Abstract
Multi-center preclinical studies can facilitate the discovery of biomarkers of antiepileptogenesis and thus facilitate the diagnosis and treatment development of patients at risk of developing post-traumatic epilepsy. However, these studies are often limited by the difficulty in harmonizing experimental protocols between laboratories. Here, we assess whether the production of traumatic brain injury (TBI) using the lateral fluid-percussion injury (FPI) in adult male Sprague-Dawley rats (12 weeks at the time of injury) was harmonized between three laboratories - located in the University of Eastern Finland (UEF), Monash University in Melbourne, Australia (Melbourne) and The University of California, Los Angeles, USA (UCLA). These laboratories are part of the international multicenter-based project, the Epilepsy Bioinformatics Study for Antiepileptogenesis Therapy (EpiBioS4Rx). Lateral FPI was induced in adult male Sprague-Dawley rats. The success of methodological harmonization was assessed by performing inter-site comparison of injury parameters including duration of anesthesia during surgery, impact pressure, post-impact transient apnea, post-impact seizure-like behavior, acute mortality (< 72 h post-injury), time to self-right after the impact, and severity of the injury (assessed with the neuroscore). The data was collected using Common Data Elements and Case Report Forms. The acute mortality was 15% (UEF), 50% (Melbourne) and 57% (UCLA) (p < 0.001). The sites differed in the duration of anesthesia, the shortest being at UEF < Melbourne < UCLA (p < 0.001). The impact pressure used also differed between the sites, the highest being in UEF > Melbourne > UCLA (p < 0.001). The impact pressure associated with the severity of the functional deficits (low neuroscore) (P < 0.05) only at UEF, but not at any of the other sites. Additionally, the sites differed in the duration of post-impact transient apnea (p < 0.001) and time to self-right (P < 0.001), the highest values in both parameters was registered in Melbourne. Post-impact seizure-like behavior was observed in 51% (UEF), 25% (Melbourne) and 2% (UCLA) of rats (p < 0.001). Despite the differences in means when all sites were compared there was significant overlap in injury parameters between the sites. The data reflects the technical difficulties in the production of lateral FPI across multiple sites. On the other hand, the data can be used to model the heterogeneity in human cohorts with closed-head injury. Our animal cohort will provide a good starting point to investigate the factors associated with epileptogenesis after lateral FPI.
Keywords: Biomarkers, Multicenter study, Post-traumatic epileptogenesis, Post-traumatic epilepsy, Traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is caused by a bump, blow, or jolt to the head that disrupts the normal function of the brain (Menon et al., 2010). The severity of a TBI may range from “mild” (i.e. a brief change in mental status or consciousness) to “severe” (i.e. an extended period of unconsciousness or memory loss after the injury) (Maas et al., 2017). TBI is common worldwide, and occurs once every 20 s in Europe and the USA, with up to 20% of subjects developing post-traumatic epilepsy over the course of their lifetime (https://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf, accessed 02 October 2018; https://www.center-tbi.eu/; accessed 02 October 2018). Other long-term effects of TBI include: impaired thinking or memory, movement, sensation (e.g., vision or hearing), or emotional functioning (e.g., personality changes, depression) (Wilson et al., 2017; Maas et al., 2017). These issues not only affect individuals but can have lasting effects on families and communities (Galanopoulou et al., 2013; DeWitt et al., 2018).
There are currently no established antiepileptogenic therapies following TBI, and their development is seriously impeded by the lack of biomarkers for epileptogenesis, which would facilitate the identification of individuals at the highest risk (Engel et al., 2013; Pitkanen and Immonen, 2014; Pitkanen et al., 2014, 2016; Pitkanen et al., 2018). This applies both to pre-clinical and clinical studies. Animal models provide essential tools to expand our understanding of post-traumatic epilepsy (PTE). These models are critical to establish causal mechanisms for the development of PTE; facilitate hypothesis testing; enable systematic exploration of pathophysiology and biomarkers; and conduct rigorously controlled experiments to evaluate new therapeutics that will ultimately lead to better informed medical decisions and improved outcomes for patients (Maas et al., 2017; Pitkänen et al., 2017).
Despite the benefits of pre-clinical evaluation of PTE in animal models, there is increasing concern that results from preclinical target validation or drug testing studies are often not replicable in independent studies (Prinz et al., 2011; Landis et al., 2012). There are multiple reasons for the failure of replication and translation of positive preclinical results; lack of study design rigor, publication of inadequately powered studies, and inadequate study blinding treatment at a stage that may not be clinically relevant. Other reasons include: publication bias favoring positive studies, measures of statistical significance with questionable clinical relevance, and failure to address issues related to translation of findings to the clinical setting (Prinz et al., 2011; Galanopoulou et al., 2013; Simonato et al., 2013, 2014; Brady et al., 2018).
Another challenge to PTE research is the significant heterogeneity of preclinical and clinical TBI and PTE (DeWitt et al., 2018). One strategy to help overcome this problem is to embrace and capitalize on the inherent heterogeneity within TBI and PTE models, by studying and correlating the variability of the injury and development of PTE with different outcomes of interest. This requires large-scale multi-center pre-clinical trials in the context of consortiums to enable enough animals to be included in study groups to have sufficient power to validly identify the outcomes of interest. This approach may be particularly useful to trial prospective biomarkers, and novel antiepileptogenic and disease modifying therapies (Simonato et al., 2014). Moreover, to further reduce variability between the different PTE models, pre-clinical researchers must also standardize aspects of data collection and provide more complete and comparable data across studies (Galanopoulou et al., 2013; Simonato et al., 2014). This means incorporating objective indicators of injury, recovery, and endpoints into their studies, particularly when investigating biomarkers of antiepileptogenesis and disease modifying therapies.
The fluid-percussion injury (FPI) model is one of the most widely characterized and frequently applied methods to induce PTE (Brady et al., 2018). Specifically, a lateral FPI has been reported by a number of laboratories to induce PTE in 30–50% of rats, a proportion that is similar to the incidence of PTE reported in patients with TBI (Annegers et al., 1998; Herman, 2002; Englander et al., 2003; Kharatishvili et al., 2006, 2007; Christensen et al., 2009; Shultz et al., 2013). FPI induces a mixed focal-diffuse brain injury pattern that models human closed-head TBI (Thompson et al., 2005; Kabadi et al., 2010; Xiong et al., 2013). The FPI model is a versatile technique because the force of the fluid pulse, severity of the injury and the impact location can be modified (Millen et al., 1985; Armstead and Kurth, 1994; Kabadi et al., 2010; Xiong et al., 2013; Pitkänen et al., 2017; Johnstone et al., 2018). Accordingly, it also leads to pathophysiological outcomes, especially related to moderate-severe FPI that induces significant neuronal death, vascular injury, axonal damage, mossy fiber sprouting, neuroinflammation, and proteinopathies, many of which progress and persist chronically and may contribute to epilepsy development (Kharatishvili and Pitkanen, 2010; Bao et al., 2012; Shultz et al., 2013, 2015; Wright et al., 2017).
As described in the introductory section of this Virtual Special Issue, the EpiBioS4Rx project is a major effort to identify novel biomarkers to predict PTE and identify clinically translatable antiepileptogenesis treatments for PTE (https://epibios.loni.usc.edu/). For this, we have utilized a rigorous standardized FPI rat model for TBI leading to PTE in our laboratories, and present here the harmonization and monitoring procedures critical for the success of such multicentre-international collaborative projects.
2. Materials and method
2.1. Study sites
Three sites from the international multicenter-based project, the Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx), were involved in the standardization of lateral FPI model and post-injury monitoring protocols for biomarker discovery. These sites include: The University of Eastern Finland (UEF), Monash University in Melbourne, Australia (Melbourne) and The University of California, Los Angeles, USA (UCLA).
2.2. Common data elements (CDEs) and case report forms (CRFs)
Prior to the commencement of the project, all the study sites had agreed on some common data elements (CDE) presented in case report forms (CRF). The goal of the CDEs was to ensure standardized system of data is collection for every rat in all sites (See Supplementary material for CEDs used in this study). This will facilitate analysis of the harmonization process between the labs and be used to check for variability when interpreting the epilepsy outcome.
2.3. Animals
In the three study sites, adult male Sprague Dawley rats (300–350 g at the time of TBI) were used in all experiments. In UEF and Melbourne, rats were individually housed in a controlled environment (temperature 22 ± 1 °C; humidity 50–60%; lights on from 07:00 to 19:00 h). At UCLA, the rats were housed in pairs during the quarantine period, and thereafter, in single cages until the end of the experiment, under controlled environment (temperature 22 ± 1 °C; humidity 40–70 %; lights on from 06:00 to 18:00 h). Pellet food and water were provided ad libitum for the duration of the study in all the sites (see Table 3 for pellet code). In UEF, all animal procedures were approved by the Animal Ethics Committee of the Provincial Government of The Southern Finland, and carried out in accordance with the guidelines of the European Community Council Directives 2010/63/EU. In Melbourne, animal procedures were approved by the Florey Animal Ethics Committee (ethics number 17–014 UM) and at UCLA, by the University of California Los Angeles Institutional Animal Care and Use Committee (protocol 2000–153).
Table 3.
Materials used at the experimental sites.
| Material | Site | Catalogue Number | Vendor | Country |
|---|---|---|---|---|
| Rats | UEF | Sprague Dawley (SD) | Envigo Laboratories B.V. | The Netherlands |
| Melbourne | Sprague Dawley (SD) | In house bred | Australia | |
| UCLA | Sprague Dawley (SD) | Charles River | USA | |
| Anesthesia system | UEF | Somnosuite # SS6069B | Kent Scientific | USA |
| Melbourne | Somnosuite # SS6069B | Kent Scientific | USA | |
| UCLA | Matrix VIP 3000 Vaporizer # 91305430 | Patterson Veterinary | ||
| Trephine | UEF | #18004–50 | Fine Science Tools GmbH | Germany |
| Melbourne | Model 300 | Dremmel | Australia | |
| UCLA | #18004–50 | Fine Science Tools GmbH | Germany | |
| Tissue adhesive | UEF | 3 M Vetbond | 3 M Deutschland GmbH | Germany |
| Melbourne | Octyl cyanoacrylate (N/A) | Bostik | Australia | |
| UCLA | 3 M Vetbond | 3 M Deutschland GmbH | Germany | |
| Dental acrylate | UEF | Selectaplus #10009210, Selectaplus #D10009102 | DeguDent | Germany |
| Melbourne | AVSCV00500 | Vertex | The Netherlands | |
| UCLA | SNAP Liquid (P16-02-65), SNAP Powder (P16-02-60) | Pearson Dental | USA | |
| Fluid-percussion device | UEF | Model FP 302 | AmScien Instruments | USA |
| Melbourne | Model FP 302 | AmScien Instruments | USA | |
| UCLA | Model FP 302 | AmScien Instruments | USA | |
| Buprenorphine | UEF | Not applicable | Orion Pharma | Finland |
| Melbourne | Not applicable | Indivior Pty Ltd | Australia | |
| UCLA | Not applicable | AgriLabs | USA | |
| Medical Oxygen | UEF | Not applicable | Not applicable | Not applicable |
| Melbourne | Not applicable | Mediquip Medical Equipment & Supplies | Australia | |
| UCLA | Not applicable | Not applicable | Not applicable | |
| Food Pellet | UEF | 2016S (TeklanDiet) | Envigo Laboratories B.V. | The Netherlands |
| Melbourne | 102108 | Barastoc | Australia | |
| UCLA | LabDiet 5001* | LabDiet | St. Loius, MO, USA |
2.4. Pre-injury handling
In UEF and UCLA, all rats were quarantine for one week upon arrival at the animal facility (see Table 3 for the animal vendor). In Melbourne, the animals were bred in the Biological Research Facilities, The Royal Melbourne Hospital, University of Melbourne.
Animals were housed in individual cages at the end of the one-week quarantine period until the end of the experiment. One week prior to injury, baseline blood sampling and physiological monitoring was performed. Daily monitoring involved assessing the weight, temperature and signs of any disease or discomfort; which included: general appearance; hair, coat and skin abnormalities; bowel and gastrointestinal function, body condition score (Hickman and Swan, 2010) and external bleeding (if any).
2.5. Induction of Lateral fluid percussion injury (FPI)
TBI was induced by lateral fluid-percussion injury (FPI) (McIntosh et al., 1989). Briefly, rats were placed in the induction chamber and anesthesia was induced - using 5% isoflurane in room air as carrier gas (UEF and Melbourne, Somnosuite # SS6069B, Kent Scientific; UCLA, Matrix VIP 3000 Vaporizer # 91305430, Patterson Veterinary). Once the rat was fully anesthetized, as evaluated by the absence of pain reflex, the animal was placed into a stereotaxic frame with lambda and bregma at the same horizontal level. A heating pad was placed in the ventral surface of the animal and the body temperature was continuously monitored using a rectal probe (maximum temperature was set at 38 °C). Isoflurane anesthesia was maintained at 1.9% throughout the surgery. In Melbourne, rats received buprenorphine (Indivior Pty Ltd., Australia) at the beginning of the surgery, as instructed by the local animal ethics committee.
The fur over the skull was shaved and using aseptic technique and a 5-mm diameter craniotomy centered AP −4.5 mm from the bregma; ML 2.5 mm over the left cortex was performed using a hand-held trephine (UEF and UCLA, #18004–50, Fine Science Tools GmbH, Germany), leaving the dura intact. In Melbourne, craniotomy was performed using a motorized drill (Dremmel 300, Australia) connected to a jewelry burr (Maillefer, Switzerland: size 6). A plastic female Luer-Lock connector made from an 18G needle ending was inserted into the craniotomy vertical to the surface of the skull, and its edges were carefully sealed with a tissue adhesive (UEF and UCLA: 3M Vetbond, 3M Deutschland GmbH, Germany; Melbourne: octyl cyanoacrylate, Bostik, Australia). Luer-Lock was anchored to the skull with dental acrylate (UEF, Selectaplus powder #10009210; Selectaplus liquid CN #D10009102, DeguDent, Germany; Melbourne, AVSCV00500, Vertex, The Netherland; UCLA, SNAP Liquid #P16-02-65; SNAP Powder #P16-02-60, Pearson Dental, UCLA) which surrounded also a frontally inserted anchoring dental screw (Ø 1 mm, #BN82213, Bossard). (See Table 3 for additional detail on materials and vendors)
TBI was induced with a fluid-percussion device equipped with a straight tip (UEF, Melbourne and UCLA: AmScien Instruments, Model FP 302, Richmond, VA, USA). The pressure level was adjusted to produce severe TBI with an expected 20–30% post-impact mortality within the first 48 h (Pitkanen and McIntosh, 2006; Liu et al., 2016).
The rat was removed from the device and placed on a heating pad immediately after the impact. Occurrence of immediate post-impact behavioral seizures and duration of apnea were monitored. In Melbourne, if apnea is greater than 10 s after FPI, animals received medical oxygen (Mediquip Pvt Ltd. Australia) at 0.5 ml/min to aid oxygenation until the animal display a regular breathing pattern. Dental cement, screw, and Luer-Lock connector were detached from the skull. The time to self-right after TBI, defined in all experimental sites as the time from impact to the time when the animal rolls over and stand on all four limbs, was recorded.
2.6. Post-injury monitoring
After rats righted themselves, they were re-anesthetized, placed on the stereotaxic frame and the scalp incision was sutured. Animals randomized to the EEG follow-up study, anesthesia was induced again and intracranial and skull electrode were implanted. At the end of the surgery, rats received 0.05 mg/Kg of buprenorphine (Orion Pharma, Finland) for post-operative analgesia. Treatment was repeated there-after based on the animal’s well-being.
Rats received powdered pellet (ad libitum) and 10 ml of 0.9% NaCl (twice a day, s.c.) for the first 3 days after injury; or until the rat was able to eat solid pellets and drink on its own (see Table 3 for food pellet code and vendor). In addition, Melbourne rats received rodent milk powder, mixed with powdered pellet and water, until they recovered their pre-injury weight. UCLA animals received 2.5 mg/Kg of Flu-Nix (Fluxinin meglumine, AgriLabs, USA) for post-operative analgesia. Treatment was repeated every 12 h during the 3 days. In UCLA, rats received chow added with trimethoprim and sulfadiazine (TMS) pellet (ad libitum). In all sites rats received 10 ml of 0.9% NaCl (twice a day, s.c.) for the first 3 days after injury.
The well-being of the rats was assessed by monitoring of several physiological parameters over the course 30 days and monthly there-after, or on an as-needed basis as described in the Pre-injury handling section.
2.7. Assessment of acute post-injury functional impairment
Post-injury somatomotor deficits were assessed based on the composite neuroscore as previously described (Kharatishvili et al., 2009). Rats were scored on a 0 (severely impaired) to 4 (normal) nominal scale for (i) left and right contraflexion, (ii) left and right hindlimb flexion, (iii) left and right lateral pulsion, and (iv) ability to stand on an inclined board in a vertical and horizontal (left and right) position. The maximum possible score was 28. Baseline neuroscore was performed 2 days prior to injury. For the baseline inclined board assessment, the angle at which the rat was able to maintain a steady posture was given a maximal score of 4. The neuroscore was later on assessed at 2, 7, 14, 21 and 28 days after injury. For the post-injury incline board assessment, the injury score was determined by the ability of the rat to stand at an angle similar to that at the baseline (4 = no difference in the angle; 3 = 2.5° less than the baseline; 2 = 5° less than the baseline; 1 = 7.5° less than the baseline; 0 = 10° less than the baseline).
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism (V. 5.03). First, all data was tested for normal distribution using D’Agostino & Pearson’s omnibus normality test. If not normally distributed, then a non-parametric test was used to compare the variable. The chi-square test was used to assess differences between the sites in percentage of mortality and the percentage of animals with post-impact seizure-like behavioral manifestations. The Kruskal-Wallis test was used to assess differences between the sites in impact pressure, apnea and time to self-right. When differences were found, the Dunn’s multiple comparison test or the Mann-Whitney U test was used as a post-hoc test. The repeated measure ANOVA (RMA) was used to assess differences between the sites in neuroscore, body weight and temperature follow-up. Differences between sites at each time point was assessed using the Kruskal-Wallis test followed by the Dunne’s multiple comparison test or Mann-Whitney U test.
3. Results
3.1. Acute mortality following lateral FPI and impact pressure
At all sites, two independent cohorts of rats were prepared: one for long-term MRI follow-up and another for video-EEG follow-up. For MRI follow-up, the rats were sutured after TBI and subsequently randomized for magnetic resonance imaging (MRI) follow-up cohort (MRI-group). The EEG follow-up cohort received cortical and intracranial electrodes (EEG-group). Details of electrode implantation and EEG follow-up have been described in the papers, “Harmonization of pipeline for automated seizure detection for phenotyping of post-traumatic epilepsy in a preclinical multicenter study on post-traumatic epileptogenesis” (Casillas-Espinosa et al., 2019) and “Harmonization of pipeline for detection of HFOs in a rat model of post-traumatic epilepsy in preclinical multicenter study on post-traumatic epileptogenesis” (Santana Gomez et al., 2019).
At UEF, 98 rats were used in the MRI-group. The experiments were performed in three cohorts. Of these, 84 received TBI and 14 were sham-operated. The acute mortality (< 72 h post-TBI) for the MRI-group was 14% (12/84) (see Table 1 for details). Furthermore, 11 rats experienced broken dura after the impact and were excluded. In the EEG-group, the experiment was performed in two cohorts. Altogether 37 TBI and 8 sham-operated rats were operated, among which the acute mortality was 16% (6/37). Four rats experienced broken dura after the impact. The mean impact pressure was similar between the MRI (2.88 ± 0.15 atm) and EEG-groups (2.79 ± 0.14 atm) (p > 0.05). Since there was no difference in the impact pressure between the groups, both groups were pooled together for further analysis. The data indicated that there is a positive correlation between the magnitude of the impact pressure (2.84 ± 0.14 atm; range, 2.44–3.13 at m) and the angle of the pendulum (20.35 ± 0.37° degree; range, 19.5 – 22°) (r = 0.597, p < 0.001).
Table 1.
Mortality following lateral FPI in all sites.
| UEF | Melbourne | UCLA | |||||
|---|---|---|---|---|---|---|---|
| MRI-group | EEG-group | MRI-group | EEG-group | MRI-group | EEG-group | ||
| Enrolled | TBI | 84 | 37 | 48 | 32 | 31 | 32 |
| Sham | 14 | 8 | 14 | 7 | 11 | 7 | |
| Alive - in | TBI | 44 | 25 | 20 | 12 | 14 | 13 |
| Sham | 13 | 8 | 10 | 5 | 9 | 7 | |
| Alive - Ex | TBI | 28 | 6 | 6 | 0 | 0 | 0 |
| Sham | 1 | 0 | 0 | 2 | 0 | 0 | |
| Mortality | TBI | 12 (14%) | 6 (16%) | 20 (42%) | 19 (59%) | 17 (55%) | 19 (59%) |
| Sham | 0 | 0 | 2 | 0 | 2 | 0 | |
Abbreviation: Alivein, included in study; Aliveex, excluded from study (reason being high animal number than expected).
At Melbourne, 62 rats were recruited into the MRI-group. The rats were divided into five cohorts. Together there was 48 TBI and 14 sham-operated rats. The acute mortality (all cohort together) was 42% (20/48). Also, 2 sham-operated rats died unexpected (see Table 1). One rat experienced a broken dura after the impact and was not included in the analysis. Thirty-nine rats were recruited (32 TBI and 7 shams) in the EEG-group. There was 59% (19/32) mortality after TBI as shown in Table 1. No rats experience a broken dura after the impact in the EEG-group. The mean impact pressure in the MRI-group (2.62 ± 0.26 atm) was higher than that in the EEG-group (2.41 ± 0.21 atm) (p < 0.05). Furthermore, the pendulum in the FPI device used for the impact was applied only at two angles, 17.5° or 16.5°. In the MRI group, 40% rats were injured with the pendulum at 17.5° (2.72 ± 0.29 atm; range, 2.2–3.33 at m) and 60% at 16.5° (2.54 ± 0.19 atm, range, 2.3–3.01 atm). In the EEG group, all the rats were injured at 16.5° (2.41 ± 0.21 atm; range, 2.01–2.8 at m). The high number of rats in the MRI group injured at 17.5° resulted in a higher mean impact pressure when compared to the EEG group, as stated above. A correlation analysis between angle of the pendulum and impact pressure could not be performed since only 2 angles were used. Nonetheless, the mean impact pressure in both groups (MRI and EEG groups together) was lower than that in UEF (p < 0.001).
In UCLA, 42 rats (31 TBI and 11 shams) were used in the MRI-group. Experiments were performed in four cohorts. The acute mortality (all cohorts together) was 55% (17/31). In the EEG-group, 39 rats (32 TBI and 7 shams) were randomized and the post-impact mortality was 59% (19/32) as shown in Table 1. No animal from both groups experienced a broken dura following the impact. The mean impact pressure was similar between the MRI-group (2.47 ± 0.21 atm) and EEG-group (2.37 ± 0.18 atm). When both groups were pooled together, a correlation analysis of the impact angle of the pendulum in the FPI (20.55 ± 1.2°; range, 19° - 23°) and the resulting impact pressure (2.4 ± 0.21 atm; range, 3.31 – 2.1 atm) showed that the higher the angle the higher the pressure (r = 0.691, p < 0.0001). There was no difference in the mean angle of the pendulum between UCLA and UEF (20.35 ± 0.37°vs. 20.55 ± 1.2°, p > 0.05). However, the impact pressure (both groups together) was lower than that at UEF (2.4 ± 0.21 atm vs. 2.84 ± 0.14 atm, p < 0.001), but similar to that in Melbourne (2.4 ± 0.21 atm vs. 2.53 ± 0.21 atm, p > 0.05).
When the acute post-impact mortality was compared between the sites, Chi-square analysis revealed that the mortality in the MRI-group and EEG-group differed between the sites (p < 0.0001). However, the mortality was similar between the MRI-group and EEG-group at all the sites (p > 0.05).
3.2. Duration of anesthesia exposure during surgery
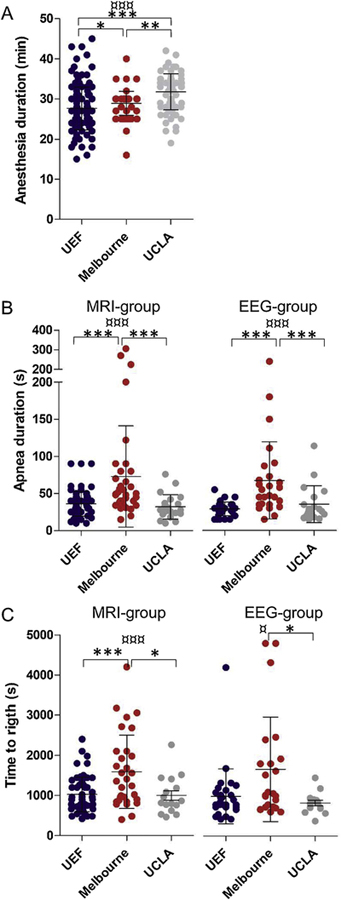
In order to determine whether the duration of exposure to anesthesia can affect the lateral FPI induced brain injury and functional outcome between the sites, the time when the rats started the induction of anesthesia until the anesthesia was discontinued (before impact) was analyzed. There was no difference in the mean duration of anesthesia between the MRI-group and the EEG-group at all sites (p > 0.05), thus both groups were pooled together for further analysis. The mean duration of anesthesia differed between the sites, being 26.98 ± 6.68 min at UEF, 28.91 ± 2.97 min in Melbourne, and 31.78 ± 4.51 min at UCLA (p < 0.001) (Fig. 1A). Further analysis showed that the duration was shorter at UEF than in Melbourne (p < 0.05) and UCLA (p < 0.001). Similarly, the duration in Melbourne was shorter than that at UCLA (p < 0.001) (Fig. 1A).
Fig. 1.
Dot plots showing anesthesia duration, apnea duration and time to righting reflex, analyzed at the three sites, University of Eastern Finland (UEF), University of Monash, Melbourne, Australia Melbourne and University of California Los Angeles (UCLA). (A) The anesthesia duration differed between the three sites. Note that the duration was particularly low at UEF as compared to Melbourne and UCLA. (B) The apnea duration in MRI-group and EEG-group at all sites. Note the long duration in Melbourne in both groups. (C) The duration to self-right after the impact. Note the long duration in Melbourne. * p < 0.05, ** p < 0.01, *** p < 0.001 (Dunne’s test and ManWhitney U test, comparison between 2 sites) and ¤ p < 0.05, ¤¤¤ p < 0.001 (Kruskal-Wallis test, comparison between all sites). Data is presented as mean ± SD.
3.3. Incidence of post-impact seizure-like behavior
At all sites, immediate post-impact seizure-like behaviors were observed in a subpopulation of animals. The behaviors included hind limb jerks or movements, tail rotation, tail erection and strong jerks of the lower torso. At UEF, 52% (42/81) of rats in the MRI-group showed post-impact seizure-like behavior with a mean duration of 17.7 ± 11.6 s. In the EEG-group 51% (19/37) showed post-impact seizures with a mean duration of 13.11 ± 7.8 s. In Melbourne, 26% (8/31) and 24% (7/29) in the MRI-group and EEG-group, respectively, developed post-impact seizure-like behavior. At UCLA, none of the rats in the MRI-group (0/31) and only 1 rat (1/29) in the EEG-group showed post-impact seizure-like behavior. The duration of the behavior was not assessed in Melbourne and UCLA. Statistical analysis showed that the occurrence of post-impact seizure-like behavior was similar between MRI-group and EEG-group at all the sites (p > 0.05). However, there was a difference in the total number of observed seizure-like behaviors (all behavioral types described above) between the sites in the MRI-group and EEG-group (p < 0.0001). When both groups (MRI and EEG-groups) were pooled together for further analysis, the data revealed that the percentage of rats with acute post-impact seizure-like behavior was higher at UEF as compared to Melbourne (p < 0.0001) and UCLA (p < 0.0001). Similarly, the occurrence of seizure-like behavior was more frequent in Melbourne as compared to UCLA (p < 0.0001).
3.4. Duration of post-impact apnea
The period of apnea at all three sites was defined as “the time after impact from when the rat stopped breathing to the time when spontaneous breathing returned”. In Melbourne, all rats received intermittent medical oxygen (see “method” section) supply until regular breathing was restored. The mean apnea duration in the MRI-group differed between sites, being 36.1 ± 16.6 s (n = 75) in UEF, 72.9 ± 68.3 s (n = 36) in Melbourne and 32.05 ± 16.54 s (n = 21) at UCLA (p < 0.0001) (Fig. 1B). Similarly, the mean apnea duration for the rats in the EEG-group differed between the sites (UEF 29.1 ± 9.2 s, n = 34; Melbourne 67.5 ± 10.2 s, n = 26; and UCLA 35.6 ± 5.7 s, n = 19) (p < 0.0001) (Fig. 1B). At all experimental sites, the apnea duration was similar between the MRI and EEG groups, and thus, both groups were pooled for further analysis. A post-hoc analysis revealed that the mean apnea duration was similar between UEF and UCLA (34.1 ± 15.1 s vs. 33.7 ± 20.7 s p > 0.05). However, for rats in Melbourne the apnea was 2.1 times longer than at UEF (70.6 ± 61.6 vs. 34.1 ± 15.1, p < 0.001) and UCLA (70.6 ± 61.6 s vs. 33.7 ± 20.7 s, p < 0.001). Several rats had apnea duration longer than 60 s at UEF (n = 3), Melbourne (n = 14) and UCLA (n = 4) (Fig. 1B). To assess whether the long apnea duration in these rats was a source of variability, the apnea duration between the sites was compared without these outliers. There was still a significant difference in the apnea duration between the sites (p < 0.001), being 32.36 ± 11.79 s (n = 106) in UEF, 40.37 ± 11.84 s (n = 41) in Melbourne and 28.33 ± 11.69 s (n = 36) in UCLA. Consequently, the apnea time will be used as a co-factor in future data analysis.
To further investigate whether the duration of the transient apnea was associated with the magnitude of the impact, a correlation analysis was performed between the apnea duration and the magnitude of the pressure of the impact. Data indicates that the higher the pressure the higher the apnea time at UEF (r = 0.345, p < 0.01, n = 88) and Melbourne (r = 0.539, p < 0.001, n = 49) but not at UCLA (r = 0.232, p > 0.05, n = 30).
3.5. Time to self-right and pain reflex
The time for the rat to self-right after TBI, defined as the time from impact to when the rat rights itself, was monitored at all sites. In the MRI-group, the mean duration to self-right after impact differed between the sites, being 1025 ± 434 s at UEF, 1589 ± 914 s in Melbourne, and 1000 ± 472 s at UCLA (p < 0.0001) (Fig. 1C). Similarly, in the EEG-group the time to self-right differed between the sites being 991 ± 685 s at UEF, 1665 ± 1303s in Melbourne, and 824 ± 267 s at UCLA (p < 0.05) (Fig. 1C). Further analysis revealed that the time to self-right for the MRI-group and EEG-group was similar between UEF and UCLA. However, in Melbourne, the time to self-right for the MRI-group was longer than that at UEF (p < 0.001) or UCLA (p < 0.05). In addition, in Melbourne, the time to self-right for the EEG-group was similar to that at UEF (p > 0.05) but higher than that at UCLA (p < 0.05). (Fig. 1C). There was no difference between the MRI-group and the EEG-group at all of the sites, thus, both groups where pooled together for each site for further analysis. The time to self-right did not differ between UEF and UCLA (1014 ± 523.9 s vs. 917.9 ± 394 s). However, for the rats in Melbourne it was still 1.6 and 1.8 times higher than that at UEF (1621 ± 1088s vs. 1014 ± 523.9, p < 0.001) and UCLA (1621 ± 1088s vs. 917.9 ± 394 s, p < 0.001) respectively.
To determine whether the duration to self-right is associated with the severity of the impact, a correlation analysis was performed between the time to self-right (MRI and EEG groups pooled together) and the magnitude of the impact. No correlation was found at UEF (r = 0.167, p > 0.05) and UCLA (r = 0.164, p > 0.05) sites. At Melbourne however, there was weak positive correlation between the magnitude of the impact and the time to self-right (r = 0.339, p < 0.05).
3.6. Post-injury follow-up
3.6.1. Post-injury acute functional impairment
The composite neuroscore was used to assess acute functional impairment (Kharatishvili et al., 2009). The neuroscore was performed in the MRI-group at all sites. However, for the EEG-group, the neuroscore was performed only in Melbourne and at UCLA. The UEF site did not perform the neuroscore to reduce the risk of fall-off of the electrode headset due to repeated disconnection of the animal from the recording system.
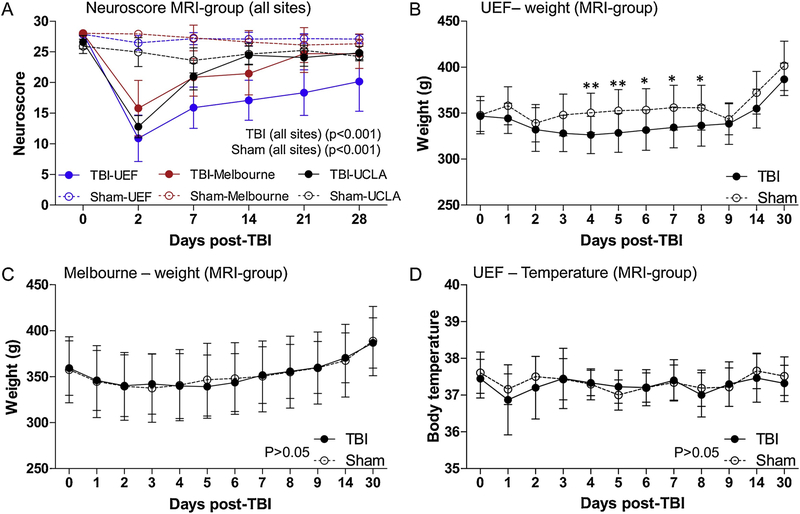
A repeated-measure ANOVA(RMA) of all rats included in the MRI follow-up with complete neuroscore evaluation (base line, 2 d, 7 d, 14 d, 21 d and 28 d), showed that the neuroscore differed between sites over time (p < 0.001). The baseline neuroscore for the sham operated animals in the MRI-group was similar between UEF and Melbourne (p > 0.05). Whereas, the baseline neuroscore in UCLA was lower than that at UEF (p < 0.05) and Melbourne (p < 0.05). After sham-operation, the neuroscore for sham rats was similar between UEF and Melbourne at all time-points (p > 0.05) (Fig. 2A and Table 2). The neuroscore of the sham rats at UCLA was lower at all time-points compared to that at UEF, except at 2 d post-TBI, (p > 0.05) (Fig. 2A and Table 2). Similarly, the neuroscore for sham rats at UCLA was lower as compared to that in Melbourne at all time-points, except 21 d post-TBI (p > 0.05) (Fig. 2A and Table 2).
Fig. 2.
Line plot demonstrating progression of neuroscore, weight and body temperature after TBI. (A) The neuroscore for the MRI-group at the different sites. Note the variability in the neuroscore between the sites across time points. Repeated measure analysis of rats with complete neuroscore analysis (0–28 d) shows that the neuroscore over time is different between the sites. In addition, the neuroscore was different between the sites at individual time points (see also Table 2 for details). (B) Body weight follow-up of the MRI-group at UEF. The weight of the TBI rats decreased by 3 d post-injury, then recovered to control levels by 14 d post-TBI. (C) Body weight follow-up of rats in MRI-group in Melbourne. There was no difference in the post-injury weight progression between TBI and sham-operated rats at any time point, as assessed using the repeated measure ANOVA (RMA) (D) Temperature follow-up of MRI-group rats at UEF. RMA showed no difference in temperature between TBI and sham rats after TBI, at any time point. Data are presented as mean ± SD. *p < 0.05, **p < 0.01 (Mann-Whitney test).
Table 2.
Neuroscore of rats in the MRI and EEG groups at the different sites.
| MRI | Site | Baseline | 2 d | 7 d | 14 d | 21 d | 28 d |
|---|---|---|---|---|---|---|---|
| Mean (range, n) | Mean (range, n) | Mean (range, n) | Mean (range, n) | Mean (range, n) | Mean (range, n) | ||
| Sham | 1 | 28 (28 – 26, n = 13)¤¤¤ | 27 (28 – 25, n = 13)¤¤¤ | 27 (28 – 25, n = 13) ¤¤¤ | 28 (28 – 25, n = 13) ¤¤¤ | 27 (28 – 26, n = 13) ¤¤¤ | 27 (28 – 26, n = 13) ¤¤¤ |
| 2 | 28 (28 – 28, n = 9) | 28 (28 – 27, n = 10) | 28 (28 – 22, n = 9) | 28 (28 – 24, n = 8) | 28 (28 – 20, n = 10) | 27 (28 – 24, n = 8) | |
| 3 | 26 (28 – 24, n = 9)*+ | 25 (28 – 21, n = 9) | 23 (27 – 21, n = 9)** | 25 (27 – 22, n = 9)** | 25 (26 – 24, n = 9)* | 24 (26 – 24, n = 8)** | |
| TBI | 1 | 28 (28 – 28, n = 45) ¤¤¤ | 11 (21 – 1,11= 45)¤¤¤ | 16 (24 – 8, n = 45) ¤¤¤ | 17 (24 – 10, n = 45) ¤¤¤ | 18 (26 – 12, n = 45) ¤¤¤ | 20 (28 – 11, n = 45) ¤¤¤ |
| 2 | 28 (28 – 28, n = 18)*** | 17 (22 – 6, n = 20)*** | 21 (27 – 17, n = 19)*** | 20 (27 – 16, n = 13)*** | 25 (28 – 17, n = 20)*** | 25 (28 – 20, n = 15)*** | |
| 3 | 27 (28 – 24, n = 14) | 13 (17 – 10, n = 14)+ | 21 (25 – 17, n = 14)*** | 25 (26 – 22, n = 14)*** | 24 (26 – 22, n = 14)*** | 25 (27 – 23, n = 11)*** | |
| EEG | |||||||
| Sham | 2 | 28 (28 – 28, n = 3) | 23 (23 – 20, n = 3) | 27 (28 – 26, n = 3) | 26 (27 – 25, n = 3) | 27 (27 – 27, n = 2) | 26 (28 – 26, n = 3) |
| 3 | 26 (26 – 25, n = 7) | 24 (26 – 22, n = 7) | 24 (26 – 23, n = 7)+ | 24 (26 – 20, n = 7) | 23 (25 – 21, n = 7)++ | 23 (24 – 22, n = 7)+++ | |
| TBI | 2 | 28 (28 – 28, n = 10) | 12 (16 – 7, n = 8) | 17 (20 – 13, n = 8) | 17 (22 – 15, n = 8) | 19 (24 – 16, n = 6) | 22 (26 – 21, n = 7) |
| 3 | 27 (28 – 25, n = 13) | 11 (15 – 9, n = 13) | 19 (21 – 17, n = 13) | 24 (28 – 22, n = 9)+ | 25 (27 – 22, n = 9)+ | 23 (26 – 21, n = 9) |
Site 1, University of Eastern Finland (UEF); Site 2, University of Monash (Melbourne); Site 3, University California (UCLA). Abbreviation: d, day; EEG, electroencephalography; n, number of animals; TBI, traumatic brain injury. Statistical significances:
p < 0.001, comparison between all sites (Kruskal-Wallis test);
p < 0.05,
p < 0.01,
p < 0.001 comparison with UEF (Dunn’s multiple comparison and Mann-Whitney U test);
p < 0.05,
p < 0.01,
p < 0.001, comparison between Melbourne (Dunn’s multiple comparison and Mann-Whitney U test).
For the TBI rats in the MRI-group, RMA revealed that the neuroscore of the rats in the MRI group over the 28-d follow-up period (only for animals with a complete data) differed between the sites across time (p < 0.001). Though the baseline neuroscore was the same at all sites (p > 0.05) (Fig. 2A and Table 2), the post-TBI neuroscore at UEF was lower when it was compared to Melbourne, at all time-points (Fig. 2A and Table 2). Similarly, the neuroscore at UEF was lower when compared to UCLA, at all time-points except at 2 d post-TBI (p > 0.05) (Fig. 2A and Table 2). The neuroscore in Melbourne and UCLA was similar at all time-points, except at 2 d (p < 0.05) (Fig. 2A and Table 2).
For the EEG-group, the composite neuroscore (performed only in Melbourne and UCLA) of the sham-operated rats was similar between Melbourne and UCLA, at all time-points except at 7 d (p < 0.05), 21 d (p < 0.01) and 28 d (p < 0.001) (Table 2). A repeated-measure ANOVA could not be performed for the sham rats because in UCLA there were only 2 rats with a complete data set. In the TBI group, the neuroscore of the EEG-group differed between the sites over time (p < 0.05). Further analysis at the different time-points showed that the neuroscore was similar between Melbourne and UCLA, at all time-points except at 14 d and 21 d post-TBI (p < 0.001) (Table 2).
In order to assess whether the severity of the acute functional impairment at 2 d post-TBI is associated with the magnitude of the impact, we performed a correlation analysis between the neuroscore and the impact pressure. The data revealed a weak negative correlation, i.e. the higher the impact pressure, the lower the neuroscore (more impaired) at the UEF site (r = −0.313, p < 0.05) but not in Melbourne (r = −0.237, p > 0.05) or UCLA (r = −0.189, p > 0.05). Furthermore, to determine whether longer anesthesia duration will associated with severity of the acute injury, we correlated the duration of anesthesia and the neuroscore at 2 d. There was no correlation seen at any of the sites (p > 0.05).
3.6.2. Post-injury body weight follow-up
The body weight was used as an index of recovery after TBI. The body weight of the rat was registered at the baseline at all the sites. The post-injury body weight was available in the database only at UEF and Melbourne. The body weight was recorded during the first 9 days post-injury and then on days 14 and 30. At UEF, the body weight was assessed only for the MRI-group to avoid repetitive disconnection of rats from the EEG-monitoring system. There was no difference in the body weight between the sham-operated and the TBI rats at baseline, 1 d and 2 d post-TBI. However, on 3 d post-TBI the body weight of the TBI rats decreased to 94% of that in sham rats (328 ± 19.7 g vs. 348 ± 22.7 g) (p < 0.05) and stayed below 94% of that in shams until 9 d post-TBI (338.5 ± 22.6 g vs. 343.3 ± 16.3 g, p > 0.05). (Fig. 2B).
At Melbourne, the TBI and sham rats in the MRI-group did not differ in weight throughout the follow-up period (p > 0.05) (Fig. 2C). When rats in the MRI-group at UEF and Melbourne were compared, the body weight of the sham-operated rats was similar at all time-points and the weight of the TBI rats was also similar at all time-points, except at 8 d (336.4 ± 22.3 g vs. 355.7 ± 2.4 g, p < 0.05) and 9 d (338.5 ± 22.6 g vs. 360 ± 28.4 g, p < 0.05) post-injury, where the weight of the rats at UEF were lower compared to those in Melbourne. The body weight of the TBI and sham rats recruited in the EEG-group in Melbourne were different only at 6 d post-TBI (350.4 ± 27.8 g vs. 411 ± 1.4 g, p < 0.05).
3.6.3. Post-injury temperature follow-up
The core temperature was assessed only in UEF in rats belonging to the MRI-group. The body temperature was assessed at the same time-point as the weight (see above) using a rectal probe. The mean body temperature was similar between sham (range, 36.2–38.6 °C) and TBI (range, 32.8–38.7 °C) rats at all of the time points (p > 0.05) (Fig. 2D).
4. Discussion
Harmonization of experimental procedures across different experimental sites is crucial in studies aimed at establishing robust clinically translatable epileptogenesis biomarkers using a multicenter-based approach. Our objective was to assess the success of harmonization of the procedures, used in the production of the lateral FPI model, across the different laboratories. The harmonization of the lateral FPI model of PTE involved three laboratories located at the University of Eastern Finland (UEF), Monash University (Melbourne, Australia) and The University of California Los Angeles (UCLA, USA). The main findings demonstrate (1) marginal differences between the sites in the core independent variables, such as the impact pressure and the duration of anesthesia; (2) Moderate to severe injury severity at all sites, as assessed with the neuroscore, even though sites differed in other outcome measures including acute mortality, transient apnea duration and time to self-right.
4.1. Differences in acute mortality between experimental sites is not associated with impact pressure
A key objective in the harmonization of the lateral FPI model between the experimental sites was to achieve a moderate to severe TBI with acute mortality (< 72 h post-TBI) between 20–30%. A moderate-severe TBI was selected because it has been reported by a number of laboratories to induce PTE in a proportion of rats that is similar to the incidence of PTE reported in patients with TBI (Annegers et al., 1980; Herman, 2002; Frey, 2003; Englander et al., 2003). The overall mortality at UEF was about 20%, whereas at Melbourne and UCLA it was above 40%, even though the impact pressure at UEF was about 10% and 15% higher than that in Melbourne and UCLA, respectively. The average impact pressure in the group of rats with acute impact-related mortality was 3%, 2% and 8% higher than that in the group of rats that survived at UEF, Melbourne and UCLA respectively (data not shown). This is in agreement with previous data showing that high impact pressure is associated with increased mortality in the lateral FPI model (McIntosh et al., 1989; Kharatishvili et al., 2009). This data suggests that the higher acute mortality in Melbourne and UCLA is not due to the magnitude of the impact pressure. Additionally, at UEF, the higher the impact pressure the more severe the injury. However, this was not the case with Melbourne and UCLA. The subjectivity in the assessment of the neuroscore (as discussed below) may have contributed to lack of correlation between the impact pressure and the severity of the injury in Melbourne and UCLA. Nonetheless, since the injures were more severe at UEF, it will be interesting to see whether the incidence of epilepsy will also be higher at UEF.
There are several other reasons that might explain the variability in mortality, including rat strain, age, the experimenter performing the injury procedures and deviations in original experimental protocol. First, to eliminate the issue of strain-related mortality, a common variability among studies, was that all sites used Sprague Dawley (SD) rats (Tan et al., 2009; Reid et al., 2010). However, variability within the same strain is also a common phenomenon. O’Bryant et al. (2011) found that breeder and batch-to-batch differences within rats from the same breeding facility were strong contributing factors to experimental variability (O’Bryant et al., 2011). The rats at UEF were acquired from Envigo Laboratories B.V. (Melderslo, The Netherlands) in 3 batches (3 cohorts). For example, the mortality in MRI group of the first cohorts in UEF was 25% but later got stable at 9% and 10% respectively in the last 2 cohorts (data not shown). This data reflects more of a learning curve rather than a batch effect due to the fact that the UEF site was switching to an isoflurane anesthesia protocol. The rats used in Melbourne were in-house breed (Biological Research Facilities, Melbourne Medical School, The Royal Melbourne Hospital-Parkville, Australia), and the rats used in UCLA where purchased from Charles River, USA. Our data suggests that there may be genetic and environmental influences in their response to TBI, which may change during a long-lasting experiment. The genetic diversity can be used as positive aspect in this study, since it could correspond with the genetic variability in TBI patients. The possibility of age-related differences as a potential source of variability was eliminated by using rats of the same age at the beginning of the experiment at all the sites. It is also possible that factors such as biometric pressure and room temperature can influence the impact pressure and subsequently the mortality, however, this remains to be shown. Another factor that may contribute to the variability in mortality is the inter-experimenter variability in handling and performance of the surgery and TBI. The latter can be managed if there is common training of personnel involved in the multicenter study. Additionally, the administration of buprenorphine before surgery in Melbourne (as requested by the local ethics committee) may have significantly contributed to increased mortality. However, this needs to be further explored.
Taken together the data suggests that the variability in mortality between the sites is not associated with the impact pressure. Additional factors need to be considered to reliably decipher the source of the observed variation in mortality.
4.2. Duration of transient apnea was associated with severity of impact but varied between the sites
To establish further that the harmonization of the lateral FPI model production in the different sites was on track we compared the transient apnea duration between the sites. The duration of the transient apnea has been suggested as an indicator of injury severity (Igarashi et al., 2007). Previous studies using this TBI model have reported incidence of apneic episodes ranging from 10 to 60 s, (Dixon et al., 1987; Kharatishvili et al., 2006). The average transient apnea time was comparable between UEF and UCLA, but in Melbourne, it was twice as long as that of the other sites. Contrary to previous studies, we found a weak but positive correlation between the transient apnea duration and magnitude of the impact (impact pressure) in UEF and Melbourne, but not at UCLA (Dixon et al., 1987; Kharatishvili et al., 2006). The difference between our study and previous reports might be due to the number of animals involved. In this study, UEF had 105 rats and Melbourne 62 rats, whereas in the study by Dixon et al., as well as Kharatishvili et al., there were 20 and 48 rats, respectively. This gives a higher statistical power to our study. At UCLA where the total number of rats used was 40, no correlation was observed. It should be noted that the UCLA site had just started performing the lateral FPI model. It is possible that the limited experience in model production compromised the analysis of apnea duration. Additionally, rats in Melbourne received buprenorphine before the surgery, and medical oxygen after the impact to improve survival. It remains to be investigated whether these procedures may have confounded the assessment of post-impact transient apnea in Melbourne. Furthermore, it is very likely that the breeding-related genetic and environmental factors may also have played a role in the response to injury, as discussed above.
We also wanted to establish whether disparities in the duration of the surgeries might also contribute to the variability observed in the transient apnea duration, even though there is limited evidence in the literature to suggest any relationship. Nonetheless, we did not find any correlation between the duration of anesthesia and the transient apnea duration. This suggests that the long post-impact transient apnea duration observed in Melbourne may not be related to the anesthesia duration.
Taken together, our data indicates that the duration of the post-impact transient apnea is associated with the magnitude of the impact pressure but not with the duration of pre-injury anesthesia exposure.
4.3. All experimental sites produced injuries with moderate to severe severity but with variable post-injury body weight progression profile
The production of rats with moderate to severe injury was a fundamental goal in the harmonization of the injury model production. The acute functional impairment, as assessed using the neuroscore, was used a measure of severity of the injury. The neuroscore is effective at detecting acute sensorimotor deficits following lateral FPI (Niskanen et al., 2013). Moreover, reports further suggest that lateral FPI-induced deficits are pronounced in animals with severe injury, as compared to those with moderate or mild injury (injury severity based on impact pressure) when assessed at 2 or 3 d post-TBI (McIntosh et al., 1989; Kharatishvili et al., 2009). The neuroscore at 2 d at all the sites was 13–17, indicating that all sites were able to produce moderate-severe injury. However, the 2 d neuroscore was lower at UEF when compared to Melbourne and UCLA. This data is corroborated by the fact that impact pressure at UEF was higher than that at the other sites. Despite the fact that the sites show differences in the absolute neuroscore at 2 d, the relative difference between TBI and shams, as well as the temporal profile of the neuroscore, was similar between the sites. It is worth noting that the increased injury severity observed at UEF was associated with acute post-impact seizure-like behaviors in a large proportion of animals, which ranged from tonic jerks, to tail rotation, to movement of the lower body. Since these behaviors were not systematically defined and monitored at the beginning of the study, it is possible that they may have remained unreported by the investigators, accounting for the differences in the number of observations reported between the sites.
Another factor that can influence the neurological outcome is the duration of anesthesia. In fact, Gaidhani et al. (2017) recently reported that duration of isoflurane anesthesia was a significant source of variability in the assessment of infarct volume and neurological deficits in a transient suture middle cerebral artery occlusion model of ischemic stroke in rats. They demonstrated an inverse relationship between the anesthesia duration and severity of brain injury and neurological deficits (Gaidhani et al., 2017). This data suggests that longer anesthesia may be protective. Though the anesthesia duration was longer in Melbourne and UCLA than that at UEF, there was no association with significant changes in the neuroscore at any of the sites. Whether the longer isoflurane anesthesia resulted in neuroprotection remains to be shown. Nonetheless, it should be considered that the subjective nature of the neuroscore test is also a source for variability in neuroscore between the study sites. This can be seen in the baseline neuroscore values for naïve rats, which was different between the sites, and particularly low at UCLA. This clearly demonstrates experimenter-associated variability.
In this study, body weight follow-up was used as a measure of assessing post-injury recovery. Previous reports suggest that there is usually a 15–20% body weight loss following lateral FPI, and increased weight loss correlates with injury severity (Adelson et al., 1996; Moinard et al., 2005; Wang et al., 2013; Eakin et al., 2015). At UEF there was a clear drop in the body weight during the first week post-TBI, which subsequently regained their pre-injury (baseline) weight as well as sham levels by 9 d post-injury. This pattern was not evident in Melbourne, where the TBI and sham rats showed similar body weight progression at all follow-up time points. The discrepancy in the post-injury weight progression between the sites cannot be attributed to post-injury treatment since, at all the sites, rats where treated with supportive food and fluid replacement with saline (0.9% NaCl) administration. It is unclear whether the addition of rodent milk powder to the powdered pellet in Melbourne had any effect on the body weight. Furthermore, in all sites, all rats received buprenorphine after surgery in the first few days after injury. Whether other factors, including genetic and environmental influences could contribute to the observed difference, remains to be shown.
Taken together our data shows that the severity of the injury was similar between the sites, ranging from moderate to severe injury. Furthermore, the temporal profile of the injury severity was similar between the sites. Nevertheless, the differences in the absolute values of injury severity at individual time-points may be related to subjective differences in performing the neuroscore test.
4.4. Conclusion
Our data demonstrates that variability in the injury harmonization parameters can, to a lesser extent, relate to site-specific experimental protocol differences, and to a greater extent to breeder-related genetic and breeder/site-specific environmental influences. Furthermore, there is inherent variability between experimenters. On the other hand, the variability can be used to model the genetic and exposomal heterogeneity in humans exposed to closed-head injury that is the objective of using the lateral FPI. The follow-up of the animals will demonstrate whether the variability in procedures will be beneficial for our multi-center biomarker study. That is, will the variability in observed injury generate a subpopulation of animals with a high risk of epileptogenesis. Furthermore, the biomarker discovered will tolerate a certain level of experimental variability, which will be inevitable in future studies with different laboratories. The results presented show the first analysis of the ongoing study. It will be interesting to see whether the level of variability reduces between the sites during the course of the study. Nonetheless, the data presented here demonstrates that all sites were able to produce a consistent and comparable moderate-severe TBI (see Immonen et al., in this volume). In our ongoing follow-up of these rats we will see if the proportion of rats that develop post-traumatic epilepsy will be comparable across sites.
Overall, our data demonstrates the relevance of performing standardization (harmonization) in preclinical multicenter studies, and indicates which injury parameters are critical in establishing a harmonized injury model. Furthermore, it reiterates the need to consider local animal ethics committee recommendations, animal genetic background and experimenter training, in designing future multi-center based studies.
Supplementary Material
Acknowledgements
This research was supported by the National Institute of Neurological Disorders and Stroke (NINDS) Center without Walls of the National Institutes of Health (NIH) under Award Number U54NS100064 (EpiBioS4Rx).
Footnotes
This article is part of a special issue ‘Discovery of diagnostic biomarkers for post-traumatic epileptogenesis – an interim analysis of procedures in preclinical multicenter trial EpiBios4Rx’.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eplepsyres.2019.01.006.
References
- Adelson PD, Robichaud P, Hamilton RL, Kochanek PM, 1996. A model of diffuse traumatic brain injury in the immature rat. J. Neurosurg 85, 877–884. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Kurland LT, Laws ER Jr., 1980. The incidence, causes, and secular trends of head trauma in Olmsted County, Minnesota, 1935–1974. Neurology 30, 912–919. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Coan SP, Rocca WA, 1998. A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med 338, 20–24. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Kurth CD, 1994. Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J. Neurotrauma 11, 487–497. [DOI] [PubMed] [Google Scholar]
- Bao F, Shultz SR, Hepburn JD, Omana V, Weaver LC, Cain DP, Brown A, 2012. A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats. J. Neurotrauma 29, 2375–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RD, Casillas-Espinosa PM, Agoston DV, Bertram EH, Kamnaksh A, Semple BD, Shultz SR, 2018. Modelling traumatic brain injury and posttraumatic epilepsy in rodents. Neurobiol. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas-Espinosa PM, de Abreu PA, Santana-Gomez C, Paananen T, Smith G, Peruca P, Ali I, Ciszek R, Ndode-Ekane XE, Immonen R, Puhakka N, Staba RJ, Pitkänen A, O’Brien TJ, 2019. Harmonization of pipeline for automated seizure detection for phenotyping of post-traumatic epilepsy in a preclinical multi-center study on post-traumatic epileptogenesis. Epilepsy Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M, 2009. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 373, 1105–1110. [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Hawkins BE, Dixon CE, Kochanek PM, Armstead W, Bass CR, Bramlett HM, Buki A, Dietrich WD, Ferguson AR, Hall ED, Hayes RL, Hinds SR, LaPlaca MC, Long JB, Meaney DF, Mondello S, Noble-Haeusslein LJ, Poloyac SM, Prough DS, Robertson CS, Saatman KE, Shultz SR, Shear DA, Smith DH, Valadka AB, VandeVord P, Zhang L, 2018. Pre-clinical testing of therapies for traumatic brain injury. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL, 1987. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg 67, 110–119. [DOI] [PubMed] [Google Scholar]
- Eakin K, Rowe RK, Lifshitz J, 2015. Modeling Fluid Percussion Injury: Relevance to Human Traumatic Brain Injury. [PubMed] [Google Scholar]
- Engel J Jr., Pitkanen A, Loeb JA, Dudek FE, Bertram EH 3rd, Cole AJ, Moshe SL, Wiebe S, Jensen FE, Mody I, Nehlig A, Vezzani A, 2013. Epilepsy bio-markers. Epilepsia 54 (Suppl. 4), 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, Hughes R, Bergman W, 2003. Analyzing risk factors for late posttraumatic seizures: a pro-spective, multicenter investigation. Arch. Phys. Med. Rehabil 84, 365–373. [DOI] [PubMed] [Google Scholar]
- Frey LC, 2003. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia 44 (Suppl 10), 11–17. [DOI] [PubMed] [Google Scholar]
- Gaidhani N, Sun F, Schreihofer D, Uteshev VV, 2017. Duration of isoflurane-based surgical anesthesia determines severity of brain injury and neurological deficits after a transient focal ischemia in young adult rats. Brain Res. Bull 134, 168–176. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Simonato M, French JA, O’Brien TJ, 2013. Joint AES/ILAE translational workshop to optimize preclinical epilepsy research. Epilepsia 54 (Suppl 4), 1–2. [DOI] [PubMed] [Google Scholar]
- Herman ST, 2002. Epilepsy after brain insult: targeting epileptogenesis. Neurology 59,S21–6. [DOI] [PubMed] [Google Scholar]
- Hickman DL, Swan M, 2010. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J. Am. Assoc. Lab. Anim. Sci 49, 155–159. [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Potts MB, Noble-Haeusslein LJ, 2007. Injury severity determines Purkinje cell loss and microglial activation in the cerebellum after cortical contusion injury. Exp. Neurol 203, 258–268. [DOI] [PubMed] [Google Scholar]
- Johnstone MR, Sun M, Taylor CJ, Brady RD, Grills BL, Church JE, Shultz SR, McDonald SJ, 2018. Gambogic amide, a selective TrkA agonist, does not improve outcomes from traumatic brain injury in mice. Brain Inj. 32, 257–268. [DOI] [PubMed] [Google Scholar]
- Kabadi SV, Hilton GD, Stoica BA, Zapple DN, Faden AI, 2010. Fluid-percussion-induced traumatic brain injury model in rats. Nat. Protoc 5, 1552–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkanen A, 2010. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 90, 47–59. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A, 2006. A model of post-traumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140, 685–697. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Immonen R, Grohn O, Pitkanen A, 2007. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain 130, 3155–3168. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Sierra A, Immonen RJ, Grohn OH, Pitkanen A, 2009. Quantitative T2 mapping as a potential marker for the initial assessment of the severity of damage after traumatic brain injury in rat. Exp. Neurol 217, 154–164. [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD, 2012. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zheng P, Wright DK, Dezsi G, Braine E, Nguyen T, Corcoran NM, Johnston LA, Hovens CM, Mayo JN, Hudson M, Shultz SR, Jones NC, O’Brien TJ, 2016. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 139, 1919–1938. [DOI] [PubMed] [Google Scholar]
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Buki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang JY, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Soderberg J, Stanworth SJ, Stein MB, von Steinbuchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K, InTBIR Participants and Investigators, 2017. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL, 1989. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244. [DOI] [PubMed] [Google Scholar]
- Menon DK, Schwab K, Wright DW, Maas AI, 2010. Demographics and clinical assessment working group of the international and interagency initiative toward common data elements for research on traumatic brain injury and psychological health. Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil 91, 1637–1640.21044706 [Google Scholar]
- Millen JE, Glauser FL, Fairman RP, 1985. A comparison of physiological responses to percussive brain trauma in dogs and sheep. J. Neurosurg 62, 587–591. [DOI] [PubMed] [Google Scholar]
- Moinard C, Neveux N, Royo N, Genthon C, Marchand-Verrecchia C, Plotkine M, Cynober L, 2005. Characterization of the alteration of nutritional state in brain injury induced by fluid percussion in rats. Intensive Care Med. 31, 281–288. [DOI] [PubMed] [Google Scholar]
- Niskanen JP, Airaksinen AM, Sierra A, Huttunen JK, Nissinen J, Karjalainen PA, Pitkanen A, Grohn OH, 2013. Monitoring functional impairment and recovery after traumatic brain injury in rats by FMRI. J. Neurotrauma 30, 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant AJ, Allred RP, Maldonado MA, Cormack LK, Jones TA, 2011. Breeder and batch-dependent variability in the acquisition and performance of a motor skill in adult Long-Evans rats. Behav. Brain Res. 224, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Immonen R, 2014. Epilepsy related to traumatic brain injury. Neurotherapeutics 11, 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, McIntosh TK, 2006. Animal models of post-traumatic epilepsy. J. Neurotrauma 23, 241–261. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Kemppainen S, Ndode-Ekane XE, Huusko N, Huttunen JK, Grohn O, Immonen R, Sierra A, Bolkvadze T, 2014. Posttraumatic epilepsy – disease or comorbidity? Epilepsy Behav. 38, 19–24. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Loscher W, Vezzani A, Becker AJ, Simonato M, Lukasiuk K, Grohn O, Bankstahl JP, Friedman A, Aronica E, Gorter JA, Ravizza T, Sisodiya SM, Kokaia M, Beck H, 2016. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 15, 843–856. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kyyriäinen J, Andrade P, Pasanen L, Ekolle Ndode-Ekane X, 2017. Epilepsy after traumatic brain injury In: Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshe SL (Eds.), Models of Seizure and Epilepsy, 2nd edition Academic Press, Elsevier Inc., London, pp. 661–681. [Google Scholar]
- Pitkanen A, Ekolle Ndode-Ekane X, Lapinlampi N, Puhakka N, 2018. Epilepsy bio-markers - toward etiology and pathology specificity. Neurobiol. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz F, Schlange T, Asadullah K, 2011. Believe it or not: how much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov 10 712–c1. [DOI] [PubMed] [Google Scholar]
- Reid WM, Rolfe A, Register D, Levasseur JE, Churn SB, Sun D, 2010. Strain-related differences after experimental traumatic brain injury in rats. J. Neurotrauma 27, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana Gomez C, Andrade P, Hudson M, Paananen T, Ciszek R, Smith G, Ali I, Ndode-Ekane XE, Casillas-Espinosa PM, Immonen R, Puhakka N, Jones N, Perucca P, Pitkänen A, O’Brien TJ, Staba R, 2019. Harmonization of pipeline for detection of HFOs in a rat model of post-traumatic epilepsy in preclinical multicenter study on post-traumatic epileptogenesis. Epilepsy Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Cardamone L, Liu YR, Hogan RE, Maccotta L, Wright DK, Zheng P, Koe A, Gregoire MC, Williams JP, Hicks RJ, Jones NC, Myers DE, O’Brien TJ, Bouilleret V, 2013. Can structural or functional changes following traumatic brain injury in the rat predict epileptic outcome? Epilepsia 54, 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Wright DK, Zheng P, Stuchbery R, Liu SJ, Sashindranath M, Medcalf RL, Johnston LA, Hovens CM, Jones NC, O’Brien TJ, 2015. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138, 1297–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato M, French JA, Galanopoulou AS, O’Brien TJ, 2013. Issues for new antiepilepsy drug development. Curr. Opin. Neurol 26, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato M, Brooks-Kayal AR, Engel J Jr., Galanopoulou AS, Jensen FE, Moshe SL, O’Brien TJ, Pitkanen A, Wilcox KS, French JA, 2014. The challenge and promise of anti-epileptic therapy development in animal models. Lancet Neurol. 13, 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AA, Quigley A, Smith DC, Hoane MR, 2009. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J. Neurotrauma 26, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK, 2005. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chen YS, Lin BS, Chio CC, Hu CY, Kuo JR, 2013. The neuronal protective effects of local brain cooling at the craniectomy site after lateral fluid percussion injury in a rat model. J. Surg. Res 185, 753–762. [DOI] [PubMed] [Google Scholar]
- Wilson L, Stewart W, Dams-O’Connor K, Diaz-Arrastia R, Horton L, Menon DK, Polinder S, 2017. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 16, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DK, O’Brien TJ, Shultz SR, Mychasiuk R, 2017. Sex matters: repetitive mild traumatic brain injury in adolescent rats. Ann. Clin. Transl. Neurol 4, 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2013. Animal models of traumatic brain injury. Nat. Rev. Neurosci 14, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.