Abstract
Purinergic receptors, especially P2RX, are associated to the severity of symptoms in patients suffering from depressive and bipolar disorders, and genetic deletion or pharmacological blockade of P2RX7 induces antidepressant-like effect in preclinical models. However, there is scarce evidence about the alterations in P2RX7 or P2RX4 levels and in behavioral consequences induced by previous exposure to stress, a major risk factor for depression in humans. In the present study, we evaluated the effect of imipramine (IMI) on P2RX7 and P2RX4 levels in dorsal and ventral hippocampus as well as in the frontal cortex of rats submitted to the pretest session of learned helplessness (LH) paradigm. Repeated, but not acute administration of IMI (15 mg/kg ip) reduced the levels of both P2RX7 and P2RX4 in the ventral, but not in dorsal hippocampus or frontal cortex. In addition, we tested the effect of P2RX7/P2RX4 antagonist brilliant blue G (BBG: 25 or 50 mg/kg ip) on the LH paradigm. We observed that repeated (7 days) but not acute (1 day) treatment with BBG (50 mg) reduced the number of failures to escape the shocks in the test session, a parameter mimicked by the same regimen of IMI treatment. Taken together, our data indicates that pharmacological blockade or decrease in the expression of P2RX7 is associated to the antidepressant-like behavior observed in the LH paradigm after repeated drug administration.
Keywords: P2X7 receptor, P2X4 receptor, Learned helplessness, Brilliant blue G, Imipramine
Introduction
Adenosine triphosphate (ATP)-mediated signaling has been recently involved in the behavioral effects of stress and neurobiology of depression (Burnstock et al., 2011; Sperlagh et al., 2012). ATP effects are mediated by the activation of P2 receptors (P2R), classified in two major families: P2RX are ligand-gated ion channels, while P2RY are G protein-coupled receptors. Among those, P2RX have been associated to several processes that are dysfunctional in stress response and depression neurobiology, such as neurotransmitter release, cognition, sleep, energy levels, appetite, immune and endocrine system (Burnstock et al., 2011).
Accordingly, clinical evidence associates a P2RX7 polymorphism that results in a mutation in the protein (Q460R), with higher severity of symptoms in patients with major depressive disorder (MDD) (Hejjas et al., 2009), which was recently confirmed by a meta-analysis study (Czamara, Müller-Myhsok & Lucae, 2018).
Pre-clinical studies indicate that knocking out of P2RX7 leads to antidepressant-like phenotype in the forced swimming (FST) and tail suspension (TST) tests (Basso et al., 2009; Boucher et al., 2011; Csölle et al., 2013a, 2013b). Corroborating this idea, systemic treatment with P2RX7 antagonist induces antidepressant-like effects in both FST and TST (Csölle et al., 2013a; Pereira et al., 2013). However, these two tests do not involve a previous exposure to stress, a major factor in triggering depressive behavior (Hammen, 2005). Indeed, a positive correlation between previous exposure to stressful events and the severity of the first depressive episodes has been demonstrated (Post, 1992). Two studies monitoring 1,942 adult women during 9 years (Kendler, Gardner & Prescott, 2002), and 2,935 adult men during 2–4 years (Kendler, Gardner & Prescott, 2006) elaborated a model to predict depressive episodes based on the patient’s history. According to these findings, MDD would be the result of the interaction between risk factors from multiple domains, including stress exposure (Kendler, Gardner & Prescott, 2002, 2006).
In this scenario, animal models that address the behavioral consequences of stress rise as useful tools to study the neurobiology of depression as well as to investigate potential new antidepressant drugs. One of the most prominent, the learned helplessness (LH) paradigm, presents good face validity since the exposure to inescapable foot shocks leads to endocrine, neuroanatomical and neurochemical changes observed in depression such as decreased hippocampal volume, diminished neurogenesis, impaired monoaminergic neurotransmission and hypothalamic pituitary adrenal axis imbalance (Pryce et al., 2011). In addition, helplessness has been found in depressed patients which turned it into the focus of preclinical and clinical depression research (Seligman, 1975; Pryce et al., 2011). The LH predictive validity is supported by the lack of responsiveness to acute treatment with classical antidepressants, as observed in FST (Saarelainen et al., 2003), but it requires about 7 days of continuous treatment to induce observable effects (Petty, Kramer & Wilson, 1992). Moreover, LH is not sensitive to anxiolytic drugs (diazepam, lorazepam) or to stimulants (amphetamine, caffeine), which further supports that the model exhibits good predictive validity (Sherman, Sacquitne & Petty, 1982; Martin & Puech, 1996).
Despite previous evidence indicating that P2RX7 blockade might induce antidepressant-like effect, it is not clear if P2RX7 antagonists are effective in the LH model. Moreover, it has not yet been investigated if repeated treatment with these drugs is required to induce antidepressant-like effects. Thus, the aim of the present study was to: (1) evaluate the effect of acute and repeated treatment with the P2RX7 antagonist brilliant blue G (BBG) in animals submitted to the LH model; (2) to investigate if the stress and the antidepressant effect would be associated with altered P2RX7 levels in the hippocampus and frontal cortex, brain regions highly implicated in the neurobiology of depression (Liu et al., 2017). In addition, P2RX4 levels were also evaluated since these receptors may form heterodimers with P2RX7 (Guo et al., 2007), P2RX4 activation modulates depressive-related behaviors (Bortolato et al., 2013) and BBG may also block P2RX4 (Jiang et al., 2000).
Methods
Animals
Male Wistar rats weighting 250–280 grams (about 8 weeks old) were obtained from the Central Vivarium of the University of São Paulo, campus of Ribeirão Preto, Brazil. The animals were kept under standard conditions: temperature (24 ± 1 °C), light cycle (lights on from 6:00 a.m. to 6:00 p.m.), free access to food and water. The animals were kept in groups of 4/cage (41 × 34 × 16 cm) during the habituation period (at least 1 week prior to the beginning of experiments) and isolated (30 × 20 × 13 cm) during the experimental procedure. The welfare of the animals was assessed daily. The cages and bedding were changed every 2 days as well as food and water replacement. Animals were randomly assigned to the different experimental groups and experiments were conducted from 7 am to 6 pm, with randomization of treatment conditions. All procedures were developed in accordance with Brazilian Council for Animal Experimentation (CONCEA), they were approved by the ethical committee for animals use of the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo (CEUA, Protocol Number 13.1.1506.53.0), and all efforts were made to minimize animal suffering.
Based on previous experiments, the sample size required for LH experiments is about 10 animals per group (Stanquini et al., 2017). In the present study, the total number of animals used was 126 male rats, an average of 8.4 animals/group.
Drugs and reagents
The following drugs were freshly prepared before use and administered intraperitoneally (ip): BBG (#B0770; Sigma Aldrich, St. Louis, MO, USA), a P2RX7/P2RX4 antagonist (Jiang et al., 2000), diluted in sterile isotonic saline and 2% tween 80, administered at 25 or 50 mg/kg/day doses (Csölle et al., 2013a; Carmo et al., 2014). Imipramine (IMI; #I7379; Sigma Aldrich, St. Louis, MO, USA), a tricyclic antidepressant, diluted in sterile isotonic saline, administered at 15 mg/kg/day dose (Stanquini et al., 2017). Chloral hydrate (Vetec) was used as a sedative for sample collection, at 5% concentration and administered at one ml/100g volume.
Learned helplessness
The apparatus consisted of an acrylic box (30.7 × 33.3 × 54 cm, INSIGHT Apparatus—EP111, Brazil), with two compartments of equal sizes separated by a wall with a central opening, through which the animals could cross from one compartment to another. The apparatus has a metal grid on the floor, which can deliver foot shocks. The behavioral tests took place in a sound attenuated, temperature-controlled (25 ± 1 °C) room.
The experimental procedure was conducted similarly to previously described (Stanquini et al., 2017). Male Wistar rats were exposed to a pre-test (PT) session, consisting of 40 inescapable foot shocks (0.4 mA, 10 s duration, 30–90 s interval). 7 days later, the rats were submitted to the test session (T), when 30 escapable foot shocks (0.4 mA, 10 s, 30–90 s interval), preceded (5s) by a warning tone (60dB, 670Hz) were applied. In the test session, the animals could avoid (by crossing from one compartment to the other during tone presentation) or escape (by interrupting the shock when crossing the shuttle box during shock application) from the shocks. Inescapable stress exposure in PT leads to helplessness behavior, reflected as failure to avoid/escape the shocks in the test session. Antidepressant treatment decreases the number of failures to avoid/escape shocks (Sherman, Sacquitne & Petty, 1982). Therefore, the number of escape failures was registered in the present work as a parameter indicative of LH behavior. The number of inter trial crossings (ITC) were also recorded as a parameter of locomotor activity (Geoffroy & Christensen, 1993).
Sample collection, preparation and western blotting analysis
The animals were deeply anesthetized with chloral hydrate 5% and decapitated. The frontal cortex, ventral and dorsal hippocampus were dissected on ice for posterior analysis. Considering the longitudinal axis of the rat brain, dorsal and ventral hippocampus were respectively divided in the two-thirds higher and one-third lower of the total hippocampus due their differences in anatomical connections, patterns of gene expression and behavioral functionality (Bannerman et al., 2014; Strange et al., 2014). Samples were mechanically homogenized in lysis buffer (137 mM NaCl, 20 mM Tris–HCl pH 7.6, 10% glycerol), supplemented with protease inhibitor cocktail (#P2714; Sigma Aldrich, St. Louis, MO, USA). The homogenate was centrifuged for 15 min, 9,000×g, 4 °C. The supernatant was collected and stored at −80 °C.
The levels of P2RX7 and P2RX4 were determined in frontal cortex, ventral and dorsal hippocampus samples by western blotting (WB). Briefly, quantification of total proteins was used to determine the amount of each sample was submitted to analysis (Bradford, 1976). Then, 30 micrograms of proteins from each sample were resolved in SDS-PAGE (12% polyacrylamide gel) and transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% bovine serum albumin solution in tris buffered saline with tween 20 (TBST) buffer and incubated with primary antibody against P2RX7 (1:200, #APR-004; Alomone Labs, Jerusalem, Israel), P2RX4 (1:500, #APR-002; Alomone Labs, Jerusalem, Israel) or GAPDH (1:1,000, #sc-25778; Santa Cruz, Dallas, TX, USA), overnight, 4 °C.
The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit, 1:2,000, #7074; Cell Signaling, Danvers, MA, USA) for 1 h at room temperature, and washed with TBST buffer. The HRP activity was developed with chromogenic reagent 4-chloro-naphthol (#NEL300001EA; Perkin Elmer, Waltham, MA, USA). Finally, the membranes were air dried and scanned, the optical density was analyzed with the Image Studio Lite program (version 5.2) and the values for the P2RX7 or P2RX4 were normalized by the GAPDH value in the corresponding sample and expressed as a percentage of the control group (vehicle-injected).
Experimental design
The animals assigned to WB analysis were maintained in the animal house for at least 1 week before the beginning of the experimental protocol. Animals were brought to the lab on day 1 and taken individually to experimental rooms, where they were exposed to PT. Right after, animals received the administration of IMI or vehicle. After that, they were housed in individual cages (30 × 19 × 13 cm) and taken back to the animal house. Every day, at 11:00 am, the animals were brought to the lab experimental rooms, where they were weighted and injected with drug or vehicle, according to the groups they had been randomly assigned to. Acutely treated animals received the administration of vehicle during 6 days and, in the 7th day, they were injected with IMI. On the last day, animals received the last injection and returned to their homecages where they remained undisturbed for 1 h. Following, they were anesthetized and euthanized for frontal cortex, dorsal and ventral hippocampus dissection. The last treatment injection and sample collection were performed in random order to avoid circadian influences on the analysis.
The animals submitted to LH paradigm were kept in groups of four/cage (41 × 34 × 16 cm) for at least 1 week before the beginning of the experiment. For behavioral experiments, animals were brought to the lab on day 1 and taken individually to experimental rooms, where they were exposed to PT. Immediately after completion of PT, animals received the administration of drug (BBG or IMI) or vehicle. After that, they were housed in individual cages (30 × 19 × 13 cm) and taken back to the animal house. Every day, at 11:00 am, the animals were brought to the lab experimental rooms, where they were weighed and injected with drug or vehicle, according to the groups they had been randomly assigned to. Acutely treated animals received the administration of vehicle during 6 days and, on the 7th day, they were injected with drug (BBG or IMI). 1 h after the last injection, the animals were exposed to the T session of LH paradigm.
Statistical analysis
The number of escape failures and ITC in the LH were analyzed by Kruskal–Wallis test followed by Dunn’s post hoc. Data from WB was analyzed by one-way ANOVA followed by Fisher’s post hoc test. Values of p < 0.05 were considered statistically significant. All data used in the present study is available in FigShare under CC-BY license (DOI 10.6084/m9.figshare.6989063).
Results
Experiment 1: effect of imipramine in the P2RX7 and P2RX4 levels of rats exposed to LH model
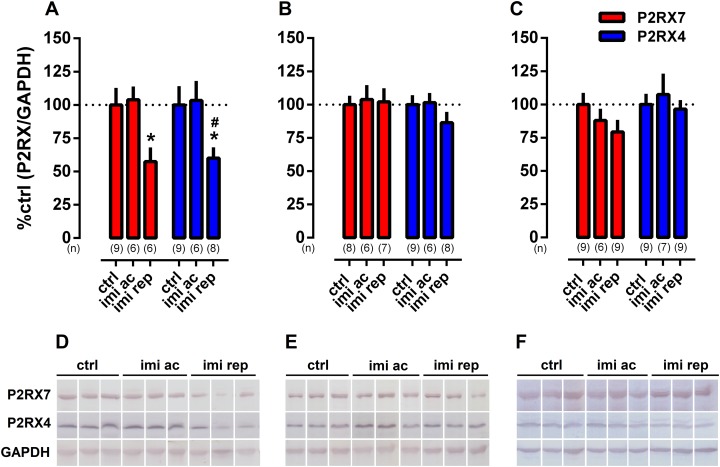
One-way ANOVA indicated a significant effect of IMI treatment on P2XR7 (F(2,18) = 4.169, p = 0.0325) and P2RX4 (F(2,20) = 3.627, p = 0.0453) levels in ventral hippocampus. Fisher’s test showed that repeated IMI-treatment significantly reduced the level of both receptors in comparison to vehicle-treated groups (p < 0.05), as found in Fig. 1A.
Figure 1. Effect of repeated treatment with imipramine on the levels of P2RX7 and P2RX4 in the (A) ventral hippocampus, (B) dorsal hippocampus and (C) frontal cortex.
Representative WB bands of (D) ventral hippocampus, (E) dorsal hippocampus and (F) frontal cortex. Values are expressed as mean ± SEM and the sample size of each experimental group (n) is depicted under the respective columns. *p < 0.05 from ctrl (vehicle-treated) group; #p < 0.05 from imi ac group.
In samples from dorsal hippocampus (Fig. 1B) or frontal cortex (Fig. 1C), no effect of treatment with IMI was observed.
Experiment 2: effect of BBG and imipramine treatment in the LH paradigm
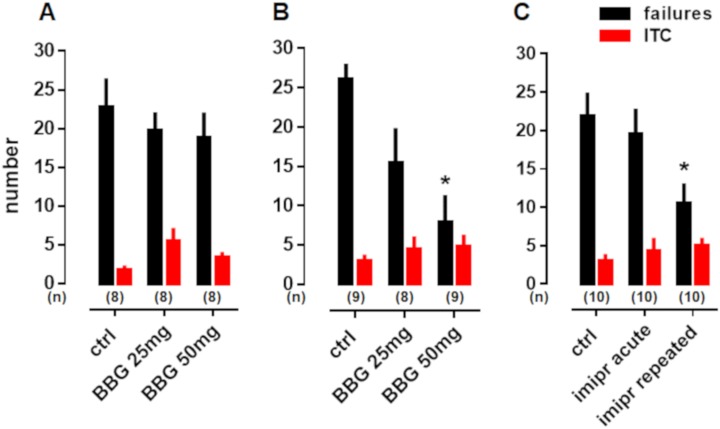
Kruskal–Wallis test showed that acute treatment with BBG altered neither the number of failures (H = 1.75, p = 0.42) nor the ITC (H = 4.52, p = 0.10) of animals exposed to the LH model (Fig. 2A). However, repeated treatment with BBG decreased the number of escape failures (H = 10.53, p = 0.0052) without changing the ITC (H = 0.27, p = 0.87) of animals exposed to the LH model treated with BBG 50mg/kg (Dunn’s p < 0.05) (Fig. 2B).
Figure 2. Effect of BBG and imipramine treatment in the LH paradigm.
(A) Acute treatment with BBG did not alter the number of failures (black) or the ITC (red) of animals exposed to the LH paradigm. (B) Repeated treatment with BBG 50 mg/kg decreased the number of failures without changing the ITC of rats submitted to this model. (C) Repeated but not acute administration of imipramine decreased the number of failures in the LH paradigm without altering the ITC. Values are expressed as mean ± SEM and the sample size of each experimental group (n) is depicted above the respective column. *p < 0.05 from ctrl (vehicle-treated) group.
Repeated but not acute treatment with IMI (15 mg/kg) significantly decreased the number of escape failures (H = 7.949, p = 0.019) and induced no alterations in the ITC (H = 1.41, p = 0.49) of animals exposed to the LH paradigm (Fig. 2C).
Discussion
In the present study, we report that the antidepressant-like effect of IMI is associated with the attenuation of P2RX7 and P2RX4 levels in ventral hippocampus of rats exposed to LH. Repeated but not acute administration of IMI, an antidepressant consistently tested in the LH model (Sherman, Sacquitne & Petty, 1982; Takamori, Yoshida & Okuyama, 2001; Joca, Padovan & Guimarães, 2003; Stanquini et al., 2017), decreased the number of escape failures of animals exposed to this paradigm, as well as P2RX7 and P2RX4 levels in the ventral hippocampus of stressed animals.
Based on the findings described above, we further assessed if acute and repeated treatment with P2RX7/P2RX4 antagonist, BBG, could induce antidepressant effects in animals exposed to the LH model. By doing that, we assessed if repeated treatment with BBG would be required to induce antidepressant effects. The LH model was chosen based on its fulfillment of face, construct and predictive validity criteria (Sherman, Sacquitne & Petty, 1982; Willner, 1984; Maier et al., 1986; Petty, Kramer & Wilson, 1992; Nankai et al., 1995; Centeno & Volosin, 1997; Fleshner et al., 1998; Maier & Watkins, 2005; Hajszan et al., 2009). Similar to the clinical scenario, and contrary to usual protocols of FST and TST, the antidepressant-like effect on LH model is observed only after repeated pharmacological treatment, i.e., administration of classical antidepressant drugs during 7–21 days (Petty, Kramer & Wilson, 1992; Nankai et al., 1995; Mansbach, Brooks & Chen, 1997; Hajszan et al., 2009). We observed that only the repeated (7 days) treatment with BBG decreased the number of escape failures. None of the treatments changed the number of ITC performed by animals exposed to the LH. The ITC was evaluated as a proxy of the locomotor activity since alterations in this parameter can interfere in the animals’ behavior during the LH test (Geoffroy & Christensen, 1993). Altogether, these results are interpreted as an antidepressant-like effect induced by BBG (Sherman, Sacquitne & Petty, 1982).
Previous studies indicated that BBG exerts antidepressant-like effect in animals submitted to the FST and TST (Csölle et al., 2013a). Chronic treatment with A-804598, a selective P2RX7 antagonist, was able to block the anhedonic effects of chronic unpredictable stress (Iwata et al., 2016). Moreover, mice lacking P2RX7 showed decreased LH behavior (Otrokocsi, Kittel & Sperlágh, 2017). Our results further support these findings and add important information regarding the antidepressant effect induced by P2RX7 blockade, since none of the previous studies assessed the effects induced by the acute treatment. In light of that, our findings suggest that repeated treatment with BBG is required to induce antidepressant effects, similarly to conventional monoaminergic antidepressants.
In fact, the antidepressant effect induced by P2RX antagonists is dependent on the facilitatory action upon monoamine signaling in the central nervous system (CNS) since depletion of serotonin or noradrenaline blocked the effects induced by pyridoxalphosphate-6-azophenyl-2,4-disulphonoic acid (Diniz et al., 2017). Moreover, chronic blockade of P2RX7 have also shown to increase brain-derived neurotrophic factor (BDNF) levels in mice hippocampus (Csölle et al., 2013a) as well as activate BDNF-TRKB signaling pathway in ventral hippocampus of stressed rats (Ribeiro et al., 2019), an effect that is central to the mechanism of action induced by monoaminergic drugs (Saarelainen et al., 2003; Rantamäki et al., 2007). Therefore, it is plausible to suggest that P2RX7 blockade could promote antidepressant effects as a result of monoaminergic and BDNF signaling facilitation.
In addition, activation of the tropomyosin receptor kinase B (TRKB) receptor-mediated signaling by antidepressants could partially counteract the consequences of P2RX7 activity, putatively through epigenetic mechanisms (Duclot & Kabbaj, 2015), such as chemical modifications in the DNA or histones (Vialou et al., 2013; Nestler, 2014). These mechanisms are also found to regulate P2RX7 gene transcription (Zhou et al., 2009; Shin et al., 2015). Therefore, the activation of TRKB receptors would result in an epigenetic-mediated decrease in transcription and, consequently, expression of P2RX7, contributing to the antidepressant-like effect.
P2RX7 are cation channels activated by high concentrations of ATP, with an EC50 around one mM (Donnelly-Roberts et al., 2009), which is released after stress exposure (Volonte et al., 2012; Jiang et al., 2013). P2RX7 are widely expressed in the CNS including brain regions involved in stress response such as frontal cortex and hippocampus (Jimenez-Mateos et al., 2018). For this reason, we focused our molecular analysis in these two brain regions. These receptors were assumed to be expressed in nerve terminals but an ongoing debate suggests that the observed effects would be an indirect result of P2RX7 activation in glial cells (for a detailed discussion see (Illes, Khan & Rubini, 2017)).
P2RX7 stimulation leads to the activation of neuronal nitric oxide synthase (NOS1) (Pereira et al., 2013), which in turn provides a positive feedback to glutamate release. P2RX7 activation also promotes potassium efflux, thus stimulating the nucleotide-binding, leucine-rich repeat, pyrin domain containing 3 (NLPR3) inflammasome and caspase 1 (CASP1), leading to interleukin release (mainly IL-1β and IL-6). Therefore, enhancing neuroimmune response, axonal degeneration, cell death and inhibiting neurogenesis through NFkB signaling (Iwata et al., 2016). The whole process could be positively fed by the decrease in BDNF signaling promoted by P2RX7 activation (Csölle et al., 2013a). Therefore, is plausible to suppose that the behavioral effects of antidepressants potentially involve the inhibition/repression of P2RX7.
P2RX4 are cation channels activated by ATP and also widely expressed in the CNS, including neurons and glial cells (Soto et al., 1996; Stokes et al., 2017). According to electron microscopy evidence, this receptor can be found on post- or pre-synaptic terminals (Rubio & Soto, 2001), and high levels of P2rx4 mRNA was detected in the dentate gyrus’ granule cells and in CA1/CA3 pyramidal neurons as well as astrocytes in rat hippocampus (Soto et al., 1996; Kukley et al., 2001). Hippocampal P2RX4 have been associated to induction of NMDA-dependent long-term potentiation (LTP) (Sim et al., 2006; Choi et al., 2010). Severe stress decreases LTP in the rodent hippocampus, which has been associated with cognitive impairment (Foy et al., 1987; Shors et al., 1989; Kim & Diamond, 2002). However, stress also increases LTP (Joels & Krugers, 2007), which could be associated to the decrease in P2RX4 levels after antidepressant treatment. P2RX4 expressed in microglia (Tsuda et al., 2003; Ulmann et al., 2008) have been involved in the activation and migration of these cells to injury sites (Guo, Trautmann & Schluesener, 2005; Schwab, Guo & Schluesener, 2005).
In this context, stress might activate hippocampal P2RX4 increasing microglia/neuroimmune response (Guo, Trautmann & Schluesener, 2005; Schwab, Guo & Schluesener, 2005) as well as LTP and synaptic plasticity strengthening, contributing to the formation of aversive memories (Sim et al., 2006; Baxter et al., 2011). Such effects are frequently associated to depressive-related behaviors and can be prevented or reverted by antidepressant treatment (Veith et al., 1994; Bliss & Cooke, 2011; Kreisel et al., 2014). Accordingly, we observed that the repeated treatment with IMI decreased the P2RX4 expression in ventral hippocampus.
P2RX4 and P2RX7 are activated by ATP, which levels are efficiently controlled by an extracellular enzymatic chain collectively called of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases) (Yegutkin, 2014). The treatment with three different doses (100, 250 or 300 μM) of antidepressants (fluoxetine, sertraline, nortriptyline or clomipramine) decreased E-NTPDases activity in hippocampal and cortical synaptosomes of rats (Pedrazza et al., 2007, 2008).
Ectonucleoside triphosphate diphosphohydrolase 1, also known as cluster of differentiation 39, is the rate-limiting enzyme of a cascade which contributes to extracellular adenosine production through the hydrolysis of ATP/ADP to AMP (Yegutkin, 2014) and antidepressant treatment seems to modulates this enzyme activity. Chronic treatment (10 mg/kg/day i.p. during 14 days) with nortriptyline promoted a decrease in NTPDase1 transcript levels in the hippocampus and induced an increase of gene expression for NTPDase1 in cerebral cortex while the same treatment regimen with fluoxetine produced an enhancement for NTPDase1 transcript levels in hippocampus and cerebral cortex of rats (Pedrazza et al., 2008).
These results indicate that antidepressants decrease E-NTPDases activity in the cortex and hippocampus which probably leads to increased levels of ATP. Although at first sight these results are contrary to our hypothesis, E-NTPDases activity measurements do not predict the ATP action upon P2RX4 or P2RX7.
Conclusion
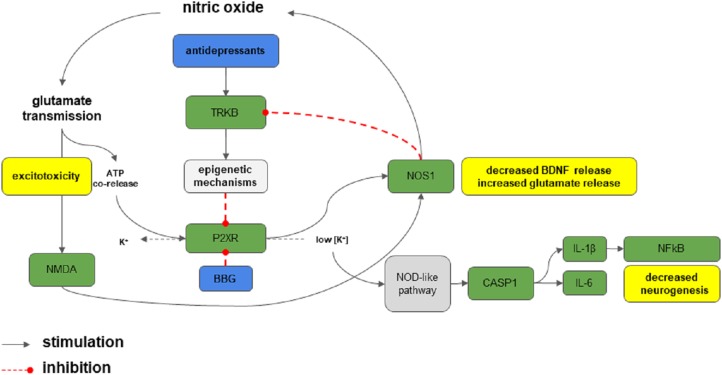
As shown in Fig. 3, excessive/sustained release of ATP during stress exposure could trigger the activation of P2RX7 and/or P2RX4 in brain regions important for stress and depression. In turn, this facilitates neurochemical and molecular processes that hinders behavioral adaptation to stress (i.e., increased inflammation and cell death through caspase), thus leading to behavioral consequences, such as LH and depression (Burnstock et al., 2011; Sperlagh et al., 2012). The increased nitric oxide production, by activation of P2RX7 or NMDA for example, could blunt TRKB activation, through a decrease of BDNF release (Canossa et al., 2002) or putative nitration of the receptor (Biojone et al., 2015).
Figure 3. P2RX7 signaling in stress.
Stress elicits massive glutamate and ATP release. The activation of P2RX7 receptors by ATP activates nitric oxide (NO) production, which provides a positive feedback to glutamate release. This process leads to excitotoxicity. P2RX7 activation also promotes potassium efflux, stimulating the nucleotide-binding, leucine-rich repeat, pyrin domain containing 3 (NLPR3) inflammasome and caspase 1 (CASP1), production of interleukins (IL-6 and IL-1β), and activation of NF-κB exacerbating neuroimmune response, axonal degeneration and cell death, and inhibition of neurogenesis. The whole process is positively fed by the decrease in BDNF signaling promoted by P2RX7. Antidepressants, putatively through TRKB-dependent epigenetic mechanisms could counteract such effects by decreasing P2RX7 expression. Green boxes: proteins or targets involved; blue: drugs or compounds; gray: process or pathways: red: deleterious effects.
In this scenario, attenuation of the P2RX7/P2RX4 levels by antidepressant could be part of the mechanisms that contribute to their behavioral/therapeutic effects and the selective blockade of these receptors could represent a new strategy to develop novel antidepressant drugs. Although our data supports this hypothesis, further studies are required to elucidate the P2RX7 and P2RX4 role in stress response. Altogether our data shows that inhibition of P2RX4- and P2RX7-mediated signaling by BBG or IMI induces antidepressant-like effects.
Acknowledgments
Authors are thankful for the technical assistance of Flavia Salata (University of São Paulo).
Funding Statement
This study was funded by the State of São Paulo Research Foundation (2013/01737-7) and Aarhus University Research Foundation (AU-UDEAS initiative: eMOOD, and a Mobility Stipend to Deidiane Elisa Ribeiro). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Gregers Wegener received lecture/consultancy fees from H. Lundbeck A/S, Servier SA, Astra Zeneca AB, Eli Lilly A/S, Sun Pharma Pty Ltd, Pfizer Inc, Shire A/S, HB Pharma A/S, Arla Foods A.m.b.A., Alkermes Inc, and Mundipharma International Ltd. All other authors declare no conflict of interest.
Author Contributions
Deidiane Elisa Ribeiro conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Plinio C. Casarotto conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Laura Staquini performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Maria Augusta Pinto e Silva performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Caroline Biojone conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Gregers Wegener conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Samia Joca conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All procedures were developed in accordance with Brazilian Council for Animal Experimentation (CONCEA), they were approved by the ethical committee for animals use of the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo (CEUA, Protocol Number 13.1.1506.53.0), and all efforts were made to minimize animal suffering.
Data Availability
The following information was supplied regarding data availability:
All data used in the present study is available in FigShare: Casarotto, Plinio (2019): data. figshare. Dataset. https://doi.org/10.6084/m9.figshare.6989063.v3.
References
- Bannerman et al. (2014).Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JNP, Monyer H, Seeburg PH. Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews Neuroscience. 2014;15(3):181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Basso et al. (2009).Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behavioural Brain Research. 2009;198(1):83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Baxter et al. (2011).Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. European Journal of Neuroscience. 2011;34(2):213–220. doi: 10.1111/j.1460-9568.2011.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biojone et al. (2015).Biojone C, Casarotto PC, Joca SR, Castrén E. Interplay between nitric oxide and brain-derived neurotrophic factor in neuronal plasticity. CNS & Neurological Disorders—Drug Targets. 2015;14(8):979–987. doi: 10.2174/1871527314666150909113727. [DOI] [PubMed] [Google Scholar]
- Bliss & Cooke (2011).Bliss TVP, Cooke SF. Long-term potentiation and long-term depression: a clinical perspective. Clinics. 2011;66(Suppl_1):3–17. doi: 10.1590/S1807-59322011001300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato et al. (2013).Bortolato M, Yardley MM, Khoja S, Godar SC, Asatryan L, Finn DA, Alkana RL, Louie SG, Davies DL. Pharmacological insights into the role of P2X4 receptors in behavioural regulation: lessons from ivermectin. International Journal of Neuropsychopharmacology. 2013;16(5):1059–1070. doi: 10.1017/S1461145712000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher et al. (2011).Boucher AA, Arnold JC, Hunt GE, Spiro A, Spencer J, Brown C, McGregor IS, Bennett MR, Kassiou M. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience. 2011;189:170–177. doi: 10.1016/j.neuroscience.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Bradford (1976).Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnstock et al. (2011).Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Progress in Neurobiology. 2011;95(2):229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Canossa et al. (2002).Canossa M, Giordano E, Cappello S, Guarnieri C, Ferri S. Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(5):3282–3287. doi: 10.1073/pnas.042504299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo et al. (2014).Carmo MRS, Menezes APF, Nunes ACL, Pliássova A, Rolo AP, Palmeira CM, Cunha RA, Canas PM, Andrade GM. The P2X7 receptor antagonist brilliant blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology. 2014;81:142–152. doi: 10.1016/j.neuropharm.2014.01.045. [DOI] [PubMed] [Google Scholar]
- Centeno & Volosin (1997).Centeno VA, Volosin M. Chronic treatment with desipramine: effect on endocrine and behavioral responses induced by inescapable stress. Physiology & Behavior. 1997;62(4):939–944. doi: 10.1016/S0031-9384(97)00255-2. [DOI] [PubMed] [Google Scholar]
- Choi et al. (2010).Choi DC, Maguschak KA, Ye K, Jang S-W, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csölle et al. (2013a).Csölle C, Andó RD, Kittel Á, Gölöncsér F, Baranyi M, Soproni K, Zelena D, Haller J, Németh T, Mócsai A, Sperlágh B. The absence of P2X7 receptors (P2rx7) on non-haematopoietic cells leads to selective alteration in mood-related behaviour with dysregulated gene expression and stress reactivity in mice. International Journal of Neuropsychopharmacology. 2013a;16(1):213–233. doi: 10.1017/S1461145711001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csölle et al. (2013b).Csölle C, Baranyi M, Zsilla G, Kittel A, Gölöncsér F, Illes P, Papp E, Vizi ES, Sperlágh B. Neurochemical changes in the mouse hippocampus underlying the antidepressant effect of genetic deletion of P2X7 receptors. PLOS ONE. 2013b;8(6):e66547. doi: 10.1371/journal.pone.0066547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czamara, Müller-Myhsok & Lucae (2018).Czamara D, Müller-Myhsok B, Lucae S. The P2RX7 polymorphism rs2230912 is associated with depression: a meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018;82:272–277. doi: 10.1016/j.pnpbp.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Diniz et al. (2017).Diniz CR, Rodrigues M, Casarotto PC, Pereira VS, Crestani CC, Joca SR. Monoamine involvement in the antidepressant-like effect induced by P2 blockade. Brain Research. 2017;1676:19–27. doi: 10.1016/j.brainres.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts et al. (2009).Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. British Journal of Pharmacology. 2009;157(7):1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot & Kabbaj (2015).Duclot F, Kabbaj M. Epigenetic mechanisms underlying the role of brain-derived neurotrophic factor in depression and response to antidepressants. Journal of Experimental Biology. 2015;218(1):21–31. doi: 10.1242/jeb.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner et al. (1998).Fleshner M, Nguyen KT, Cotter CS, Watkins LR, Maier SF. Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity. American Journal of Physiology. 1998;275(3):R870–R878. doi: 10.1152/ajpregu.1998.275.3.R870. [DOI] [PubMed] [Google Scholar]
- Foy et al. (1987).Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behavioral and Neural Biology. 1987;48(1):138–149. doi: 10.1016/S0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Geoffroy & Christensen (1993).Geoffroy M, Christensen AV. Psychomotor stimulants versus antidepressants in the learned helplessness model of depression. Drug Development Research. 1993;29(1):48–55. doi: 10.1002/ddr.430290106. [DOI] [Google Scholar]
- Guo et al. (2007).Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Molecular Pharmacology. 2007;72(6):1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- Guo, Trautmann & Schluesener (2005).Guo L-H, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. Journal of Neuroimmunology. 2005;163(1–2):120–127. doi: 10.1016/j.jneuroim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Hajszan et al. (2009).Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biological Psychiatry. 2009;65(5):392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen (2005).Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1(1):293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hejjas et al. (2009).Hejjas K, Szekely A, Domotor E, Halmai Z, Balogh G, Schilling B, Sarosi A, Faludi G, Sasvari-Szekely M, Nemoda Z. Association between depression and the Gln460Arg polymorphism of P2RX7 gene: a dimensional approach. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B(2):295–299. doi: 10.1002/ajmg.b.30799. [DOI] [PubMed] [Google Scholar]
- Illes, Khan & Rubini (2017).Illes P, Khan TM, Rubini P. Neuronal P2X7 receptors revisited: do they really exist? Journal of Neuroscience. 2017;37(30):7049–7062. doi: 10.1523/JNEUROSCI.3103-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata et al. (2016).Iwata M, Ota KT, Li X-Y, Sakaue F, Li N, Dutheil S, Banasr M, Duric V, Yamanashi T, Kaneko K, Rasmussen K, Glasebrook A, Koester A, Song D, Jones KA, Zorn S, Smagin G, Duman RS. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biological Psychiatry. 2016;80(1):12–22. doi: 10.1016/j.biopsych.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2013).Jiang L-H, Baldwin JM, Roger S, Baldwin SA. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Frontiers in Pharmacology. 2013;4:55. doi: 10.3389/fphar.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2000).Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Molecular Pharmacology. 2000;58(1):82–88. doi: 10.1124/mol.58.1.82. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mateos et al. (2018).Jimenez-Mateos EM, Smith J, Nicke A, Engel T. Regulation of P2X7 receptor expression and function in the brain. Brain Research Bulletin. 2018;151:153–163. doi: 10.1016/j.brainresbull.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Joca, Padovan & Guimarães (2003).Joca SRL, Padovan CM, Guimarães FS. Activation of post-synaptic 5-HT1A receptors in the dorsal hippocampus prevents learned helplessness development. Brain Research. 2003;978(1–2):177–184. doi: 10.1016/S0006-8993(03)02943-3. [DOI] [PubMed] [Google Scholar]
- Joels & Krugers (2007).Joels M, Krugers HJ. LTP after stress: up or down? Neural Plasticity. 2007;2007(6):93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler, Gardner & Prescott (2002).Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry. 2002;159(7):1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kendler, Gardner & Prescott (2006).Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. American Journal of Psychiatry. 2006;163(1):115–124. doi: 10.1176/appi.ajp.163.1.115. [DOI] [PubMed] [Google Scholar]
- Kim & Diamond (2002).Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kreisel et al. (2014).Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Molecular Psychiatry. 2014;19(6):699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Kukley et al. (2001).Kukley M, Barden JA, Steinhauser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36(1):11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2017).Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, Cui R. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plasticity. 2017;2017(5):6871089. doi: 10.1155/2017/6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier et al. (1986).Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behavioral Neuroscience. 1986;100(5):669–674. doi: 10.1037/0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier & Watkins (2005).Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience & Biobehavioral Reviews. 2005;29(4–5):829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mansbach, Brooks & Chen (1997).Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. European Journal of Pharmacology. 1997;323(1):21–26. doi: 10.1016/S0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Martin & Puech (1996).Martin P, Puech AJ. Antagonism by benzodiazepines of the effects of serotonin-, but not norepinephrine-, uptake blockers in the learned helplessness paradigm in rats. Biological Psychiatry. 1996;39(10):882–890. doi: 10.1016/0006-3223(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Nankai et al. (1995).Nankai M, Yamada S, Muneoka K, Toru M. Increased 5-HT2 receptor-mediated behavior 11 days after shock in learned helplessness rats. European Journal of Pharmacology. 1995;281(2):123–130. doi: 10.1016/0014-2999(95)00222-7. [DOI] [PubMed] [Google Scholar]
- Nestler (2014).Nestler EJ. Epigenetic mechanisms of depression. JAMA Psychiatry. 2014;71(4):454–456. doi: 10.1001/jamapsychiatry.2013.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otrokocsi, Kittel & Sperlágh (2017).Otrokocsi L, Kittel Á, Sperlágh B. P2X7 receptors drive spine synapse plasticity in the learned helplessness model of depression. International Journal of Neuropsychopharmacology. 2017;20(10):813–822. doi: 10.1093/ijnp/pyx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazza et al. (2008).Pedrazza EL, Rico EP, Senger MR, Pedrazza L, Zimmermann FF, Sarkis JJF, Bogo MR, Bonan CD. Ecto-nucleotidase pathway is altered by different treatments with fluoxetine and nortriptyline. European Journal of Pharmacology. 2008;583(1):18–25. doi: 10.1016/j.ejphar.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Pedrazza et al. (2007).Pedrazza EL, Senger MR, Pedrazza L, Zimmermann FF, De Freitas Sarkis JJ, Bonan CD. Sertraline and clomipramine inhibit nucleotide catabolism in rat brain synaptosomes. Toxicology in Vitro. 2007;21(4):671–676. doi: 10.1016/j.tiv.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Pereira et al. (2013).Pereira VS, Casarotto PC, Hiroaki-Sato VA, Sartim AG, Guimarães FS, Joca SRL. Antidepressant- and anticompulsive-like effects of purinergic receptor blockade: involvement of nitric oxide. European Neuropsychopharmacology. 2013;23(12):1769–1778. doi: 10.1016/j.euroneuro.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Petty, Kramer & Wilson (1992).Petty F, Kramer G, Wilson L. Prevention of learned helplessness: in vivo correlation with cortical serotonin. Pharmacology Biochemistry and Behavior. 1992;43(2):361–367. doi: 10.1016/0091-3057(92)90163-A. [DOI] [PubMed] [Google Scholar]
- Post (1992).Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149(8):999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Pryce et al. (2011).Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, Meinlschmidt G. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacology & Therapeutics. 2011;132(3):242–267. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Rantamäki et al. (2007).Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Männistö PT, Castrén E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cγ signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Ribeiro et al. (2019).Ribeiro DE, Müller HK, Elfving B, Eskelund A, Joca SRL, Wegener G. Antidepressant-like effect induced by P2X7 receptor blockade in FSL rats is associated with BDNF signalling activation. Journal of Psychopharmacology. 2019:269881119872173. doi: 10.1177/0269881119872173. [DOI] [PubMed] [Google Scholar]
- Rubio & Soto (2001).Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. Journal of Neuroscience. 2001;21(2):641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen et al. (2003).Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castrén E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. Journal of Neuroscience. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, Guo & Schluesener (2005).Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. Journal of Neuroimmunology. 2005;163(1–2):185–189. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Seligman (1975).Seligman MEP. Helplessness: on depression, development, and death. A series of books in psychology. New York: WH Freeman/Times Books/Henry Holt & Co; 1975. [Google Scholar]
- Sherman, Sacquitne & Petty (1982).Sherman AD, Sacquitne JL, Petty F. Specificity of the learned helplessness model of depression. Pharmacology Biochemistry and Behavior. 1982;16(3):449–454. doi: 10.1016/0091-3057(82)90451-8. [DOI] [PubMed] [Google Scholar]
- Shin et al. (2015).Shin Y-H, Kim M, Kim N, Choi S-K, Namkoong E, Choi S-Y, Lee J-H, Cha S, Park K. Epigenetic alteration of the purinergic type 7 receptor in salivary epithelial cells. Biochemical and Biophysical Research Communications. 2015;466(4):704–710. doi: 10.1016/j.bbrc.2015.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors et al. (1989).Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244(4901):224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Sim et al. (2006).Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. Journal of Neuroscience. 2006;26(35):9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto et al. (1996).Soto F, Garcia-Guzman M, Karschin C, Stuhmer W. Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochemical and Biophysical Research Communications. 1996;223(2):456–460. doi: 10.1006/bbrc.1996.0915. [DOI] [PubMed] [Google Scholar]
- Sperlagh et al. (2012).Sperlagh B, Csolle C, Ando RD, Goloncser F, Kittel A, Baranyi M. The role of purinergic signaling in depressive disorders. Neuropsychopharmacologia Hungarica. 2012;14:231–238. [PubMed] [Google Scholar]
- Stanquini et al. (2017).Stanquini LA, Biojone C, Guimarães FS, Joca SR. Repeated treatment with nitric oxide synthase inhibitor attenuates learned helplessness development in rats and increases hippocampal BDNF expression. Acta Neuropsychiatrica. 2017;30:127–136. doi: 10.1017/neu.2017.28. [DOI] [PubMed] [Google Scholar]
- Stokes et al. (2017).Stokes L, Layhadi JA, Bibic L, Dhuna K, Fountain SJ. P2X4 receptor function in the nervous system and current breakthroughs in pharmacology. Frontiers in Pharmacology. 2017;8:291. doi: 10.3389/fphar.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange et al. (2014).Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Takamori, Yoshida & Okuyama (2001).Takamori K, Yoshida S, Okuyama S. Repeated treatment with imipramine, fluvoxamine and tranylcypromine decreases the number of escape failures by activating dopaminergic systems in a rat learned helplessness test. Life Sciences. 2001;69(16):1919–1926. doi: 10.1016/S0024-3205(01)01279-6. [DOI] [PubMed] [Google Scholar]
- Tsuda et al. (2003).Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Ulmann et al. (2008).Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. Journal of Neuroscience. 2008;28(44):11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veith et al. (1994).Veith RC, Lewis N, Linares OA, Barnes RF, Raskind MA, Villacres EC, Murburg MM, Ashleigh EA, Castillo S, Peskind ER, Pascually M, Halter JB. Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Archives of General Psychiatry. 1994;51(5):411–422. doi: 10.1001/archpsyc.1994.03950050071008. [DOI] [PubMed] [Google Scholar]
- Vialou et al. (2013).Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annual Review of Pharmacology and Toxicology. 2013;53(1):59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte et al. (2012).Volonte C, Apolloni S, Skaper SD, Burnstock G. P2X7 receptors: channels, pores and more. CNS & Neurological Disorders—Drug Targets. 2012;11(6):705–721. doi: 10.2174/187152712803581137. [DOI] [PubMed] [Google Scholar]
- Willner (1984).Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83(1):1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Yegutkin (2014).Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Critical Reviews in Biochemistry and Molecular Biology. 2014;49(6):473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2009).Zhou L, Luo L, Qi X, Li X, Gorodeski GI. Regulation of P2X7 gene transcription. Purinergic Signalling. 2009;5(3):409–426. doi: 10.1007/s11302-009-9167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
All data used in the present study is available in FigShare: Casarotto, Plinio (2019): data. figshare. Dataset. https://doi.org/10.6084/m9.figshare.6989063.v3.