Abstract
Background and purpose
Stroke is the leading cause of mortality and disability in China. Precise aetiological classification, imaging and biological markers may predict the prognosis of stroke. The Third China National Stroke Registry (CNSR-III), a nationwide registry of ischaemic stroke or transient ischaemic attack (TIA) in China based on aetiology, imaging and biology markers, will be considered to clarify the pathogenesis and prognostic factors of ischaemic stroke.
Methods
Between August 2015 and March 2018, the CNSR-III recruited consecutive patients with ischaemic stroke or TIA from 201 hospitals that cover 22 provinces and four municipalities in China. Clinical data were collected prospectively using an electronic data capture system by face-to-face interviews. Patients were followed for clinical outcomes at 3 months, 6 months and 1–5 year annually. Brain imaging, including brain MRI and CT, were completed at baseline. Blood samples were collected and biomarkers were tested at baseline.
Results
A total of 15 166 stroke patients were enrolled, among which 31.7% patients were women with the average age of 62.2±11.3 years. Ischaemic stroke was predominant (93.3%, n=14 146) and 1020 (6.7%) TIAs were enrolled.
Conclusions
CNSR-III is a large scale nationwide registry in China. Data from this prospective registry may provide opportunity to evaluate imaging and biomarker prognostic determinants of stroke.
Keywords: stroke, registry, transient ischaemic attack, biomarker, outcome
Introduction
Stroke is the second leading cause of death worldwide and the leading cause of mortality and disability in China.1 2 Being the most common types of cerebrovascular events in China, ischaemic stroke or transient ischaemic attack (TIA) account for about 70% of all strokes.3
Current guidelines for secondary prevention of ischaemic stroke treatment are recommended based on the potential aetiology and concomitant disease.4 Therefore, there’s of great significance to clarify the cause and pathogenesis through the standard stroke diagnosis process evaluation. Previous studies indicated that stroke of undetermined aetiology also had a substantial burden of stroke recurrence rates, but have few atherosclerotic markers and no excess of cardioembolic markers.5 6 Although aetiology of ischaemic stroke was assessed, most previous studies had no specific requirements for examination process and many patients didn’t receive complete evaluation thus didn’t get clear cause.3 7 8 Furthermore, recent studies showed that imaging and biological markers are determinant factors to predict the prognosis of stroke,9–11 and even classify the efficacy of treatment.12 13 It’s urgent to establish a prospective registry of stroke patients based on aetiological classification, imaging and biological markers through comprehensive clinical evaluation to clarify the pathogenesis and prognostic factors of ischaemic stroke.
The Third China National Stroke Registry (CNSR-III) is a nationwide clinical registry of ischaemic stroke or TIA in China based on aetiology, imaging and biology markers. In this report, we look at rationale, study design, baseline data, as well as the strengths and potential limitations of the CNSR-III study.
Methods
Overview of the CNSR III
The CNSR-III is a nationwide prospective registry for patients presented to hospitals with acute ischaemic cerebrovascular events between August 2015 and March 2018 in China. The CNSR-III was designed to establish Chinese ischaemic cerebrovascular disease aetiology classification, identify the imaging and biological markers for the prognosis of the ischaemic cerebrovascular events and facilitate evaluation and identification of patients at high risk in early phase.
Specific aims
The primary objectives of the CNSR-III were to establish Chinese ischaemic cerebrovascular disease aetiology classification cohort based on standard diagnosis procedures, identify imaging and biological markers for prognosis of the patients with ischaemic stroke and further establish the predictive model of stroke recurrence based on imaging and biological markers in ischaemic cerebrovascular diseases. The secondary objectives included evaluation of the association of chronic kidney disease (CKD), abnormal glucose regulation, oxidative stress, homocysteine, intestinal flora, heart rate variability and outcome of ischaemic cerebrovascular diseases and its subtypes.
Site selection
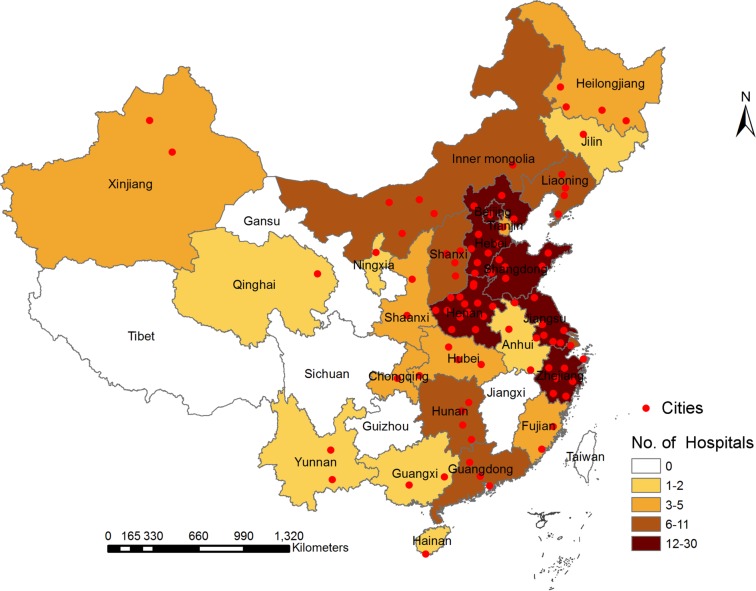
The project was carried out in multicentres to reduce selection bias and facilitate comparison of regional differences of the country. The steering committee attempted to ensure nationwide representation of the sample from each area in Mainland China. A total of 201 study sites from 22 provinces and four municipalities, including 163 grade III (central hospitals for certain district or city, usually teaching hospitals) and 38 grade II (hospitals serving several communities) urban hospitals, were selected from voluntary sites after comprehensive assessment. The sites with adequate research personnel, relevant research experience and qualified equipment, and proved commitment to the study were selected to participate in this study.
The geographical locations of the selected sites are illustrated in figure 1. The hospital characteristics of the participating hospitals are listed in table 1. The complete list of CNSR-III members and sites could be found in online appendix S1.
Figure 1.
The geographical locations of participating hospitals in the Third China National Stroke Registry.
Table 1.
Characteristics of participating hospitals in CNSR-III (n=201)
| Characteristics | |
| Hospital type | |
| Grade II | 38 (18.9) |
| Grade III | 163 (81.1) |
| Infrastructures | |
| Beds in total (n) | 1263 (1000–2000) |
| Beds in Neurology department (ND) (n) | 108 (72-180) |
| Stroke out-patients clinic | 172 (85.6) |
| Emergency department | 201 (100.0) |
| NICU | 144 (71.6) |
| Stroke unit | 155 (77.1) |
| Inpatients’ rehabilitation | 199 (99.0) |
| Neurological rehabilitation department | 158 (78.6) |
| Personnel | |
| Neurological rehabilitators | 176 (87.6) |
| Speech therapist | 151 (75.1) |
| Physiotherapist | 193 (96.0) |
| Diagnostic procedures | |
| Brain CT scan 24/7 | 201 (100.0) |
| Digital subtraction angiography | 185 (92.0) |
| Extracranial duplex sonography | 195 (97.0) |
CNSR-III, the Third China National Stroke Registry; NICU, neonatal intensive care unit.
svn-2019-000242supp001.pdf (209.5KB, pdf)
Patient enrolment
The registry recruited consecutive patients from August 2015 to March 2018 who met the following criteria:
Age older than 18.
Ischaemic stroke or TIA.
Within 7 days from the onset of symptoms to enrolment.
Informed consent from patient or legally authorised representative (primarily spouse, parents, adult children, otherwise indicated).
Patients who had silent cerebral infarction with no manifestation of symptoms and signs or who refused to participate in the registry were excluded.
Acute ischaemic stroke was diagnosed according to the WHO criteria14 and confirmed by MRI or brain CT.
Baseline data collection and data management
The baseline data of included patients were collected by trained research coordinators following a standard data collection protocol that was developed by the steering committee. The study investigators and research coordinators were trained before the kick-off meeting. Trained research coordinators at each site identified eligible patients, obtained informed consent, enrolled consecutive patients, and collected data by face-to-face interviews with the patients.
Information including prehospital care, prestroke modified Rankin Scale (mRS), National Institutes of Health Stroke Scale (NIHSS) score, Age, Blood pressure, Clincial features, Duration of symptoms and presence of Diabetes (ABCD2) score was collected through a direct interview by trained research coordinators at admission. Aetiology classification of ischaemic stroke was performed according to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.15 Other data were extracted from medical records that include patient demographics, medical history, family history, previous medication, physical examination, primary diagnosis, laboratory tests and risk factor assessment. At discharge, the research coordinators extracted the auxiliary examination and recorded standard aetiological evaluation result, medication, vascular related operation and surgical procedures, final diagnosis, NIHSS and mRS score, economic burden, cerebrovascular events during hospitalisation. Details of information collected at admission and discharge are presented in online appendix S2.
An electronic data capture system (EDC) was developed and used for data collection. Each participating hospital site entered data using an electronic signature (unique username and password). Trained research coordinators can store data in Pads and then upload the data later. The system can remind the researchers of patients’ follow-up spot, provide feedback of uploaded data timely and facilitate data quality monitoring. All data elements from each patient were automatically checked for completeness, correct coding, value range and logical error through EDC. All data changes made had an electronic audit trail with electronic signature and date. All laboratory test and auxiliary examination results were uploaded to the EDC as pictures. Considering that researchers had varying levels of comfort with the mobile technology, paper-based case report forms were also offered as a supplement if necessary. Independent data monitoring was also performed through EDC by an independent contract research organisation throughout the study period. All data were de-identified before data analysis.
Biological and urine sample collection
Blood and urine samples are collected in face-to-face visits from 171 study sites which had prior experience collecting samples for genetic and biomarker studies and agreed to participate in the pre-specified genetic and biomarker substudies. Fasting blood samples were collected in serum-separation tubes and EDTA anticoagulation blood collection tube within 24 hours of admission, at 3 months, 12 months for all patients. For patients with minor stroke (NIHSS ≤3) or TIA, additional blood samples were collected on the seventh day. Blood samples were sent to the laboratory to extract serum, plasma and white blood cells.
Random urine samples were collected by the first day of enrolment (within 24 hours) and at 3 months follow-up.
All the blood and urine samples were frozen in cryotube at − 80 ℃ refrigerator. The the samples were transported through cold chain to the centre laboratory in Beijing Tiantan Hospital, where all serum specimens were stored at −80℃ untill testing was performed.
Imaging data collection
The following protocols are recommended for all patients: brain imaging, including brain MRI (T1 weighted, T2 weighted, Fluid-attenuated Inversion Recovery (FLAIR), Diffusion-Weighted Imaging (DWI) with Apparent Diffusion Coefficient (ADC) maps, Magnetic Resonance Angiography (MRA), T2*/SWI) or CT (if contraindicated to MRI), and at least one vascular assessment for intracranial (MRA, Computed Tomography Angiography (CTA) or Digital Subtraction Angiography (DSA)) and extra-cranial arteries (Carotid Doppler, CTA, Contrast-Enhanced Magnetic Resonance Angiography (CE-MRA) or DSA); heart examination, including 12-lead ECG, precordial echocardiography, cardiac monitoring for ≥24 hours with automated rhythm detection; image data were collected inDICOM format on discs and analysed by the image research centre in Beijing Tiantan Hospital.
Follow-up data collection and data management
Patients were followed up at 3 months, 6 months and 1–5 year annually. Patients were interviewed face-to-face at 3 months and contacted over the telephone by trained research coordinators at 6 months and 1–5 year annually. Information including functional status, cardiovascular/cerebrovascular events, compliance of recommended secondary prevention medication and risk factor control was queried at each follow-up. The mRS was used to assess patients’ functional dependence, and European Quality of Life Scale-5 Dimensions (EQ-5D) to describe the quality of life. Any death, stroke recurrence, cardiovascular events, systemic embolism and intravascular operation during the follow-up periods were recorded. Confirmation of cerebrovascular events were sought from the treating hospital, and suspected recurrent cerebrovascular events without hospitalisation were judged by independent endpoint judgement committee. Each case fatality was either confirmed on a death certificate from the attended hospital or the local citizen registry. The definitions of cerebrovascular events are presented in online appendix S3.
Primary outcomes
Stroke recurrence: new ischaemic stroke and recurrent haemorrhagic stroke (intracerebral haemorrhage and subarachnoid haemorrhage).
Cardiovascular death: Cardiovascular death defined as ischaemic stroke, haemorrhagic stroke, sudden cardiac death, acute myocardial infarction, death directly caused by heart failure and other cardiovascular death (including: cardiac arrhythmias unrelated to sudden cardiac death, pulmonary embolism, cardiovascular intervention (unrelated to acute myocardial infarction) aortic aneurysm rupture or peripheral arterial disease).
Post stroke disability: mRS score >3.
Secondary outcomes
Non-fatal myocardial infarction: diagnostic criteria for myocardial infarction: cardiac markers, cTn elevated with dynamic change for the best, accompanied by one evidence of myocardial ischemia as follows: including symptoms, ECG changes and imaging.
Non-fatal heart failure: initial myocardial injuries due to various causes result in changes in cardiac structure and function, ultimately lead to ventricular pumping dysfunction.
Vascular related operations and surgery: carotid artery stenting; carotid endarterectomy; intracranial stenting; coronary stenting and balloon dilatation.
Systemic embolism events: arterial embolism (except for central nervous system, coronary artery and pulmonary artery) leading to clinical ischaemic events. Evidence of embolisation found by surgical specimens, autopsy, angiography or other objective examination approves, such as: mesenteric artery embolisation, splenic artery embolism.
Subgroup of the CNSR-III
Besides the main cohort, several subgroups were designed in CNSR-III for specific objectives of the study.
The Impairment of CognitiON and Sleep quality for patients after acute ischaemic stroke (AIS) or TIA (ICONS)
A total of 40 study sites with the experience of cognition and sleep research participated in the ICONS substudy. The cognition, sleep scales and related drug information at different stages afterAIS or TIA was supplemented and completed. The main aim of ICONS was to investigate the occurrence, variation trend and influencing factors of cognitive impairment and sleep disorder in Chinese patients who had AIS and TIA.
High resolution MRI in intracranial arterial substudy
A total of 30 study sites with the capability of 3D high-resolution black-blood vessel wall MRI (HR-MRI) participated the HR-MRI sub-study. HR-MRI were performed on 3.0 T Philips (Philips, Best, the Netherlands) or GE (GE, Milwaukee, Wisconsin, USA) scanners for all the patients in these sites with uniform scanning protocol during hospitalisation. Intracranial atherosclerotic stenosis and plaque images were collected through HR-MRI sequences. The main aim of the HR-MRI substudy was to investigate the association of intracranial atherosclerotic stenosis characteristics and the outcome of patients who had a stroke/TIA.
Genetic substudy
Although lots of stroke risk loci have been identified and replicated by large-scale genetic association studies to date,16 only few studies found a genetic contribution to recurrent strokes. A recurrent stroke shares many common risk factors with an ischaemic stroke.17 A previous study also showed that rare variants may also contribute to risk of complex diseases.18 In this study, blood samples were collected from the 171 study sites that participated in the genetic substudy and potential single-nucleotide polymorphisms (SNPs) were tested to identify loci that were associated with pathogenesis or outcomes of ischaemic stroke and TIA.
Biomarker substudy
Several biomarkers were tested using the blood and urine samples from the 171 study sites that participated in the biomarker substudy to investigate the association between the biomarkers and the outcome of the patients. Abbreviations and explanation of these biomarkers were listed in in online appendix S4.
Atherosclerotic plaque stability and coagulation
Biomarkers of atherosclerotic plaque stability (IL-1β, IL-6, TNF-α, hs-CRP, MCP-1, MMP-9 and sCD40L), thrombosis (t-PA, PAI-1 and vWF), fibrinogen and D-dimner at baseline and 3 months will be tested to evaluated the association between plasma these markers and outcome of stroke and TIA.
Homocysteine
Four biomarkers will be measured, including total homocysteine, two coenzymes—folate and vitamin B12— involved in homocysteine metabolism, and methylmalonic acid as a functional indicator of B12 status. We will aim to describe the prevalence of hyperhomocysteinemia, folate deficiency and vitamin B12 deficiency in patients with acute ischaemic stroke or TIA and test the hypothesis that elevated level of plasma total homocysteine in acute and/or recovery phase will predict the prognosis of stroke.
Renal function
Circulating or urinary relevant biomarkers for renal function, including serum creatinine, serum cystatin C, urine albumin-to-creatinine ratio, neutrophil gelatinase–associated lipocalin, uromodulin, Kidney Injury Molecule-1 and uric acid, will be tested at baseline and 3 months to determine the association of CKD and novel renal biomarkers with 1-year stroke outcomes in patients with ischaemic stroke or TIA.
Diabetes and glucose metabolism
Advanced Glycation End Products (AGEs), soluble receptor for AGEs (sRAGE) and endogenous secreted receptor for AGEs (esRAGE) will be measured to evaluate the association between AGE–RAGE axis and prognosis of ischaemic stroke. Fasting glucose, HbA1c, fasting insulin and C petide will be measured to evaluate the association between abnormal glucose regulation and prognosis of ischaemic stroke.
Proprotein convertase subtilisin/kexin type 9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an enzyme encoded by the PCSK9 gene in humans on chromosome 1.19 PCSK9 will be measured to evaluate the association between level of PCSK9 and prognosis of ischaemic stroke.
Inositol 1,4,5-triphosphate
Inositol 1,4,5-triphosphate (IP3) levels were determined using the Inositol-1,4,5-Trisphosphate (3 hour) Radioreceptor Assay Kit. Correlation between the PAR4 Thr/Ala-120 variation and plasma IP3 concentration in stroke patients will be evaluated.
Lipid and lipoprotein level and functions
Lipid level such as serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG) and lipoprotein (a), and lipoprotein function, such as cholesterol efflux capacity, will be measured to evaluate association of lipid level or functions and prognosis of ischaemic stroke and TIA.
Gut microbial metabolites
Dietary choline, gut microbes and TMAO directly may increase platelet hyperreactivity, thrombosis potential, atherosclerosis and outcomes of stroke.20 These markers will be measured by using mass spectrometry to investigate the role of gut microbial metabolites in the pathogenesis and prognosis of ischaemic stroke, and the effect on drug metabolism.
Statistical analysis
At each follow-up, a patient was considered as a dropout if the patient clearly refused to further participate in the study or could not be reached after three phone contact attempts at three different days. Patients who were not eligible at the baseline were excluded from the study. As we collected the data through EDC with automatic check for completeness of data, the missing rates for covariates were expected to be low. If necessary, medians and most common categories were considered as replacement of missing values for continuous and categorical variables, respectively.
Proportions were used to describe the categorical variables, means with SD or median with the IQR were used for continuous variables. t-Test or Mann-Whitney test will be performed to compare continuous variables, while as Χ2 test or fisher’s exact test will be performed to compare the categorical variables. The association of most imaging and biological markers with the occurrence of vascular events will be performed by univariate and multivariate Cox proportional hazard regression model and HRs with their 95% CIs will be evaluated. For the outcome of poor functional outcome, univariate and multivariate logistic regression will be performed and ORs with their 95% CI will be evaluated. A two-sided p value <0.05 was considered to be statistically significant.
In this article, the analyses focused on the baseline characteristics of the included hospital and patients. Proportions were used to describe the categorical variables, means with SD or median with the IQR were used for continuous variables. All analyses were conducted with SAS V.9.4.
Results
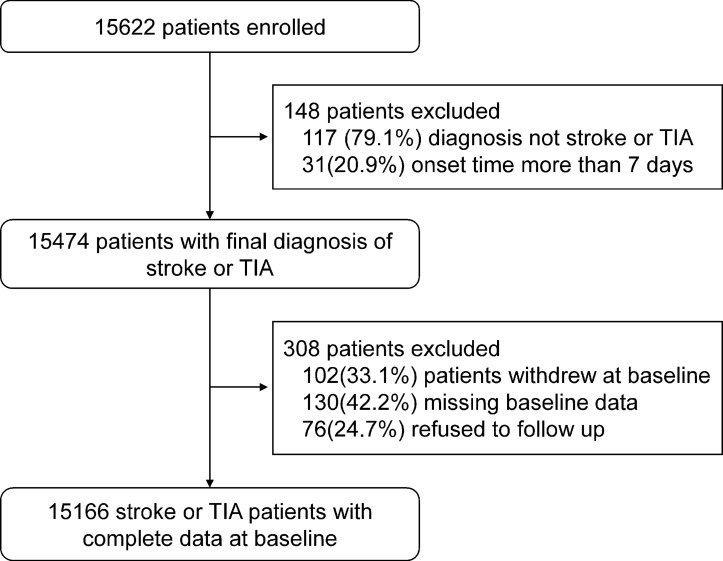
The patient recruitment phase has been completed. We initially screened 15 622 patients according to the inclusion criteria. There were 15 474 stroke patients who consented and were enrolled from 201 participating sites between August 2015 and March 2018. After excluding 102 patients who withdrew at baseline, 130 patients with missing baseline data and 76 patients who refused to follow-up, a total of 15 166 patients were eligible and had complete information at baseline. The detailed patient enrolment flow chart is shown in figure 2.
Figure 2.
Flow chart of patient enrolment in the Third China National Stroke Registry. TIA, transient ischaemic attack.
Baseline characteristics of the included patients are presented in table 2. Overall, 31.7% patients were women with the average age of 62.2±11.3 years. Among the patients, 31.3% were current smokers and 14.0% were heavy drinkers, 20.8% had a history of ischaemic stroke and 62.6% were hypertensive. The median time from disease onset to enrolment was 2.0 days. Overall, 1020 (6.7%) patients had TIA and 14 146 (93.3%) patients had ischaemic stroke, among which 51.7% had minor stroke (NIHSS ≤3).
Table 2.
Baseline characteristics of the included patients in CNSR-III (n=15 166)
| Characteristics | |
| Age (yr), mean±SD | 62.2±11.3 |
| Female, n(%) | 4802 (31.7) |
| Ethnicity (non-Han), n (%) | 440 (2.9) |
| Education, n(%) | |
| Elementary or below | 4292 (28.3) |
| Middle school | 4405 (29.1) |
| High school or above | 4282 (28.2) |
| Unkown | 2187 (14.4) |
| Current smoker, n(%) | 4752 (31.3) |
| Heavy drinker, n(%)* | 2126 (14.0) |
| Medical history, n(%) | |
| Ischaemic stroke | 3149 (20.8) |
| Coronary heart diseases | 1608 (10.6) |
| Atrial fibrillation | 1019 (6.7) |
| Hypertension | 9494 (62.6) |
| Diabetes mellitus | 3510 (23.1) |
| Hypercholesterolemia | 1191 (7.9) |
| Days from event onset to enrolment, median (IQR) | 2.0 (1.0–4.0) |
| NIHSS at admission, median (IQR) | 3 (1–6) |
| Stroke type | |
| TIA | 1020 (6.7) |
| IS | 14 146 (93.3) |
| NIHSS 0–3 | 7319 (51.7) |
| NIHSS≥4 | 6827 (48.3) |
*Heavy drinker was defined as ≥2 standard alcohol consumption/per day.
CNSR-III, the Third China National Stroke Registry; IS, ischaemic stroke; NIHSS, National Institutes of Health Stroke Scale score; TIA, transient ischaemic attack.
We compared the CNSR-III with the CNSR-I3 and the CNSR-II21 for area coverage, target population, the patient sizes and disease subtypes (table 3). The CNSR-III targeted the similar population with the CNSR-I and the CNSR-II but only focused on ischaemic stroke and TIA. Most important, comprehensive imaging and biological sample were collected and patients will be followed up for 5 years in the CNSR-III.
Table 3.
Comparison of CNSR-III with CNSR-I and CNSR-II
| Characteristic | CNSR-I | CNSR-II | CNSR-III |
| Published year | 2011 | 2016 | - |
| Study year | 2007–2008 | 2012–2013 | 2015–2018 |
| Patients (n) | 21 902 | 25 018 | 15 166 |
| Sites (n) | 132 | 219 | 201 |
| Age(yr), mean±SD | 63.8±12.9 | 64.3±12.2 | 62.2±11.3 |
| Female (%) | 38.8 | 37.4 | 31.7 |
| Onset to admission (days) | ≤14 | ≤7 | ≤7 |
| Disease subtypes | |||
| IS (%) | 66.4 | 78.4 | 93.3 |
| TIA (%) | 6.2 | 7.9 | 6.7 |
| ICH (%) | 23.4 | 12.1 | - |
| SAH (%) | 3.4 | 1.2 | - |
| Imaging and biological sample collection | No | No | Yes |
| Follow-up (years) | 1 | 1 | 5 |
CNSR, China National Stroke Registry; ICH, intracerebral haemorrhage; IS, ischaemic stroke; SAH, subarachnoid haemorrhage; TIA, transient ischaemic attack.
Discussion
The CNSR-III enrolled 15 166 patients from August 2015 to March 2018. The study profile and patient baseline characteristics were reported. Compared with the CNSR-I and the CNSR-II, the CNSR-III was unique, being a national registry of ischaemic stroke and TIA with comprehensive aetiological evaluation, imaging and biological sample collection, which provided the opportunity to identify and evaluate the imaging and biological markers for the prognosis of ischaemic cerebrovascular events.
Another strength of the CNSR-III was that the study used an EDC to capture data, which provide the opportunity of simultaneous automatic data check for completeness and logical correction of the uploaded data. Therefore, the rate of missing data was low and the quality of the data has been highly improved.
There were several limitations in this study. Selection bias were unavoidable in this study. The selection of participating sites in the CNSR-III was by convenience in nature, although the sample covered most areas of China. Similar to previous studies, the selected sites in this study mostly represented the hospitals with more medical resources and expertise than the low-level hospitals which were excluded from the study. The patient characteristics in the selected hospitals could be different from those in the excluded hospitals.
The CNSR-III was also limited by the fact that the follow-ups after 6 months were performed by phone interviews. Although it was performed by trained research coordinators following a standard protocol, the accuracy of self-reported clinical events needs to be evaluated, especially the mild clinical events might be underestimated.
The CNSR-III was a nationwide prospective registry of ischaemic cerebrovascular diseases designed to evaluate the aetiology, imaging and biological markers for the prognosis of ischaemic stroke and TIA. Data from the CNSR-III will provide evidences of prognostic determinants of stroke, and further explore comprehensive multi-dimensioned prediction model of outcome of stroke based on imaging and biological markers.
Footnotes
Contributor: YoW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YoW, JJ, XM, YiW and RJ. Supplying patients: ZW, XH, SW, ZJ and YC. Drafting of the manuscript: YoW, YP and HL. Critical revision of the manuscript for important intellectual content: YiW, XZ and HL. Statistical analysis: YP, HL and YJ. Study supervision and organisation of the project: YoW, XM, JL, WL, ZL, XZ, XY and CW.
Funding: This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2016YFC0901001, 2016YFC0901002, 2017YFC1310901, 2017YFC1310902, 2018YFC1311700 and 2018YFC1311706), and grants from Beijing Municipal Commission of Health and Family Planning (No.2016-1-2041, SML20150502).
Competing interests: None declared.
Ethics approval: The protocol of the CNSR-III study was approved by ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2015-001-01) and all participating centres.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Zhou M, Wang H, Zhu J, et al. . Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 2016;387:251–72. 10.1016/S0140-6736(15)00551-6 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Cui L, Ji X, et al. . The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke 2011;6:355–61. 10.1111/j.1747-4949.2011.00584.x [DOI] [PubMed] [Google Scholar]
- 4. Kernan WN, Ovbiagele B, Black HR, et al. . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 5. Li L, Yiin GS, Geraghty OC, et al. . Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015;14:903–13. 10.1016/S1474-4422(15)00132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ntaios G, Vemmos K, Lip GY, et al. . Risk Stratification for Recurrence and Mortality in Embolic Stroke of Undetermined Source. Stroke 2016;47:2278–85. 10.1161/STROKEAHA.116.013713 [DOI] [PubMed] [Google Scholar]
- 7. Wei JW, Heeley EL, Wang JG, et al. . Comparison of recovery patterns and prognostic indicators for ischemic and hemorrhagic stroke in China: the ChinaQUEST (QUality Evaluation of Stroke Care and Treatment) Registry study. Stroke 2010;41:1877–83. 10.1161/STROKEAHA.110.586909 [DOI] [PubMed] [Google Scholar]
- 8. Echouffo-Tcheugui JB, Xu H, Matsouaka RA, et al. . Diabetes and long-term outcomes of ischaemic stroke: findings from Get With The Guidelines-Stroke. Eur Heart J 2018;39:2376–86. 10.1093/eurheartj/ehy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amarenco P, Lavallée PC, Labreuche J, et al. . One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N Engl J Med 2016;374:1533–42. 10.1056/NEJMoa1412981 [DOI] [PubMed] [Google Scholar]
- 10. Pan Y, Meng X, Jing J, et al. . Association of multiple infarctions and ICAS with outcomes of minor stroke and TIA. Neurology 2017;88:1081–8. 10.1212/WNL.0000000000003719 [DOI] [PubMed] [Google Scholar]
- 11. Lin J, Zheng H, Cucchiara BL, et al. . Association of Lp-PLA2-A and early recurrence of vascular events after TIA and minor stroke. Neurology 2015;85:1585–91. 10.1212/WNL.0000000000001938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jing J, Meng X, Zhao X, et al. . Dual Antiplatelet Therapy in Transient Ischemic Attack and Minor Stroke With Different Infarction Patterns: Subgroup Analysis of the CHANCE Randomized Clinical Trial. JAMA Neurol 2018;75:711–9. 10.1001/jamaneurol.2018.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Wang Y, Wang D, et al. . Glycated albumin predicts the effect of dual and single antiplatelet therapy on recurrent stroke. Neurology 2015;84:1330–6. 10.1212/WNL.0000000000001421 [DOI] [PubMed] [Google Scholar]
- 14. Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407–31. 10.1161/01.STR.20.10.1407 [DOI] [PubMed] [Google Scholar]
- 15. Adams HP, Bendixen BH, Kappelle LJ, et al. . Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 16. Malik R, Chauhan G, Traylor M, et al. . Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018;50:524–37. 10.1038/s41588-018-0058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams SR, Hsu FC, Keene KL, et al. . Shared genetic susceptibility of vascular-related biomarkers with ischemic and recurrent stroke. Neurology 2016;86:351–9. 10.1212/WNL.0000000000002319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tennessen JA, Bigham AW, O’Connor TD, et al. . Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 2012;337:64–9. 10.1126/science.1219240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ference BA, Robinson JG, Brook RD, et al. . Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N Engl J Med 2016;375:2144–53. 10.1056/NEJMoa1604304 [DOI] [PubMed] [Google Scholar]
- 20. Zhu W, Gregory JC, Org E, et al. . Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016;165:111–24. 10.1016/j.cell.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z, Wang C, Zhao X, et al. . Substantial Progress Yet Significant Opportunity for Improvement in Stroke Care in China. Stroke 2016;47:2843–9. 10.1161/STROKEAHA.116.014143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2019-000242supp001.pdf (209.5KB, pdf)