Abstract
This article comments on:
Fabre D, Yin X, Dingkuhn M, Clément-Vidal A, Roques S, Rouan L, Soutiras A, Luquet D. 2019. Is triose phosphate utilization involved in the feedback inhibition of photosynthesis in rice under conditions of sink limitation? Journal of Experimental Botany 70, 5773–5785.
Keywords: Feedback, gas exchange, photosynthesis, source sink ratio, triose phosphate utilization
Photosynthesis in plants occurs in the chloroplast, which is considered an endosymbiotic organelle living within a host. Metabolism in the endosymbiont and host must be aligned and plants can only grow as fast as chloroplasts can provide resources. However, it is also true that photosynthesis can only be as fast as the plant can use resources from the chloroplast. The interaction between the chloroplast and the rest of the plant has fascinated researchers for many years Fabre et al. (2019) have exciting new data about source sink effects on photosynthesis, which is best predicted by the triose phosphate utlization limitation of photosynthesis but Kumarathunge et al. (2019) suggest that this behavior can be ignored.
Simple end product feedback, for example sucrose inhibition of sucrose-phosphate synthase or the Calvin-Benson cycle has not been demonstrated, but a number of more subtle mechanisms have been found. For example, the sucrose-phosphate synthase phosphorylation state can change (Huber and Huber, 1996) as a result of interaction with sucrose-sensitive SNRKs (sucrose non-fermenting receptor kinase). Trehalose 6-phosphate has emerged as a sucrose signaling surrogate that can affect SnRK1 and also stimulate starch synthesis and inhibit its breakdown (Lunn et al., 2006, Martins et al., 2013, Paul et al., 2018).
An important method used to study photosynthesis is measurement of CO2 uptake by leaves. Gas exchange analysis can be interpreted with the widely used model of Farquhar et al. (1980). An extension of this model considers the condition in which photosynthesis is limited by how fast carbon is exported from the Calvin-Benson cycle, a form of end-product feedback (Sharkey, 1985). Since most carbon leaves the cycle as triose phosphate this is called the triose phosphate utilization (TPU) limitation. It would seem logical that the TPU limitation would increase when the source-sink ratio is increased. Fabre et al. (2019) used rice to test this. They pruned rice plants to decrease sinks and used elevated CO2 to increase source capacity. They found a strong relationship between source sink balance and the capacity for TPU (see Box 1). There was reasonable correlation between sucrose in leaves and TPU capacity (higher sucrose was correlated with lower TPU). Photosynthesis declined during the day as did TPU.
Box 1.
A central part of the work of Fabre et al., 2019 is assessing what is typically called the “A/Ci” curve, net CO2 assimilation rate (A) as a function of CO2 in the intercellular airspaces (Ci). By using Ci, effects of changes in stomatal conductance are removed. It is much better to assess an A/Cc curve, where Cc is the CO2 concentration in the chloroplast, removing all diffusion resistance effects. In this way biochemical processes of photosynthesis are more readily apparent. There are specific recommendations for measuring and assessing A/Ci curves (Long and Bernacchi, 2003, Sharkey, 2016). I make several recommendations here for best practices.
First, the “right” way to measure A/Ci curves is the way that is most likely to illuminate the part of photosynthesis you wish to study. Do you want to measure steady state photosynthesis or do you want to take a snapshot? As seen in the data of Fabre et al. (2019), photosynthetic properties of leaves change all day. There are many processes that change such as activation states of enzymes, ionic balance in the stroma and lumen, etc. Very rapid measurements are more likely to give a picture in sharp focus. The fastest method is the RACiR method described by Stinziano et al. (2017). However, I also like to assess chlorophyll fluorescence and so usually make discrete measurements.
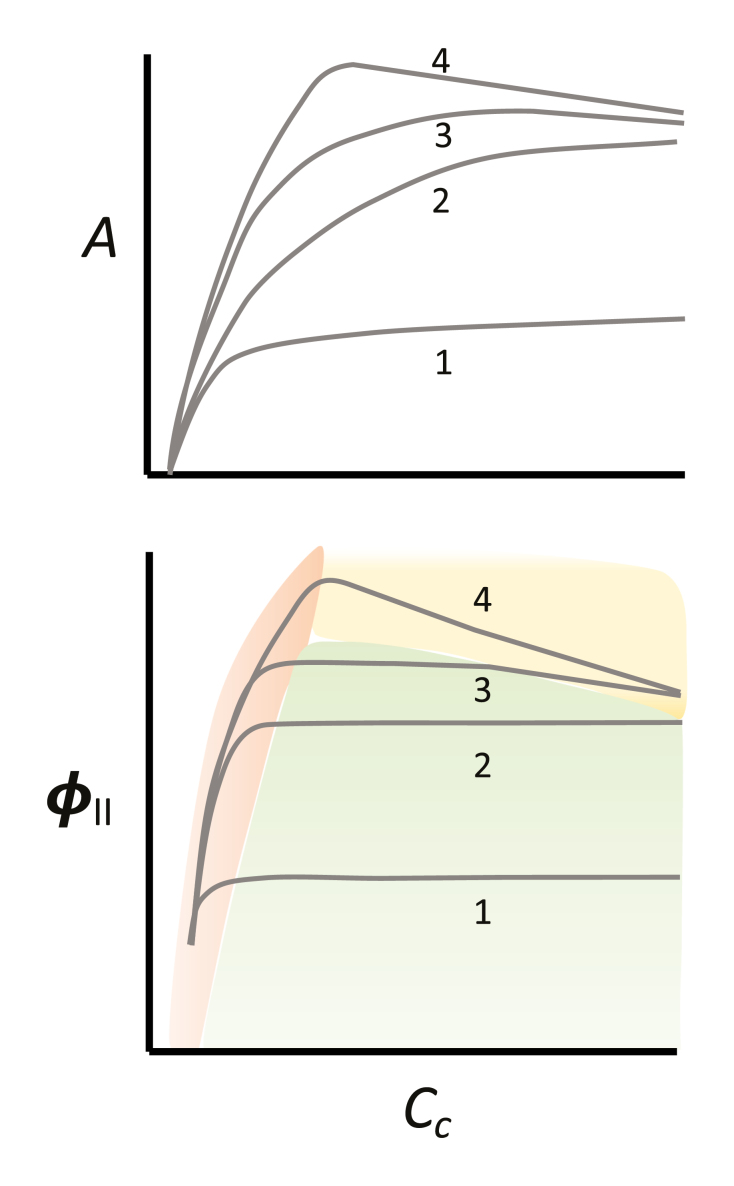
A second issue is light intensity. At saturating light Fabre et al. (2019) saw a rubisco limitation (photosynthetic electron transport increasing with CO2) give way to a TPU limitation (photosynthetic electron transport decreasing with CO2) with no obvious RuBP-regeneration-limited points (photosynthetic electron transport independent of CO2). This behavior is quite common at saturating light (depicted as lines 4 in Box 2). If it is important to see an RuBP regeneration limitation then it is useful to use less than saturating light (lines 3 and even better 2 in figure B1). If light is low enough, all points on the curve may be RuBP regeneration limited (lines 1 of Figure B1), slightly higher there may be both rubisco-limited and RuBP-regeneration-limited data (lines 2 of Figure B1). RuBP regeneration-limited points are especially useful if the curve is used to estimate mesophyll conductance. There is no information about mesophyll conductance in TPU limited data and very little in rubisco-limited data, so it is good to have a number of RuBP-regeneration-limited data points for estimating mesophyll conductance.
Third, there is debate about the sequence of CO2 that should be imposed, high to low, low to high, or start in the middle and go down, jump back to the middle and go up. A continuous and rapid progression through the CO2 concentrations works best. The ‘start in the middle and go both ways’ method often results in noise in the RuBP regeneration part of the curve, where it is most disruptive, especially for estimating the mesophyll conductance.
Fourth, use white light if available or a 50:50 mix of red and blue. Although a lower amount of blue light is sufficient for some stomatal opening, in practice, stressed plants and plants in which the stomata have already opened several times, may require more than 10% blue light. It is important to account for the reduced effectiveness of blue light (McCree, 1971), probably a result of non-photosynthetic absorption of blue light. If this is not done then there is an error introduced proportional to the amount of blue light used (Evans et al., 2017).
However, in essentially all situations, TPU was not the apparent limiting factor for photosynthesis at the expected operating conditions when assessed by gas exchange analysis. It is usually found that TPU is not limiting under physiological conditions and TPU is often ignored for this reason (Kumarathunge et al., 2019). Typically, around 30% of A/Ci curves show an obvious TPU limitation (Kumarathunge et al., 2019) although switching to a low oxygen atmosphere or measuring the electrochromic shift of the carotenoid absorption spectrum (caused by the electrical gradient of the proton motive force across the thylakoid membrane) may detect TPU under a broader range of conditions (Yang et al., 2016). The more important implication, based on Fabre et al. (2019), is that, while TPU is the best reflection of changes in the photosynthetic apparatus in response to source-sink variations, it is not itself limiting.
One consequence of ignoring TPU is that it may lead to inaccurate estimates of the capacity for photosynthetic electron transport (Jmax). This is clear in the data of Figure 2B reported by Fabre et al. (2019). Chlorophyll fluorescence quenching analysis showed that, at low CO2, electron transport increased with CO2 (indicative of rubisco limitation) but then abruptly switched to decline with further increases in CO2 (indicative of TPU). Electron transport was never independent of CO2, the behavior that indicates J limitation/RuBP regeneration. In this case, the maximum rate of electron transport is set by TPU, not by electron transport or Calvin-Benson cycle reactions per se.
When plants are put into TPU-limited conditions for a long time (hours to days), the TPU limitation is observable at first but then other components of photosynthesis are adjusted so that TPU is no longer the apparent limitation. For example, Pammenter et al. (1993) found that holding leaves in 1500 μmol photons m-2 s-1 and 35 Pa CO2 (~350 ppm) for four hours had no effect on the gas exchange characteristics of leaves of Phaseolus vulgaris or Xanthium strumarium. However, if the CO2 partial pressure were increased to 150 Pa (~1500 ppm) resulting in TPU limitation, photosynthesis was reduced because of changes to the capacity for electron transport as measured by chlorophyll quenching analysis. Ordinarily, it would be expected that high CO2 would be less damaging than normal CO2 but the opposite was found. On the other hand, holding leaves in low CO2 caused a reduction in activity of sucrose-phosphate synthase and therefore a reduction in TPU (Vassey et al., 1991). In other words, TPU-limited leaves undergo changes so that most of the time TPU is (1) not limiting, but also (2) just slightly in excess of the photosynthetic rate under physiological conditions.
A similar situation occurs when photosynthesis is limited by ribulose bisphosphate (RuBP) regeneration. When a leaf is first switched to low light, inhibiting RuBP regeneration, the concentration of RuBP falls as expected. However, very soon, rubisco deactivates and the RuBP concentration increases and is no longer the limiting factor. Mott et al. (1984) showed that measuring quickly after a change to limiting light resulted in measured RuBP values that accurately predicted photosynthetic rate but in the ‘steady state’, RuBP pool size did not predict photosynthetic rate, even though the leaves behaved as though they were RuBP-regeneration limited. So, when ‘RuBP regeneration’ limits the rate of photosynthesis, the concentration of RuBP remains high, and thus not limiting. It is possible that carbamylated rubisco with no RuBP bound is more susceptible to degradation or that deactivation keeps the concentration of phosphoglyceric acid in check preventing an upset to the pH regulation in the stroma. For these reasons it may be advantageous for rubisco deactivation to maintain high RuBP levels even though production of RuBP is the limiting process.
Similarly, TPU limitation should result in low RuBP concentration, but steady state RuBP pools are higher following induction of TPU limitation and rubisco is deactivated (Sharkey et al., 1986). TPU limitation causes changes that make it appear that TPU is not limiting, just as rubisco deactivation makes it appear that RuBP is not limiting photosynthesis limited by RuBP regeneration.
If these things are true, then what is the meaning of ‘RuBP regeneration limited’ or ‘TPU limited’ photosynthesis? These describe gas exchange behavior and reflect the process setting the rate of photosynthesis. They do not describe the immediate mechanism by which the rate is set but do describe how photosynthetic rate will change in response to changes in light, gas composition, or temperature. These behaviors are seen in CO2 response curves, especially when supplemented with chlorophyll quenching analysis to estimate PSII activity (see Box 2).
Box 2.
Figure legend Idealized A/Cc and ϕII/Cc curves. At low light (growth light for Arabidopsis (~100 μmol m-2 s-1) (curves 1) photosynthesis increases with CO2 primarily because of changing the ratio of carboxylation to oxygenation. Electron transport as reflected in ϕII is often independent of CO2 except at very low CO2. At a higher photon flux density (for example between 300 and 400 μmol m-2 s-1) (curves 2) ϕII increases with CO2 at low CO2 (rubisco limited photosynthesis) but then becomes independent of CO2. At a higher photon flux (for example between 400 and 600 μmol m-2 s-1 depending on the species, growth conditions etc.) all three limitations are seen. At low CO2, ϕII (photosynthetic electron transport) increases with CO2, then it is independent of CO2 (RuBP-regeneration-limited photosynthesis), then it declines with more CO2, a clear indication of TPU limitation (curves 3). A fourth condition is frequently seen as depicted in curves 4. In this case, the rubisco limited condition gives way directly to the TPU limitation. Fabre et al. (2019) report this behavior in Figure 2. It is not always best to measure A/Cc curves at saturating light since there may be no data that is RuBP-regeneration-limited. Box 3 provides protocols that may be useful in measuring A/Ci curves.
It may be that if TPU is too high it is difficult to develop a low luminal pH that can regulate photosynthetic electron transport (Avenson et al., 2005) whereas if TPU is too low it may cause excess feedback at the photosystems and result in damage (Kiirats et al., 2009). For these reasons TPU is adjusted to normally be just a little higher than photosynthetic rate allowed by other processes or other processes are adjusted to be a little less than what would otherwise be allowed by TPU. This makes it easy to connect the source/sink treatments with TPU, as expected, and as demonstrated by Fabre et al. (2019) and justifies using TPU to constrain global modeling of how photosynthesis may change with increasing CO2 (Lombardozzi et al., 2018, but see Kumarathunge et al., 2019).
Box 3.
Standard practices for measuring gas exchange
Sharkey lab protocols
These suggestions for measuring gas exchange will be appropriate for >90% of the measurements that might be made. These protocols can easily be adapted to address specific issues. Most of the recommendations, especially related to fluorescence, are the default settings on a LI-COR 6800. If using an instrument from a different company you may need to make minor adjustments.
Plant growth: Arabidopsis should be grown with 12 or fewer hours (8 to 10 ideal) light of at minimum 100 μmol m-2 s-1. Longer daylengths results in smaller leaves that often do not give useful data.
Other plants (beans, tomato, wheat, tobacco) should be grown in large pots, with 400 or more μmol photons m-2 s-1.
Unhealthy plants can be hard to measure and give confusing results.
Single measurement of A
Leaf temperature 23°C for Arabidopsis, 25°C for everything else
Humidity 22 mmol mol-1 in the reference channel (watch dew points to ensure no condensation)
CO 2 set sample to 410 ppm (increasing each year)
Light set to growth light intensity or 1000 μmol m-2 s-1 (saturating) Use 50:50 blue red LEDs if those are the only colors available. Use white light when available.
Match
Measure
If you want to measure photosynthesis as a function of time, be sure to match every ten min.
Use the multiphase flash for fluorescence (the default of the LI-COR 6800)
If you want to measure Fv/Fm, keep the leaf in the dark for at least 20 min. Most people will not need this.
Light response curve
Make a single measurement as above, then
Disable matching
Program the sequence of light changes desired
CO 2 response curve
Make a single measurement as above, then
Set light to EITHER 1. Growth light level, OR 2. subsaturating (400 μmol m-2 s-1) (useful to determine gm) OR 3. saturating (1000 μmol m-2 s-1)
Set CO2 to control on the reference channel
Allow early match, match at every CO2 concentration (do not allow early match when measuring isoprene)
Set minimum wait time to 90 seconds, maximum to 180 seconds
Set CO2 concentrations 50, 100, 200, 300, 350, 400, 450, 500, 550, 600, 700, 800, 1000, 1200, 1500, 420.
To save time delete (in order of preference) 1200, 1500, 700
Sometimes it is useful to go high to low, especially if stomatal closure is causing problems.
We do not recommend starting at 400 and going down, returning to 400 and then going up
Leave fluorescence measurement enabled but do not measure Fo’ unless specifically needed.
References
- Avenson TJ, Cruz JA, Kanazawa A, Kramer DM. 2005. Regulating the proton budget of higher plant photosynthesis. Proceedings of the National Academy of Sciences, USA 102, 9709–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Morgan PB, von Caemmerer S. 2017. Light quality affects chloroplast electron transport rates estimated from chl fluorescence measurements. Plant and Cell Physiology. doi: 10.1093/pcp/pcx103. [DOI] [PubMed] [Google Scholar]
- Fabre D, Yin X, Dingkuhn M, Clément-Vidal A, Roques S, Rouan L, Soutiras A, Luquet D. 2019. Is triose phosphate utilization involved in the feedback inhibition of photosynthesis in rice under conditions of sink limitation? Journal of Experimental Botany 70, 5771–5783. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Huber SC, Huber JL. 1996. Role and regulation of sucrose-phosphate synthase in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 431–444. [DOI] [PubMed] [Google Scholar]
- Kiirats O, Cruz JA, Edwards GE, Kramer DM. 2009. Feedback limitation of photosynthesis at high CO2 acts by modulating the activity of the chloroplast ATP synthase. Functional Plant Biology 36, 893–901. [DOI] [PubMed] [Google Scholar]
- Kumarathunge DP, Medlyn BE, Drake JE, Rogers A, Tjoelker MG. 2019. No evidence for triose phosphate limitation of light saturated leaf photosynthesis under current atmospheric CO2 concentration. Plant, Cell & Environment. doi: 10.1111/pce.13639. [DOI] [PubMed] [Google Scholar]
- Lombardozzi DL, Smith NG, Cheng SJ, Dukes JS, Sharkey TD, Rogers A, Fisher R, Bonan GB. 2018. Triose phosphate limitation in photosynthesis models reduces leaf photosynthesis and global terrestrial carbon storage. Environmental Research Letters 13, 074025. [Google Scholar]
- Long SP, Bernacchi CJ. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54, 2393–2401. [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks Janneke HM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei M-R, Stitt M. 2006. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochemical Journal 397, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins MCM, Hejazi M, Fettke J, et al. . 2013. Feedback inhibition of starch degradation in Arabidopsis leaves mediated by trehalose 6-phosphate. Plant Physiology 163, 1142–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCree KJ. 1971. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agricultural Meteorology 9, 191–216. [Google Scholar]
- Mott KA, Jensen RG, O’Leary JW, Berry JA. 1984. Photosynthesis and ribulose 1,5-bisphosphate concentrations in intact leaves of Xanthium strumarium L. Plant Physiology 76, 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammenter NW, Loreto F, Sharkey TD. 1993. End product feedback effects on photosynthetic electron transport. Photosynthesis Research 35, 5–14. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Gonzalez-Uriarte A, Griffiths CA, Hassani-Pak K. 2018. The role of trehalose 6-phosphate in crop yield and resilience. Plant Physiology 177, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. 1985. O2-insensitive photosynthesis in c3 plants: its occurrence and a possible explanation. Plant Physiology 78, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. 2016. What gas exchange data can tell us about photosynthesis. Plant, Cell & Environment 39, 1161–1163. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Seemann JR, Berry JA. 1986. Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to changing partial pressure of O2 and light in Phaseolus vulgaris. Plant Physiology 81, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinziano JR, Morgan PB, Lynch DJ, Saathoff AJ, McDermitt DK, Hanson DT. 2017. The rapid A-Ci response: photosynthesis in the phenomic era. Plant, Cell & Environment 40, 1256–1262. [DOI] [PubMed] [Google Scholar]
- Vassey TL, Quick WP, Sharkey TD, Stitt M. 1991. Water stress, carbon dioxide, and light effects on sucrose-phosphate synthase activity in Phaseolus vulgaris. Physiologia Plantarum 81, 37–44. [Google Scholar]
- Yang JT, Preiser AL, Li Z, Weise SE, Sharkey TD. 2016. Triose phosphate use limitation of photosynthesis: short-term and long-term effects. Planta 243, 687–698. [DOI] [PubMed] [Google Scholar]