated macrophages. Biochem J 340(2):549–553. 10.1042/bj3400549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaparakis M, Walduck AK, Price JD, Pedersen JS, van Rooijen N, Pearse MJ, Wijburg OLC, Strugnell RA (2008) Macrophages are mediators of gastritis in acute Helicobacter pylori infection in C57BL/6 mice. Infect Immun 76:2235–2239. 10.1128/IAI.01481-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R (2006) Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3:309–319. 10.1016/j.cmet.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Lech M, Anders H-J (2013) Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta - Mol Basis Dis 1832:989–997. 10.1016/j.bbadis.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Lewis ND, Asim M, Barry DP, Singh K, de Sablet T, Boucher J-L, Gobert AP, Chaturvedi R, Wilson KT (2010) Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages. J Immunol 184(5):2572–2582. 10.4049/jimmunol.0902436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ND, Asim M, Barry DP, de Sablet T, Singh K, Piazuelo MB, Gobert AP, Chaturvedi R, Wilson KT (2011) Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J Immunol 186:3632–3641. 10.4049/jimmunol.1003431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-P, Lasbury ME, Wang S-H, Zhang C, Durant PJ, Murakami Y, Matsufuji S, Lee C-H (2009) Pneumocystis mediates overexpression of antizyme inhibitor resulting in increased polyamine levels and apoptosis in alveolar macrophages. J Biol Chem 284:8174–8184. 10.1074/jbc.M805787200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtnekert J, Kawakami T, Parks WC, Duffield JS (2013) Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol 13:555–564. 10.1016/j.coph.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsalata M, Orlando A, Russo F (2014) Pharmacological and dietary agents for colorectal cancer chemoprevention: Effects on polyamine metabolism. Int J Oncol 45:1802–1812. 10.3892/ijo.2014.2597 [DOI] [PubMed] [Google Scholar]
- Mandal A, Das S, Kumar A, Roy S, Verma S, Ghosh AK, Singh R, Abhishek K, Saini S, Sardar AH, Purkait B, Kumar A, Mandal C, Das P (2017) L-arginine uptake by cationic amino acid transporter promotes intra-macrophage survival of Leishmania donovani by enhancing arginase-mediated polyamine synthesis. Front Immunol 8:839 10.3389/fimmu.2017.00839 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Ou J, Peng Y, Zhang X, Chen Y, Hao L, Xie G, Wang Z, Pang X, Ruan Z, Li J, Yu L, Xue B, Shi H, Shi C, Liang H (2016) Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat Commun 7:11716 10.1038/ncomms11716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM (2003) The many faces of macrophage activation. J Leukoc Biol 73:209–212. 10.1189/jlb.0602325 [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737. 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini A, Caldarera CM, Giordano E (2014) Chromatin remodeling by polyamines and polyamine analogs. Amino Acids 46:595–603. 10.1007/s00726-013-1550-9 [DOI] [PubMed] [Google Scholar]
- Peek RM Jr, Fiske C, Wilson KT (2010) Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev 90:831–858. 10.1152/physrev.00039.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE (2009) Mammalian polyamine metabolism and function. IUBMB Life 61:880–894. 10.1002/iub.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE (2006) Regulation of ornithine decarboxylase. J Biol Chem 281: 14529–14532. 10.1074/jbc.R500031200 [DOI] [PubMed] [Google Scholar]
- Pegg AE, McCann PP (1982) Polyamine metabolism and function. Am J Physiol Physiol 243:C212–C221. 10.1152/ajpcell.1982.243.5.C212 [DOI] [PubMed] [Google Scholar]
- Saulnier Sholler GL, Gerner EW, Bergendahl G, MacArthur RB, VanderWerff A, Ashikaga T, Bond JP, Ferguson W, Roberts W, Wada RK, Eslin D, Kraveka JM, Kaplan J, Mitchell D, Parikh NS, Neville K, Sender L, Higgins T, Kawakita M, et al. (2015) A Phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PLoS One 10:e0127246–e0127246. 10.1371/journal.pone.0127246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D, Stampfel G, Stark A (2014) Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet 15:272–286. 10.1038/nrg3682 [DOI] [PubMed] [Google Scholar]
- Singh K, Coburn LA, Asim M, Barry DP, Allaman MM, Shi C, Washington MK, Luis PB, Schneider C, Delgado AG (2018) Ornithine decarboxylase in macrophages exacerbates colitis and promotes colitis-associated colon carcinogenesis by impairing M1 immune responses. Cancer Res 78:4303–4315. 10.1158/0008-5472.CAN-18-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss-Ayali D, Conrad SM, Mosser DM (2007) Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol 82:244–252. 10.1189/jlb.0307191 [DOI] [PubMed] [Google Scholar]
- Szabó C, Southan GJ, Wood E, Thiemermann C, Vane JR (1994) Inhibition by spermine of the induction of nitric oxide synthase in J774.2 macrophages: requirement of a serum factor. Br J Pharmacol 112:355–356. 10.1111/j.1476-5381.1994.tb13078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J, Bogaert P, van Hengel J, Guérin CJ, Berx G, Movahedi K, Van den Bergh R, Pereira-Fernandes A, Geuns JMC, Pircher H, Dorny P, Grooten J, De Baetselier P, Van Ginderachter JA (2009) Alternatively activated macrophages engage in homotypic and heterotypic interactions through IL-4 and polyamine-induced E-cadherin/catenin complexes. Blood 114:4664–4674. 10.1182/blood-2009-05-221598 [DOI] [PubMed] [Google Scholar]
- Van den Bossche J, Lamers WH, Koehler ES, Geuns JMC, Alhonen L, Uimari A, Pirnes-Karhu S, Van Overmeire E, Morias Y, Brys L, Vereecke L, De Baetselier P, Van Ginderachter JA (2012) Pivotal Advance: Arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J Leukoc Biol 91:685–699. 10.1189/jlb.0911453 [DOI] [PubMed] [Google Scholar]
- Wilson KT, Crabtree JE (2007) Immunology of Helicobacter pylori: insights Into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 133:288–308. 10.1053/j.gastro.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Wroblewski LE, Peek RM Jr, Wilson KT (2010) Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23:713–739. 10.1128/CMR.00011-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496:445–455. 10.1038/nature12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang Y (2017) Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol 10:58 10.1186/s13045-017-0430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeramian A, Martin L, Serrat N, Arpa L, Soler C, Bertran J, McLeod C, Palacín M, Modolell M, Lloberas J (2006) Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J Immunol 176:5918–5924. 10.4049/jimmunol.176.10.5918 [DOI] [PubMed] [Google Scholar]
- Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ (1997) Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med 185:1759–1768. 10.1084/jem.185.10.1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Gu J, Liu R, Wei S, Wang Q, Shen H, Dai Y, Zhou H, Zhang F, Lu L (2018) Spermine alleviates acute liver injury by inhibiting liver-resident macrophage pro-inflammatory response through ATG5-dependent autophagy. Front Immunol 9:948 10.3389/fimmu.2018.00948 [DOI] [PMC free article] [PubMed] [Google Scholar]