Abstract
Background:
It is unknown to what extent higher maternal blood pressure (BP) in early postpartum impacts the relationship between higher maternal weight status and greater infant weight gain in early postpartum.
Objective:
To evaluate the mediating role of higher maternal BP at 1-month postpartum on the association between higher maternal weight status at 1-month postpartum and greater infant weight gain over 6-months postpartum.
Methods:
Participants were 169 Hispanic mother-infant pairs. Maternal BMI and BP were assessed at 1-month postpartum. Infant weight was measured at 1 and 6-months postpartum to calculate weight-for-age z-scores (WAZ). Multiple linear regression models were used for prediction and Sobel’s test was used to determine mediation.
Results:
Controlling for maternal pre-pregnancy BMI, age, delivery mode, infant sex, and infant birth weight revealed that both maternal BMI (β=0.29) and BP (β=0.32) predicted infant WAZ gain (both P≤0.03). However, the relationship between infant WAZ gain and maternal BMI was no longer significant after further adjustment for maternal BP, which remained significant (P<0.05). Maternal BP explained 23.6% (Sobel’s T=2.01) of the association between maternal BMI at 1-month and infant WAZ gain over 6-months.
Conclusion:
Our data suggest that higher maternal weight status at 1-month postpartum is related to greater infant weight gain over 6-months postpartum, and this relationship is mediated by higher maternal BP at 1-month postpartum.
Keywords: Growth, blood pressure, obesity, pediatrics, mother, infant
INTRODUCTION
Infancy is the most rapid period of growth during postnatal life. However, higher rates of infant weight gain have been associated with subsequent obesity,1 cardiovascular disease,2 and type-2 diabetes,3 particularly in the first 6 months after birth.1 Moreover, an NIH Workshop on the Prevention of Obesity in Infancy and Early Childhood concluded that there is still a lack of knowledge regarding antecedents of excessive infant weight gain and future adiposity.4 Filling in these gaps may help explain differential responses to obesity treatment.
One topic identified as a high priority for further research was family and caregiver characteristics that influence infant weight gain, particularly those of the mother. Mothers may influence infant weight gain through shared genetics, environments, and behaviors.5,6 Of note, maternal obesity has been examined in relation to infant obesity risk: mothers who were classified as obese before pregnancy (BMI ≥30 kg/m2) were more likely to have a child classified as obese (BMI >97th percentile) within 2 years of age.7,8 Furthermore, mothers with excessive weight gain during pregnancy were more likely to have a child classified as overweight (BMI ≥95th percentile) at 3 years of age.9 There is also evidence that higher maternal blood pressure (BP) may be related to infant obesity risk: among normotensive and hypertensive women, higher maternal BP during gestation was associated with overweight status at ages 4 to 7 years.10
Although maternal obesity and higher BP may occur separately, they are also complex concomitant conditions.11 It is unknown whether the maternal obesity-hypertension phenotype is particularly influential on infant weight gain. A potential hypothesis is that when mothers have excess weight along with elevated BP in early postpartum, the infant may be more susceptible to greater weight gain, and presumably a greater risk for obesity later in childhood.12,13 This notion is supported by few studies in mother-infant pairs that suggest maternal physiological adaptations to support pregnancy may persist long after pregnancy,14 and contribute to greater infant weight gain.9,10 To our knowledge, no studies have examined the extent to which higher maternal BP in early postpartum influences the relationship between higher maternal weight status and greater infant weight gain in early postpartum. Therefore, the purpose of this study was to evaluate the mediating role of higher maternal BP at 1-month postpartum on the association between higher maternal weight status at 1-month postpartum and greater infant weight gain over 6-months postpartum.
METHODS
Subjects
Participants were 169 mother-infant pairs recruited from maternity clinics affiliated with the University of Southern California in Los Angeles County beginning in 2016 as part of a longitudinal study on early growth and development. Inclusion criteria were as follows: 1) self-identified Hispanic ethnicity; 2) ≥18 years old at time of delivery; 3) gave birth to a healthy, term, singleton newborn; 4) enrolled within 1-month postpartum; 5) intended to breastfeed for 3-months postpartum; and 6) able to read English or Spanish at a 5th grade level to understand study procedures. Mothers were excluded if they reported taking medications or reported they had a diagnosed medical condition that could affect physical or mental health, nutritional status, or metabolism, current use of tobacco (i.e., >1 cigarette per week) or other recreational drugs, and a clinical diagnosis of fetal abnormalities. The University of Southern California’s Institutional Review Board approved all study procedures. Participants provided written informed consent prior to data collection.
Study design
Mother-infant dyads completed two study visits at 1 and 6-months postpartum. Historical health-related information was collected regarding family health history, maternal health history, maternal pre-pregnancy BMI, age at delivery, delivery mode, infant sex, and infant birth weight. Maternal BP was assessed at 1-month postpartum using an automated sphygmomanometer. The average of two measurements was used for systolic and diastolic values, with the systolic value representing maternal BP in the analyses. Maternal weight was measured using an electronic scale and standing height was measured using a stadiometer. Each measurement was taken in duplicate. The average of these measurements was used to calculate maternal BMI at 1-month postpartum, and to classify mothers as normal weight, overweight, and obese.15
Infant weight was measured by weighing the mother holding the infant, and then calculating the difference in the mother’s weight with and without holding the infant. The average of two measurements was used to calculate weight-for-age z scores (WAZ) at 1 and 6-months postpartum based on WHO standards.16 Change in infant WAZ (primary outcome variable) and change in absolute weight (secondary outcome variable) from 1 to 6-months postpartum were used to denote infant weight gain outcomes.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation (SD) for continuous variables and as frequency (percentage) for categorical variables. Normal distribution and homogeneity of variances were confirmed by Shapiro–Wilks W and Levene’s tests, respectively. Differences between maternal BMI status groups at 1-month postpartum (normal weight vs. overweight vs. obese) were tested by analysis of variance with polynomial contrast for continuous variables and by Mantel–Haenszel linear-by-linear association chi-square tests for categorical variables.
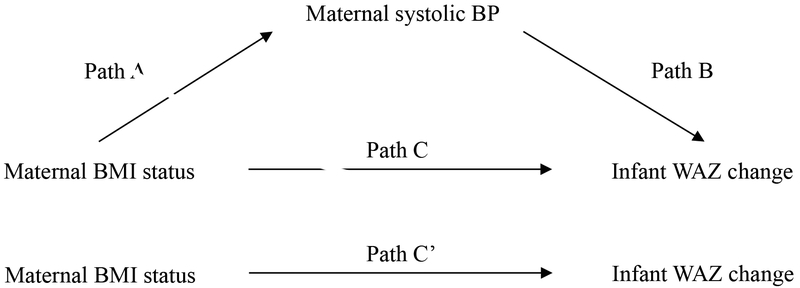
Mediation analysis hypothesizes that the association between exposure and outcome is mediated by a mechanism factor: the mediator, as shown in Figure 1. To be considered a mediator, the following criteria need to be satisfied: 1) the exposure variable is significantly associated with the outcome variable (Path C); 2) the exposure variable is significantly associated with the mediator variable (Path A); 3) the mediator variable is significantly associated with the outcome variable, after adjustment for the exposure variable (Path B); and 4) after adjustment for the potential mediator, a previously significant relationship between the exposure variable and outcome variable is no longer significant (Path C`).17 We performed linear regression analyses to investigate the following associations: 1) between maternal BMI status (exposure) and infant WAZ gain (outcome); and 2) between maternal BMI status and the potential mediator (systolic BP). We then examined the extent to which the association between maternal BMI status and infant WAZ gain was explained (i.e., potentially mediated) by the potential mediator considered. This was done by quantifying, in percentage, the attenuations in the magnitude of the linear regression coefficient reflecting the association between maternal BMI status and infant WAZ gain after adjustment for the systolic BP measurement. Before analyses, the distributions of maternal BMI status, maternal systolic BP, infant WAZ gain, and infant absolute weight gain were assessed and found to be normal. We also confirmed that the residuals of the outcome, exposure, and mediator variables were independent of each other and that there was no misspecification of causal order or misspecification of causal direction: infant WAZ gain (outcome) could not affect maternal BMI status (exposure) or maternal systolic BP (mediator). The mediation effect was tested for significance using Sobel’s test.18 The same analyses were conducted replacing infant WAZ gain with absolute weight gain as the outcome. All models were adjusted for the following covariates: maternal pre-pregnancy BMI, age at delivery, delivery mode, infant sex, and infant birth weight. All analyses were conducted with SPSS software (version 24; IBM SPSS Statistics, Chicago, IL). Statistical significance was set at P value <0.05.
Figure 1. Hypothesized mediation models of whether maternal blood pressure (BP) is a factor linking maternal body mass index (BMI) status at 1-month postpartum and greater infant WAZ change over 6-months postpartum.
Path A indicates the path from maternal BMI status (exposure) to maternal systolic BP (mediator). Path B indicates the path from maternal systolic BP (mediator) to infant WAZ change (outcome). Path C indicates the path from maternal BMI status (exposure) to infant WAZ change (outcome). Path C’ indicates the path from maternal BMI status (exposure) to infant WAZ change (outcome) when controlled for maternal systolic BP (mediator).
Results
The sample was composed of 169 Hispanic mother-infant dyads. In total, mothers were 28.6 ± 6.0 years old at delivery and had a pre-pregnancy BMI of 28.1 ± 5.6 kg/m2, a 1-month postpartum BMI of 29.8 ± 5.0 kg/m2, a 1-month postpartum systolic BP of 107 ± 10 mm Hg, and a 1-month postpartum diastolic BP of 70.0 ± 8.0 mm Hg. The percentages of mothers who were overweight and obese at 1-month postpartum were 35.5% and 46.2%, respectively.
Characteristics of the mother-infant dyads grouped by maternal BMI status at 1-month postpartum are described in Table 1. Distribution of infant sex, infant birth weight, infant weight at 1-month, and infant weight at 6-months did not differ between groups. However, significant linear upward trends in maternal pre-pregnancy BMI and maternal BMI and BP at 1-month postpartum were found across groups (normal weight vs. overweight vs. obese; all P<0.01). Significant linear upward trends across groups were also observed for infant change in absolute weight and change in WAZ (both P=0.03).
Table 1.
Characteristics of the Hispanic mother-infant pairsa
| Maternal postpartum BMI statusb | |||||
|---|---|---|---|---|---|
| Total | Normal weight |
Overweight | Obese | Pc | |
| n | 169 | 31 | 60 | 78 | |
| Age at delivery (years) | 28.6 ± 6.0 | 26.5 ± 6.5 | 28.9 ± 5.5 | 29.1 ± 6.0 | 0.04 |
| BMI, pre-pregnancy (kg/m2) | 28.1 ± 5.6 | 21.3 ± 1.7 | 26.5 ± 3.4 | 32.1 ± 4.7 | <0.01 |
| BMI, 1-month (kg/m2) | 29.8 ± 5.0 | 23.0 ± 1.2 | 27.9 ± 1.2 | 34.1 ± 3.5 | <0.01 |
| Systolic BP, 1-month (mm Hg) | 107 ± 10 | 99 ± 7.0 | 103 ± 8.0 | 112 ± 10 | <0.01 |
| Diastolic BP, 1-month (mm Hg) | 70 ± 8.0 | 66 ± 6.6 | 68 ± 7.0 | 74 ± 7.0 | <0.01 |
| Caesarean section delivery (%)d | 25.4 | 25.8 | 18.3 | 30.8 | 0.25 |
| Female (%)d | 53.8 | 48.4 | 58.3 | 52.6 | 0.64 |
| Birth weight (kg) | 3.4 ± 0.4 | 3.3 ± 0.5 | 3.4 ± 0.4 | 3.4 ± 0.4 | 0.50 |
| Breastfed, 1-month (%)d | 99.4 | 100 | 98.3 | 100 | 0.40 |
| Breastfed, 6-months (%)d | 79.4 | 82.6 | 82.0 | 75.5 | 0.65 |
| Weight, 1-month (kg) | 4.6 ± 0.5 | 4.6 ± 0.5 | 4.6 ± 0.4 | 4.5 ± 0.5 | 0.57 |
| Weight, 6-months (kg) | 8.0 ± 0.8 | 7.8 ± 0.7 | 8.1 ± 0.7 | 8.1 ± 0.9 | 0.13 |
| Change in weight, 1-6 months (kg) | 3.4 ± 0.7 | 3.2 ± 0.6 | 3.4 ± 0.7 | 3.6 ± 0.7 | 0.03 |
| WAZ, 1-month | 0.48 ± 0.74 | 0.52 ± 0.90 | 0.56 ± 0.69 | 0.38 ± 0.74 | 0.50 |
| WAZ, 6-months | 0.51 ± 0.84 | 0.21 ± 0.86 | 0.56 ± 0.75 | 0.57 ± 0.91 | 0.12 |
| Change in WAZ, 1-6 months | 0.03 ± 0.85 | −0.30 ± 0.79 | 0.00 ± 0.84 | 0.19 ± 0.86 | 0.03 |
BP, blood pressure; BMI, body mass index; WAZ, weight-for-age z score.
Values are mean ± SD or %.
Normal weight, overweight, and obese groups based on maternal BMI at 1-month.
Tests of significance between groups were calculated with ANOVA.
Tests of significance between groups were based on chi-square test.
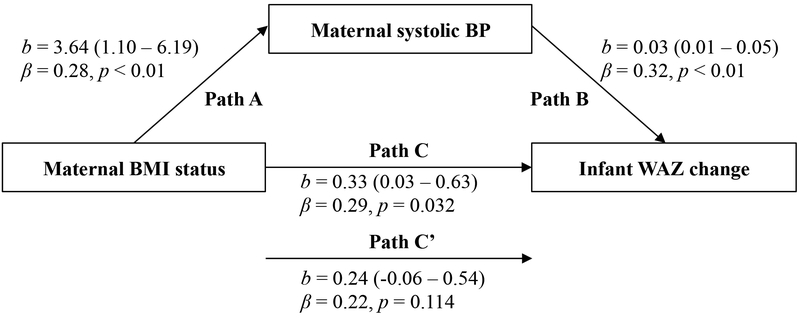
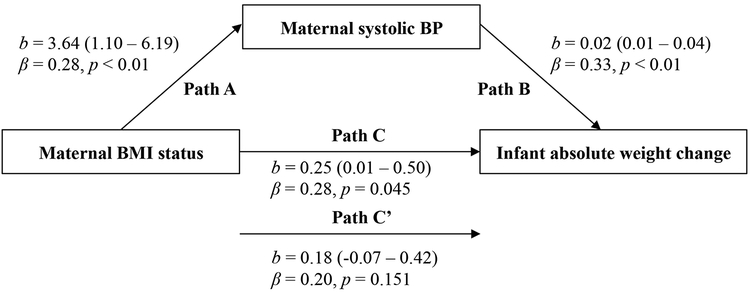
Infant WAZ gain between 1 and 6-months postpartum was predicted by maternal BMI status (β=0.29) and systolic BP (β=0.32) (both P≤0.03), after controlling for maternal pre-pregnancy BMI, age, delivery mode, infant sex, and infant birth weight. When maternal systolic BP was added to the model as a control variable, the association between infant WAZ gain and maternal BMI status was no longer significant (Figure 2). Mediation analysis revealed that maternal systolic BP explained 23.6% (Sobel’s T=2.01) of the association between infant WAZ gain and maternal BMI status. Similar results were observed after substituting infant absolute weight gain as the outcome (Figure 3); mediation analysis revealed that maternal systolic BP explained 24.8% (Sobel’s T=2.08) of the association between infant absolute weight gain and maternal BMI status.
Figure 2. Mediation models of the pathway from maternal body mass index (BMI) status at 1-month postpartum to infant weight-for-age z score (WAZ) change over 6-months postpartum via maternal blood pressure (BP).
Path A indicates the path from maternal BMI status (exposure) to maternal systolic BP (mediator). Path B indicates the path from maternal systolic BP (mediator) to infant WAZ change (outcome). Path C indicates the path from maternal BMI status (exposure) to infant WAZ change (outcome). Path C’ indicates the path from maternal BMI status (exposure) to infant WAZ change (outcome) when controlled for maternal systolic BP (mediator). Unstandardized (b) coefficients with 95% confidence intervals and standardized (B) coefficients with p values are presented. All models are adjusted for the following covariates: maternal pre-pregnancy BMI, age at delivery, delivery mode, infant sex, and infant birth weight.
Figure 3. Mediation models of the pathway from maternal body mass index (BMI) status at 1-month postpartum to infant absolute weight change over 6-months postpartum via maternal blood pressure (BP).
Path A indicates the path from maternal BMI status (exposure) to maternal systolic BP (mediator). Path B indicates the path from maternal systolic BP (mediator) to infant absolute weight change (outcome). Path C indicates the path from maternal BMI status (exposure) to infant absolute weight change (outcome). Path C’ indicates the path from maternal BMI status (exposure) to infant absolute weight change (outcome) when controlled for maternal systolic BP (mediator). Unstandardized (b) coefficients with 95% confidence intervals and standardized (B) coefficients with p values are presented. All models are adjusted for the following covariates: maternal pre-pregnancy BMI, age at delivery, delivery mode, infant sex, and infant birth weight.
DISCUSSION
In this study of Hispanic mother-infant pairs, we found that higher maternal weight status at 1-month postpartum predicted greater infant weight gain over 6-months postpartum, a significant risk factor for obesity,1 cardiovascular disease,2,5 and type-2 diabetes.3 Of note, we found that higher maternal BP at 1-month postpartum mediated this association between mothers and their infants. To our knowledge, this is the first study to show that higher maternal weight status and greater infant weight gain measured in early postpartum was mediated via higher maternal BP in early postpartum. This information may provide greater insight into the potential for infant obesity risk by using maternal factors collected in early postpartum.
While the relationship between maternal weight status and BP in early postpartum has not been examined in relation to infant weight gain, the influence of maternal weight status before and during pregnancy — and the influence of maternal BP during pregnancy — have been examined separately in relation to infant weight gain. For example, studies have shown that maternal obesity before pregnancy presented a two to threefold increase in overweight risk at ages 12 and 24 months,8,7 while excessive maternal weight gain during pregnancy presented a fourfold increase in overweight risk at age 36 months.9 In one of the few studies to have examined maternal weight status after pregnancy, Robinson and colleagues found that greater maternal weight gain within 13 months postpartum was associated with increased odds of overweight at age 5 years.12 Our results linking higher maternal weight status to greater infant weight gain indicate that this relationship is evident even earlier, within months of delivery.
As briefly mentioned, the impact of maternal BP during pregnancy has also been examined in relation to infant weight gain. For example, Zhang and colleagues found that maternal hypertension during pregnancy was associated with 1.9 increased odds of overweight and obesity at ages 1 to 5 years.19 Moreover, a recent study found that higher maternal BP (below the threshold for hypertension) in the second and third trimesters was associated with a 49% and 14% higher risk, respectively, of overweight at ages 4 to 7 years.10 Ultimately, these studies suggest that higher maternal weight status and BP, particularly prior to delivery, may predispose infants to greater obesity risk.9,10 Here, we provide additional evidence that excess maternal weight along with elevated BP after delivery may also be problematic for greater infant weight gain.
We postulate that there are two potential mechanisms for the association we observed in early postpartum. The first is that undiagnosed elevated maternal BP during pregnancy persisted after pregnancy (and was reflected in our measure at 1-month postpartum), and initial exposure impacted infant weight gain. Women experience vascular, renal, and metabolic adaptations to support pregnancy: these are accentuated in obesity and have the potential to alter BP long-term.20,21,14 For example, the Generation R Study found that higher maternal lipids in pregnancy were associated with higher maternal BP in pregnancy, and higher maternal BP in pregnancy was sustained for years after delivery.14 Because we did not measure maternal BP during gestation and our participants did not report having diagnosed hypertension, more work is need to monitor mothers pre and post delivery and assess infant outcomes. There is the potential for maternal measures collected in early postpartum to be a proxy for predicting infant obesity risk in the absence of maternal measures collected in pregnancy.
Another potential mechanism is that higher maternal weight status and BP in early postpartum reflect dietary patterns that inform greater infant weight gain via breast milk composition. The maternal obesity-hypertension phenotype has been attributed to dietary (e.g., high-fat and high-fructose consumption)22–24 and subsequent metabolic (e.g., hyperleptinemia and hypertriglyceridemia) factors.23,25 The same dietary and metabolic factors are detectable in breast milk, and have been shown to influence infant obesity risk through breast milk exposure.26–29 Findings from our prior works revealed that maternal consumption of a sugar-sweetened beverage during lactation increased breast milk fructose concentrations,30 which were related to increased infant fat mass over 6 months.26 We postulated that this was due to infant exposure to elevated triglycerides, which has been proposed in other studies.25 Increased breast milk leptin concentrations have also been linked to early weight gain,27 although findings are less conclusive.28,29 Because infants in our study were predominantly breastfed, it may be that higher maternal weight status and BP were a reflection of dietary and metabolic factors that were transmitted to the infant via breast milk, resulting in greater infant weight gain.
This study is not without limitations. The cross-sectional design cannot be used to establish causality. Although cross-sectional studies can use mediation models, a stronger case is made with longitudinal data, particularly when variables that come earlier in the causal model are measured before those that come later. Adding a randomized design to longitudinal data collection makes causal mediation claims even stronger by eliminating many possible confounders. We did not assess maternal weight status or BP before or during pregnancy, and information regarding maternal weight status and BP prior to delivery was based on self-report. We did not assess biochemical parameters of metabolic abnormalities in breast milk (e.g., insulin) or blood (e.g., glucose), nor did we include information on maternal dietary intake. Therefore, we can only speculate as to how higher maternal weight status and BP in early postpartum may inform infant obesity risk. Although multiple covariates were carefully adjusted for in our analyses, residual confounding caused by incomplete categorization, misclassification, or unmeasured factors may exist. Because other metabolic abnormalities of the mother in early postpartum might be correlated with both maternal BP and infant weight gain in early postpartum, we cannot rule out the possibility that other maternal metabolic abnormalities might confound the associations we observed.
Another important limitation is that our relatively small sample size limited the power to examine the potential mediating role of maternal BP within maternal BMI strata. Given the complex association between obesity and hypertension, it is possible that the mediation effect may have been valid only in mothers who were overweight or obese. However, this proposition warrants further investigation in a larger cohort. Finally, our study findings are limited to Hispanic mothers and infants living in the Southwestern United States: therefore, differences in socioeconomic status, built environment, food choice, and eating behaviors of our study population may limit generalizability of our study findings. Given that Hispanic mothers and infants tend to have a higher susceptibility to obesity, our results may provide insight into this racial/ethnic disparity.31
In conclusion, findings from this study revealed that higher maternal weight status at 1-month postpartum was related to greater infant weight gain over 6-months postpartum, and this relationship was mediated by higher maternal BP at 1-month postpartum. Future studies should monitor higher maternal BP on a continuous scale before, during, and after pregnancy to better screen for infant obesity risk. They should also explore dietary and metabolic factors that underlie the maternal obesity-hypertension phenotype to impact the infant. This may inform interventions to improve the quality of breast milk by maternal dietary or pharmacological means,28 and therefore reduce infant obesity risk.
ACKNOWLEDGEMENTS
Funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIH R01 DK110793) and the Gerber Foundation. MIG was responsible for funding acquisition, study design, and oversight. PKB was responsible for manuscript conceptualization and statistical analysis. NKP and JHR provided statistical support. All authors contributed to the interpretation of the data. PKB was responsible for writing the manuscript. All authors were involved in reviewing and editing the manuscript. All authors had final approval of the submitted version.
The authors thank the participating mothers for their commitment to this research. We also thank Carla Flores, Danielle Garcia, Rosa Rangel, Paola Garcia, Elizabeth Campbell, and Claudia Rios for coordination and recruitment for this project.
ABBREVIATIONS:
- (BP)
Blood pressure
- (BMI)
body mass index
- (WAZ)
weight-for-age z score
Footnotes
CONFLICTS OF INTEREST STATEMENT
The authors have nothing to disclose.
REFERENCES
- 1.Taveras EM, Rifas-Shiman SL, Sherry B, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–998. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–1809. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350(9):865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng JC, Taveras EM, Birch L, Yanovski SZ. Prevention of obesity in infancy and early childhood: a National Institutes of Health workshop. JAMA Pediatr. 2015;169(5):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauder KA, Kaar JL, Starling AP, Ringham BM, Glueck DH, Dabelea D. Predictors of Infant Body Composition at 5 Months of Age: The Healthy Start Study. J Pediatr. 2017;183:94–99 e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger PK, Lavner JA, Smith JJ, Birch LL. Differences in early risk factors for obesity between African American formula-fed infants and White breastfed controls. Pilot Feasibility Stud. 2017;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridgman SL, Azad MB, Persaud RR, et al. Impact of maternal pre-pregnancy overweight on infant overweight at 1 year of age: associations and sex-specific differences. Pediatr Obes. 2018. [DOI] [PubMed] [Google Scholar]
- 8.Bider-Canfield Z, Martinez MP, Wang X, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes. 2017;12(2):171–178. [DOI] [PubMed] [Google Scholar]
- 9.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322 e321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng JS, Liu H, Ong KK, et al. Maternal Blood Pressure Rise During Pregnancy and Offspring Obesity Risk at 4 to 7 Years Old: The Jiaxing Birth Cohort. J Clin Endocrinol Metab. 2017;102(11):4315–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–619. [DOI] [PubMed] [Google Scholar]
- 12.Robinson CA, Cohen AK, Rehkopf DH, et al. Pregnancy and post-delivery maternal weight changes and overweight in preschool children. Prev Med. 2014;60:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rossem L, Wijga AH, Gehring U, Koppelman GH, Smit HA. Maternal Gestational and Postdelivery Weight Gain and Child Weight. Pediatrics. 2015;136(5):e1294–1301. [DOI] [PubMed] [Google Scholar]
- 14.Adank MC, Benschop L, Peterbroers KR, et al. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long-term postpartum? Am J Obstet Gynecol. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081. [DOI] [PubMed] [Google Scholar]
- 16.WHO Multicentre Growth Reference Study Group. WHO child growth standards: growth velocity based on weight, length and head circumference. 2009. [Google Scholar]
- 17.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 18.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models Sociological Methodology. Washington DC: American Sociological Association; 1982:290–312. [Google Scholar]
- 19.Zhang S, Wang L, Leng J, et al. Hypertensive disorders of pregnancy in women with gestational diabetes mellitus on overweight status of their children. J Hum Hypertens. 2017;31(11):731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin U, Davies C, Hayavi S, Hartland A, Dunne F. Is normal pregnancy atherogenic? Clin Sci (Lond). 1999;96(4):421–425. [DOI] [PubMed] [Google Scholar]
- 21.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285(12):1607–1612. [DOI] [PubMed] [Google Scholar]
- 22.Fiorino P, Americo ALV, Muller CR, et al. Exposure to high-fat diet since post-weaning induces cardiometabolic damage in adult rats. Life Sci. 2016;160:12–17. [DOI] [PubMed] [Google Scholar]
- 23.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10(5):512–516. [DOI] [PubMed] [Google Scholar]
- 24.Toop CR, Gentili S. Fructose Beverage Consumption Induces a Metabolic Syndrome Phenotype in the Rat: A Systematic Review and Meta-Analysis. Nutrients. 2016;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco JG, Fernandes TP, Rocha CP, et al. Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol. 2012;590(21):5503–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goran MI, Martin AA, Alderete TL, Fujiwara H, Fields DA. Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients. 2017;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savino F, Sardo A, Rossi L, Benetti S, Savino A, Silvestro L. Mother and Infant Body Mass Index, Breast Milk Leptin and Their Serum Leptin Values. Nutrients. 2016;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields DA, George B, Williams M, et al. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes. 2017;12 Suppl 1:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunner S, Schmid D, Zang K, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. 2015;10(1):67–73. [DOI] [PubMed] [Google Scholar]
- 30.Berger PK, Fields DA, Demerath EW, Fujiwara H, Goran MI. High-fructose corn syrup-sweetened beverage intake increases 5-hour breast milk fructose concentrations in lactating women. The Journal of Nutrition. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimbro RT, Brooks-Gunn J, McLanahan S. Racial and ethnic differentials in overweight and obesity among 3-year-old children. Am J Public Health. 2007;97(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]