Abstract
Social context is increasingly recognized as essential for understanding complex human outcomes even among geneticists who focus on genetic influences. These outcomes typically involve multiple genes, multiple environmental factors, and the interactions between the two. In this paper, we propose a conceptual framework for gene-environment interaction and show how the interaction can be tested empirically using a sample of MZ twin, DZ twins, and full siblings. We test the hypothesis that the genetic contribution to adolescent drinking depends on the drinking behavior of their friends, using a sample of clusters of siblings and their friends from Add Health. Our analysis has yielded evidence supporting the gene-environment interaction hypothesis. High levels of alcohol use by one’s best friend or among one’s friends tend to bring about higher levels of genetic contribution to alcohol use. Lower levels of alcohol use by one’s best friend or among one’s friends tend to suppress the level of genetic contribution to alcohol use. Our findings suggest that friend behavior might be a particularly important environmental moderator of the expression of genetic disposition for adolescent drug use, smoking, dietary habits, and risky sexual behavior. Subsequent studies of these behaviors that use non-DNA twin samples or DNA measures of genetic variants should investigate peer influence as a significant environmental moderator.
INTRODUCTION
Decades of efforts in molecular genetics have discovered more than a thousand genes responsible for Mendelian human outcomes—outcomes mostly determined by alleles of a single gene (Risch 2000; Botstein and Risch 2003). Examples of such human outcomes include Huntington’s disease, cystic fibrosis, hereditary non-polyposis colon cancer, and heritable breast cancers. These diseases are rare in human populations and therefore explain relatively small portions of overall disease prevalence.
Molecular genetic efforts have been much less successful on Non-Mendelian or complex human outcomes. Many of these outcomes, including reading disability, smoking, alcohol use, drug use, and obesity, are of interest to sociologists. The links between genetic heritage and complex human outcomes are enormously complicated, typically involving multiple genes, environmental factors, and the interaction between the two.
There has been an increasing recognition that social scientists’ expertise in social context is indispensable for understanding many of complex human outcomes (Caspi et al 2002, 2003). The success of the Human Genome Project (Collins et al 2003a) and the HapMap Project1 (The International HapMap Consortium 2003, 2005) is improving the design and effectiveness of genetic studies. These advances, however, do not lessen the need to understand the environmental part of the puzzle. On the contrary, inadequate understanding of environment has increasingly become the bottleneck for the rapid technological advances in molecular genetics. Recently, the HapMap project (The International HapMap Consortium 2005) and the National Human Genome Research Institute (Collins et al 2003b) called for heavy investment in lifestyle factors and environmental exposures and in longitudinal studies of adequate size that would obtain such information.
In addition to contributing to work that focuses on genetic influences, sociologists may also be interested in incorporating the advances in molecular genetics into sociological thinking. Genes may be an important component of outcomes relevant to sociologists. Taking genetic heritage into account promises a fuller understanding of social outcomes and a more precise understanding of the roles of social context.
The objective of this study is two fold. First, we develop a conceptual framework for gene-environment interactions and indicate how the interaction can be tested empirically using a sample of MZ twins, DZ twins, and full siblings. Second, we apply this framework to test the hypothesis that the genetic contribution to alcohol use among adolescents depends on the drinking behavior of their friends. Data come from a sample of clusters of siblings as well as their friends collected by the National Longitudinal Study of Adolescent Health (Add Health) (Harris et al. 2003).
BACKGROUND
Adolescent Drinking
Alcohol consumption among the young remains a major public health problem in the United States (Johnston, et al, 2003). The project Monitoring the Future, a nationally representative survey of 8th, 10th, and 12th graders, reveals a high prevalence of alcohol use and a trend toward earlier onset. Nearly 30 percent of 8th graders reported consuming alcohol in the previous 30 days, and 7 percent reported having been drunk during that time. About 29 percent of 12th graders reported binge drinking (i.e. having five or more drinks in a row) during the two-week period prior to the study. More noteworthy, the trend shows the onset of alcohol use at younger ages. Between 1987 and 1996, the average age at onset decreased from about 18 to 16 years by 1996 (Office of National Drug Control Policy, 1997). In 1999, a third of youth surveyed reported beginning to drink before the age of 13 (CDC, 2000).
The initiation of alcohol use prior to age 15 is associated with an increased risk of alcohol-related problems later in life (Grant & Dawson, 1997). Alcohol abuse among adolescents is correlated with a variety of other risk behaviors, such as poor school performance, substance abuse, sexual activity, violence, delinquency, drinking and driving, tobacco use, and suicide (Windle, 1999; 2003). Heavy drinking is associated with Type II diabetes (e.g., Wannamethee, Shaper, Perry, & Alberti, 2002) and coronary artery disease, cardiac arrhythmias, and stroke, among other disorders (Puddey, Rakic, Dimmitt & Beilin, 1999). Heavy drinking is also linked to morbidity and mortality through an increased risk of accidents (e.g., Hingson & Howland, 2002).
Sociological Theories of Peer Influence, Genes, and Adolescent Drinking
‘Imitation’ is a significant theme in Durkheim’s classic Suicide (1897/1951) and defined as the social process spreading unstable and transitory social currents. Although Durkheim (1897/1951: 138–142) did not find empirical support for it as an explanation for suicide, he recognized imitation as a potentially potent force influencing social behavior. Peer influence occupies a particularly important place in Coleman’s The Adolescent Society (1961) in which he focused on the leading crowd, or the most popular students, in a school who both reflect and influence the normative climate of the school. Durkheim’s imitation corresponds closely to a prominent and long-standing theoretical tradition in the sociology of crime and deviant behavior (Allen and Wilder 1973; Festinger 1954; Akers 1977, 1997; Sutherland and Cressey 1984). A number of mechanisms within this tradition link peer influence to individual behavior, but the main argument is that associating with delinquent peers breeds delinquency.
The empirical evidence linking peer delinquent behavior and ego delinquent behavior such as drinking, substance abuse, and sexual behavior is among the strongest and the most widely reported in the social sciences (Kandel 1975; Brown and Theobald 1999; Billy, Rodgers, and Udry 1984; Billy and Udry 1985; Ennett and Bauman 1994; Yamaguchi and Kandel 1987; Haynie 2001; Haynie 2002; Haynie and Osgood 2005; Warr 1993; Warr and Stafford 1991). Peers and friends are particularly important for adolescents who spend twice as much time each week with peers outside the family as do they with parents (Brown 1990). One well-known position argues that socialization mainly takes place in the peer groups of childhood and adolescence and in comparison parents do not have any important long-term effects on child development (Harris 1995). All of the contemporary social theories on peer influence and delinquency emphasize social proximity between the individual and his or her peers. However, an individual is not equally influenced by everyone in the population. The amount of influence depends on the strength of the social ties involved and most theories predict the largest influence from the best friend. In our analysis, we focus on the drinking behavior of best friend as a critical source of peer influence.
Twin and adoption studies have indicated the presence of genetic influences on various aspects of alcohol use and dependence, explaining about 50–60% of the variance in the alcohol measures (McGue, 1999; Tyndale 2003; Dick and Foroud 2003). Twin studies have shown that genetic factors play an important role in the initiation of drinking, early alcohol use, frequency of intoxication, frequency of alcohol consumption, average quantity consumed when drinking, the observed longitudinal stability in frequency and in quantity of alcohol consumed per drinking occasion, alcohol metabolism measures such as time to peak blood alcohol concentration, and alcohol dependence (Heath 1995). Efforts to identify specific candidate allelic variants have focused on polymorphisms in the ALDH genes and the ADH genes and polymorphisms in the dopaminergic system, the GABAergic system, and serotonergic system (Tyndale 2003; Dick and Foroud 2003).
Peer influence may strengthen or moderate the genetic disposition of youth for alcohol abuse. Twin studies show that about half of the variance in the alcoholism-related measures is attributable to environmental factors indicating the crucial role environments play in alcoholism. Certain environments may promote the expression of genetic predispositions for adolescent drinking whereas others may suppress it.
Gene-Environment Interactions
Most social scientists are familiar with the concept of statistical interaction in a regression context. The following regression equation estimates the effects of a college degree, age, and their interaction on income:
Without the interaction term, β1 would represent the additional income a college degree holder has over someone without a college degree; this effect would be the same at all ages. The interaction term allows the effect of college degree depends on age. It is plausible that the advantage from a college degree increases over age (experience). If the interaction term is significant, the effect of a college degree will be age-specific and calculated from β1 and β3. The dependence between a college degree and age is mutual, that is, the interaction also implies that the age effect depends on a college degree.
The concept of a gene-environment interaction (Kendler et al. 2005) is similar to the above example in the sense that the effect of a gene depends on an environment and vice versa; but it does not have to be expressed by a statistical interaction term in a regression model. Phenylketonuria or PKU is a classic case in point (Hunter 2005). Children having two doses of the defective gene from mothers and fathers are affected. In healthy children, a specific enzyme converts phenylalanine to a substance called tyrosine. In affected children, the enzyme is either missing or defective because of the defective gene. Therefore, the children have too little tyrosine and too much phenylalanine in their bodies and the excess of phenylalanine would cause brain damage in all these patients. However, patients who are treated from early infancy with a low-phenylalanine diet escape brain damage and usually develop intelligence and behavior patterns within the normal range. In this case, PKU can be successfully treated by altering the environment in spite of the defective genes the children receive before birth.
Studies based on animal experiments have also provided compelling evidence for such interactions. For example, Bennett et al. (1998) found interactions between rearing methods and the serotonin transporter gene (5-HTT) with respect to a number of behavior indicators among rhesus monkeys. The 5-HTT is a candidate gene for impaired serotonergic functions with each monkey possessing either the short (LS) or the long (LL) 5-HTT allele. The adult rhesus monkeys under study were genotyped regarding the 5-HTT polymorphism. Some of these monkeys were peer-reared for their first six months while the others were reared by their biological mothers. Bennett et al. (1998) reported dramatically different consequences of having the LS allele between peer-reared and mother-reared monkeys. While peer-reared subjects with the LS allele showed deficits in serotonin metabolism and excessive alcohol consumption, mother-reared monkeys with the same allele displayed normal serotonin metabolism, lowered risk for excessive alcohol consumption, and high social dominance. These results suggest the short allele of the 5-HTT gene may well result in psychopathology among monkeys with inadequate early rearing, but may be adaptive among subjects with mother rearing.
Influential social-science examples come from recent work by Caspi et al. (2002; 2003). The 2002 article investigated the role of genotype in violent behavior among maltreated children. Boys who were maltreated early in life are at risk of becoming violent offenders. But not all children respond to maltreatment in the same way. The study found that a functional polymorphism2 in the gene encoding the neurotransmitter-metabolizing enzyme monoamine oxidase A (MAOA) modifies the effect of maltreatment. Only maltreated children with a genotype generating low levels of MAO A expression tended to develop the violent behavior problem. Maltreated children with a genotype that produces high levels of MAO A activity were less affected. Some of Capsi et al.’s work (2003) has been replicated (Kim-Cohen et al. 2006) and others (Caspi et al. 2002) has not (Haberstick et al. 2005).
Numerous studies have attempted to detect gene-environment interactions using twin or immigration data in the absence of molecular genetic measures. Guo and Stearns (2002) investigated how family SES affects the realization of genetic potential for intellectual development using the sibling data from the Add Health. They showed that parental unemployment and the racial status of African Americans have a statistically significant effect on the proportional contributions of heritability and shared environment. A disadvantaged environment appears to have prevented African American children from fully realizing their genetic potential for intellectual development.
Turkheimer et al (2003) analyzed scores on the Wechsler Intelligence Scale in a sample of 7-year-old twins from the National Collaborative Perinatal Project. The analyses allowed for the additive effects of genotype, shared environment, and nonshared environment to interact with socioeconomic status. Results demonstrated that the proportions of IQ variance attributable to genes and environment vary with SES. The models suggest that in impoverished families, 60% of the variance in IQ is accounted for by the shared environment, and the contribution of genes is close to zero; in affluent families, the result is almost exactly the opposite.
Using an immigration design, studies have found that because of lifestyle and diet, U.S.-born Asians are twice as likely as Asian immigrants to suffer from prostate cancer (Cook et al. 1999). Other studies have shown that Asian American adolescents born in the U.S. are more than twice as likely to be obese as are adolescents who immigrated into the U.S. recently (Popkin and Udry 1998). Assuming that the genetic heritage between Asian immigrants and US-born Asians is comparable, then the observed differences between the two groups might be reasonably attributed to the different environments in their early life.
METHODS
Twin Studies
The twin study is based on two observations. First, monozygotic (MZ) and dizygotic (DZ) twins share different portion of amount of DNA (genes). MZ twins grow from a single fertilized ovum and genetically, they are identical. DZ twins grow from two different ova and they share, on average, 50% of their DNA, the same as full siblings. Second, MZ and DZ twins are assumed to share the same amount of environmental influences. The twin study enables us to separate the relative importance of genetic and environmental influences. Under the twin research design, if MZ twins on average appear to be more similar than DZ twins with regard to a trait, researchers conclude that the trait is influenced by genetic effects. The within-pair similarity is often measured by a correlation, which is a standardized within-pair covariance.
The twin research design is based on two assumptions. The equal environments assumption requires that the environments for identical twins are no more similar than the environments for fraternal twins. If the environments for identical twins are more similar and thus make them more similar than fraternal twins, genetic influences would be overestimated. More similar treatment of identical twins in certain aspects of life (e.g., dressing alike), however, does not disqualify the twin design automatically. What is crucial is whether the special way identical twins are treated might affect the outcome of interest, which is drinking in this particular analysis. For example, if dressing alike or unalike does not affect drinking, dressing more alike in identical twins will not alter the results of a study focusing on adolescent drinking. In our analysis, the assumption seems reasonable that parental influences such as the availability of alcohol in the home do not differ between the two types of twin pairs.
The second assumption assumes little or no assortative mating, which refers to the tendency that like marry like with respect to personal traits such as height, intelligence, and personality. Children of assortative mating parents would be more likely to receive the same genes for some traits than children of non-assortative mating parents. Consequently, assortative mating would exaggerate genetic similarity for fraternal twins, but it would not affect genetic similarty for identical twins. In a twin study, a violation of the assumption would deflate the estimated genetic influence, which is calculated as the difference between the similarity for identical twin pairs and the similarity for fraternal twin pairs. The violations of the equal environments assumption and the assumption of assortative mating would thus have opposite effects and tend to cancel each other to a certain extent.
A Design for Assessing Gene-Friends Interaction for Alcohol Use
Ideally, we would like to observe pairs of siblings in one of the three types of environments: (Type 1) an alcohol-prevalent environment in which alcohol is easily available and alcohol consumption among adolescents are accepted; (Type 2) an alcohol-free environment in which alcohol is unavailable and alcohol consumption among adolescents are deemed unacceptable; and (Type 3) an environment in which one sibling in a sib pair is subject to the influence from an alcohol-prevalent environment and the other sibling in the pair is subject to the influence from an alcohol-free environment.
Because alcohol is easily available and routinely used by people with whom they closely associate, including their peers, adolescents in such an alcohol-prevalent environment (Type 1) should have a much better chance to develop their genetic predisposition for alcohol use. In an alcohol-free environment (Type 2), adolescents with a genetic predisposition for alcohol use will have a greater difficulty in expressing the predisposition. In an alcohol-free environment, the adolescents’ ability to ‘niche-pick’ or find environments suitable for their genetic tendencies for alcohol use is lower than in an alcohol-prevalent environment. A discordant environment (Type 3), with two opposing forces, would tend to pull the two twins in opposite directions, thus suppressing genetic manifestation for one twin, facilitating it for the other twin, and producing a lower estimate of heritability than that in the Type-1 environment. When the predisposition for alcohol use is more easily expressed, we expect identical twins to be more similar to each other than fraternal twins in alcohol consumption, leading to a higher estimate of heritability.
The heritability expressed in an alcohol-prevalent environment may be taken as a ‘standard’ or ‘baseline’, against which the heritability expressed in an alcohol-free environment can be compared. The difference between the two “expressed” heritabilities or the “unexpressed” portion of the heritability in an alcohol-free environment is an estimate of how much of a genetic predisposition for alcohol use has been suppressed. The assumption for this reasoning is that the heritability estimate should be about the same in all environments in the absence of gene-environment interactions. Then the difference in the estimate between two environments may reflect an environmental moderation of genetic influences in one of the environments. This reasoning lies behind all studies of gene-environment interactions when DNA measures are unavailable.
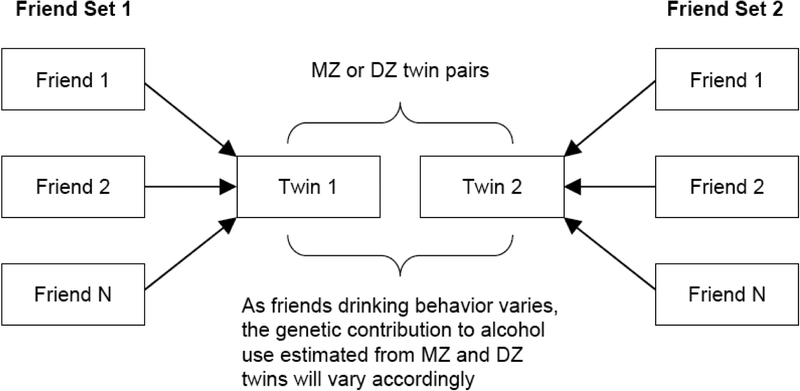
To apply this reasoning to our analysis, we examined the genetic contribution to adolescent alcohol use in three types of friend influence: concordant/high, concordant/low, or discordant (Table 1). The first two types of influence correspond to an alcohol-prevalent environment and an alcohol-free environment, respectively. In the two concordant types, the friends of twin 1 and twin 2 exhibit similar patterns of drinking behavior. In the concordant-high type, both sets of friends within each pair of twins have a relatively high-level of alcohol use. In the concordant-low type, both sets of friends have a relatively low-level of alcohol use. In the discordant group, the friends of twin 1 differ in drinking behavior from that of the friends of twin 2; one set of friends has a relatively high-level of alcohol use and the other set a relatively low one. Figure 1 further illustrates our research design for detecting the moderation of genetic contribution to adolescent alcohol use by friends’ drinking behavior.
Table 1.
Research design: Moderation of genetic contribution to adolescent alcohol use by friends’ drinking behavior
| Three Types of Friend Influence | Data Used | Level of Expressed Genetic Contribution to Alcohol use or estimated h2 |
|---|---|---|
| Concordant-High on Drinking | MZ, same-sex DZ twins, & same-sex full siblings | Baseline |
| Concordant-Low on Drinking | MZ, same-sex DZ twins, & same-sex full siblings | Lower |
| Discordant Drinking | MZ, same-sex DZ twins, & same-sex full siblings | Lower |
Figure 1.
Research Design: Detecting the Moderation of Genetic Contribution to Drinking Behavior
The three types of friends’ drinking behavior represent three types of environments that are expected to influence the level of genetic expression for alcohol use. Specifically, we expect a statistically significant genetic contribution (heritability) to alcohol use among the concordant/high pairs of siblings, a statistically non-significant genetic contribution or a smaller significant genetic contribution among the concordant/low siblings, and a statistically nonsignificant genetic contribution or a smaller significant genetic contribution among the discordant siblings.
It should be pointed out that none of the friend-influence environments in Table 1 corresponds to the idealized alcohol-free environment discussed in the section on research design. Virtually all adolescents in the United States are exposed to alcohol consumption. The lack of a truly alcohol-free environment in observational data makes it more difficult to detect any gene-environment interaction. However, a pair of siblings may, indeed, have friends that have different drinking behavior. When we do have the data, we ask the question: Does an adolescent’s friend make a difference over and above the larger societal influence?
Subjects
Add Health.
We used data from the National Longitudinal Study of Adolescent Health (Add Health), which started as a nationally representative sample of more than 20,000 adolescents in grades 7–12 in 1995 in the United States (Harris et al. 2003). The respondents have since been followed by two additional in-home interviews in 1996 (Wave II) and 2002 (Wave III). Add Health is school-based and the adolescents are from 134 schools. The school sample is stratified by region, ethnic mix, size, urbanicity (urban/suburban/rural), and school type (public/private/parochial).
The Add Health Sibling Sample.
We used the sibling sample within the Add Health Study, which deliberately incorporated a behavior-genetic design as a component in an otherwise traditional survey. The sibling sample is composed of six groups: monozygotic twins, dizygotic twins, full biological siblings, half biological siblings, cousins, and biologically unrelated adolescents living in the same household. Our analysis sample consists of pairs of adolescents who are genetically related to varying degrees including MZ twins, DZ twins, and full siblings. These data represent pairs of adolescents who took the same questionnaire and who share the same home environment, and in most cases the same school and same neighborhood. This design creates a rare opportunity to explore the relative contributions of genetics and environment to health and health behaviors in a national longitudinal sample.
When the twin sample was first collected at Wave I, the classification of the twins into monozygotic and dizygotic pairs was based on self-reported confusability of appearance. At Wave III in 2002, DNA samples were collected from a subset of the Add Health sample. The subset consists of 2,574 MZ twins, DZ twins, and full biological siblings. For these individuals, genomic DNA was isolated from buccal cells using a modification of published methods (Freeman et al. 1997). Then the zygosity of the twins was re-determined at the DNA level through a comparison of their match on 12 unlinked short tandem repeat (STR) (http://www.cpc.unc.edu/proiects/addhealth/_).
Add Health has approximately 3,200 related respondents that include MZ twins, same-sex DZ twins, different-sex DZ twins, full siblings, half siblings, and cousin. Our sample starts with 1,247 pairs of sibling pairs that includes all MZ twin pairs, but only like-sex DZ twin pairs and like-sex full sibling pairs. Whereas all identical twin pairs are of the same sex, a portion of fraternal twins and full siblings are of unlike sex. To eliminate the potential impact of sex, we decide to restrict the comparisons to those between like-sex identical twins and like-sex fraternal twins and full siblings. Our final analysis sample includes 600 pairs of MZ twin pairs, DZ twin pairs, and full sibling pairs after eliminating pairs that have missing data on friends, siblings’ drinking behavior, or siblings’ friends’ drinking behavior.
Measures of Adolescent Drinking.
At the first Wave of Add Health, the respondents were asked about their frequency of alcohol use. To protect confidentiality and reduce nonresponses, this section of the interview was self-administered by audio-CASI (Computer Assisted Self Interview). The sensitive questions were read to respondents by means of audio headphones. Respondents were given instructions on how to complete their answers on the computer. The respondents answered the question, “During the past twelve months, on how many days did you drink alcohol?” Respondents’ answers were recorded as 0, never; 1, one or two days in the past twelve months; 2, once a month or less; 3, two or three days a month; 4, one or two days a week; 5 three to five days a week; or 6, everyday or almost everyday. This seven-point scale is simplified in the analysis to a six-point scale in which categories 5 and 6 of the original measure are collapsed. The drinking score of an individual is recorded as 0, 1, 2, 3, 4, and 5 for never drinks, once in the last yr, once a month, 2–3 times last month, 1–2 a week, and 3 or more times a week, respectively.
Measures of Friend Influence.
Almost all studies of friend influences use data based on the respondent’s perceptions of a friend’s behavior instead of the actual behavior of a friend. In other words, these studies have data only on respondents; the data on their friends are reported by these respondents. However, perceptions of others’ behavior have been considered unreliable because the reporters tend to project their own behavior onto others (Bauman and Ennett 1996). Comparison of measures on perception of behavior with measures on actual behavior has shown this projection tendency for sexual behavior among boys (Wilcox and Udry 1986) and for smoking and drinking behavior among male and female adolescents (Fisher and Bauman 1988). The perception bias can be corrected with data that measure friends directly. Our Add Health data include as respondents not only a large number of genetically related individuals, but also their friends.
Respondents nominated same-sex and different-sex friends in both the in-school questionnaire and the in-home interview. The in-school respondents were asked to nominate up to five male and five female friends, starting with the closest friend, including girlfriends and boyfriends. The in-home interviewers requested the names of the best male- and the best female-friend from most of these adolescents. The in-home respondents who were in the saturated sample were asked to name five friends of each sex starting with boyfriends or girlfriends if any. Adolescents who participated in both the school and the home data collections could potentially have made 20 friendship nominations—10 for each sex.
To construct our analysis data, we searched for the best friend and other friends from these multiple nominations for each adolescent in our genetic pair samples using the following procedure. We searched for the nominations in the in-home interview first. When the information was unavailable in the home data, we went to the school interview. If a friend had missing data on drinking, then we moved on to the next nominated friend(s) listed during the home interview and the school interview.
Measures of Peer Drinking Influences.
Because these nominated friends are themselves respondents in the Add Health Study, measures of these individuals’ drinking behavior can be readily constructed as the respondent’s friends’ drinking behavior. To test for the robustness of the friend drinking measure, we constructed three measures of their drinking influence: (1) Peers as the best friend: we use the drinking score of the friend nominated as the best friend in the chosen set of friends. (2) Peers defined as maximum possible exposure among friends: we use the highest drinking score found among the nominated friends. And (3) Peers defined as all nominated friends: we use average friends’ score, that is, a weighted average in which greater weight is given to first-nominated friends. The first-nominated or best friend is given a greater weight because we assume best friends tend to have a larger influence than the other friends.
Each pair of siblings are subject to the influence of one of the three types of peer drinking influence: (1) Discordant drinking influences: the two siblings in a sib pair are subject to different levels of drinking influence; discordant influences are defined when the two sets of friends’ drinking scores are two points or greater away from each other (1 vs. 3; 2 vs. 4). (2) Concordant/low drinking influences: the two siblings in a sibling pair are subject to similarly low levels of drinking influence; concordant/low influences are defined when the two sets of friends score zero or one. (3) Concordant/high drinking influences: the two siblings in a sibling pair are subject to similarly high levels of drinking influence; concordant/high influences are defined when the two sets of friends score 2 or higher. These cut-off points are somewhat arbitrary; the concordant high group may not differ sufficiently from the concordant low or discordant group. However, our ability to vary the cut-off points is restricted by the moderate sample sizes.
Analytic Strategy
Our objective is to estimate heritability (h2) for each of the three environments defined by friend influence. Of the several approaches that are used to estimate heritability: the classic approach described by Falconer and Mackay (1996, p171–4), the structural equation model via Mx (Neale and Cardon 1992), and the multilevel model (Guo and Wang 2002), we chose the classic approach for its simplicity. Regardless of a particular approach, heritability for an outcome variable is defined as the proportion of genetic variance over the total variance. Guo and Wang (2002) showed that the heritability estimates from the classic approach, SEM, and the multilevel model are quite similar. Falconer and Mackay (1996) show that heritability is equal to twice the difference between the MZ correlation and the DZ correlation estimates.
Our job was, therefore, to calculate the correlations in alcohol use for MZ twins, DZ twins, and DZ twins plus full siblings by type of friend influence (concordant/high, concordant/low, and discordant), using the method of correlation coefficient, ρ = Cov(Y1,Y2) / σ1σ2, where Y1,Υ2,σ1, and σ2 are twin 1 ‘s drinking, twin 2’s drinking, the standard deviation of twin 1 ‘s drinking, and the standard deviation of twin 2’s drinking, respectively. Then, the correlation estimates were used to calculate heritability by type of friend influence (concordant/high, concordant/low, and discordant).
To perform hypothesis testing or construct confidence intervals for heritability estimates, we used the bootstrapping method (Efron and Tibshirani 1993), which re-samples the data by pair (without breaking up the pairs) with replacement for a large number of times (2000 or more), each time re-estimating the correlation coefficients for MZ twins, DZ twins, and full siblings by type of friend influence as well as the heritability estimates by type of friend influence. Then we rank the estimates of heritability within each type of friend influence. The 90% confidence interval for the heritability estimates can be constructed by the 5th and 95th percentiles of the ranked heritability estimates.
The bootstrapping method is a modern, computationally intensive, general method for statistical inference (Efron and Tibshirani 1993; Chernick 1999). Bootstrapping can be used to study the properties of an estimator (e.g., the variance of an estimator) and the results are asymptotically equivalent to the classic statistical approaches under certain conditions. Its main advantage is simplicity. In our case, deriving the confidence interval for a correlation coefficient via analytical methods is complicated; applying bootstrapping is a computer-intensive, but straightforward procedure.
We carried out these analyses in three samples: (1) MZ twins and same-sex DZ twins; (2) MZ twin pairs, same-sex DZ twin pairs, and same-sex full sibling pairs; and (3) random pairs. The second sample that combines the same-sex DZ twins and the full siblings provides more power for hypothesis testing. The third sample of random pairs was created by first breaking up the observed pairing between each sibling and the drinking score of his or her friend, and then pairing each sibling with the drinking score of a randomly-chosen individual in the sibling sample. The third sample is used as a “placebo” group where the friend drinking behavior is randomly assigned. In the third sample, the pattern of friend drinking behavior is not expected to be related to heritability estimates. The findings from the third sample will provide further evidence for whether friend drinking patterns systematically influence heritability.
RESULTS
The overall heritability estimate of the alcohol measure using all MZ and DZ twins is 0.402. Table 2 reports descriptive statistics for Add Health Wave II sample including the sample size in pairs of siblings by type of siblings and friend drinking behavior, the mean age by type of siblings, the respondents’ mean alcohol use by type of siblings, and the friend mean alcohol use by type of siblings and pattern of friend drinking behavior. Our analysis sample consists of 127 pairs of MZ twins, 101 pairs of same-sex DZ twins, and 372 pairs of same-sex biological full siblings. The number of MZ-twin pairs is small in part because we are able to include a twin pair in the analysis only if information is available for both members of a twin pairs as well as for the friends of both members of the twin pair.
Table 2.
Mean level of adolescent drinking and number of pairs by type of sibling and pattern of friends’ drinking behavior and other descriptive statistics of Add Health Wave II Sample
| MZ Twins (pairs) | Same-sex DZ Twins (pairs) | Same-sex Full Siblings (pairs) | All | |
|---|---|---|---|---|
| Sample Size in Pairs | 127 | 101 | 372 | 600 |
| Age | 16.01 | 15.81 | 16.23 | 16.14 |
| Mean of Alcohol Use | ||||
| Respondent’s alcohol use | 1.02 | 1.11 | 1.19 | 1.07 |
| Friend’s average | 1.08 (127) | 1.2 (101) | 1.16 (372) | 1.15 |
| Concordant high | 2.06 (46) | 2.15 (29) | 1.69 (93) | 1.83 |
| Concordant low | 0.32 (56) | 0.36 (46) | 0.36 (171) | 0.35 |
| Discordant | 1.78 (25) | 1.88 (26) | 1.96 (108) | 1.92 |
| Best friend’s use | 1.12 (127) | 1.23 (101) | 1.17 (372) | 1.17 |
| Concordant high | 2.60 (41) | 2.60 (32) | 2.28 (81) | 2.41 |
| Concordant low | 0.34 (51) | 0.34 (42) | 0.35 (145) | 0.34 |
| Discordant | 1.81 (35) | 2.02 (27) | 1.86 (146) | 1.87 |
| Maximum exposure | 1.34 (127) | 1.51 (101) | 1.58 (372) | 1.52 |
| Concordant high | 2.94 (47) | 2.89 (33) | 2.47 (116) | 2.64 |
| Concordant low | 0.38 (49) | 0.40 (38) | 0.40 (101) | 0.40 |
| Discordant | 1.87 (31) | 2.02 (30) | 2.16 (155) | 2.10 |
On average, our respondents were aged about 16 regardless of type of siblings. The average score of alcohol use among the respondents is 1.07, which is close to the mean alcohol use among the average friends (1.15) and the mean alcohol use among the best friends (1.17). By design, the mean alcohol use among the highest drinking friends (1.52; maximum exposure) is considerable higher. Consistent with the research design, the average alcohol use is the highest for the concordant-high group, the lowest for the concordant-low group, and in between for the discordant group.
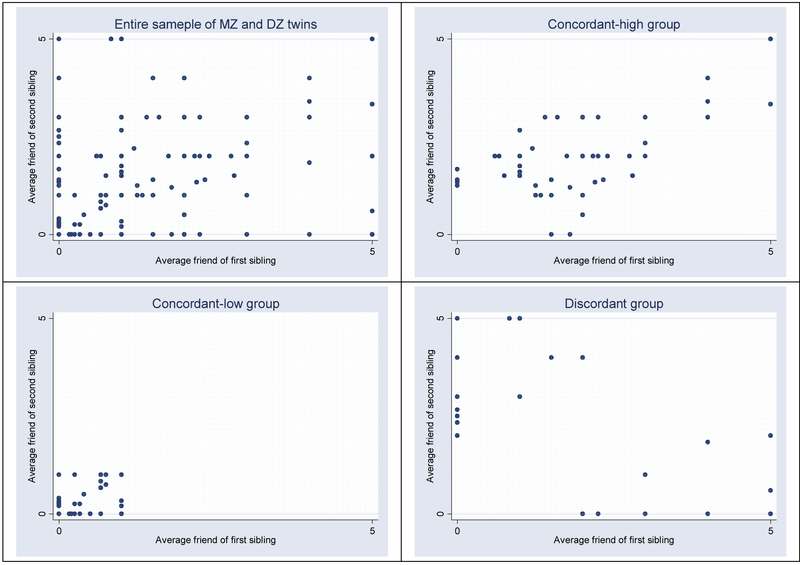
Panel 1 of Figure 2 plots the pair-wise drinking score of the friends of all sibling pairs including MZ twins, DZ twins, and full siblings. The other three panels of Figure 2 describe the division of our entire sample into three groups that differ by pattern of friend drinking behavior: Concordant high, Concordant low, and discordant. Panels 1–4 describe what constitutes our three friend influence environments. Panel 2 of Figure 2 shows the drinking scores of the friends of the sibling pairs who score two or above and who are defined as concordant high. Panel 3 of Figure 2 shows the drinking scores of the friends of the sibling pairs who score 0 or 1 and who are defined as concordant low. Panel 4 of Figure 2 shows the drinking scores of the friends of the sibling pairs who are two points away or more from each other. They are defined as discordant.
Figure 2.
Scatterplot of twins’ friends’ drinking behavior
Table 3 presents heritability estimate h2 and its 90% bootstrapping confidence interval for drinking across three types of friend influence (concordant/high, concordant/low, and discordant) for three samples, with friend influence measured as the weighted average drinking score of one’s friends, the drinking score of the best friend, and the maximum drinking score among one’s friends. The results from our twin samples are consistent with our hypothesis. The confidence interval of the heritability estimate in the concordant-high group is above zero for all three types of friend drinking: (0.12, 1), (0.02, 1), and (0.29, 1), respectively, for average friends, best friends, and maximum exposure; the three corresponding mean heritabilities are 0.76. 0.77, and 0.98. For the concordant/low group, the three mean heritability estimates are 0.47, 0.45, and 0.53, respectively, but all three corresponding confidence intervals are wide and include zero, indicating that the heritability estimates may not differ from zero. For the discordant group, the bootstrapping estimates of heritability are generally small in absolute values and fall on both sides of zero, suggesting (1) the underlying heritability is zero or very close to zero and (2) this heritability cannot be precisely estimated. We set the three estimates to zero without providing a confidence interval.
Table 3.
Estimated h2 (heritability) and its 90% confidence interval for adolescent drinking by friend drinking behavior
| Friend drinking (pairs) | Twins and Same-sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MZ & same-sex DZ Twins | Full Siblings | Random Pairs | |||||||
| h2 | CI | h2 | CI | h2 | CI | ||||
| Average friend | |||||||||
| Concordant High | 0.76 | 0.12 | 1.00 | 0.87 | 0.47 | 1.00 | 0.38 | 0.00 | 1.00 |
| Concordant Low | 0.47 | 0.00 | 0.98 | 0.49 | 0.10 | 0.87 | 0.39 | 0.00 | 0.98 |
| Discordant | 0.00 | - | - | 0.00 | - | - | 0.36 | 0.00 | 1.00 |
| Best friend | |||||||||
| Concordant High | 0.77 | 0.02 | 1.00 | 1.00 | 0.70 | 1.00 | 0.33 | 0.00 | 1.00 |
| Concordant Low | 0.45 | 0.00 | 0.94 | 0.57 | 0.20 | 0.91 | 0.40 | 0.00 | 0.93 |
| Discordant | 0.00 | - | - | 0.00 | - | - | 0.36 | 0.00 | 1.00 |
| Maximum exposure | |||||||||
| Concordant High | 0.98 | 0.29 | 1.00 | 0.93 | 0.45 | 1.00 | 0.37 | 0.00 | 1.00 |
| Concordant Low | 0.53 | 0.00 | 1.00 | 0.49 | 0.08 | 0.86 | 0.42 | 0.00 | 1.00 |
| Discordant | 0.03 | 0.00 | 0.74 | 0.33 | - | - | 0.35 | 0.00 | 1.00 |
For the discordant group, many of the bootstrapping estimates of heritability fall below zero, suggesting that the underlying heritability is zero or very close to zero. We set the three estimates to zero without providing a confidence interval.
Refer to Table 2 for sample sizes.
Results from the samples that combine twins and full siblings are broadly similar to those from the twin sample except that the estimated confidence intervals have all narrowed: from (0.12, 1) to (0.47, 1), (0.02, 1) to (0.7, 1), and (0.29, 1) to (0.45, 1) for the concordant-high group probably because of the increased sample size when the full siblings are added. For the concordant-low group, the mean estimates are very similar to those from the twin sample (0.49 vs. 0.47, 0.57 vs. 0.45, and 0.49 vs.0.53), but the three confidence intervals are all located above zero (0.10–0.87, 0.20–0.91, and 0.08–0.86), suggesting that the heritability for the concordant/low group is located above zero, but below that for the concordant/high group. The three heritability estimates for the discordant group remain zero.
In the analysis of random pairs, the mean correlations, which range 0.33–0.42, reflect the average correlation of the entire sample (but the precision is based on a subset of the sample), and are almost the same across the randomly assigned “friend drinking groups”. The sharp differences between the analysis of observed friend’s drinking pattern and the analysis of randomly assigned friend drinking pattern provide an independent piece of evidence that the level of heritability for adolescent drinking may depend on friend drinking behavior.
DISCUSSION
Genetic factors are often considered incompatible with sociological analysis which by definition focuses on social context. Recent mapping of the human genome has led to new information about the association of genomics with diseases and other human outcomes. Yet, it is becoming clear that few diseases or human conditions are caused purely by genetic factors; most are the result of interactions between genetic and environmental factors (Collins et al 2003b). Therefore, in order to expand our understanding of social outcomes and the role of social context, it is imperative to explore the interactions between social context and genetic factors. In this article, we describe a framework and a piece of empirical test that show how both social context and genetic factors can be integrated in a sociologically informed analysis.
In this study, we show that twin data can be used to estimate the interaction between environmental factors and the latent genetic influence when DNA data are unavailable. The analysis supports our gene-environment interaction hypothesis that the genetic contribution to adolescent drinking is subject to the influence of friends’ drinking behavior. Higher levels of drinking by friends tend to bring about higher levels of genetic contribution to this behavior. Lower levels of drinking by friends tend to suppress the level of genetic contribution to alcohol use.
Any investigation of friend influence must address the difficult issue of selection. It has long been recognized that adolescents have a tendency to select friends who are similar to themselves (Kandel 1978; Bauman and Ennett 1996). Although Harris (1995) considers peers the dominant influence in child development, she notes that adolescents tend to sort themselves into groups of like-minded individuals and that the similarity among adolescents in the same peer group cannot be attributed entirely to group influences. Scarr and McCartney (1983) argued that children are not only influenced by their environment, they also actively participate in its creation. Children tend to seek the environment that matches their own genetic propensities. Friends and peers are a natural part of this environment. The same selection issue is discussed by Cleveland et al. (2005) in a behavior genetic study on friend influence and adolescent risky behaviors.
Although friend selection as a general problem has remained unresolved, a careful examination of our research design has suggested that our findings are likely to be more conservative because of selection. That is, the evidence in the absence of selection could be stronger than the evidence we have obtained with selection.
Friend selection implies that an adolescent tends to choose a best friend among those whose drinking behavior matches his or her genetic propensity for alcohol use. We know that DZ twins are 50% similar genetically, but unlike the 50% genetic similarity between a parent and his or her child, the 50% similarity for DZ twins is an average. A particular pair of DZ twins could be 0%–100% similar genetically. It is possible that more genetically similar DZ twins are more likely to be classified into the concordant groups and that more genetically dissimilar DZ twins are more likely to be assigned to the discordant group. This problem only affects DZ twins because the amount of genetic material shared within MZ twin pairs does not vary by pairs.
What would be the potential impact of this selection on our findings? For the discordant group, the heritability estimate for alcohol use would be overestimated because (1) the DZ twin pairs in this group are more dissimilar than the average DZ twins in the sample and the DZ correlation would be underestimated; (2) the MZ correlation remains unaffected by the selection; and (3) the heritability estimate depends on the difference between the DZ and MZ correlation estimates. For the concordant/high group, the heritability would be underestimated because (1) the DZ twin pairs in the concordant-high are more similar than the average DZ twins in the sample and the DZ correlation would be overestimated; (2) the MZ correlation remains unaffected by the selection; and (3) the heritability estimate depends on the difference between the DZ and MZ correlation estimates.
Recall that we hypothesize that the heritability in the concordant-high group is significantly higher than the heritability in the discordant group. The selection bias in both the discordant and concordant/high groups would, thus, make it more difficult for us to obtain evidence for our hypothesis. In other words, because of the selection, our results are likely to be an underestimation of the actual influence of friend drinking.
Classic (Durkheim 1897/1951) and contemporary (Allen and Wilder 1977; Festinger 1954; Akers 1977, 1997; Sutherland and Cressey 1984; Haynie and Osgood 2005) social theories have always viewed peer influence as vitally important in the development of deviant behavior. This view has been repeatedly supported empirically (Kandel 1975; Yamaguchi and Kandel 1987; Haynie 2001; Haynie 2002; Warr 1993; Warr and Stafford 1991). Our analysis has confirmed the importance of peer influence for deviant behavior by demonstrating that peer drinking behavior moderates genetic contribution to adolescent alcohol use.
Our study has a number of limitations or potential limitations.
We only focused on the immediate friends of the respondents. Future studies should investigate whether the gene-environment interaction is influenced by the structural properties of peer networks such as its density and the centrality and popularity of the adolescents involved (Haynie 2001). Durkheim’s theory implies that social integration and anomie may contextualize social imitation such that imitation becomes a more potent social force when social integration becomes weaker. The theory suggests that future investigation should also take into account other forms of social integration, familial and religious.
One limitation is the moderate sample size, especially the sample sizes for MZ and DZ twins. Our analysis sample consists of 127, 101, and 372 pairs of MZ twins, DZ twins, and full siblings, respectively. The small number of available MZ and DZ twin pairs make it difficult to produce credible test results for group differences in heritability estimates. An insignificant result could come from either a genuine insignificant group difference or a lack of statistical power.
More MZ twin pairs (31%) than DZ twin pairs (17%) nominate a common third party as a best friend. This MZ-DZ difference, however, does not necessarily confound our main finding. First, “best friend” is only one of the three types of friend influence (best friend, average friend, and maximum exposure) we tested. Only when “best friend” is considered, a common third friend automatically defines a concordant pair. The weight of the best friend is reduced when friend influence is defined by average friend influence or maximum friend influence. Second, even in the case of “best friend”, a common friend only defines a concordant pair; whether the concordant pair is concordant high or concordant low depends on the drinking scores of the best friend. In actuality, if there is a genetic contribution to friendship formation (Guo 2006), we would expect that MZ twins are more likely to share a common best friend than DZ twins.
With continuing advances in molecular genetic technology and the success of the Human Genome Project (Collins et al 2003a) and the HapMap Project (The International HapMap Consortium 2003, 2005), the number of genetic polymorphisms in humans that are available for research on gene-environment interactions are increasing rapidly. Our findings suggests that peer behavior is likely to be an important environmental moderator of the expression of genetic disposition for adolescent drug use, smoking, dietary habits, and risky sexual behavior. Future studies on these behaviors that use non-DNA twin samples or DNA measures of genetic variants should consider peer influence as a significant environmental moderator. By identifying the underlying genetic contribution to risky adolescent behavior and by determining the social, cultural, and other environmental circumstances under which genetic factors are expressed, prevention science may better target interventions to groups at greatest risk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The International HapMap Project is a collaborative effort by scientists and funding agencies from Japan, the United Kingdom, Canada, China, Nigeria, and the United States. The purpose of the project is to identify and catalog genetic similarities and differences in human beings. The information from the HapMap will facilitate researchers in finding genes that affect health, disease, and individual responses to medications and environmental factors.
One of two or more alternate forms (alleles) of a chromosomal locus that differ in nucleotide sequence or have variable numbers of repeated nucleotide units
REFERENCES
- Akers RL, 1973. Deviant Behaviour: A Social Learning Approach. Wadsworth, Belmont, CA. [Google Scholar]
- Akers R, 1997. Criminological Theories: Introduction and Evaluation, 2nd edition Roxbury, Los Angeles, CA. [Google Scholar]
- Allen VL, and Wilder DA, 1977. Social comparison, self-evaluation, and conformity to the group In: Suis JM, and Miller RL, (Ed.) Social Comparison Processes: Theoretical and Empirical Perspectives. Hemisphere Publishing, Washington, D.C.£: pp. 187–208. [Google Scholar]
- Bauman K, and Ennett S, 1996. On the Importance of Peer Influence for Adolescent Drug Use: Commonly Neglected Considerations. Addiction 91, 185–198. [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long J, Lorenz J, Shoaf SE, Champoux M, Suomi SJ, Linnoila M, and Higley JD, 1998. Serotonin Transporter Gene Variation, Strain, and Early Rearing Environment Affect CSF 5-HIAA Concentrations in Rhesus Monkeys (Macaca Mulatta). American Journal of Primatology 32, 95–104. [Google Scholar]
- Billy JOG, Rodgers JL, and Udry JR, 1984. Adolescent Sexual Behavior and Friendship Choice. Social Forces 62, 653–678. [Google Scholar]
- Billy JOG, and Udry JR, 1985. The Influence of Male and Female Best Friends on Adolescent Sexual Behavior. Adolescence 20, 21–32. [PubMed] [Google Scholar]
- Botstein D, and Neil R, 2003. Discovering Genotypes Underlying Human Phenotypes: Past Successes for Mendelian Disease, Future Approaches for Complex Disease. Nature Genetics 33 Suppl, 228–237. [DOI] [PubMed] [Google Scholar]
- Brown BB, 1990. Peer Groups and Peer Cultures In: Feldman SS, and Elliot GR, (Ed.), At the Threshold: The Developing Adolescent. Harvard University Press, Cambridge, pp. 171–196 [Google Scholar]
- Brown BB, and Theobald W, 1999. How Peers matter: A Research Synthesis of Peer Influences on Adolescent Pregnancy In: Peer Potential: Making the Most of How Teens Influence Each Other. National Campaign to Prevent Teen Pregnancy, Washington, D.C. [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, and Poulton R, 2002. Role of genotype in the cycle of violence in maltreated children. Science 297,851–854. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, McClay J, Mill J, Martin J, Braithwaite A, and Poulton R, 2003. Influence of life stress on depression: moderation in the 5-HTT gene. Science 301, 386–389. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention), 2000. Youth Risk Behavior Surveillance—United States, 1999. Morbidity and Mortality Weekly Report 49(SS-5), 1–94. [PubMed] [Google Scholar]
- Chernick Michael R. 1999. Bootstrap Methods, A practitioner’s guide. Wiley Series in Probability and Statistics. [Google Scholar]
- Cleveland HH, Wiebe RP, and Rowe DC, 2005. Sources of Exposure to Smoking and Drinking Friends among Adolescents: A Behavioral-Genetic Evaluation, Journal of Genetic Psychology 166, 153–169. [PubMed] [Google Scholar]
- Coleman JS, 1961. The Adolescent Society: The Social Life of the Teenager and Its Impact on Education. Free Press of Glencoe, New York. [Google Scholar]
- Collins FS, Green ED, Guttmacher AE, Guyer MS, on behalf of US National Human Genome Research Institute, 2003. A vision for the future of genomics research. Nature 422, 835–847. [DOI] [PubMed] [Google Scholar]
- Collins FS, Morgan M, and Patrinos A, 2003. The Human Genome Project: Lessons from Large-scale Biology. Science 300, 286–290. [DOI] [PubMed] [Google Scholar]
- Cook LS, Goldoft M, Schwartz SM, and Weiss NS, 1999. Incidence of Adenocarcinoma of the Prostate in Asian Immigrants to the United States and their Descendants. Journal of Urology 161, 152–155. [PubMed] [Google Scholar]
- Dick DM, and Foroud T, 2003. Candidate Genes for Alcohol Dependence: A Review of Genetic Evidence from Human Studies. Alcoholism: Clinical and Experimental Research 27, 868–879. [DOI] [PubMed] [Google Scholar]
- Durkheim E, 1951. Suicide: A Study in Sociology. Free Press, New York. [Google Scholar]
- Efron B, and Tibshirani RJ, 1993. An Introduction to the Bootstrap. Chapman & Hall, New York. [Google Scholar]
- Ennett ST, and Bauman KE,. 1994. The Contribution of Influence and Selection to Adolescent Peer Group Homogeneity: The Case of Adolescent Cigarette Smoking. Journal of Personality and Social Psychology 67, 653–663. [DOI] [PubMed] [Google Scholar]
- Falconer D, and Mackay T, 1996. Introduction to Quantitative Genetics, 4th Edition Longman, New York. [Google Scholar]
- Festinger L, 1954. A Theory of Social Comparison Processes. Human Relations 7, 117–140. [Google Scholar]
- Fisher LA, and Bauman KE, 1988. Influences and Selection in the Friend-Adolescent Relationship: Findings from Studies of Adolescent Smoking and Drinking. Journal of Applied Social Psychology 18, 289–314. [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, and Plomin R, 1997. DNA by Mail: An Inexpensive and Noninvasive Method for Collecting DNA Samples from Widely Dispersed Populations. Behavior Genetics 27, 251–257. [DOI] [PubMed] [Google Scholar]
- Grant BF, and Dawson DA, 1997. Age at Onset of Alcohol Use and Its Association with DSM-IV Alcohol Abuse and Dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse 9, 103–10. [DOI] [PubMed] [Google Scholar]
- Guo G, and Stearns E, 2002. The Social Influences on the Realization of Genetic Potential for Intellectual Development. Social Forces 80, 881–910. [Google Scholar]
- Guo G, and Wang JM, 2002. The Mixed or Multilevel Model for Behavior Genetic Analysis. Behavior Genetics 32, 37–49. [DOI] [PubMed] [Google Scholar]
- Guo G 2006. Genetic similarity shared by best friends among adolescents. Twin Research and Human Genetics 9:113–121. [DOI] [PubMed] [Google Scholar]
- Haberstick B, Lessem JM, Hopfer CJ, Smolen A, Ehringer MA, Timberlake D, Hewitt JK, 2005. Monoamine Oxidase a (Maoa) and Antisocial Behaviors in American Journal of Medical Genetics B, Neuropsychiatrie Genetics 135B, 59–64. [DOI] [PubMed] [Google Scholar]
- Harris KM, Florey F, Tabor J, Bearman PS, Jones J, and Udry JR, 2003. The National Longitudinal Study of Adolescent Health: Research Design. <http://www.cpc.unc.edu/projects/addhealth/design>.
- Harris JR, 1995. Where is the Child’s Environment? A Group Socialization Theory of Development. Psychological Review 102, 458–490. [Google Scholar]
- Haynie DL, 2001. Delinquent Peers Revisited: Does Network Structure Matter? American Journal of Sociology 106, 1013–1057. [Google Scholar]
- Haynie DL, 2002. Friendship Networks and Adolescent Delinquency: The Relative Nature of Peer Delinquency. Journal of Quantitative Criminology 18, 99–134. [Google Scholar]
- Haynie DL, and Osgood DW, 2005. Reconsidering Peers and Delinquency: How Do Peers Matter? Social Forces 84, 1109–1130. [Google Scholar]
- Heath AC, 1995. Genetic Influences on Drinking Behavior in Humans In: Begleiter H and Kissin B. (Ed.), The Genetics of Alcoholism. Oxford University Press, New York, pp. 82–121. [Google Scholar]
- Hingson RW, and Howland J, 2002. Comprehensive Community Interventions to Promote Health: Implications for College-age Drinking Problems. Journal of Studies on Alcohol 14(Suppl.), 226–240. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, 2005. Gene-environment interactions in human diseases. Nature Reviews Genetics 6, 287–298. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, 2003. Genetic associations: false or true? Trends in Molecular Medicine 9, 135–138. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, and Bachman JG, 2003. Monitoring the Future National Survey Results on Drug Use, 1975–2002: Vol. 1. Secondary School Students. (NIH Pub. No. 03–5375). National Institute of Drug Abuse, Bethesda, MD. [Google Scholar]
- Kandel DB, 1975. Stages in Involvement in Adolescent Drug Use. Science 190, 912–914. [DOI] [PubMed] [Google Scholar]
- Kandel DB, 1978. Homophily, Selection, and Socialization in Adolescent Friendships. American Journal of Sociology 84, 427–436. [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, and Riley B, 2005. The Interaction of Stressful Life Events and a Serotonin Transporter Polymorphism in the Prediction of Episodes of Major Depression: A Replication. Archives of General Psychiatry 62, 529–535. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Rutter M, Polo Tomas M, and Moffitt TE, 2006. The Caregiving Environments Provided to Children by Depressed Mothers with or without an Antisocial History. American Journal of Psychiatry 163, 1009–1018. [DOI] [PubMed] [Google Scholar]
- McGue M, 1999. The Behavioral Genetics of Alcoholism. Current Directions in Psychological Science 8, 109–115. [Google Scholar]
- Neale M and Cardon L, 1992. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers, Dordrecht, the Netherlands. [Google Scholar]
- Office of National Drug Control Policy. The National Drug Control Strategy, 1997. <http://www.ncjrs.org/htm/chapter2.htm.>
- Popkin BM, and Udry JR, 1998. Adolescent Obesity Increases Significantly in Second and Third Generation U.S. Immigrants: The National Study of Adolescent Health. Journal of Nutrition 128, 701–706. [DOI] [PubMed] [Google Scholar]
- Puddey IB, Rakic V, Dimmitt SB, and Beilin LJ, 1999. Influence of Pattern of Drinking on Cardiovascular Disease and Cardiovascular Risk Factors — A Review. Addiction 94, 649–663. [DOI] [PubMed] [Google Scholar]
- Risch NJ, 2000. Searching for Genetic Determinants in the New Millennium. Nature 405, 847–856. [DOI] [PubMed] [Google Scholar]
- Scarr S, and McCartney K, 1983. How People Make Their Own Environments: A Theory of Genotype Greater Than Environment Effects. Child Development 54, 424–435. [DOI] [PubMed] [Google Scholar]
- Sutherland EH, and Cressey DR, 1984. Differential Association Theory In: Kelly DH, (Ed.), Deviant Behavior, 2nd edition St. Martins Press, New York, [Google Scholar]
- The International HapMap Consortium, 2005. A Haplotype Map of the Human Genome. Nature 437, 1299–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International HapMap Project, 2003. The International HapMap Project. Nature 426, 789–796. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, and Gottesman II, 2003. Socioeconomic Status Modifies Heritability of IQ in Young Children. Psychological Science 14, 623–628. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, 2003. Genetics of Alcohol and Tobacco Use in Humans. Annals of Medicine 35, 94–121. [DOI] [PubMed] [Google Scholar]
- Wannamethee G, and Shaper AG, 1992. Alcohol and Sudden Cardiac Death. British Heart Journal 68, 443–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr M, 1993. Parents, Peers and Delinquency. Social Forces 72, 247–264. [Google Scholar]
- Warr M, and Stafford M, 1991. The Influence of Delinquent Peers: What They Think or What They Do? Criminology 29, 851–866. [Google Scholar]
- Wilcox S, and Udry JR, 1986. Autism and Accuracy in Adolescent Perceptions of Friends’ Sexual Attitudes and Behavior. Journal of Applied Social Psychology 16, 361–374. [Google Scholar]
- Windle M, 1999. Alcohol Use Among Adolescents. Sage Publications, Thousand Oaks, CA. [Google Scholar]
- Windle M, 2003. Alcohol Use Among Adolescents and Young Adults. Epidemiology in Alcohol Research 27, 79–86. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, and Kandel D, 1987. Drug Use and Other Determinants of Premarital Pregnancy and Its Outcome: A Dynamic Analysis of Competing Life Events. Journal of Marriage and the Family 49, 257–270. [Google Scholar]