Abstract
This article comments on:
Garg A, Kirchler T, Fillinger S, Wanke F, Stadelhofer B, Stahl M, Chaban C. 2019. Targeted manipulation of bZIP53 DNA-binding properties influences Arabidopsis metabolism and growth. Journal of Experimental Botany 70, 5659–5671.
Keywords: bZIP, development, stress, metabolism, interactome, posttranslational modification, homo/heterodimerization
Basic (region) leucine zipper (bZIP) transcription factors are involved in different metabolic pathways, developmental cues and stress responses in eukaryotic organisms. Using a targeted manipulation approach, Garg et al. (2019) investigated the effect of the bZIP53 protein in the regulation of dimerization and direct binding to the DNA, thus affecting plant amino acid metabolism and development in Arabidopsis plants.
The bZIP family of transcriptional regulators are characterized by the presence of a basic DNA-binding domain, also responsible for nuclear localization, adjacent to a leucine zipper domain involved in dimerization. In Arabidopsis, this family includes 78 members divided into 13 groups (A to M) (Jakoby et al., 2002; updated in Dröge-Laser et al., 2018). bZIP transcription factors are involved in different developmental programmes in all phases of plant life, from seed maturation, dormancy and germination, to photomorphogenesis and senescence. Several key bZIP regulators are also involved in metabolic reprogramming and biotic and abiotic stress responses. However, their functional characterization is often impeded by the high redundancy and overlapping roles among the different members.
In their study, Garg et al. (2019) reveal that dominant-negative versions of different bZIPs from groups C and S1 maintain their dimerization partners and nuclear localization, while disrupt DNA binding to their cis regulatory elements.
Edgetic alleles enable bZIP functional analysis
The involvement of bZIP transcription factors in a plethora of different metabolic, developmental and stress-related processes (i) makes it difficult to study each of their functions without affecting others and (ii) reduces the possibilities of their use as biotechnological tools. For the study of the physiological relevance of bZIP protein-protein and bZIP-DNA interactions, the use of null alleles or overexpression lines has traditionally been used. If we represent bZIP signal transduction pathways as transcriptional networks in which the different elements involved make up nodes, we obtain something similar to what we can observe in Box 1. When a bzip loss-of-function allele is used, we completely eliminate the node and all of its interactions, which makes individual evaluation of each interaction difficult (Box 1). For this reason, the identification of edgetic alleles, versions of bZIPs that have lost the ability to interact with one or more proteins (or DNA) while maintaining their interaction with other partners, is particularly useful (Box 1) (Dreze et al., 2009; Sahni et al., 2013). With this strategy it is also possible to evaluate the robustness of the network when its complexity is reduced.
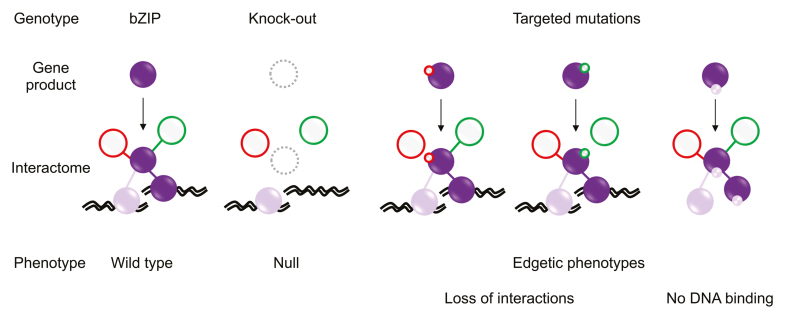
Box 1. Representation of the advantages of using edgetic alleles in the study of the bZIP signaling network.
In a schematic representation of the different bZIP genotype-phenotype associations, the possible phenotypes resulting from network perturbations caused by knock-out (dotted circles) or different targeted mutation types compared to bZIP wild type, are represented. The edgetic strategy applied to a bZIP of interest (purple node) may affect its interactome or DNA binding itself. Red and green colors represent specific edges and their perturbations, while purple and violet colors represent bZIP homo and heterodimers, respectively.
In their current work, Garg et al. (2019) found that phosphorylation-mimicking substitution of conserved serines in the DNA-binding domain of bZIP53 monomeric subunit suffices for the disruption of the interaction of both, bZIP homo- and heterodimers, with cognate DNA.
Prototypic bZIPs with functional and regulatory relevance
The past two decades have witnessed a growing body of evidence confirming the bZIP interactome in plants, based on the several posttranslational protein modifications they undergo (Box 2). The bZIP family is able to homo and heterodimerize with other partners that results in an enormous regulatory flexibility regarding target-side selection or protein interactions. Different posttranslational modifications that impact on bZIP functionality include sumoylation, ubiquitination and consequent proteasomal degradation, redox status (i.e. S-glutathionylation and S-nitrosylation of specific Cys residues), together with phosphorylation and dephosphorylation (revised for ABI5 in Yu et al., 2015; Skubacz et al., 2016). Aditionally, bZIP association with members of the Mediator (MED) machinery and AFP-related Topless (TPL) co-repressors alter the transcriptional regulation of target genes. Quintessential examples of different bZIP group members modified through these posttranslational modifications are highlighted here.
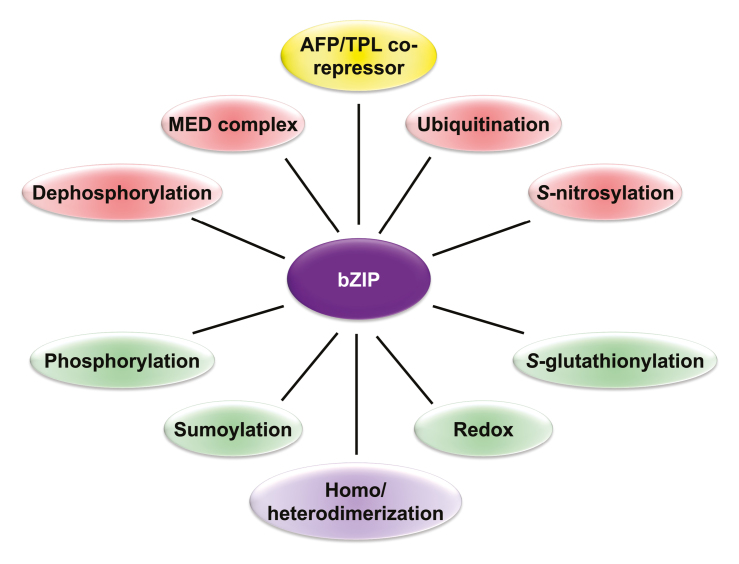
Box 2. Postulated bZIP interactome based on the posttranslational protein modifications.
The ability of the bZIP family to homo and heterodimerize with other bZIPs expands their regulatory network of targets and interactors (purple). Different posttranslational modifications may positively (green) or negatively (red) affect bZIP protein stability. Among the positive effectors, sumoylation by SUMO E3 ligases has been reported, while ubiquitination by the corresponding E3 ubiquitin ligases promotes bZIP destabilization. Similarly, alterations modulating the bZIP redox status (i.e. by redoxins) include S-glutathionylation and S-nitrosylation of specific Cys residues. In addition, phosphorylation and dephosphorylation of several bZIP members exerted by specific protein kinases and phosphatases is a well-known mechanism impacting on bZIP functionality. Finally, members of the Mediator (MED) machinery and AFP-related Topless (TPL) co-repressors (yellow) have the ability to physically associate with bZIPs and alter the transcriptional regulation of target genes.
The Arabidopsis bZIP members of the C- and S1-groups have a proposed function in plant energy management during development and stress trade-off (Dröge-Laser et al., 2018). This network is usually formed by heterodimers that synergistically control metabolic reprogramming during stress responses through direct interaction with G-box or ACTCAT cis-elements. Indeed, Garg et al. (2019) also attempted to understand the role of bZIP heterodimerization, mainly between bZIP53 from group S1 and bZIP10 from group C. Interestingly, the bZIP63 group C member is phosphorylated by SnRK1, both in vitro and in vivo, upon energy deprivation (Mair et al., 2015). This posttranslational modification causes significant enhancement of bZIP63 heterodimerization with group S1 bZIP1 and bZIP11 leading to increased bZIP63-dependent gene activation and the formation of a ternary C/S1/SnRK1 complex (Pedrotti et al., 2018).
Members of the group A of bZIP transcription factors are involved in different developmental cues including embryo development, seed maturation and germination, and stress responses (Jakoby et al., 2002). Among them, ABI5 is a key player in ABA-triggered processes (Finkelstein and Lynch, 2000; Lopez-Molina et al., 2001) able to bind the ABRE (ACGTGG/TC) cis-element and regulated by phosphorylation and dephosphorylation events (Yu et al., 2015). ABI5 is also sumoylated by the SUMO E3 ligase SIZ1 (Miura et al., 2009) and regulated through S-nitrosylation of Cys153 residue. This latter modification targets ABI5 to the proteasome by promoting the interaction with CULLIN4-based and KEEP ON GOING E3 Ligases (Albertos et al., 2015).
TGA transcription factors form group D of the bZIPs, according to their conserved TGACG DNA binding motif. In the Arabidopsis genome ten members of the TGA family are present (TGA1-TGA10), falling into five clades (Jakoby et al. 2002). The TGA family of bZIP transcription factors, which bind specifically to SA response elements, interact with NPR1 to modulate gene expression. TGA1 and TGA4 are regulated through Cys residues sensitive to the cellular redox state that form an intramolecular disulfide bond in the absence of SA, preventing their interaction with NPR1 (Després et al., 2003). The Cys residues 260 and 266 of TGA1 are regulated by both S-nitrosylation and S-glutathionylation, affecting protein conformation and preventing formation of disulphide bonds (Lindermayr et al., 2010). At the same time, NPR1 ameliorates not only the DNA binding activity of the reduced TGA1 (Despres et al., 2003), but also the DNA binding activity of TGA1-SNO. At the biochemical level, the ability of PERIANTHIA (PAN)/TGA8 to bind DNA (and, specifically, AG-like elements) is altered according to cell redox conditions. PAN contains an N-terminal end with five Cys residues able to form intramolecular disulfide bridges, consistent with the regulatory mechanism of other TGAs (Gutsche and Zachgo, 2016). In addition, a sixth Cys residue at the C-terminal end, Cys340, is essential for PAN function because it undergoes a specific S-glutathionylation (Li et al., 2009; Gutsche and Zachgo, 2016). In this redox context, the ROXY1 CC-type glutaredoxin negatively regulates PAN protein activity (Li et al., 2009).
The most representative member of group H, HY5, is controlled by posttranslational mechanisms in particular. Thus, HY5 interaction with COP1 results in its ubiquitination and degradation in darkness. Interestingly, HY5 protein is phosphorylated preventing COP1-dependent degradation, but only the non-phosphorylated version is physiologically active and binds DNA (Gangappa and Botto, 2016). The interactome of HY5 comprises PIF bHLH factors, MYBs and bZIP groups G, C and S1.
Finally, other bZIPs studied in detail regarding their posttranslational modifications, dimerization and DNA binding properties include GBF1 (bZIP41) from group G, VIP1 (bZIP51) from group I, bZIP34 and bZIP61 from group E and bZIP60 from group K; as recently reviewed in Dröge-Laser et al. (2018).
Stepping forward
Although the path has been paved by establishing the targeted manipulation of bZIP transcription factors for their functional analysis, many fundamental and applied questions remain to be answered.
The bZIP family of transcriptional factors in Arabidopsis is large and redundant, while Marchantia polymorpha has only one or two members in most of the groups and no orthologs for groups M and K (Table 1). Translational biology using low redundancy species (i.e. Marchantia polymorpha) offers a powerful approach for understanding fundamental processes in plant development. As we move away from Arabidopsis to other model species and crops, it is important to have a clear vision not only of bZIP function and target specificity, but also of gene abundance and the evolutionary signaling pathways for translational biology.
Table 1.
bZIP transcription factors in the low redundancy species Marchantia polymorpha. Classification of the plant bZIP family in Marchantia polymorpha according to the updated database (http://marchantia.info/) with high similarity to Arabidopsis thaliana
| Gene symbol | Mapoly ID | Arabidopsis Correspondence |
|---|---|---|
| MpBZIP1 | Mapoly0001s0021.1 | Group H |
| MpBZIP2 | Mapoly0007s0056.1 | Group I |
| MpBZIP3 | Mapoly0012s0172.1 | Group C |
| MpBZIP4 | Mapoly0015s0005.1 | Group G |
| MpBZIP5 | Mapoly0016s0098.1 | Group J |
| MpBZIP6 | Mapoly0019s0040.1 | Group E |
| MpBZIP7 | Mapoly0022s0095.1 | Group B |
| MpBZIP8 | Mapoly0026s0039.1 | Group D |
| MpBZIP9 | Mapoly0034s0126.1 | Group S |
| MpBZIP10 | Mapoly0046s0102.1 | Group I |
| MpBZIP11 | Mapoly0069s0009.1 | Group A |
| MpBZIP12 | Mapoly0072s0050.1 | Group A |
| MpBZIP13 | Mapoly0122s0020.1 | Group F |
| MpBZIP14 | Mapoly0130s0030.1 | Group B |
| MpBZIP15 | Mapoly0737s0001.1 | Group S |
Future research should reveal if precise amino acid substitutions in bZIP targets will lead to the design of more accurate molecular tools for stress tolerance, development and metabolic improvement in crops. To this end, it is also of utmost importance to use CRISPR-Cas technology to generate and introduce targeted mutations in those key residues detrimental for bZIP protein function. Understanding how bZIP interaction dynamics changes under metabolic, developmental or stress conditions would result in the identification of useful targets that could be manipulated to increase plant biomass yields and resistance to different stresses relevant to the agricultural field. There is no doubt that this study is a stepping stone towards more exciting research to come.
Acknowledgements
Research of OL is supported by the projects EcoSeed Impacts of Environmental Conditions on Seed Quality ‘EcoSeed-311840’ ERC.KBBE.2012.1.1-01, BIO2017-85758-R and CSD2007-00057 (TRANSPLANTA) from the Ministerio de Ciencia, Innovación y Universidades (MICIU), SA093U16 and SA313P18 from Junta de Castilla y León.
References
- Albertos P, Romero-Puertas MC, Tatematsu K, Mateos I, Sánchez-Vicente I, Nambara E, Lorenzo O. 2015. S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nature Communications 6, 8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR. 2003. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. The Plant Cell 15, 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreze M, Charloteaux B, Milstein S, et al. 2009. ‘Edgetic’ perturbation of a C. elegans BCL2 ortholog. Nature Methods 6, 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge-Laser W, Snoek BL, Snel B, Weiste C. 2018. The Arabidopsis bZIP transcription factor family-an update. Current Opinion in Plant Biology 45, 36–49. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. 2000. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. The Plant Cell 12, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF. 2016. The multifaceted roles of HY5 in plant growth and development. Molecular Plant 9, 1353–1365. [DOI] [PubMed] [Google Scholar]
- Garg A, Kirchler T, Fillinger S, Wanke F, Stadelhofer B, Stahl M, Chaban C. 2019. Targeted manipulation of bZIP53 DNA-binding properties influences Arabidopsis metabolism and growth. Journal of Experimental Botany 70, 5657–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsche N, Zachgo S. 2016. The N-Terminus of the floral Arabidopsis TGA transcription factor PERIANTHIA mediates redox-sensitive DNA-Binding. PloS One 11, e0153810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby M, Weisshaar B, Dröge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, Parcy F; bZIP Research Group 2002. bZIP transcription factors in Arabidopsis. Trends in Plant Science 7, 106–111. [DOI] [PubMed] [Google Scholar]
- Li S, Lauri A, Ziemann M, Busch A, Bhave M, Zachgo S. 2009. Nuclear activity of ROXY1, a glutaredoxin interacting with TGA factors, is required for petal development in Arabidopsis thaliana. The Plant Cell 21, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindermayr C, Sell S, Müller B, Leister D, Durner J. 2010. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. The Plant Cell 22, 2894–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH. 2001. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 98, 4782–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair A, Pedrotti L, Wurzinger B, et al. 2015. SnRK1-triggered switch of bZIP63 dimerization mediates the low-energy response in plants. elife 4, e05828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Lee J, Jin JB, Yoo CY, Miura T, Hasegawa PM. 2009. Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proceedings of the National Academy of Sciences, USA 106, 5418–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrotti L, Weiste C, Nägele T, et al. 2018. Snf1-RELATED KINASE1-controlled C/S1-bZIP signaling activates alternative mitochondrial metabolic pathways to ensure plant survival in extended darkness. The Plant Cell 30, 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni N, Yi S, Zhong Q, Jailkhani N, Charloteaux B, Cusick ME, Vidal M. 2013. Edgotype: a fundamental link between genotype and phenotype. Current Opinion in Genetics & Development 23, 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubacz A, Daszkowska-Golec A, Szarejko I. 2016. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Frontiers in Plant Science 7, 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Wu Y, Xie Q. 2015. Precise protein post-translational modifications modulate ABI5 activity. Trends in Plant Science 20, 569–575. [DOI] [PubMed] [Google Scholar]