Abstract
Perivascular adipocytes residing in the vascular adventitia are recognized as distinct endocrine cells capable of responding to inflammatory stimuli and communicating with the sympathetic nervous system and adjacent blood vessel cells, thereby releasing adipocytokines and other signaling mediators to maintain vascular homeostasis. Perivascular adipocytes exhibit phenotypic heterogeneity (both white and brown adipocytes) and become dysfunctional in conditions such as diet-induced obesity, thus promoting vascular inflammation, vasoconstriction and smooth muscle cell proliferation to potentially contribute to the development of vascular diseases such as atherosclerosis, hypertension, and aortic aneurysms. Although accumulating data have advanced our understanding of the role of perivascular adipocytes in modulating vascular function, their impact on vascular disease, particularly in humans, remains to be fully defined. This brief review will discuss the mechanisms whereby perivascular adipocytes regulate vascular disease, with a particular emphasis on recent findings and current limitations in the field of research.
Keywords: perivascular adipocyte, adipose tissue, adipokine, inflammation, vascular disease
Graphical Abstract
1. Introduction
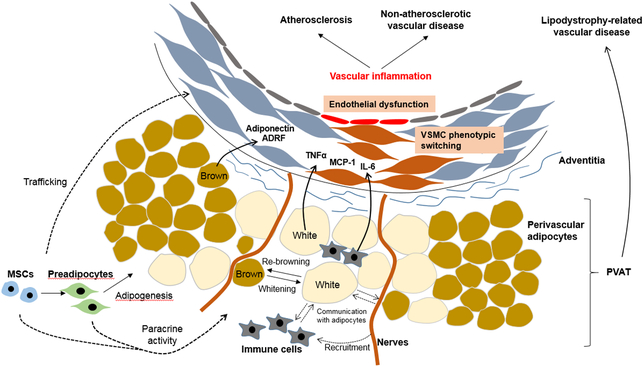
Most blood vessels, except the cerebral vasculature, are surrounded by perivascular (PV) adipose tissue (PVAT), which was long considered to function primarily as a connective tissue to support vascular structure. PVAT is now recognized as a physiologically independent endocrine tissue that maintains vascular homeostasis, and mounting evidence suggests that PVAT becomes dysfunctional under certain disease states, including diet-induced obesity (DIO), wherein it plays a pathogenic role in vascular disease.1 PVAT consists of many types of cells, dominated by mature adipocytes and preadipocytes, and to a lesser extent resident mesenchymal stem cells (MSCs), endothelial cells and inflammatory cells. PVAT-derived MSCs can differentiate into multiple cell lineages, including adipocytes, osteoblasts and endothelial cells. Infiltration of inflammatory cells (e.g. macrophages, T cells) is increased in inflamed PVAT, which likely contributes to the pathogenesis of vascular disease via “outside-in” signaling.
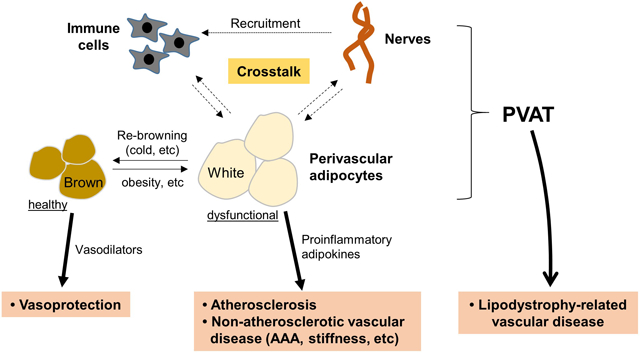
Perivascular adipocytes communicate with other cells in PVAT, and with vascular cells, by directly transferring adipocytokines and by secreting exosomes that contain adipocytokines and other signaling molecules, including microRNAs (miRs). Chemokines such as monocyte chemoattractant protein-1 (MCP-1, also known as CCL2) and RANTES (known as CCL5) produced by PV adipocytes recruit macrophages and other immune cells into PVAT. The sympathetic nervous system was suggested to interact with PV adipocytes and/or preadipocytes, which express receptors for neurocytokines including neuropeptide Y, to modulate PVAT function.2 In physiological conditions, adipocytokines released from PV adipocytes play an important role in regulating vasomotor tone and vascular homeostasis, while dysfunctional PV adipocytes under inflammatory conditions produce cytokines such as tumor necrosis factor-α (TNFα), interleukin-6 (IL-6) and MCP-1 to promote vascular disease. PV adipocytes exhibit significant phenotypic heterogeneity, displaying features of white or brown adipocytes depending on the anatomic location.3 In animal models, brown-like PV adipocytes may exhibit anti-atherosclerotic properties by promoting non-shivering thermogenesis and fatty acid metabolism, but brown-like perivascular adipocytes are observed less frequently in adult humans.4 PV adipocytes surrounding human coronary arteries are primarily white-like, but they are more heterogeneous in shape, smaller in size and exhibit impaired adipogenic differentiation as compared with subcutaneous (SQ) or perirenal adipocytes, suggesting a unique phenotype which may govern their role in vascular disease.5
This review focuses on key concepts pertaining to PV adipocyte biology in vascular function and disease. For general information regarding pathophysiologic function of PVAT, the readers are referred to recent comprehensive review articles.6,7
2. Brief overview of perivascular adipocyte biology
2.1. Developmental origin and regional differences of PV adipocytes
Adipose tissue depots are comprised of progenitor cells that can be traced to unique embryonic lineages, which likely contributes to the specialized functions of the individual depots.8 The origin of PV adipocytes remains to be fully elucidated; however, current evidence primarily obtained in rodent models suggests that these cells may be derived from vascular smooth muscle cell (VSMC) progenitors, neural crest cells, and other unidentified lineages, depending on their precise location along the vascular tree.9-11 The phenotype of PV adipocytes surrounding the thoracic aorta in rodents resembles brown adipocytes, with a multilocular appearance and round nuclei, whereas the abdominal aorta is mainly surrounded by white adipocytes. However, mechanisms whereby these distinct PV adipocyte populations differentially regulate vascular function and pathophysiology are unclear. Human adipocytes from SQ adipose tissue (SQAT) and pericoronary PVAT exhibit distinct gene expression patterns related to both early development and vascular functions.5 For example, engrailed-1 (En-1), the homeodomain transcription factor (Emx-2), and homeobox A10 protein (Hox-A10), genes related to development, are differentially expressed in human PV adipocytes. Functionally, human pericoronary PV adipocytes exhibit increased capacity to attract immune cells and to induce angiogenesis, which suggests a possible role in promoting coronary atherosclerosis.6
2.2. Paracrine activities to regulate vascular function
PV adipocytes secrete factors that can promote either vasoconstriction or vasodilation.12 Under physiological conditions, PV adipocytes primarily exert vasodilatory effects by releasing adipocyte-derived relaxing factor (ADRF), which promotes vasodilation through endothelium-dependent or -independent mechanisms, including activating VSMC potassium channels. In addition, adiponectin produced by PV adipocytes may regulate AMP-activated protein kinase to favorably modulate vascular tone and remodeling. Moreover, omentin secreted from healthy PV adipocytes may exert vasoprotection by inhibiting NADPH oxidase (NOX) activity and NF-κB signaling. Thus, under physiologic conditions, PV adipocytes may promote vascular health and homeostasis. On the other hand, in conditions such as DIO, PV adipocytes become dysfunctional, leading to reduced adiponectin production and nitric oxide (NO) bioavailability, and loss of vasodilatory function. Moreover, proinflammatory cytokines produced by inflamed PVAT during DIO enhance vasoconstriction and impair endothelium-dependent relaxation. These inflammatory cytokines can also promote VSMC proliferation and migration. For example, IL-6 increases the expression of vascular angiotensin II receptor type 1 (AT1) receptors and mediates medial hypertrophy through VSMC phenotype switching, while MCP-1 exerts paracrine effects on VSMC to promote neointimal formation. Using PVAT transplantation coupled with wire injury, Manka et al. demonstrated that MCP-1 locally released by PVAT contributes to neointimal formation in the mouse carotid artery.13 Transplantation of similar quantities of subcutaneous adipose tissue did not significantly impact neointimal formation, pointing to the unique properties of PVAT. MCP-1 expression was increased by 6-fold in PVAT of HFD-fed mice, and transplanted PVAT into carotid artery promoted adventitial macrophage infiltration and angiogenesis. MCP-1 gene deletion abrogated the effects of PVAT transplantation to promote angiogenesis and neointimal formation, suggesting that MCP-1 serves as an ”outside-in” molecular PVAT signal to enhance vasculopathy. However, deletion of MCP-1 in PVAT did not significantly diminish adventitial inflammatory cell recruitment, suggesting that other chemokines secreted from PV adipocytes can efficiently support chemotaxis. Leptin, also produced by PV adipocytes, has multiple opposing effects on vasorelaxation and vasoconstriction under normal conditions and thus appears to have little impact on blood pressure.14 However, in obesity, it promotes hypertension and release of proinflammatory cytokines such as TNFα, IL-6 and IL-12 from macrophages, whereas adiponectin suppresses production of TNFα and interferon-γ. Guzik et al. reported that T cell infiltration in PVAT is increased in response to angiotensin II (AngII), driven in part by RANTES expression in the vascular wall, resulting in hypertension and vascular dysfunction.15 Given that HFD increases RANTES expression in adipose tissue,16 PV adipocyte-derived chemokines could play an important role in T cell recruitment to the vascular wall, suggesting that crosstalk between PV adipocytes and immune cells is an important aspect whereby PVAT regulates vascular function.6
2.3. Interaction with sensory nerves
Like other adipose tissues, PVAT is innervated by the sympathetic nervous system (SNS).17 This SNS produces calcitonin gene-related peptide (CGRP) and other neurotransmitters which can regulate vascular tone. Bakar et al. demonstrated that SNS within PVAT plays an important role in leptin release and vasodilatation of rat mesenteric arteries, suggesting a functional interaction between SNS and PV adipocytes to regulate vasoreactivity.18 In addition, stimulation of sympathetic nerves within mesenteric PVAT may induce the release of noradrenaline, which activates adipocyte β3-adrenoceptors, leading to adiponectin release, suggesting that sympathetic nerve activation promotes PVAT-dependent vasodilation.19 Notably, while SNS activity is chronically increased in obesity, SNS activation in response to acute glucose load is blunted, suggesting this vasodilatory mechanism may be impaired in obese individuals.20 Also, leptin, released from inflamed adipocytes in obesity, can reduce adiponectin productio21 and PVAT anti-contractile function via β3-adrenoceptor internalization in PV adipocytes.18 Although the mechanisms have not been fully elucidated, these data suggest that PV adipocytes and the SNS interact to balance pro- and anti-contractile effects of PVAT.
The adrenergic system in PV adipocytes may also store and release norepinephrine to regulate vascular function. Ahmad et al. reported that, compared to other adipocytes, PV adipocytes are unique in their ability to store norepinephrine through vesicular monoamine transporters.22 Norepinephrine selectively enters PV adipocytes in PVAT through plasma membrane transporters OCT3 (organic cation transporter 3) and NET (norepinephrine transporter) and is subsequently taken up in cytosolic vesicles. These data suggest that multiple adrenergic mechanisms are operative in PV adipocytes which could be modulate vascular function and inflammation. However, whether norepinephrine storage in PV adipocytes is dysregulated in obesity remains to be investigated.
Exercise produces a myriad of beneficial cardiovascular effects that can largely mitigate the detrimental influences of obesity. Many of the beneficial effects of exercise are mediated through the SNS in skeletal muscle and the immune system. Interestingly, exercise also upregulates adrenoceptor expression in adipose tissues while reducing inflammatory cell recruitment and cytokine production, suggesting that exercise may be a promising strategy to reduce PVAT inflammation during DIO.23 Moreover, SNS activation induced by exercise could potentially promote “browning” of PVAT (discussed below). Collectively, these data suggest that crosstalk between SNS and PV adipocytes as well as immune cells plays an important role in regulating PVAT inflammation and vascular function.
2.4. Potential mechanisms of phenotypic switching (whitening and re-browning)
PV adipocytes surrounding thoracic aorta in rodents are phenotypically similar to brown adipocytes, characterized by small size, high mitochondrial content, multilocular lipid droplets and thermogenic gene expression (i.e. UCP-1), whereas abdominal PV adipocytes contain both white and brown adipocytes. On the other hand, mesenteric, carotid, and femoral artery PVAT is dominated by white adipocytes, indicating phenotypic heterogeneity of PV adipocytes depending on their anatomic location.24 Compared to rodent PVAT, phenotype of human PVAT is less well defined. Human PV adipocytes surrounding coronary arteries are white-like but small in size and inefficient at storing lipid, whereas those surrounding radial arteries are larger and exhibit high capacity to form lipid droplets, indicating phenotypic variability of human PV adipocytes depending on the associated vasculature.25
During DIO in rodents, PV adipocytes become more white-like, with a primarily unilocular appearance and large lipid droplets.26,27 While brown-like PV adipocytes, associated with non-shivering thermogenesis and combustion of fatty acids, have favorable metabolic and anti-atherosclerotic effects,9 metabolic diseases such as obesity and diabetes are characterized by PVAT inflammation that may result from PV adipocyte “whitening”.6 Interestingly, both exercise and brown adipocyte inducers (i.e. cold exposure, β-adrenergic stimulation) may enhance “re-browning” of PV adipocytes to restore protective effects on metabolism and vascular function.28,29 Using cold exposure, Pan et al. investigated the mechanisms of PV adipocyte browning. They found that PV adipocyte browning is decreased in aged mice, concomitant with miR-146b-3p downregulation, while loss of miR-146b inhibits PV adipocyte browning in young mice. Aging also reduces brown adipogenic differentiation of resident stromal cells in PVAT via loss of peroxisome proliferator-activated receptor-γ coactivator-1 α (PGC1α). These data suggest that aging potentially regulates PV adipocyte phenotypic switching via specific signaling pathways.30,31 However, the relevance of these pathways to PV adipocyte biology and vascular disease in humans is uncertain.
3. Regulatory role of perivascular adipocytes in vascular disease
3.1. Atherosclerosis
Atherosclerosis is characterized by endothelial dysfunction through reduced nitric oxide (NO) bioavailability, lipid deposition, VSMC proliferation, inflammatory cell infiltration, and vascular inflammation. While inflammatory cells such as macrophages and T cells recruited in PVAT may contribute to perivascular inflammation, mounting evidence indicates that inflamed PV adipocytes also play an important role in the pathogenesis of atherosclerosis. In physiological conditions, adipokines such as adiponectin produced by PV adipocytes can inhibit plaque formation, reduce inflammation, and improve endothelial function through endothelial NO synthase (eNOS) phosphorylation. In addition, thermogenic activity of healthy PV adipocytes can increase vascular lipid clearance. Chang et al. reported that SMC-selective PPARγ (SMPG) KO mice, which selectively lack PVAT, but not white or brown adipose tissues (WAT or BAT), exhibited impaired thermogenic activity and endothelial function. Moreover, cold exposure inhibited atherosclerosis in mice with intact PVAT, but not in SMPG KO mice, suggesting a potentially protective role of PVAT in atherosclerosis.10 However, during high fat feeding under ambient temperature, inflamed and dysfunctional PV adipocytes lose these anti-atherosclerotic properties, releasing less adiponectin and more proinflammatory adipokines such as IL-6, TNFα and MCP-1.
Fat transplantation in mice is a commonly employed method to investigate the role of PVAT in atherosclerosis, and linkages between local PVAT and atherosclerosis development have been experimentally demonstrated by several investigators using this model. Öhman et al. showed that transplantation of visceral adipose tissues isolated from obese mice increased endothelial dysfunction and atherosclerosis in recipient low density lipoprotein receptor (LDLR) knockout (KO) mice,32 suggesting a pro-atherogenic role in obesity. Mechanistically, Li et al. reported that adiponectin/apolipoprotein E (apoE) double KO mice exhibited increased perivascular inflammation and atherosclerosis development,33 indicating that adiponectin is a key regulator of PVAT inflammation in atherosclerosis. Because the transplanted adipose tissues also contain immune cells, the specific role of PV adipocytes is difficult to ascertain. Thus, developing more specific in vivo experimental models to examine the precise mechanisms whereby PV adipocytes regulate atherosclerosis and other vascular diseases is required in the future.
3.2. Remote effects on endothelial dysfunction and atherosclerosis
Proximity of PV adipocytes to vascular adventitia facilitates biotransfer of their adipocytokines to the vascular wall. Thus, local inflammation triggered by dysfunctional PV adipocytes is presumed to be the predominant mechanism whereby PV adipocytes contribute to the pathogenesis of atherosclerosis. However, human pericoronary perivascular adipocytes were reported to secrete significantly more pro-inflammatory cytokines compared with adipocytes derived from other depots in the same patients; in particular, PV adipocytes secreted approximately 50-fold more MCP-1 compared with perirenal adipocytes and 3-fold more MCP-1 than omental adipocytes.34 Thus, it is possible that accumulation of dysfunctional PV adipocytes could conceivably contribute to systemic inflammation in obesity, thereby augmenting endothelial dysfunction and atherosclerosis at remote sites. This hypothesis was tested by Horimatsu et al., who demonstrated that transplanting 50 mg of PVAT (but not SQ or epididymal adipose tissue) harvested from DIO mice onto the abdominal aorta of recipient LDLR KO mice augmented endothelial dysfunction and atherosclerosis in the remote thoracic aorta. These effects were independent of body weight, plasma lipid levels, body composition and insulin sensitivity, and associated with vascular inflammation, suggesting a systemic inflammatory effect of PVAT in vascular disease.35 Further in-depth studies are required to elucidate the extent to which endogenous PVAT promotes systemic vascular disease, particularly in humans.
3.3. Non-atherosclerotic vascular disease
While the role of PVAT or PV adipocytes in the pathogenesis of atherosclerosis has been extensively investigated, their role in non-atherosclerotic vascular diseases, including neointimal hyperplasia, abdominal aortic aneurysm (AAA), and arterial stiffness and vasculitis, has received less attention. However, accumulating data in humans and experimental animal models suggest that dysfunctional PVAT or PV adipocytes may be potentially linked to these diseases, which are also commonly characterized by vascular wall inflammation, oxidative stress, VSMC phenotypic switching and neovascularization.
Gao et al. reported that HFD feeding increases AAA formation through eNOS uncoupling in PVAT in obese mice,36 suggesting a role for PVAT dysfunction during DIO in AAA formation. Likewise, transplanting VAT onto the abdominal aorta of ApoE KO mice promoted AAA formation via angiotensin II type 1a (AT1a) receptor signaling pathway,37 suggesting that adipose tissue surrounding the aorta play an important role in AAA pathogenesis; however, as in many prior studies, authentic PVAT was not employed in the transplantation protocol. It has also been reported that platelet-derived growth factor-D (PDGF-D), highly expressed in PVAT of obese mice, promotes AAA formation.38 Furthermore, RANTES expressed on adipocytes increases T cell infiltration which also contributes to AAA formation,15,16 suggesting that crosstalk between PV adipocytes and immune cells plays an important role in the pathogenesis of AAA.
Data in humans show that aortic stiffness is positively correlated with the quantity of PVAT, which is independent of body-mass index,39 and PVAT-derived IL-6 is associated with an increase in aortic stiffness and pulse wave velocity in humans.40,41 Several studies also suggest an association between PVAT and vasculitic syndromes, including Takayasu arteritis (TA), which is characterized by prominent granuloma accumulation in large elastic arteries. TA patients exhibit higher levels of leptin and resistin, which are associated with increased expression of inflammatory markers such as pentraxin-3.42
Taken together, these data support a linkage between PVAT and non-atherosclerotic vascular diseases; however, definitive evidence is lacking due to limitations in the currently available animal models. Importantly, most prior studies focused on the role of inflammatory cells rather than adipocytes in PVAT, so the specific contribution of PV adipocytes to these diseases remain to be determined. For additional information, the reader is referred to a recent review article pertaining to the role of PVAT in non-atherosclerotic vascular disease.43
3.4. Potential linkage with lipodystrophy-related vascular disease
Lipodystrophy, a disease state in which the amount of adipose tissue is severely diminished, promotes insulin resistance and hypertension, which is paradoxically similar to that typically seen in obese patients with excess adiposity. These metabolic and vascular disorders resulting from either excess or insufficient adipose tissue underscore the important physiological role of adipocytes. Given that PV adipocytes are adjacent to vascular adventitia and play regulatory roles in vasoreactivity and hypertension, it has been speculated that hypertension in lipodystrophic patients may be attributed to reduction or absence of adipocytes around resistance vessels. Ablation of adipose tissues in experimental animal models produced severe insulin resistance, dyslipidemia and hepatic steatosis, providing a model to study the systemic cardiovascular effects of lipodystrophy. Takemori et al. investigated the role of PVAT in vascular function using lipoatrophic A-ZIP/F1 transgenic mice, which have no WAT and dramatically reduced PVAT and BAT.44 Interestingly, these lipoatrophic mice exhibited insulin resistance and hypertension, which was linked to the absence of PVAT and upregulated expression of AT1 receptors. SMPG KO mice are completely devoid of PVAT, but have normal WAT and BAT and also display hypertension and increased arterial stiffness.10,45 On the other hand, Kugo et al. reported that hypoperfusion-induced AAA formation in rats was diminished by removal of PVAT, in conjunction with decreased number of MSCs in vascular wall.46 These data suggest that PVAT atrophy in lipodystrophic states could impact vascular function and disease states in complex ways via several mechanisms, elucidation of which may advance our understanding of hypertension, vascular stiffness and remodeling, metabolic disease, etc.
4. Clinical implications: perivascular adipose tissue imaging for detection or prediction of vascular disease
Temporal and spatial development of aortic PVAT and luminal plaque in mice can be imaged by micro computed tomography (CT), a high resolution technique that enables co-localization of atherosclerotic lesions with PVAT. Using this method, Faight et al. reported that in ApoE KO mice fed a high fat diet for 30 weeks, plaque development co-localized with luminal ostia and origins of branching arteries which traveled through the greatest areas of PVAT volume.47 In humans, PVAT also can be monitored and quantified using CT scanning and MRI. Moreover, biological functions of PVAT may be inferred from the CT imaging data. Antonopoulos et al. employed the CT fat attenuation index (FAI), an indicator of adipocyte lipid mass, which can be obtained from routine coronary CT angiograms, to quantify coronary PVAT inflammation.48 The FAI correlated with PV adipocyte size and lipid content in adipose tissues procured from the same patients. Moreover, FAI was higher in PVAT surrounding unstable plaques, and post-hoc analysis of outcome data obtained from consecutive patients undergoing angiography revealed that high PV FAI values were predictive of cardiovascular mortality,49 suggesting that imaging PVAT is a promising, noninvasive method for monitoring vascular inflammation and predicting coronary events. This methodology has been implemented in a large study to noninvasively detect plaque instability in the human coronary vasculature. Besides using imaging of PVAT to predict future risk of myocardial infarction, Ohyama et al. reported that CT coronary angiography is a useful approach to assess coronary perivascular inflammation and disease activity in patients with vasospastic angina,50 which may be exacerbated by inflammatory cytokines produced by PVAT. Furthermore, Dou et al. reported that inflammatory mediators produced in adipose tissues may be linked to local and remote (coronary) microvascular dysfunction in aged obese patients, suggesting the potential role of adipose tissues in regulating systemic vascular function.51 These emerging data suggest that imaging PVAT may help in assessment and therapeutic management of patients with diverse forms of cardiovascular disease.
5. Perspectives
Since the vasoactive effects of PVAT were first reported in 1991, extensive studies have been conducted to identify the mechanisms whereby PV adipocytes regulate vascular function and disease (Figure). While much is now known, due to the limitations of current experimental models, many questions remain unanswered to advance our understanding of the function of these unique adipocytes surrounding the vascular wall. The mechanisms that control PV adipocyte phenotype switching and communication with other cell types and systems (i.e. inflammatory cells, progenitor cells, SNS) to modulate vascular disease remain to be delineated. Also, whether and how PVAT-resident progenitor cells/preadipocytes contribute to vascular function and disease is largely unknown. Establishing more robust animal models is clearly required to answer these questions in the future. Nevertheless, accumulating data from experimental mice and humans suggest that PV adipocytes are not only important modulators of vascular function, but may also function as predictors of vascular disease and possible targets for therapeutic intervention.
Figure. Proposed mechanisms by which PV adipocytes modulate vascular function and disease.
PV adipocytes exhibit significant phenotypic heterogeneity, displaying features of white or brown adipocytes. PV adipocytes become more white-like during DIO, and exercise or brown adipocyte inducers (i.e. cold exposure, β-adrenergic stimulation) may enhance “re-browning” of PV adipocytes to restore protective effects on metabolism and vascular function. PV adipocytes communicate with other cells such as immune cells or nerves in PVAT and with vascular cells by directly transferring adipocytokines, miRNAs, etc. PV: perivascular, PVAT: perivascular adipose tissue, MSC: mesenchymal stem cell, ADRF: adipocyte-derived relaxing factor, TNFα: tumor necrosis factor-α, IL-6: interleukin-6, MCP-1: monocyte chemoattractant protein-1.
Highlights.
PV adipocytes play a fundamental role in vascular homeostasis. However, they become dysfunctional during diet-induced obesity, which disrupts the balance between pro- and anti-inflammatory adipocytokine secretion.
Accumulating data suggest that PV adipocytes play an important role not only in atherosclerosis and hypertension, but also in non-atherosclerotic vascular diseases. However, direct evidence of their involvement is lacking due to technical limitations of current experimental animal models.
While the bulk of published studies have focused on the local paracrine impact of PV adipocytes on the vascular wall, dysfunctional PV adipocytes may also be capable of promoting endothelial dysfunction and atherosclerosis remotely via their robust capacity to secrete pro-inflammatory adipocytokines into the systemic circulation.
Assessing PVAT volume and PV adipocytes size by CT imaging is a promising method for monitoring vascular inflammation and predicting the occurrence of vascular events.
Acknowledgments
Sources of funding: this study was funded by grants HL124097, HL126949, HL134354 AR070029 (N.L.W) and HL130301, HL147639 (E.J.BC) from the National Institutes of Health.
Abbreviations
- AAA
abdominal aortic aneurysm
- apoE
apolipoprotein E
- BAT
brown adipose tissue
- CCL
chemokine (C-C motif) ligand
- CT
computed tomography
- DIO
diet-induced obesity
- eNOS
endothelial NO synthase
- FAI
fat attenuation index
- IL-6
interleukin-6
- KO
knockout
- LDLR
low density lipoprotein receptor
- MCP-1
monocyte chemoattractant protein-1
- miR
microRNA
- MSC
mesenchymal stem cells
- PV
perivascular
- PVAT
perivascular adipose tissue
- SNS
sympathetic nervous system
- SQ
subcutaneous
- TNFα
tumor necrosis factor-α
- VSMC
vascular smooth muscle cell
- WAT
white adipose tissue
Footnotes
Disclosure
None.
References
- 1.Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3:e000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxton SN, Withers SB, Heagerty AM. Emerging roles of sympathetic nerves and inflammation in perivascular adipose tissue. Cardiovasc Drugs Ther. 2019;33:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, Unruh D, Blomkalns AL, Piegore MG Jr, Weintraub DS, Rudich SM, Kuhel DG, Hui DY, Weintraub NL. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosalski R, Guzik TJ. Perivascular adipose tissue inflammation in vascular disease. Br J Pharmacol. 2017;174:3496–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrand S, Stümer J, Pfeifer A. PVAT and its relation to brown, beige, and white adipose tissue in development and function. Front Physiol. 2018;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physio Endocrinol Metab. 2007;292:E298–E307. [DOI] [PubMed] [Google Scholar]
- 9.Cai X, Lin Y, Hauschka PV, Grottkau BE. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103:435–447. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012:126:1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu M, Xu L, Chen X, Han W, Ruan C, Li J, Cai C, Ye M, Gao P. Neural crest cells differentiate into brown adipocytes and contribute to periaortic arch adipose tissue formation. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119.312838. [DOI] [PubMed] [Google Scholar]
- 12.Villacorta L, Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig. 2015;21:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manka D, Chatterjee TK, Stoll LL, Basford JE, Konaniah ES, Srinivasan R, Bogdanov VY, Tang Y, Blomkalns AL, Hui DY, Weintraub NL.Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1. Arterioscler Thromb Vasc Biol. 2014;34:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltowski J Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789–801. [DOI] [PubMed] [Google Scholar]
- 15.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. [DOI] [PubMed] [Google Scholar]
- 17.Bulloch JM, Daly CJ. Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther. 2014;143:61–73. [DOI] [PubMed] [Google Scholar]
- 18.Abu Bakar H, Robert Dunn W, Daly C, Ralevic V. Sensory innervation of perivascular adipose tissue: a crucial role in artery vasodilatation and leptin release. Cardiovas Res. 2017;113:962–972. [DOI] [PubMed] [Google Scholar]
- 19.Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM. Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2018;38:880–891. [DOI] [PubMed] [Google Scholar]
- 20.Straznicky NE, Lambert GW, Masuo K, Dawood T, Eikelis N, Nestel PJ, McGrane MT, Mariani JA, Socratous F, Chopra R, Esler MD, Schlaich MP, Lambert EA. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am J Clin Nutr. 2009;89:27–36. [DOI] [PubMed] [Google Scholar]
- 21.Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7:2346–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad MF, Ferland D, Ayala-Lopez N, Contreras GA, Darios E, Thompson J, Ismail A, Thelen K, Moeser AJ, Burnett R, Anantharam A, Watts SW. Perivascular adipocytes store norepinephrine by vesicular transport. Arterioscler Thromb Vasc Biol. 2019;39:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxton SN, Withers SB, Heagerty AM. Emerging roles of sympathetic nerves and inflammation in perivascular adipose tissue. Cardiovasc Drugs Ther. 2019;33:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Circ Physiol. 2011;301:H1425–H1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol. 2014;34:1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2014;62:1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gálvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197:55–64. [DOI] [PubMed] [Google Scholar]
- 28.Boa BCS, Yudkin JS, van Hinsbergh VWM, Bouskela E, Eringa EC. Exercise effects on perivascular adipose tissue: endocrine and paracrine determinants of vascular function. Br J Pharmacol. 2017;174:3466–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bussey CE, Withers SB, Saxton SN, Bodagh N, Aldous RG, Heagerty AM. β3‐Adrenoceptor stimulation of perivascular adipocytes leads to increased fat cell‐derived NO and vascular relaxation in small arteries. Br J Pharmacol. 2018;175:3685–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan XX, Cao JM, Cai F, Ruan CC, Wu F, Gao PJ. Loss of miR-146b-3p inhibits perivascular adipocyte browning with cold exposure during aging. Cardiovasc Drugs Ther. 2018;32:511–518. [DOI] [PubMed] [Google Scholar]
- 31.Pan XX, Ruan CC, Liu XY, Kong LR, Ma Y, Wu QH, Li HQ, Sun YJ, Chen AQ, Zhao Q, Wu F, Wang XJ, Wang JG, Zhu DL, Gao PJ. Perivascular adipose tissue‐derived stromal cells contribute to vascular remodeling during aging. Aging cell. 2019;18:e12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Öhman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Wang Z, Wang C, Ma Q, Zhao Y. Perivascular adipose tissue-derived adiponectin inhibits collar-induced carotid atherosclerosis by promoting macrophage autophagy. PLoS One. 2015:10:e0124031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL., Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horimatsu T, Patel AS, Prasad R, Reid LE, Benson TW, Zarzour A, Ogbi M, Bruder do Nascimento T, Belin de Chantemele E, Stansfield BK, Lu XY, Kim HW, Weintraub NL. Rremote effects of transplanted perivascular adipose tissue on endothelial function and atherosclerosis. Cardiovasc Drugs Ther. 2018;32:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao L, Siu KL, Chalupsky K, Nguyen A, Chen P, Weintraub NL, Galis Z, Cai H. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation. Hypertension. 2012;59:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakaue T, Suzuki J, Hamaguchi M, et al. Perivascular adipose tissue angiotensin ii type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension. 2017;70:780–789. [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZB, Ruan CC, Lin JR, Xu L, Chen XH, Du YN, Fu MX, Kong LR, Zhu DL, Gao PJ. Perivascular adipose tissue–derived PDGF-D contributes to aortic aneurysm formation during obesity. Diabetes 2018;67:1549–1560. [DOI] [PubMed] [Google Scholar]
- 39.Britton KA, Wang N, Palmisano J, Corsini E, Schlett CL, Hoffmann U, Larson MG, Vasan RS, Vita JA, Mitchell GF, Benjamin EJ, Hamburg NM, Fox CS. Thoracic periaortic and visceral adipose tissue and their cross-sectional associations with measures of vascular function. Obesity. 2013;21:1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, Levy D, Keaney JF Jr, Vasan RS, Mitchell GF, Benjamin EJ. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51:1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du B, Ouyang A, Eng JS, Fleenor BS. Aortic perivascular adipose-derived interleukin-6 contributes to arterial stiffness in low-density lipoprotein receptor deficient mice. Am J Physiol Heart Circ Physiol. 2015;308:H1382–H1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. [DOI] [PubMed] [Google Scholar]
- 43.Horimatsu T, Kim HW, Weintraub NL. The role of perivascular adipose tissue in non-atherosclerotic vascular disease. Front Physiol. 2017;8:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemori K, Gao YJ, Ding L, Lu C, Su LY, An WS, Vinson C, Lee RM. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007;49:365–372. [DOI] [PubMed] [Google Scholar]
- 45.Chang L, Zhao X, Garcia-Barrio M, Zhang J, Chen YE. MitoNEET in perivascular adipose tissue prevents arterial stiffness in aging mice. Cardiovasc Drugs Ther. 2018;32:531–539. [DOI] [PubMed] [Google Scholar]
- 46.Kugo H, Moriyama T, Zaima N. The role of perivascular adipose tissue in the appearance of ectopic adipocytes in the abdominal aortic aneurysmal wall. Adipocyte. 2019;8:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faight E, Verdelis K, Ahearn JM, Shields KJ. 3D MicroCT spatial and temporal characterization of thoracic aorta perivascular adipose tissue and plaque volumes in the ApoE−/−mouse model. Adipocyte. 2018;7:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9:eaal2658. [DOI] [PubMed] [Google Scholar]
- 49.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018:392:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohyama K, Matsumoto Y, Takanami K, et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol. 2018;71:414–425. [DOI] [PubMed] [Google Scholar]
- 51.Dou H, Feher A, Davila AC, Romero MJ, Patel VS, Kamath VM, Gooz MB, Rudic RD, Lucas R, Fulton DJ, Weintraub NL. Role of adipose tissue endothelial ADAM17 in age-related coronary microvascular dysfunction. Arterioscler Thromb Vasc Biol. 2017;37:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]