Abstract
As a potential novel agent for treating pancreatic cancer, methylseleninic acid (MSeA) was evaluated in cell culture and xenograft models. Results showed that MSeA induced G1 cell cycle arrest and apoptosis in a majority of human and mouse pancreatic cancer cell lines, but G2 arrest in human PANC-1 and PANC-28 cell lines. In contrast to our previous finding in human prostate cancer LNCaP cells having a lack of P53 activation by MSeA, induction of G2 arrest in PANC-1 cells was accompanied by increased mutant P53 Ser15 phosphorylation, upregulation of P53-targets P21Cip1 and GADD45 and G2 checkpoint kinase (Chk2) activation, suggestive of DNA damage responses. A rapid inhibition of AKT phosphorylation was followed by reduced mTOR signaling and increased autophagy in PANC-1 cells attenuating caspase-mediated apoptosis execution. Furthermore, daily oral treatment with MSeA (3 mg Se/kg body weight) significantly suppressed growth of subcutaneously inoculated PANC-1 xenograft in SCID mice. Immunohistochemical analyses detected increased p-Ser15 P53, P21Cip1, pS139-H2AX (DNA damage responses) and caspase-3 cleavage and decreased pSer473AKT and Ki67 proliferative index and reduced intratumor vascular density in MSeA-treated xenograft. These results provide impetus for further research of MSeA in the therapy and/or chemoprevention of pancreatic cancer.
Keywords: Selenium, methylseleninic acid (MSeA), G2 arrest, apoptosis, autophagy, Akt-mTOR pathway
Introduction
Pancreatic cancer is the deadliest form of cancer in Western countries and is the fourth leading cause of cancer death in the United States [1]. Unfortunately, at the time of diagnosis, only 10% to 20% of pancreatic cancers are resectable. Because of the advanced stage at diagnosis, the median survival is less than 6 months and the 5-year survival rate is less than 5% [2]. One major reason for the poor prognosis for the patients is the insensitivity of pancreatic cancer to most therapies such as chemotherapy, radiotherapy and immunotherapy[3]. Therefore, novel agents for pancreatic cancer therapy are urgently needed.
In spite of the failures of selenomethionine (SeMet) supplement in men 50 years of age and older to lower their risk for prostate cancer in the SELECT study [4] and to prevent conversion of high-grade prostatic intraepithelial neoplasia to cancer in a Phase III trial [5], many preclinical studies, including ours, have suggested that selenium forms other than SeMet, especially putative methylselenol precursors such as methylseleninic acid (MSeA), have the potential to inhibit the genesis of solid cancers in different organ sites [6] [7] [8] [9]. Methylselenol has been considered an in vivo active anti-cancer metabolite [6] [7]. The overall efficacy of Se supplementation depends on a number of factors, such as the chemical form and the dosage administered, their systemic and tissue specific metabolism, and the etiology and stage of the neoplastic disease process [6] [7]. In contrast, cell culture and preclinical models have not shown anti-cancer activity of SeMet or selenized yeast (consisting mostly of SeMet), most probably because this seleno-amino acid is incorporated into general proteins in the place of methionine and does not efficiently produce methylselenol or related active anti-cancer metabolites [6] [7] [8].
Our cell culture studies have shown that MSeA inhibits the proliferation of angiogenically-stimulated vascular endothelial cells, human prostate carcinoma DU145 and PC-3 cells and androgen-dependent human prostate carcinoma LNCaP cells in vitro with G1 cell cycle arrest and caspase-mediated apoptosis [10] [11; 12] [13] [14]. Consistent with our in vitro findings, we demonstrated that MSeA suppressed tumor growth of DU145 and PC-3 xenograft in nude mice [8] and prostate primary carcinogenesis in the TRAMP mouse model [9; 15]. In addition, our previous results indicated that MSeA enhanced the apoptotic cell death induced by diverse classes of chemotherapeutic drugs [16] in part through down-regulating anti-apoptotic survival proteins such as survivin and Bcl-xL [17], highlighting a potential for MSeA as a therapeutic modality alone or in combination with other drugs.
With respect to pancreatic carcinogenesis, several studies have been performed on the role of selenium in rats and Syrian Golden hamsters between 1980 and 1990 [18; 19]. These previous reports showed that selenium might reduce or enhance pancreatic tumorigenesis depending on the form, dose, and frequency of application and duration. In later studies with sodium selenite, the incidence of azaserine-induced preneoplastic acinar lesions of the rat pancreas was decreased by sodium selenite but not by vitamin E [20]; whereas selenite altered tumor differentiation but not incidence or latency of pancreatic adenocarcinomas in Ela-TGFa p53+/− mice [21]. Our earlier work has extensively documented significant differences between selenite and methylselenol precursors in vitro in terms of genotoxicity and their in vivo efficacy against prostate xenograft models [8; 22; 23]. MSeA is a superior anti-cancer selenium form over sodium selenite in multiple organ sites and is expected to be so in pancreas as well.
Since very little work has been done to evaluate the potential for the next-generation selenium compounds for pancreatic cancer therapy, we initiated experiments using cell culture and pancreatic cancer xenograft models to inform such applications of MSeA to begin filling in the knowledge gap. We used 5 human pancreatic cancer cell lines (PANC-1, Colo357, Bxpc-3, HPAC, PANC-28) [24] and one mouse pancreatic cancer cell line (Ela-myc-1) [25] in this study. We found that MSeA induced G1 arrest and caspase-mediated apoptosis in most pancreatic cancer cell lines, while manifested a rapid G2 arrest in the PANC-1 and PANC-28 cell lines. We therefore investigated the molecular changes associated with this novel type of cellular action of MSeA within the context of daily dietary Se supplement regimen of clinical trials. In addition, our results showed an induction of autophagy by MSeA in the PANC-1 cells attenuating caspase-mediated apoptosis. Moreover, our animal data showed a significant tumor inhibitory efficacy of daily single oral administration of MSeA on PANC-1 tumor xenografts in SCID mice and concordance of a number of pharmacodynamic targets with in vitro results
Materials and methods
Chemicals
Methaneseleninic acid (same as MSeA, >95%, white powder) was purchased from Sigma Chemical Company (St. Louis, MO). Pan caspase inhibitor (z-VAD-fmk) was purchased from MP-Biomedical (Aurora, OH).
Cell culture and treatment
The cell lines used were provided by co-author Liao, who originally obtained them from the American Type Culture Collection (ATCC). PANC-1, HPAC, PANC-28 and Ela-myc-1 cells were cultured in DMEM, whereas Colo357 and Bxpc-3 cells were cultured in RPMI-1640, all supplemented with 10% FBS and 5% CO2 without antibiotics. At 24 hours after plating, the medium was changed before starting the treatment with MSeA or the other agents.
Cell growth evaluation
Cells were seeded in 6- or 12-well plates. Triplicate wells were used for each test concentration of MSeA. For the evaluation of the overall inhibitory effect of MSeA on cell number, the cells were treated daily with MSeA in fresh medium. After treatment, the culture medium was removed and the cells were fixed in 1% glutaraldehyde solution in PBS for 15 min and stained with 0.02% aqueous solution of crystal violet for 30 min as described before [16]. After washing with PBS, the cell-retained dye was solubilized with 70% ethanol. The absorbance at 570 nm with the reference filter 405 nm was evaluated using a micro plate reader (Beckman Coulter, Inc., Fullerton, CA) to estimate relative cell number.
Cell cycle and cell death/apoptosis
Cell cycle analyses were carried out with propidium iodide (PI) staining according to Krishan’s protocol [26] using a FACS Calibur flowcytometer (Becton Dickinson, San Jose, CA). Cell death was detected by either an ELISA kit for nucleosomal DNA fragmentation purchased from Roche Diagnostics Corp. (Indianapolis, IN) [27], or by an immunoblot analysis of the caspase-mediated cleavage of poly(ADP-ribose) polymerase (PARP) as described previously [10].
Caspase activity assay
The enzymatic activity of caspase-3 and 6 was assessed via a fluorescent or luminescent assay utilizing specific substrates, a caspase-specific peptide that is conjugated to the fluorescent reporter molecule 7-amino-4-trifluoromethyl coumarin (AFC) or luminogenic, Z-VDVAD-Glo™, respectively. The kits were purchased from R&D and Promega, respectively. All the procedures were performed following the manufacturers’ manuals and as described [27].
Immunoblotting
Cell lysate preparation and immunoblotting were as described previously [10; 28]. Antibody for CDC-2/Cdk1, Cyclin B1, survivin, p-AKT, c-PARP, P21Cip1, P27Kip1, GADD45, Bcl-xl, p-chk2, LC-3, Beclin1, p60S6k, mammalian target of rapamycin (mTOR), p-4EBP1 and secondary rabbit or mouse antibody were purchased from Cell Signaling Technologies, Beverly, MA.
siRNA
Specific siRNAs targeted to Beclin-1 were purchased from Santa Cruz Biotechnologies with control siRNA. The cells were transfected with 20 nmol/L siRNAs using INTERFERin transfection reagent (Polyplus-Transfection, Inc.) for 24 h and then were used for subsequent experiments with MSeA treatment. The cells were treated for 48 h with 5 μM MSeA before immunoblotting.
Flow cytometric analysis of Ser10-phosphorylated histone H3
Phosphorylation of histone H3 on Ser10 is a sensitive marker for mitotic cells, in that it increases in prophase, peaks in metaphase, and declines in anaphase [29]. The PANC-1 cells were treated for 12 or 24 h with 5μM MSeA, fixed in methanol at 4°C, suspended in 1 mL of 0.25% Triton X-100 in PBS, and then suspended in 100 μL of PBS containing a polyclonal anti–phosphor-(Ser10)-histone H3 antibody, and FITC-conjugated goat anti-rabbit antibody (1:50 dilution in PBS containing 1% BSA). Cellular fluorescence was measured using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA).
Xenograft experiment
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of University of Minnesota. Subcutaneous inoculation of 7.5×105 PANC-1 cells in 80 μL PBS was carried out in 13-week-old SCID mice. The day after inoculation, mice were treated daily (Mon. to Fri.) with water (control) or MSeA at 3 mg Se/kg body weight by oral delivery as used in previous studies [8; 9]. All mice were euthanized at 20 weeks of age for collection of xenograft tumors and biomarker analyses.
Immunohistochemical Analyses
PANC-1 xenograft tumors were dissected and fixed in 10% (v/v) neutral-buffer formalin for 24 hours and then transferred into 70% ethanol until further processing. The fixed tissues were dehydrated in ascending grades of ethanol and xylene, and then embedded in paraffin wax. Sections (4 μm) were cut with microtome and mounted on superfrost®/plus microscope slides. Immunostaining was performed by using antibodies for the proliferation marker protein (Ki67) (Thermo Scientific 1:150), p-AKT (Cell Signaling 1:100), c-Caspase83 (Cell Signaling 1:100), P21 (Santa Cruz 1:100), p-H2AX (ser139, Millipore) or CD34 antibody (Abcam 1:50). The biotinylated secondary antibody was goat anti-rabbit and rat antibody IgG (1:200 in 10% normal rabbit serum; Vector Laboratories). The slides were developed in diaminobenzidine (DAB) and counter stained with hematoxylin. The stained slides were dehydrated and mounted in permount. Images were captured and analyzed by Image-pro-plus V 6.2 software.
Statistical Analysis
Numerical results are expressed as mean ± SEM. Treatment effects were compared by ANOVA or Student’s t-test (when only 2 groups) and differences between means were considered to be significant when P < 0.05.
Results
MSeA suppressed growth of pancreatic cancer cell lines and induced apoptosis with variable efficacy in vitro
Daily treatment with MSeA decreased the cell number in a concentration-dependent manner of both the human pancreatic cancer cell line PANC-1 and mouse pancreatic cancer Ela-myc-1 cell line (Fig. 1A). We estimated the MSeA concentration that exerted 50% inhibition on cell growth (IC50) to be 2.6 μM at 5 days in PANC-1 cells and 3.2 μM at 3 days in Ela-myc-1 cells. Strong suppression efficacy of MSeA was also observed in other pancreatic cancer cell lines (Supplemental Fig. S1).
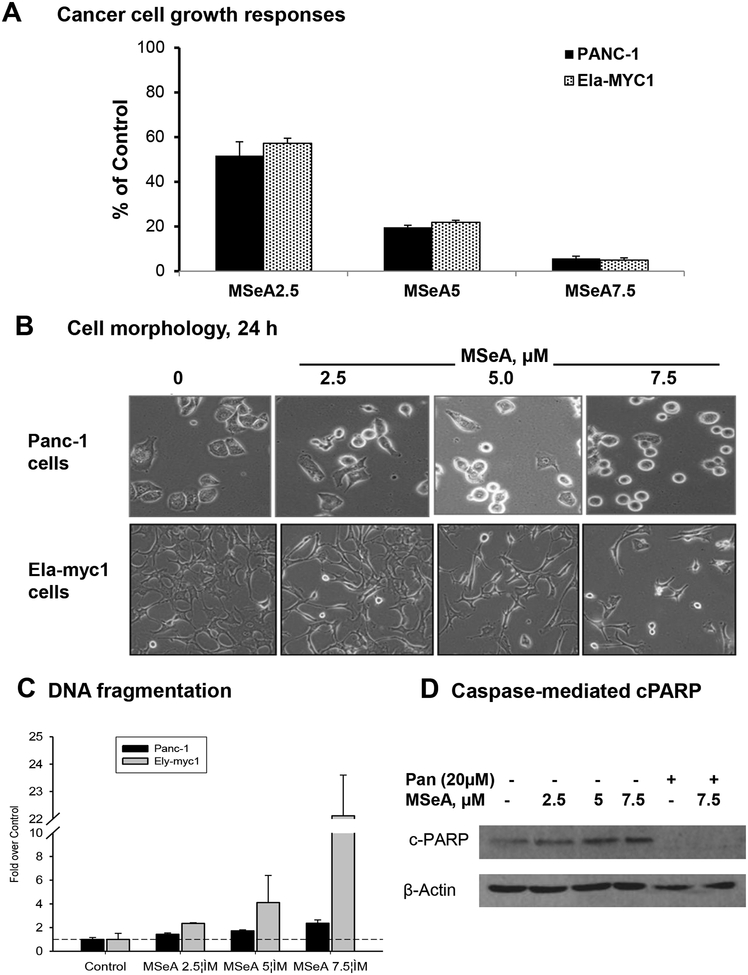
Figure 1. MSeA treatment inhibited cell growth of pancreatic cancer cell lines with variable apoptosis efficacy.
A. Effect of daily MSeA treatment (medium change) on PANC-1 (5 days) and Ela-myc1 (3 days) cell growth. Data were mean ± SEM of triple wells. Absorbance of control wells were set as 100%. B. Representative phase-contrast images of PANC-1 vs. Ela-myc cells after 24 h treatment with MSeA. C. Concentration-dependent induction of apoptotic DNA nucleosomal fragmentation detected by ELISA, after 24 h MSeA exposure, in PANC-1 and Ely-myc1 cell lines. D. Western blot detecting cleaved PARP in PANC-1 cells upon MSeA treatment for 48 h in the presence or absence of pan-caspase inhibitor, zVAD-fmk.
Acute MSeA exposure changed the human PANC-1 cancer cell morphology in a concentration-dependent manner, prominently displaying shiny rounded cells (Fig. 1B shows 24 h). On the other hand, MSeA-treated mouse Ela-myc-1 cells detached from the culture ware surface whereas the remaining adherent cells assumed elongated shapes (Fig. 1B shows 24 h). To determine whether the cellular morphology changes were differentially associated with apoptosis, we analyzed DNA nucleosomal fragmentation in PANC-1 cells and in Ela-myc1 cells after 24 h of MSeA treatment. As shown in Fig. 1C, 7.5 μM MSeA induced a modest 2.2-fold of apoptotic fragmentation in PANC-1 cells compared to control, but the same treatment induced more than 20-fold increase for the Ela-myc1 cells. Consistent with the low level of apoptotic DNA nucleosomal fragmentation in PANC-1 cells induced by MSeA, PARP cleavage status of PANC-1 cells at 48 h of MSeA exposure showed a slight elevation of c-PARP in a concentration-dependent manner (Fig. 1D). The PARP cleavage (Fig. 1D) and apoptotic DNA fragmentation (not shown) were inhibited by a caspase pan inhibitor. These results indicate lower micro-molar MSeA inhibited pancreatic cancer cell growth, but induced caspase-mediated apoptosis with variable efficacy depending on the cell lines studied.
MSeA induced G2 cell cycle arrest in select pancreatic cancer cell lines, and G1 arrest in others
Flow cytometric analyses showed that treatment with MSeA induced G1 arrest at 24 h in a concentration-dependent manner in Ela-myc-1 cells (Fig. 2A). Analyses of additional pancreatic cancer cell lines showed that MSeA induced G1 arrest in Colo357, Bxpc-3, HPAC cells at 12, 24 and 48 h (Table 1), as would be predicted from the G1 arrest effect of MSeA in vascular endothelial and many other cancer cell lines [11; 14; 22].
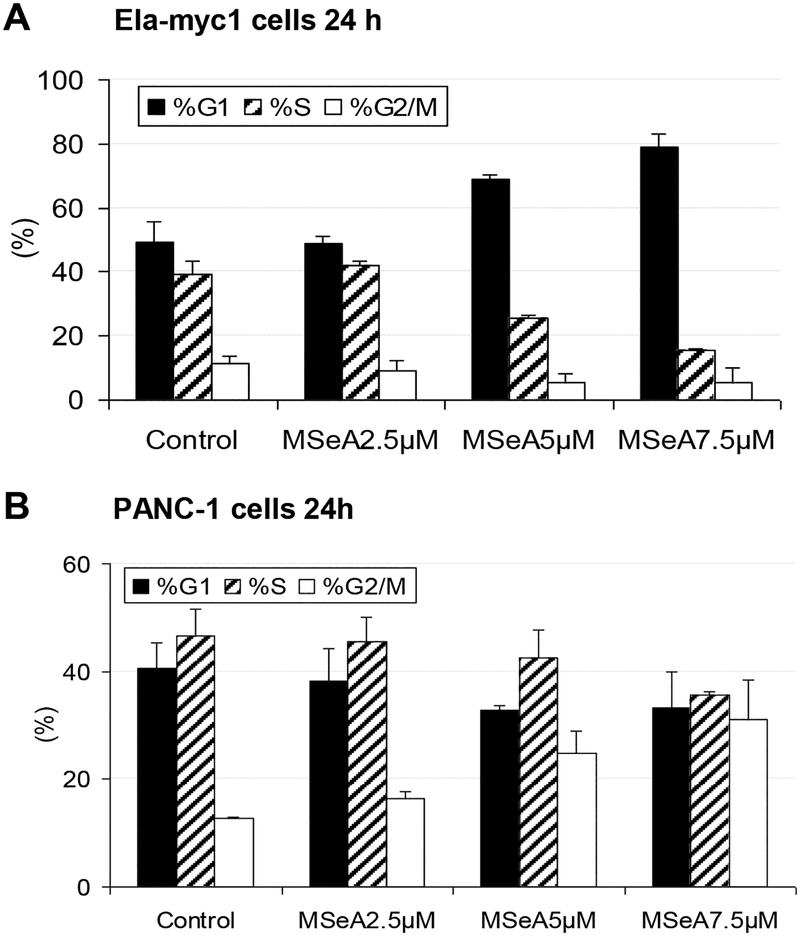
Figure 2. MSeA arrested pancreatic cancer cells in different phases of cell cycle.
Cell cycle distribution analyzed by propidium iodide staining and flowcytometry. A. Enrichment of G0/1 arrested mouse Ela-myc1 cells after 24 h MSeA treatment. B. Enrichment of “G2/M”- phase PANC-1 cells after 24 h MSeA treatment.
Table1.
Summary of cell cycle arrest patterns in different pancreatic cancer cell lines induced by MSeA
| Exposure time (h) | Treatment | Cell lines | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BxPc-3 | MiaPaca-2 | PANC28 | Colo357 | ||||||||||
| %G1 | %S | %G2/M | %G1 | %S | %G2/M | %G1 | %S | %G2/M | %G1 | %S | %G2/M | ||
| 12 | Control | 30.79 | 49.91 | 19.3 | 45.64 | 41.34 | 13.02 | 46.32 | 44.88 | 8.8 | 48.47 | 38.73 | 12.8 |
| 12 | MSeA2μM | 50.68 | 33.02 | 16.29 | 71.44 | 13.16 | 15.39 | 47.47 | 33.09 | 19.43 | 56.91 | 22.2 | 20.89 |
| 24 | Control | 38.9 | 48.72 | 12.38 | 53.9 | 31 | 15.1 | 36.37 | 45.39 | 18.24 | 51.74 | 35.24 | 13.02 |
| 24 | MSeA2μM | 60.73 | 27.52 | 11.75 | 73.76 | 12.97 | 13.27 | 27.29 | 46.26 | 26.45 | 58.43 | 36.94 | 4.63 |
| 48 | Control | 39.65 | 42.69 | 17.66 | 64.67 | 21.63 | 13.69 | 48.88 | 43.26 | 7.86 | 50.72 | 42.24 | 7.03 |
| 48 | MSeA2μM | 57.12 | 32.01 | 10.87 | 83.15 | 9.12 | 7.73 | 46.79 | 35.78 | 17.43 | 55.88 | 33.83 | 10.29 |
However, rather unexpectedly, MSeA enriched the proportion of PANC-1 cells with 4N DNA content of “G2/M” phase in a concentration-dependent manner when examined at 24 h of exposure (Fig. 2B). This enrichment of “G2/M”-phase cells could be observed at 12 h in PANC-1 cells (Fig. 3A), as well as in PANC-28 cells at 12 and 24 h of MSeA exposure (Table 1).
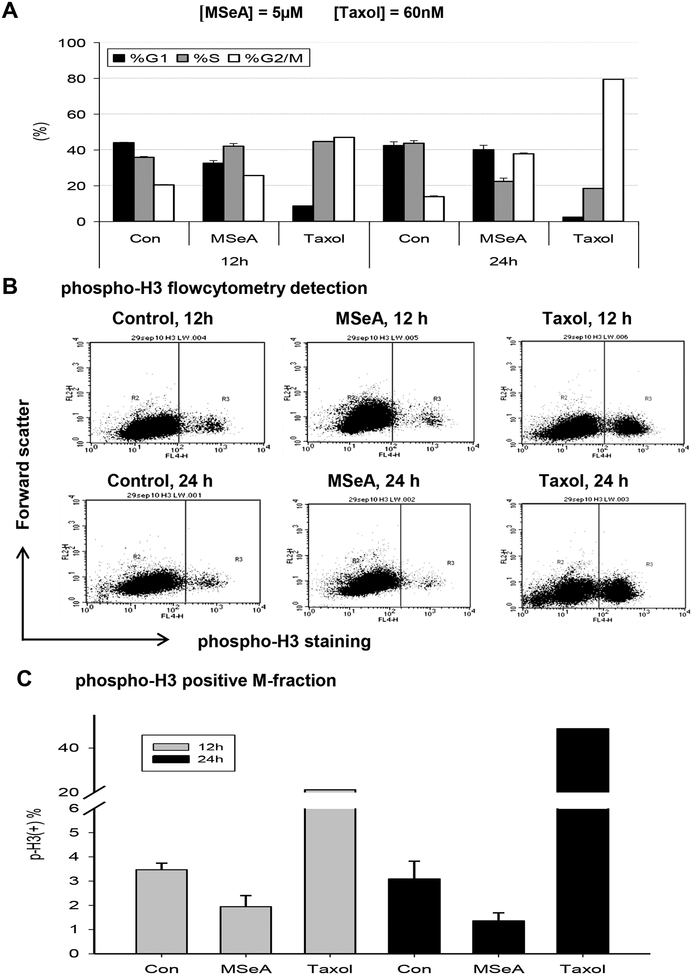
Figure 3. The G2 cell cycle arrest nature in MSeA-treated PANC-1 cells.
A. Comparison of cell cycle distribution patterns (propidium iodide staining) induced by MSeA vs. Taxol, an M-arrest inducer. B. Flow cytometric analyses of immune-labeled M phase marker phosphor-H3 in PANC-1 cells treated with 5 μM MSeA or 60 nM Taxol for 12 h and 24 h. C. Paucity of MSeA-arrested cells at M phase vs. taxol-arrested cells at M phase by phosphor-H3 labeling.
To delineate the nature of the arrest being either G2- or M-phase specific in PANC-1 cells, we examined the Ser10 phosphorylation of histone H3 (p-H3) [29] as a sensitive marker for mitotic cells. Propidium iodide flow cytometric analysis showed that the taxol-treated PANC-1 cells achieved progressive M-arrest, i.e., 44% at 12 h and 79% at 24 h from 14–21% G2/M in cycling control cells (Fig. 3A), as expected of the microtubule-targeting drug. Taxol treatment led to the accumulation of p-H3 (+) M-phase cells (Fig. 3B), accounting for approximately half of the “G2/M” population at each time point (Fig. 3C) in comparison to 3–3.4% M-cells of the 14–21% “G2/M” phase in control groups. In contrast, there were only 2% and 1.5% of p-H3 (+) M-phase cells with MSeA treatment for 12 and 24 h (Fig. 3B and C), in spite of the increased “G2/M” fractions detectable by regular flow cytometry with PI staining (Fig. 3A). Together, these data supported an induction of G2 arrest (failure to transit G2-M boundary), not M arrest by MSeA in PANC-1 cells.
Effect of MSeA on proteins involved in cell cycle and G2 checkpoint of PANC-1 cells
Due to the unexpected MSeA-induced cell cycle arrest patterns (i.e., G2 arrest) observed for some pancreatic cancer cell lines, we next surveyed molecular events affected by MSeA in PANC-1 cells. Cognizant of mutant p53 gene (codon 273 CGT→ CAT, i.e., Arg → His) [30] in PANC-1 cells, we analyzed mutant P53 protein abundance and its phosphorylation (Ser15) indicative of DNA damage response through ATM kinase, G2 checkpoint kinase Chk2 (also known to phosphorylate P53 in response to DNA damage) and selected cell cycle regulatory and checkpoint proteins, such as P21Cip1 (a known P53 transcriptional target), GADD45 (a known P53 transcriptional target), G2 Cyclin B1 and its partner kinase Cdc-2 (also known as Cdk1), as well as survival proteins Bcl-xL and survivin by immunoblotting. In G2 phase, the Cyclin B1/Cdc-2 (Cdk1) complex plays a crucial role to promote G2 transition to M phase: once the cells have acquired 4N DNA, the Cyclin B1 level promptly increases to activate the enzyme complex activity to move into mitosis [31]. GADD45 is known to bind and dissociate the Cdc2 (Cdk1)/Cyclin B complex, whereas P21Cip1 is an inhibitor of the Cdc2 (Cdk1)/Cyclin B holoenzyme activity as well as CDK4/6/Cyclin D and Cyclin E/CDK2 complexes for G1 progression.
Immunoblot of extracts of PANC-1 cells exposed to MSeA for 24 h showed increased Ser15 phosphorylation of P53 in a concentration-dependent manner without affecting total P53 protein level (Fig. 4A). The elevated p-Ser15 P53 was accompanied by increased p-Thr68 Chk2 for G2 checkpoint activation. MSeA increased P21Cip1 and GADD45 (both transcriptional targets of wild type P53) in lock-step manner with p-Ser15 P53, without affecting the expression of GADD34 (Fig. 4A) or GADD153 (neither is a known target of P53; data not shown).
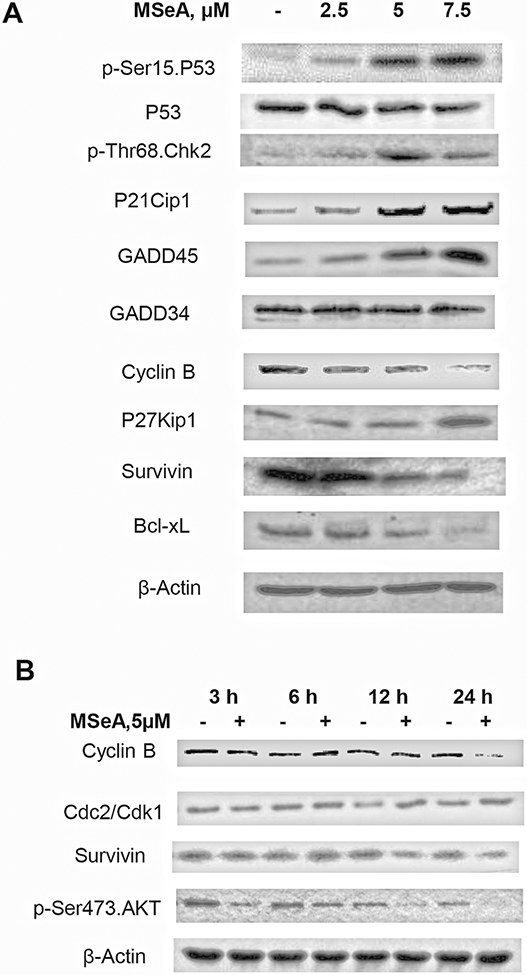
Figure 4. Western blot of protein changes involved in G2 cell cycle checkpoint, DNA damage response, cell survival and apoptosis in MSeA-treated PANC-1 cells.
A. Concentration-dependent changes of p-Ser15 P53, P21Cip1, GADD45, CyclinB, P27Kip1, and p-Thr68 Chk2, and anti-apoptotic proteins Bcl-xL and survivin in PANC-1 cells treated with MSeA for 24 h. B. Time course of MSeA-induced changes of Cyclin B, Cdc2/Cdk1, p-Ser473 AKT and survivin in PANC-1 cells. β-Actin served as loading control.
Cyclin B1 abundance was decreased by MSeA in a concentration-dependent manner (Fig. 4A) and this effect was not detectable until 24 h (Fig. 4B). In the same time course experiment, increased expression of Cdc2/Cdk1 protein was detected at 12 and 24 h of MSeA treatment (Fig. 4B), likely reflecting the increased percentage of G2-arrested cells by these time points.
The above changes were accompanied by decreased expression of Bcl-xL and survivin (Fig. 4A), which have been shown by us to be decreased in MSeA-treated prostate cancer cells [17]. In contrast to the above concentration-dependent changes across the three MSeA exposure levels, an increase of G1 CDK4/6 inhibitor P27Kip1 was only detected at the highest tested level of MSeA (Fig. 4A). Taken together, the induction of the P21Cip1/GADD45 (P53 transcriptional targets) in this mutant P53 carrying cell line, through or with the activation of G2 checkpoint responses (e.g., increased p-Thr68 Chk2) and the reduction of cyclin B would be expected to slow down G2 transition, leading to increased cells with 4N DNA content detectable by flow cytometry at 12 and 24 hours.
MSeA inhibited AKT-mammalian target of rapamycin (mTOR) pathway and activated autophagy to attenuate caspase-mediated apoptosis in PANC-1 cells
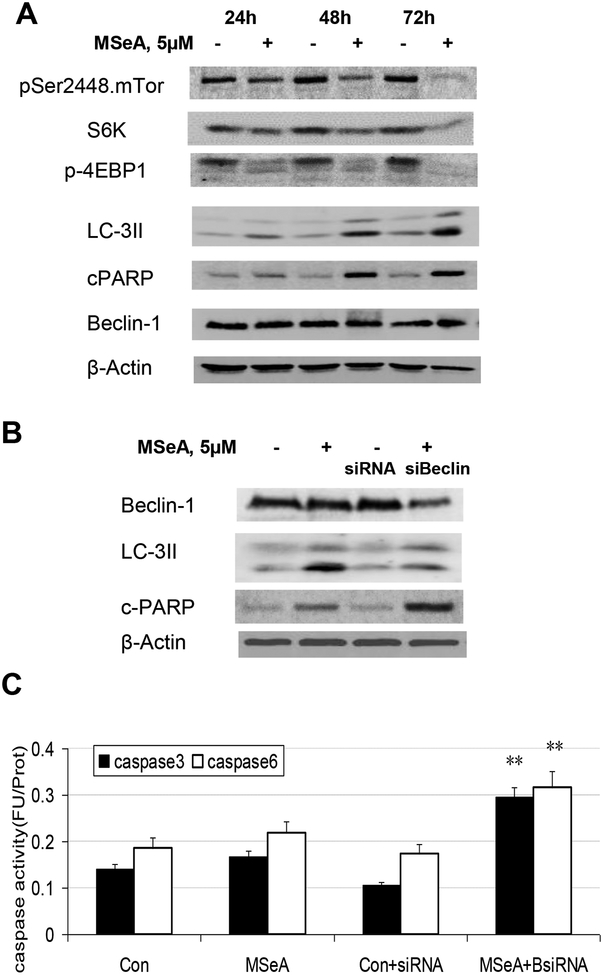
Autophagy is an evolutionarily conserved, lysosomal mediated catabolic response that is induced under nutrient-poor conditions and can be induced by inhibition of PI3K activity[32]. Since we have reported decreased phosphorylation of PI3K downstream target AKT by MSeA in prostate and endothelial cells [11] [13] [22], we examined the time course of pAKT in PANC-1 cells after acute MSeA exposure. We observed a rapid suppression of AKT Ser473 phosphorylation and subsequent diminishment of the expression of survivin, which is also a known AKT downstream target (Fig. 4B). Consistent with the decreased pAKT signaling, MSeA treatment of PANC-1 cells decreased mTOR phosphorylation at Ser2448, and mTOR downstream target proteins p-4EBP1 and S6K levels (Fig. 5A).
Figure 5. Effect of MSeA on mTOR pathway signaling and autophagy in PANC-1 cells.
A. Western blot detection of MSeA-induced changes of proteins or/and their phosphorylation of mTOR and its downstream targets and autophagy marker LC-3. B. Impact of inhibiting autophagy by knocking down Beclin-1 on MSeA-induced caspase-mediated apoptosis in PANC-1 cells after 48 h. PANC-1 cells were transiently transfected with siRNA for Beclin1. Protein lysates were probed with the antibodies against Beclin 1, LC3-II, and c-PARP. β-actin served as loading control. C. The enzymatic activities of caspase-3 and 6. Cells were treated with MSeA for 48 h. Results were plotted as mean ± SD from three independent experiments. **p<0.01 when compared with control.
The observed suppression of AKT phosphorylation and mTOR signaling led us to suspect that the rather low sensitivity of PANC-1 cells to undergo caspase-mediated apoptosis upon MSeA exposure (Fig. 1C) might involve autophagy. The conversion of LC3 to the faster migrating lipid modified LC3-II form and their incorporation into autophagosomes have been used as indicators of autophagy [33]. Immunoblotting detected an MSeA-induced increase of LC3-II protein level in PANC-1 cells treated for 24 h and beyond, in lock step with the increased PARP cleavage (Fig. 5A). MSeA treatment did not alter the level of autophagy protein Beclin-1 (Fig. 5A) [34]. The induction of autophagy response by MSeA was also observed with selected pancreatic cancer cell lines (MIAPACA-2, HPAC and BXPC-3), but not in others (e.g., Colon357, PANC-28) (Supplemental Fig. S2).
To assess the relationship between autophagy and apoptosis induced by MSeA, we knocked down Beclin-1 by siRNA. As shown in Fig. 5B, reduction of Beclin-1 attenuated autophagic LC3-II level in PANC-1 cells treated with 5μM MSeA for 48h. This was accompanied by increased apoptotic PARP cleavage (Fig. 5B) and increased caspase-3 and caspase-6 activities (Fig. 5C). Therefore, the induction of autophagy by MSeA, likely through suppression of AKT/mTOR signaling, attenuated caspase-mediated apoptosis in PANC-1 cells.
Orally administered MSeA suppressed PANC-1 xenograft tumor growth in SCID mice
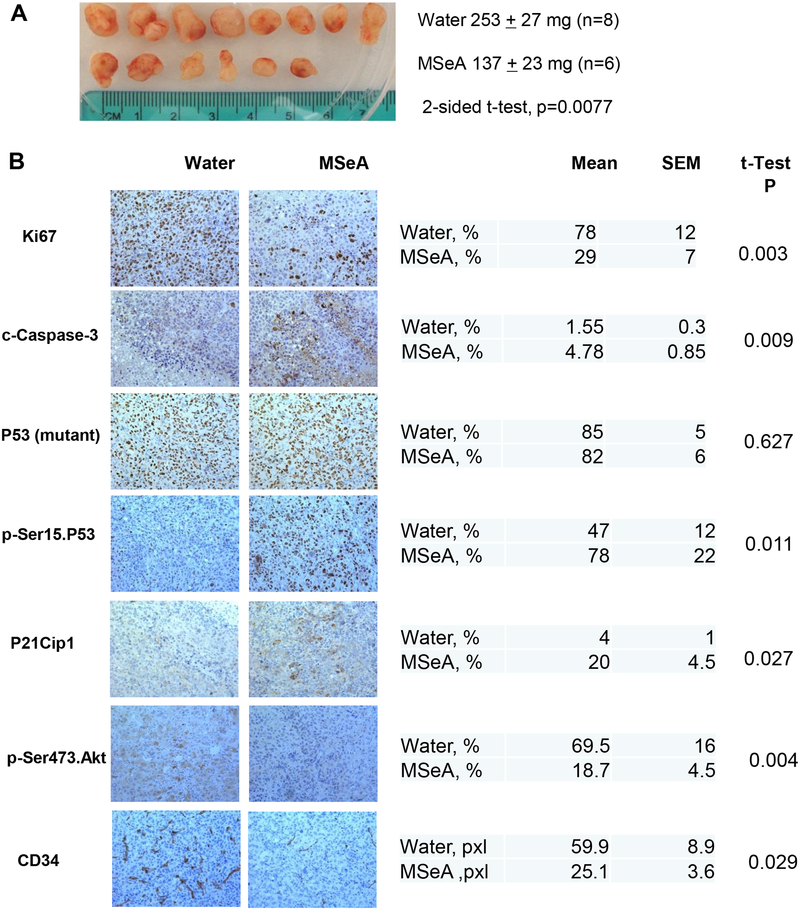
A daily single dose of 3 mg Se as MSeA per kg body weight starting the day after the inoculation of PANC-1 cells in SCID mice suppressed the growth of xenograft (tumor weight) by 46% at termination of experiment (p = 0.0077; Fig. 6A) without any effect on the body weight of the mice (33.8 g vs. 33.7 g at termination, P=0.78), as would be predicted from our previous studies using this dosing regimen [8; 9; 15; 17]. The results from immunohistochemical staining indicated that Ki67 proliferative biomarker was decreased, and cleaved (active) caspase-3 as a biomarker for apoptosis was increased in MSeA-treated xenograft tissue sections (Fig. 6B). Whereas there was detectable mutant P53 nuclear protein staining in virtually all the cancer epithelial cells in the tumor sections of both control and MSeA groups, as expected of a highly stabilized mutant P53 protein, p-Ser15 P53 was increased in MSeA-treated tumors. As a biomarker of DNA double strand breaks and damage response, nuclear staining for H2AX Ser139 phosphorylation was elevated in MSeA-treated xenografts. Consistent with in vitro upregulation of P21Cip1 by MSeA, the percentage of P21-positive cells (nuclear) was higher in MSeA group than control group.
Figure 6. Effect of daily oral-administered MSeA (3 mg Se/kg BW) on growth of subcutaneously inoculated PANC-1 xenografts in SCID mice.
A. The gross appearance of xenograft tumors from SCID mice at termination and the final tumor weights in control and MSeA-treated group. B. Immunohistochemical (IHC) analyses of biomarker changes involved in cell proliferation, apoptosis and angiogenesis in PANC-1 xenograft tumors. Representative images of IHC staining were shown in the left panel and the quantification of the IHC staining was shown in the right panel. Five to 10 pictures were randomly taken under microscope for each section and then analyzed by Image pro plus 6.2 software. For CD34, area in pixels x10−3.
In agreement with in vitro data, IHC detected decreased p-AKT in MSeA-treated tumor sections than the controls (Fig. 6B). Furthermore, IHC staining revealed decreased CD34 signals as an endothelial specific biomarker for suppressed angiogenesis in the MSeA-treated tumors (Fig. 6B). Taken together, the in vivo data showed concordance of a number of molecular changes with in vitro observations (e.g., decreased pAKT, increased p-Ser15 P53 increased P21Cip1 and increased caspase activation) and involvement of additional cellular processes such as suppressed angiogenesis in in vivo suppression of PANC-1 tumor growth.
Discussion
As a point of reference for the concentrations of MSeA evaluated in cell culture, the serum total selenium level in mice given an oral administration of MSeA (3 mg Se/kg body weight,) rose from baseline of 6 μM to a peak concentration (Cmax) of 12 μM, an increase of 6 μM [8]. In human trials, daily supplementation of 200 microgram of Se in the form of SeMet or selenized yeast resulted in an increase of total plasma Se of approximately 1 μM [4; 35]. A first-in-human single dose pharmacokinetic study for MSeC showed mean Se Cmax increment over placebo rose in a dose–response fashion from 22.8 (~0.29 μM) to 30.75 (~0.39 μM) and 63.2 (~ 0.8 μM) ng/mL for the 400, 800, and 1,200 μg dose subjects, respectively [36]. Therefore the concentrations we tested could be achievable at a pharmacological intake.
Given that the cellular and molecular effects of MSeA have been studied in prostate, mammary cancer cell lines and HUVECs [7] [10] [11; 13; 22], our current investigation with pancreatic cancer cell lines confirmed some aspects anticipated from information derived from the cancer cells of other organ sites, including caspase-mediated apoptosis, G1 arrest, and AKT inactivation. However, our data revealed also several unexpected findings and novelty of modes of action of MSeA in pancreatic cancer.
The first aspect of novelty is the observation of MSeA-induced G2 arrest in PANC-1 and PANC28 cell lines (Fig. 2 and 3, Table 1) and the molecular changes associated with this cellular effect. The G2 checkpoint response (Chk2 phosphorylation) and up-regulation of protein abundance of GADD45 and P21Cip1 in PANC-1 cells were in lock-step with increased Ser15 phosphorylation of the mutant P53 (273 Arg→ His) while several non P53-target proteins known to be involved in G1 cell cycle arrest such as GADD34 and P27Kip1 were either not affected or only at the highest exposure level of MSeA. One reasonable explanation would be that MSeA induced a P53-independent up-regulation of GADD45 and P21Cip1 on account of the mutant p53 gene in the PANC-1 cell line. Alternatively, we speculate that (a) the 273 Arg → His mutant P53 protein (e.g., by mass action ratio due to increased stability) may be partially active transcriptionally for these known P53 target genes, or (b) MSeA may, through redox or chemical actions, partially restore the P53 transcriptional activity of the mutant P53 in the PANC-1 cellular context. Resolution of these questions will increase our knowledge of how MSeA modifies or interacts with mutant P53 protein in regulating pancreatic cancer cell growth and survival specifically, and in other cancers in general.
The activation of p-H2AX/P53/GADD45/P21Cip1 and G2 checkpoint responses in PANC-1 cells by MSeA suggested DNA damage and repair responses (e.g., ATM pathway). In LNCaP prostate cancer cells with wild type P53, we have shown an absence of reactive oxygen species and lack of increased p-Ser15 P53 upon MSeA exposure, whereas the known genotoxic selenite induced P53-mediated, caspase-driven apoptosis [23; 27]. In recently published work with “normal” lung (MRC-5) and colon (CRL-1790) fibroblasts, prolonged MSeA exposure had been shown to activate ATM kinase → H2A.X → P53 → P21Cip1 DNA damage responses and cellular senescence pathway, but no cellular senescence in prostate cancer (PC-3, p53 null) or colon cancer (HT116, p53 wild type) cell lines [37] [38]. Whether organ-specific metabolism of MSeA caused such differential biochemical and cellular responses should be addressed in the future. Given the functional differences of the pancreas with other organs, such speculation may not be inconceivable.
The second aspect of novelty is the induction of autophagy by MSeA in PANC-1 and a number of other pancreatic cancer cell lines (Fig. 5, Supplement Fig S2). Whereas we are not aware of any report of this activity with MSeA in other cell lines, selenite has been reported to induce autophagy in multiple cancer cell lines [39] [40; 41; 42; 43; 44]. In our hands, MSeA-induced autophagy could be detected as increased LC3-II protein level (Fig. 5) when PANC-1 cells were treated for 24 h or longer. In fact in other pancreatic cancer cell lines and PC-3 prostate cancer cell line (data not shown), which is rather refractory to MSeA-induction of apoptosis, we found that MSeA increased LC3-II protein level. Since our siRNA approach indicated that knocking down beclin-1 protein level decreased LC3-II level in PANC-1 cells and increased PARP cleavage (Fig.5B), autophagy induction by MSeA appeared to be a survival mechanism in response to MSeA exposure to offset the apoptotic action. Such observation suggests that combining MSeA with an autophagy inhibitor drug might increase cancer-killing efficacy.
In terms of apoptosis and autophagy signaling mechanisms induced by MSeA, we confirmed that MSeA decreased AKT phosphorylation (activity) in PANC-1 cells in vitro and in vivo, and decreased survivin and Bcl-xl level, as we have found in prostate cancer cell lines and vascular endothelial cells [11; 13; 17; 22]. PI3K/AKT/mTOR pathway plays a critical role in controlling cell growth, survival and fate. The mTOR regulates many processes, including protein synthesis, ribosome biogenesis, and autophagy [45; 46]. Phosphorylated at Ser2448 by AKT, the activated mTOR transmits a positive signal to p70S6 kinase and participates in the inactivation of the eIF4E inhibitor 4E-BP1 [47]. P70S6 kinase recruits the 40S ribosomal subunit into actively translating polysomes, thereby enhancing the translation of mRNAs [48]. Phospho-4E-BP1 represses the initiation of protein translation through its association with eIF4E, the mRNA cap-binding subunit of the eIF4F complex [49]. Inhibition of mTOR stops the protein synthesis machinery by inactivating p70 S6 kinase and activating the 4E-BP1, and induces autophagy. In our hands, MSeA not only decreased AKT phosphorylation (activity) but also inhibited key mTOR targets (Fig 4 and 5) as possible cause for the induction of autophagy that attenuated apoptosis execution.
The known AKT downstream target protein survivin is a dual regulator of cell survival and mitosis, and is over-expressed in most human tumors but undetectable in most normal adult tissues [50]. Survivin binds and inhibits caspase-3, controlling the checkpoint in the G2/M-phase of the cell cycle through inhibiting apoptosis and promoting cell division [51]. Bcl-xL is an important anti-apoptosis factor, preventing apoptosis through two different mechanisms: heterodimerization with Bax family of apoptotic proteins, inhibiting their apoptotic effect and the formation of mitochondrial outer membrane pores which helps maintain a normal membrane state under stressful conditions[52]. Since we observed decreased Bcl-xL and survivin levels in MSeA-treated PANC-1 cells, mitochondrial leakage was likely triggered for releasing cytochrome C to lead to an activation of the caspase-9/caspase-3 and 6 intrinsic pathways of apoptosis. As expected, the pan caspase inhibitor blocked the cell apoptosis induced by MSeA (Fig. 1D).
More importantly, given the modest apoptosis response of PANC-1 cells to MSeA in cell culture, the growth inhibitory activity of MSeA in vivo on this very aggressive pancreatic cancer model was remarkable (Fig. 6). The MSeA treatment significantly reduced PANC-1 xenograft tumor growth in SCID mice with decreased Ki67, p-AKT and increased c-caspase-3, p-H2AX, p-Ser15 P53 and P21Cip1, consistent with key molecular changes observed in vitro. The decreased CD34, an endothelial specific marker for angiogenesis, in the MSeA-treated tumors added further support for anti-angiogenic action of MSeA that we were first to report in mammary cancer [53].
In summary, MSeA in the low micromolar range inhibited the proliferation and survival of several human pancreatic cancer cell lines and one murine cell line. In addition to the expected G1 arrest and caspase-mediated apoptosis informed from results of other cancer cell lines, we found that MSeA induced G2 arrest in a couple of human pancreatic cancer cell lines. Up-regulation of pH2AX, pSer15 P53 (both being substrates for the DNA damage response kinase ATM), and P53-targets GADD45 and P21Cip1 (indicative DNA damage responses), activation of G2 checkpoint kinase Chk2 suggest the likelihood of gentotoxic events in PANC-1 cells induced by MSeA, leading to G2 arrest. These findings add another dimension of the biochemical action and cellular consequences of MSeA, which has been assumed non-genotoxic based on published data [22; 23]. The suppression of AKT-mTOR pathway by MSeA was a likely cause of autophagy, which counter-acted against apoptosis execution. These results highlight unique cell cycle and cell fate responses to MSeA treatment in the pancreatic cancer cell lines and suggest a combination with anti-autophagy drugs might increase therapeutic efficacy of MSeA against PANC-1 and possibly cancer cells with like pathogenetic traits. The in vivo efficacy of MSeA to suppress PANC-1 xenograft growth without detectable adverse effect provided further impetus for exploiting its utility for the therapy of pancreatic cancer. In light of the increased risk of type II diabetes by SeMet in the SELECT study [4], the risk implication of MSeA for pancreatic insulin regulation should not be overlooked in future work, even though MSeA is very different from SeMet in metabolism and anti-cancer actions.
Supplementary Material
Acknowledgment
The authors thank Mr. Todd Schuster for expert help with flow cytometric analyses.
Funding supports: Grants from National Cancer Institute R01CA126880 (Lu) and R21CA155522 (Deng, Lu)
Reference
- [1].Siegel R, Naishadham D, Jemal A, Cancer statistics, 2012. CA: a cancer journal for clinicians 62 (2012) 10–29. [DOI] [PubMed] [Google Scholar]
- [2].Chue BM, Five-year survival of metastatic pancreatic carcinoma: a study of courage and hope. Gastrointestinal cancer research : GCR 3 (2009) 208–211. [PMC free article] [PubMed] [Google Scholar]
- [3].Schneider G, Siveke JT, Eckel F, Schmid RM, Pancreatic cancer: basic and clinical aspects. Gastroenterology 128 (2005) 1606–1625. [DOI] [PubMed] [Google Scholar]
- [4].Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL Jr., Baker LH, Coltman CA Jr., Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA : the journal of the American Medical Association 301 (2009) 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marshall JR, Tangen CM, Sakr WA, Wood DP Jr., Berry DL, Klein EA, Lippman SM, Parnes HL, Alberts DS, Jarrard DF, Lee WR, Gaziano JM, Crawford ED, Ely B, Ray M, Davis W, Minasian LM, Thompson IM Jr., Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Phila) 4 (2011) 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ip C, Lessons from basic research in selenium and cancer prevention. The Journal of nutrition 128 (1998) 1845–1854. [DOI] [PubMed] [Google Scholar]
- [7].Lu J, Jiang C, Selenium and cancer chemoprevention: hypotheses integrating the actions of selenoproteins and selenium metabolites in epithelial and non-epithelial target cells. Antioxidants & redox signaling 7 (2005) 1715–1727. [DOI] [PubMed] [Google Scholar]
- [8].Li GX, Lee HJ, Wang Z, Hu H, Liao JD, Watts JC, Combs GF Jr., Lu J, Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis 29 (2008) 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang L, Bonorden MJ, Li GX, Lee HJ, Hu H, Zhang Y, Liao JD, Cleary MP, Lu J, Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila) 2 (2009) 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang C, Wang Z, Ganther H, Lu J, Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer research 61 (2001) 3062–3070. [PubMed] [Google Scholar]
- [11].Wang Z, Jiang C, Ganther H, Lu J, Antimitogenic and proapoptotic activities of methylseleninic acid in vascular endothelial cells and associated effects on PI3K-AKT, ERK, JNK and p38 MAPK signaling. Cancer research 61 (2001) 7171–7178. [PubMed] [Google Scholar]
- [12].Wang Z, Jiang C, Lu J, Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Molecular carcinogenesis 34 (2002) 113–120. [DOI] [PubMed] [Google Scholar]
- [13].Hu H, Jiang C, Li G, Lu J, PKB/AKT and ERK regulation of caspase-mediated apoptosis by methylseleninic acid in LNCaP prostate cancer cells. Carcinogenesis 26 (2005) 1374–1381. [DOI] [PubMed] [Google Scholar]
- [14].Wang Z, Lee HJ, Chai Y, Hu H, Wang L, Zhang Y, Jiang C, Lu J, Persistent p21Cip1 induction mediates G(1) cell cycle arrest by methylseleninic acid in DU145 prostate cancer cells. Current cancer drug targets 10 (2010) 307–318. [DOI] [PubMed] [Google Scholar]
- [15].Wang L, Zhang J, Zhang Y, Nkhata K, Quealy E, Liao JD, Cleary MP, Lu J, Lobe-specific lineages of carcinogenesis in the transgenic adenocarcinoma of mouse prostate and their responses to chemopreventive selenium. The Prostate 71 (2011) 1429–1440. [DOI] [PubMed] [Google Scholar]
- [16].Hu H, Jiang C, Ip C, Rustum YM, Lu J, Methylseleninic acid potentiates apoptosis induced by chemotherapeutic drugs in androgen-independent prostate cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research 11 (2005) 2379–2388. [DOI] [PubMed] [Google Scholar]
- [17].Hu H, Li GX, Wang L, Watts J, Combs GF Jr., Lu J, Methylseleninic acid enhances taxane drug efficacy against human prostate cancer and down-regulates antiapoptotic proteins Bcl-XL and survivin. Clinical cancer research : an official journal of the American Association for Cancer Research 14 (2008) 1150–1158. [DOI] [PubMed] [Google Scholar]
- [18].Birt DF, Update on the effects of vitamins A, C, and E and selenium on carcinogenesis. Proc Soc Exp Biol Med 183 (1986) 311–320. [DOI] [PubMed] [Google Scholar]
- [19].Birt DF, Julius AD, Runice CE, White LT, Lawson T, Pour PM, Enhancement of BOP-induced pancreatic carcinogenesis in selenium-fed Syrian golden hamsters under specific dietary conditions. Nutrition and cancer 11 (1988) 21–33. [DOI] [PubMed] [Google Scholar]
- [20].Woutersen RA, Appel MJ, Van Garderen-Hoetmer A, Modulation of pancreatic carcinogenesis by antioxidants. Food Chem Toxicol 37 (1999) 981–984. [DOI] [PubMed] [Google Scholar]
- [21].Aichler M, Algul H, Behne D, Holzlwimmer G, Michalke B, Quintanilla-Martinez L, Schmidt J, Schmid RM, Brielmeier M, Selenium status alters tumour differentiation but not incidence or latency of pancreatic adenocarcinomas in Ela-TGF-alpha p53+/ mice. Carcinogenesis 28 (2007) 2002–2007. [DOI] [PubMed] [Google Scholar]
- [22].Jiang C, Wang Z, Ganther H, Lu J, Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Molecular cancer therapeutics 1 (2002) 1059–1066. [PubMed] [Google Scholar]
- [23].Li GX, Hu H, Jiang C, Schuster T, Lu J, Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. International journal of cancer. Journal international du cancer 120 (2007) 2034–2043. [DOI] [PubMed] [Google Scholar]
- [24].Sandgren EP, Quaife CJ, Paulovich AG, Palmiter RD, Brinster RL, Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proceedings of the National Academy of Sciences of the United States of America 88 (1991) 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Biliran H Jr., Wang Y, Banerjee S, Xu H, Heng H, Thakur A, Bollig A, Sarkar FH, Liao JD, Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clinical cancer research : an official journal of the American Association for Cancer Research 11 (2005) 6075–6086. [DOI] [PubMed] [Google Scholar]
- [26].Krishan A, Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. The Journal of cell biology 66 (1975) 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jiang C, Hu H, Malewicz B, Wang Z, Lu J, Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Molecular cancer therapeutics 3 (2004) 877–884. [PubMed] [Google Scholar]
- [28].Cho SD, Jiang C, Malewicz B, Dong Y, Young CY, Kang KS, Lee YS, Ip C, Lu J, Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Molecular cancer therapeutics 3 (2004) 605–611. [PubMed] [Google Scholar]
- [29].Guo XW, Th’ng JP, Swank RA, Anderson HJ, Tudan C, Bradbury EM, Roberge M, Chromosome condensation induced by fostriecin does not require p34cdc2 kinase activity and histone H1 hyperphosphorylation, but is associated with enhanced histone H2A and H3 phosphorylation. The EMBO journal 14 (1995) 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, Kern SE, p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer research 54 (1994) 3025–3033. [PubMed] [Google Scholar]
- [31].Widrow RJ, Rabinovitch PS, Cho K, Laird CD, Separation of cells at different times within G2 and mitosis by cyclin B1 flow cytometry. Cytometry 27 (1997) 250–254. [DOI] [PubMed] [Google Scholar]
- [32].Levine B, Klionsky DJ, Development by self-digestion: molecular mechanisms and biological functions of autophagy. Developmental cell 6 (2004) 463–477. [DOI] [PubMed] [Google Scholar]
- [33].Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T, LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. Journal of cell science 117 (2004) 2805–2812. [DOI] [PubMed] [Google Scholar]
- [34].Kametaka S, Okano T, Ohsumi M, Ohsumi Y, Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. The Journal of biological chemistry 273 (1998) 22284–22291. [DOI] [PubMed] [Google Scholar]
- [35].Clark LC, Combs GF Jr., Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr., Park HK, Sanders BB Jr., Smith CL, Taylor JR, Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA : the journal of the American Medical Association 276 (1996) 1957–1963. [PubMed] [Google Scholar]
- [36].Marshall JR, Ip C, Romano K, Fetterly G, Fakih M, Jovanovic B, Perloff M, Crowell J, Davis W, French-Christy R, Dew A, Coomes M, Bergan R, Methyl selenocysteine: single-dose pharmacokinetics in men. Cancer Prev Res (Phila) 4 (2011) 1938–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu M, Kang MM, Schoene NW, Cheng WH, Selenium compounds activate early barriers of tumorigenesis. The Journal of biological chemistry 285 (2010) 12055–12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu M, Wu RT, Wang TT, Cheng WH, Role for p53 in selenium-induced senescence. Journal of agricultural and food chemistry 59 (2011) 11882–11887. [DOI] [PubMed] [Google Scholar]
- [39].Kim EH, Choi KS, A critical role of superoxide anion in selenite-induced mitophagic cell death. Autophagy 4 (2008) 76–78. [DOI] [PubMed] [Google Scholar]
- [40].Park SH, Kim JH, Chi GY, Kim GY, Chang YC, Moon SK, Nam SW, Kim WJ, Yoo YH, Choi YH, Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicology letters 212 (2012) 252–261. [DOI] [PubMed] [Google Scholar]
- [41].Jiang Q, Li F, Shi K, Yang Y, Xu C, Sodium selenite-induced activation of DAPK promotes autophagy in human leukemia HL60 cells. BMB reports 45 (2012) 194–199. [DOI] [PubMed] [Google Scholar]
- [42].Kralova V, Benesova S, Cervinka M, Rudolf E, Selenite-induced apoptosis and autophagy in colon cancer cells. Toxicology in vitro : an international journal published in association with BIBRA 26 (2012) 258–268. [DOI] [PubMed] [Google Scholar]
- [43].Jiang Q, Wang Y, Li T, Shi K, Li Z, Ma Y, Li F, Luo H, Yang Y, Xu C, Heat shock protein 90-mediated inactivation of nuclear factor-kappaB switches autophagy to apoptosis through becn1 transcriptional inhibition in selenite-induced NB4 cells. Molecular biology of the cell 22 (2011) 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ren Y, Huang F, Liu Y, Yang Y, Jiang Q, Xu C, Autophagy inhibition through PI3K/Akt increases apoptosis by sodium selenite in NB4 cells. BMB reports 42 (2009) 599–604. [DOI] [PubMed] [Google Scholar]
- [45].Castedo M, Roumier T, Blanco J, Ferri KF, Barretina J, Tintignac LA, Andreau K, Perfettini JL, Amendola A, Nardacci R, Leduc P, Ingber DE, Druillennec S, Roques B, Leibovitch SA, Vilella-Bach M, Chen J, Este JA, Modjtahedi N, Piacentini M, Kroemer G, Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. The EMBO journal 21 (2002) 4070–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Castedo M, Ferri KF, Kroemer G, Mammalian target of rapamycin (mTOR): pro- and anti-apoptotic. Cell death and differentiation 9 (2002) 99–100. [DOI] [PubMed] [Google Scholar]
- [47].Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J, Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294 (2001) 1942–1945. [DOI] [PubMed] [Google Scholar]
- [48].Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM, RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proceedings of the National Academy of Sciences of the United States of America 95 (1998) 1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sonenberg N, Gingras AC, The mRNA 5’ cap-binding protein eIF4E and control of cell growth. Current opinion in cell biology 10 (1998) 268–275. [DOI] [PubMed] [Google Scholar]
- [50].Ambrosini G, Adida C, Altieri DC, A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature medicine 3 (1997) 917–921. [DOI] [PubMed] [Google Scholar]
- [51].Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, Villa A, Marchisio PC, Altieri DC, Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nature cell biology 1 (1999) 461–466. [DOI] [PubMed] [Google Scholar]
- [52].Minn AJ, Kettlun CS, Liang H, Kelekar A, Vander Heiden MG, Chang BS, Fesik SW, Fill M, Thompson CB, Bcl-xL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. The EMBO journal 18 (1999) 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jiang C, Jiang W, Ip C, Ganther H, Lu J, Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Molecular carcinogenesis 26 (1999) 213–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.