Abstract
Context
Understanding real-world prescribing of GH may help improve treatment of eligible patients.
Objective
Overall: to assess real-world effectiveness and safety of GH (Norditropin). This analysis: to compare clinical characteristics of GH-treated children in the United States and Europe.
Design
The American Norditropin Studies: Web-Enabled Research Program (ANSWER; 2002 to 2016, United States) and the NordiNet International Outcome Study (NordiNet IOS; 2006 to 2016, Europe) were multicenter longitudinal observational cohort studies.
Setting
Data were recorded in 207 (United States) and 469 (Europe) clinics.
Participants
Patients with GH deficiency, Turner syndrome, Noonan syndrome, idiopathic short stature, Prader–Willi syndrome, or born small for gestational age, who commenced GH treatment aged <1 year.
Intervention
GH was prescribed by treating physicians according to local practice.
Main Outcomes Measures
Baseline data and drug doses were recorded. Data on effectiveness and safety were collected.
Results
ANSWER had 19,847 patients in the full analysis set (FAS; patients with birthdate information and one or more GH prescription) and 12,660 in the effectiveness analysis set (EAS; GH-naive patients with valid baseline information). NordiNet IOS had 17,711 (FAS) and 11,967 (EAS). Boys accounted for 69% (ANSWER) and 57% (NordiNet IOS). Treatment start occurred later than optimal to improve growth. The proportion of boys treated was generally larger, children were older at treatment start, and GH doses were higher in the United States vs Europe. No new safety signals of concern were noted.
Conclusions
In most indications, more boys than girls were treated, and treatment started late. Earlier diagnosis of GH-related disorders is needed. The data support a favorable benefit–risk profile of GH therapy in children.
Two large noninterventional studies (ANSWER and NordiNet IOS) examined real-world use of GH across six indications in children. Prescribing differences were found between the United States and Europe.
GH is widely used for the treatment of short children across a number of disorders, primarily with the aim of improving linear growth toward the normal adult range. GH has been approved for the treatment of children with GH deficiency (GHD), born small for gestational age (SGA), or with Turner syndrome (TS), Noonan syndrome (NS), idiopathic short stature (ISS), or chronic renal disease, and for growth failure in Prader–Willi syndrome (PWS).
In addition to evidence from randomized controlled trials of the efficacy and safety of GH, which underpinned the various approvals, several large-scale observational studies of GH treatment (1–6) have provided important supplemental evidence, specifically on GH’s long-term safety. Data from these studies have also made it possible to derive GH response-prediction models (7, 8).
This paper reports results from the NordiNet International Outcome Study (NordiNet IOS; ClinicalTrials.gov no. NCT00960128) and the American Norditropin Studies: Web-Enabled Research Program (ANSWER; NCT01009905), two noninterventional, multicenter studies assessing the effectiveness and safety of real-world treatment with somatropin (Norditropin; Novo Nordisk A/S, Copenhagen, Denmark) in pediatric and adult patients. We report on clinical characteristics of, and GH dosing in, short children with GHD, born SGA, or with TS, NS, ISS, or PWS, followed up for up to 10 years of Norditropin treatment. These are compared between the United States and Europe with the intention that this description of current practices could help to guide future treatment decisions. In particular, sex differences in the frequency of treatment are underlined and discussed.
Effectiveness and safety results for pediatric patients from NordiNet IOS and ANSWER, as well as data on adult patients, will be reported in separate publications. Data from subsets of the patients in the two studies have been published previously (9–15).
Materials and Methods
The design and methodology of NordiNet IOS and ANSWER have been reported previously (16). Key points are summarized below and in Table 1.
Table 1.
Design and Key Features of NordiNet IOS and ANSWER
| NordiNet IOS | ANSWER | |
|---|---|---|
| Study start | 1 April 2006 | 24 June 2002 |
| Study end | 31 December 2016 | 30 September 2016 |
| No. of countries involved (no. of patients per country) | 22 [Belgium (11), Czech Republic (1213), Denmark (719), Finland (211), France (3619), Germany (7008), Hungary (86), Ireland (41), Israel (152), Italy (103), Lithuania (103), Luxembourg (2), Montenegro (85), Netherlands (152), Norway (297), Russia (156), Serbia (1299), Slovenia (281), Spain (6), Sweden (941), Switzerland (523), United Kingdom (694)]a | 1 [United States (19,847)] |
| No. of clinics | 469 | 207 |
| Important protocol amendments | Prior to protocol amendment 3 (30 September 2013), only nonserious ARs and SAEs, regardless of relatedness, MESIs, and pregnancies, were collected. The change to the protocol was due to new pharmacovigilance legislation, which came into effect in July 2012. ARs and SARs considered related to GH, and SAEs considered unrelated to GH, were included in this report | Prior to protocol amendment 2 (9 November 2011), very few events were reported to the study. This is likely to have reflected the way ARs and SARs were described in earlier versions of the protocol and revised text, with amendment 2 more clearly explaining the collection and reporting of safety information. Only events reported after this date were included in this report |
Abbreviations: AR, adverse reaction; MESI, medical event of special interest; SAE, serious adverse event; SAR, serious adverse reaction.
One additional country, Saudi Arabia, participated in the study and registered patients to the International Outcomes database; however, none of those satisfied the inclusion criteria of the study.
NordiNet IOS was a multicenter, longitudinal, observational cohort study based on systematic collection of data using the NordiNet IOS Web-based system. ANSWER was originally a postmarketing registry of adults and pediatric patients in the United States treated with Norditropin, and it was developed into a noninterventional, observational study. The two studies were complementary, with similar aims, using the same electronic platform for data management. Results of the studies are presented side by side, rather than pooled, because the reporting of effectiveness and safety endpoints differed.
In both studies, Norditropin was administered as directed by the treating physician and according to routine practice and local regulations. Approval was obtained from relevant ethics committees, written consent was obtained, and all data were pseudonymized. The studies were conducted in accordance with the Declaration of Helsinki, Guideline for Good Pharmacoepidemiology Practices, and regulatory requirements.
Pediatric patients were eligible for inclusion when they had an appropriate diagnostic condition and when treatment with Norditropin was initiated before 18 years of age. Diagnoses were determined by the participating physicians. For study purposes, patients with more than one diagnosis recorded were classified as follows: patients with a syndrome and coexisting GHD were classified by syndrome; patients recorded as both SGA and GHD (isolated or multiple pituitary hormone deficiency) were classified as GHD.
Baseline data were collected according to routine clinical practice (16). Follow-up time on GH treatment during childhood with known dose (mg/kg/d) was recorded.
In both studies, numerous effectiveness endpoints were assessed (16), with change from baseline in height SD score (SDS) as a key outcome. Data on IGF-I SDS were reported in both studies (NordiNet IOS and ANSWER).
Safety data were based on physicians’ reporting of adverse events (AEs). Adverse reactions (ARs; AEs deemed related to product), classed as serious (SARs) or nonserious (NSARs), were recorded. In NordiNet IOS, serious AEs (SAEs) not related to GH therapy were also recorded throughout the study. (For SAEs in both studies, an SAE was defined as an experience that resulted in any of the following: death, a life-threatening experience, hospitalization or prolongation of existing hospitalization, a persistent or significant disability/incapacity, or a congenital anomaly/birth defect. Important medical events that might not result in death, be life-threatening, or require hospitalization could be considered as SAEs if, based on appropriate medical judgement, they might jeopardize the patient and might require medical or surgical intervention to prevent one of the outcomes listed. The term “life-threatening” in the definition of an SAE referred to an event in which the patient was at risk for death at the time of the event. It did not refer to an event that hypothetically might have caused death if it had been more severe.) In ANSWER, however, it was not mandatory to report SAEs that were not related to GH therapy until 9 November 2011, when the protocol was amended. Fewer than 20 SAEs had been reported prior to this amendment, but subsequently the number increased. Only SAEs reported after that date were included in the analysis. In both studies, a double assessment of causality was used to identify any causal relationship to GH therapy: causality was reported by the treating physician, and all SAEs, including SARs, were also reviewed by the study sponsor. When either the original reporter or the study sponsor considered the relationship as possible or probable, the event was considered as an AR.
Statistical analysis
In both studies, the effectiveness analysis set (EAS), used to analyze effectiveness outcomes, comprised patients reported as GH-naive at baseline (however, in ANSWER, GH-naive patients could have been treated with GH for up to 6 months before enrollment) and with valid baseline height, age, and dosing information. The full analysis set (FAS), used to evaluate safety outcomes, included all patients with available birthdate information and with at least one Norditropin prescription recorded. Baseline and exposure data were summarized using descriptive statistics based on data available.
Results
Patient disposition
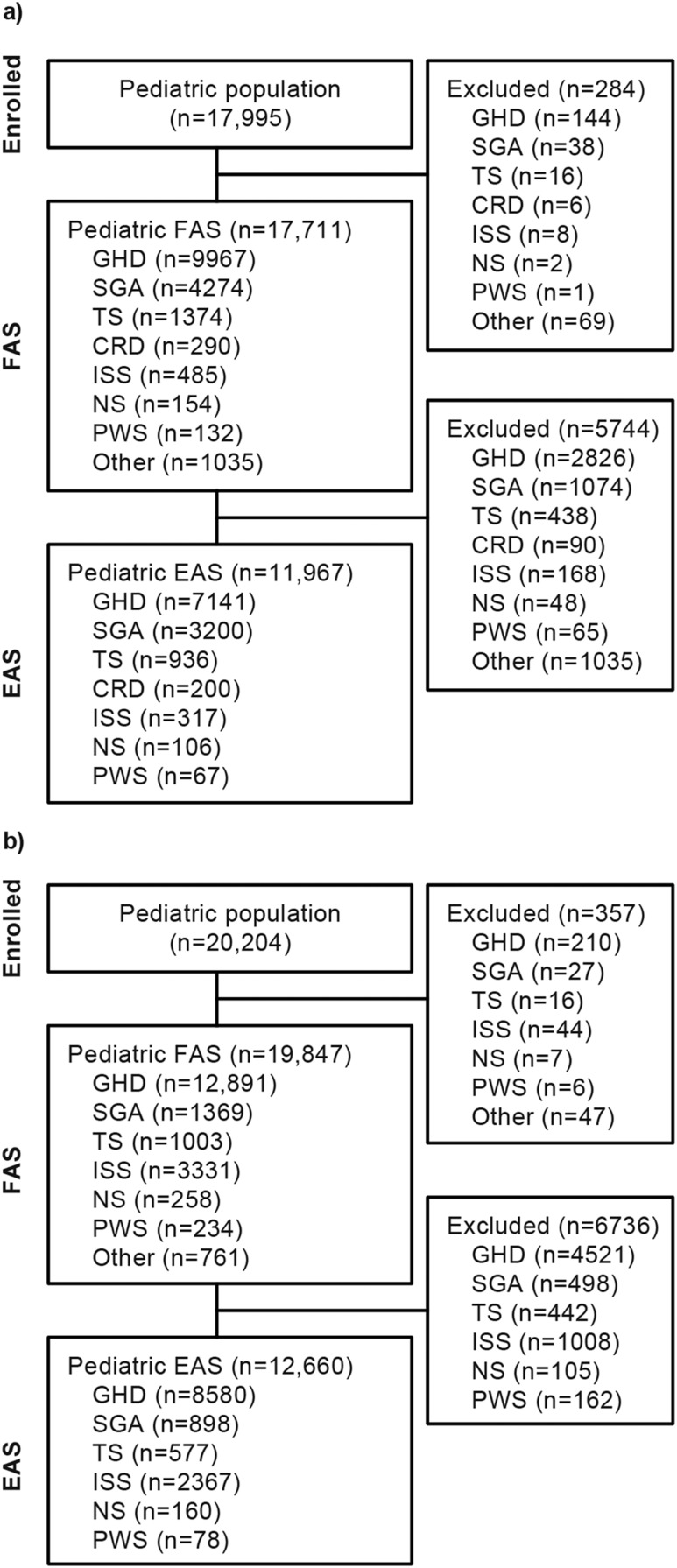
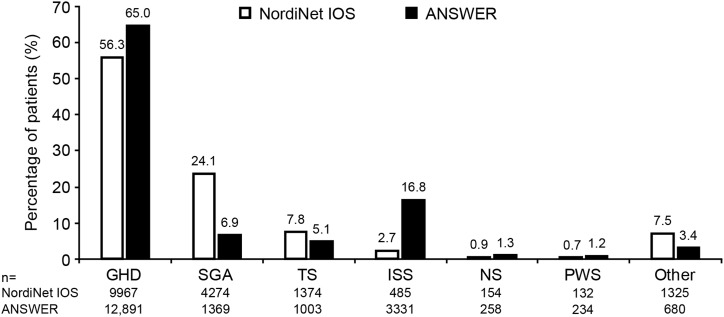
NordiNet IOS enrolled 17,995 pediatric patients, of whom 17,711 were included in the FAS and 11,967 in the EAS (Fig. 1a). The 11,967 in the EAS included 200 patients with chronic renal disease; these patients are not discussed further in this report. ANSWER enrolled 20,204 pediatric patients, of whom 19,847 were included in the FAS and 12,660 in the EAS (Fig. 1b). The most common indications in the FAS in both studies were GHD, ISS, SGA, and TS, with SGA more common in NordiNet IOS and ISS more common in ANSWER (Fig. 2). Enrollment by country is shown in Table 1.
Figure 1.
Disposition of patients in (a) NordiNet IOS and (b) ANSWER. CRD, chronic renal disease.
Figure 2.
Distribution of diagnostic groups in the two studies (FAS). “Other” indicates other diagnoses and chronic renal disease.
Patient characteristics at baseline
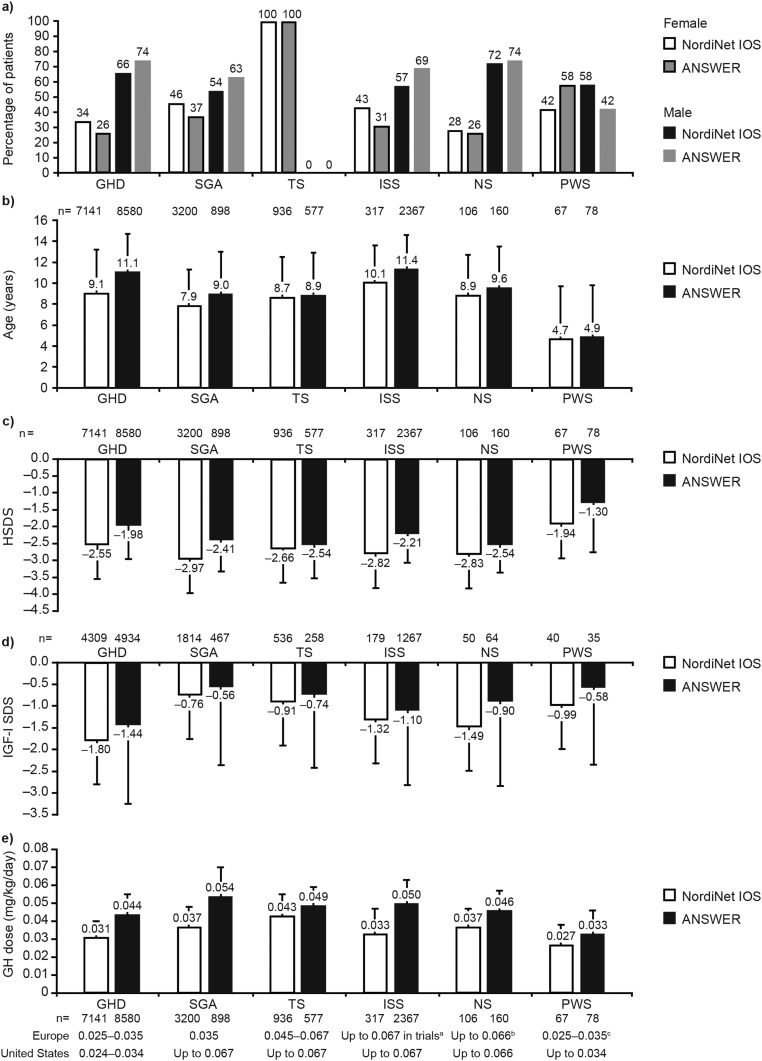
In both studies, more than half of the patients were boys, with a higher overall proportion of boys than girls in ANSWER (69%) than in NordiNet IOS (57%). Across GHD, SGA, and ISS, proportionally more boys were enrolled in ANSWER than in NordiNet IOS. In both studies, >2.5-fold as many boys as girls with NS were enrolled. For PWS, there were proportionally more girls than boys in ANSWER, but the reverse was true in NordiNet IOS [Table 2 (17); Fig. 3a].
Table 2.
Baseline Characteristics and GH Exposure of Enrolled Patients in NordiNet IOS and ANSWER
| EAS (FAS) | NordiNet IOS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GHD | SGA | TS | ISS | NS | PWS | |||||||
| 7141 (9967) | 3200 (4274) | 936 (1374) | 317 (485) | 106 (154) | 67 (132) | |||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Baseline characteristics | ||||||||||||
| Female/male, n (%) | 7141 | 2460 (34%)/ 4681 (66%) | 3200 | 1463 (46%)/ 1737 (54%) | 936 | 936 (100%)/0 | 317 | 136 (43%)/ 181 (57%) | 106 | 30 (28%)/76 (72%) | 67 | 28 (42%)/39 (58%) |
| Age at baseline, y | 7141 | 9.12 (4.12) | 3200 | 7.92 (3.37) | 936 | 8.72 (3.75) | 317 | 10.06 (3.54) | 106 | 8.86 (3.80) | 67 | 4.67 (5.00) |
| Prepubertal, % | 6415 | 84% | 2990 | 88% | 868 | 90% | 237 | 79% | 95 | 84% | 58 | 95% |
| Bone age/chronological age | 3453 | 0.79 (0.22) | 1741 | 0.79 (0.19) | 507 | 0.87 (0.16) | 127 | 0.84 (0.16) | 43 | 0.80 (0.14) | 16 | 0.80 (0.31) |
| Baseline HSDSa | 7141 | −2.55 (1.10) | 3200 | −2.97 (0.91) | 936 | −2.66 (0.93) | 317 | −2.82 (0.99) | 106 | −2.83 (1.13) | 67 | −1.94 (1.48) |
| Target HSDS (national standard)a | 6533 | −0.65 (0.98) | 2954 | −0.94 (0.95) | 856 | −0.29 (0.95) | 281 | −0.87 (0.98) | 92 | −0.67 (0.91) | 42 | 0.32 (0.84) |
| Baseline IGF-I SDS (Brabant)b | 4309 | −1.80 (1.53) | 1814 | −0.76 (1.45) | 536 | −0.91 (1.44) | 179 | −1.32 (1.63) | 50 | −1.49 (1.15) | 40 | −0.99 (1.70) |
| Baseline GH peak, ng/mL | 4045 | 4.72 (4.79) | 719 | 14.49 (12.76) | 113 | 9.64 (10.78) | 84 | 12.90 (9.89) | 32 | 8.39 (4.53) | 13 | 4.41 (5.67) |
| GH exposure and treatment follow-up | ||||||||||||
| GH dose at baseline, mg/kg/d | 7141 | 0.031 (0.009) | 3200 | 0.037 (0.011) | 936 | 0.043 (0.012) | 317 | 0.033 (0.014) | 106 | 0.037 (0.010) | 67 | 0.027 (0.011) |
| GH dose during treatment, mg/kg/d [mean (SD)] | 7141 | 0.032 (0.008) | 3200 | 0.038 (0.009) | 936 | 0.044 (0.009) | 317 | 0.038 (0.014) | 106 | 0.040 (0.009) | 67 | 0.026 (0.008) |
| GH dose during treatment, mg/kg/d [median (P10; P90)] | 7141 | 0.032 (0.024; 0.041) | 3200 | 0.035 (0.029; 0.049) | 936 | 0.044 (0.033; 0.053) | 317 | 0.034 (0.025; 0.056) | 106 | 0.039 (0.031; 0.052) | 67 | 0.027 (0.015; 0.035) |
| Treatment follow-up, y | 7141 | 3.80 (2.87) | 3200 | 3.64 (2.79) | 936 | 4.32 (2.81) | 317 | 3.33 (2.40) | 106 | 3.40 (2.93) | 67 | 4.02 (3.46) |
| Treatment follow-up, y [median (P10; P90)] | 7141 | 3.30 (0.48;7.74) | 3200 | 3.21 (0.00; 7.66) | 936 | 3.89 (0.87; 8.47) | 317 | 2.95 (0.71; 6.70) | 106 | 2.75 (0.28; 6.77) | 67 | 3.06 (0.65; 9.38) |
| PYE (FAS)c | n | PYE | n | PYE | n | PYE | n | PYE | n | PYE | n | PYE |
| 9967 | 39,967 | 4274 | 16,213 | 1374 | 6045 | 485 | 1737 | 154 | 597 | 132 | 662 | |
| EAS (FAS) | ANSWER | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GHD | SGA | TS | ISS | NS | PWS | |||||||
| 8580 (12,891) | 898 (1369) | 577 (1003) | 2367 (3331) | 160 (258) | 78 (234) | |||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Baseline characteristics | ||||||||||||
| Female/male, n (%) | 8580 | 2195 (26%)/ 6385 (74%) | 898 | 334 (37%)/ 564 (63%) | 577 | 577 (100%)/0 | 2367 | 735 (31%)/ 1632 (69%) | 160 | 41 (26%)/119 (74%) | 78 | 45 (58%)/33 (42%) |
| Age at baseline, y | 8580 | 11.09 (3.61) | 898 | 9.00 (3.98) | 577 | 8.92 (4.03) | 2367 | 11.38 (3.17) | 160 | 9.61 (3.93) | 78 | 4.91 (4.88) |
| Prepubertal, % | 7141 | 69% | 794 | 80% | 551 | 89% | 2008 | 65% | 132 | 80% | 68 | 88% |
| Bone age/ chronological age | 4004 | 0.89 (0.15) | 476 | 0.87 (0.16) | 245 | 0.90 (0.24) | 1298 | 0.90 (0.135) | 72 | 0.86 (0.24) | 15 | 1.04 (0.17) |
| Baseline HSDS (CDC)d | 8580 | −1.98 (0.98) | 898 | −2.41 (0.92) | 577 | −2.54 (0.99) | 2367 | −2.21 (0.86) | 160 | −2.54 (0.82) | 78 | −1.30 (1.46) |
| Target HSDS (CDC)d | 7440 | −0.42 (0.99) | 795 | −0.72 (0.96) | 490 | 0.03 (1.03) | 2118 | −0.69 (0.92) | 138 | −0.36 (0.94) | 65 | −0.07 (1.01) |
| Baseline IGF-I SDS (Brabant)b | 4934 | −1.44 (1.81) | 467 | −0.56 (1.80) | 258 | −0.74 (1.68) | 1267 | −1.10 (1.72) | 64 | −0.90 (1.94) | 35 | −0.58 (1.77) |
| Baseline GH peak, ng/mL | 3077 | 6.39 (4.71) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| GH exposure and treatment follow-up | ||||||||||||
| GH dose at baseline, mg/kg/d | 8580 | 0.044 (0.011) | 898 | 0.054 (0.016) | 577 | 0.049 (0.010) | 2367 | 0.050 (0.013) | 160 | 0.046 (0.011) | 78 | 0.033 (0.013) |
| GH dose during treatment, mg/kg/d [mean (SD)] | 8580 | 0.046 (0.012) | 898 | 0.054 (0.015) | 577 | 0.049 (0.010) | 2367 | 0.051 (0.013) | 160 | 0.048 (0.011) | 78 | 0.031 (0.012) |
| GH dose during treatment, mg/kg/d [median (P10; P90)] | 8580 | 0.044 (0.034; 0.061) | 898 | 0.053 (0.038; 0.071) | 577 | 0.049 (0.038; 0.059) | 2367 | 0.049 (0.039; 0.068) | 160 | 0.046 (0.035; 0.064) | 78 | 0.031 (0.018; 0.047) |
| Treatment follow-up, y | 8580 | 2.20 (15.46) | 898 | 2.24 (2.01) | 577 | 3.05 (2.36) | 2367 | 2.06 (6.02) | 160 | 2.6 (2.0) | 78 | 3.33 (3.12) |
| Treatment follow-up, y [median (P10; P90)] | 8580 | 2.06 (0.28; 5.30) | 898 | 1.62 (0.27; 5.06) | 577 | 2.52 (0.40; 6.47) | 2367 | 1.68 (0.20; 4.93) | 160 | 2.23 (0.49; 5.94) | 78 | 2.51 (0.26; 8.00) |
| PYE (FAS)c | n | PYE | n | PYE | n | PYE | n | PYE | n | PYE | n | PYE |
| 12,891 | 33,253 | 1369 | 3403 | 1003 | 3157 | 3331 | 7667 | 258 | 707 | 234 | 847 | |
EAS pertains except where FAS is also shown. Values are mean (SD) unless otherwise stated.
Abbreviations: HSDS, height SDS; NR, not reported; P10/P90, 10th/90th percentile; PYE, patient-years of exposure.
For NordiNet IOS, HSDS and target HSDS were calculated using age- and sex-specific national references.
IGF-I SDS was calculated using the Brabant et al. (17) 2003 age- and sex-specific reference (Nichols advantage).
Total PYE were 70,436 (NordiNet IOS) and 52,070 (ANSWER).
For ANSWER, HSDS and target HSDS were calculated using the U.S. Centers for Disease Control and Prevention growth tables available at www.cdc.gov/growthcharts.
Figure 3.
(a) Sex, (b) age at treatment start, (c) height SDS (HSDS), (d) IGF-I SDS, and (e) dose of GH at treatment start by diagnostic group in NordiNet IOS and ANSWER. All data are for patients included in the EAS; data are mean (SD). Approved doses (mg/kg/d) are shown below each diagnostic group. aISS is not an approved indication in Europe. bApproved in Switzerland and Japan. cApproved in Switzerland.
Sex distribution was examined by age (≥3 vs <3 years) and by GH peak at baseline (<7 or ≥7 ng/mL) for patients with GHD to determine whether it was influenced by these parameters. In both studies, the proportion of male patients was greater in older compared with younger children (Table 3), but there was no difference in sex distribution by GH peak (Table 3).
Table 3.
Sex Distribution by Age and GH Peak at Baseline for Patients With GHD in NordiNet IOS and ANSWER (EAS)
| Age at Baseline | |||
|---|---|---|---|
| <3 y | ≥3 y | Total | |
| NordiNet IOS | |||
| Female, n (% within group) | 227 (40.8) | 2233 (33.9) | 2460 (34.4) |
| Male, n (% within group) | 329 (59.1) | 4352 (66.0) | 4681 (65.5) |
| Total, n (% of all patients) | 556 (7.8) | 6585 (92.2) | 7141 (100.0) |
| ANSWER | |||
| Female, n (% within group) | 126 (37.2) | 2069 (25.1) | 2195 (25.5) |
| Male, n (% within group) | 212 (62.7) | 6173 (74.8) | 6385 (74.4) |
| Total, n (% of all patients) | 338 (3.9) | 8242 (96.1) | 8580 (100.0) |
| GH Peak at Baseline | ||||
|---|---|---|---|---|
| <7 ng/mL | ≥7 ng/mL | Missing | Total | |
| NordiNet IOS | ||||
| Female, n (% within group) | 1093 (32.7) | 238 (33.8) | 1129 (36.4) | 2460 (34.4) |
| Male, n (% within group) | 2249 (67.2) | 465 (66.1) | 1967 (63.5) | 4681 (65.5) |
| Total, n (% of all patients) | 3342 (46.8) | 703 (9.8) | 3096 (43.4) | 7141 (100.0) |
| ANSWER | ||||
| Female, n (% within group) | 457 (24.9) | 296 (23.7) | 1442 (26.2) | 2195 (25.5) |
| Male, n (% within group) | 1373 (75.0) | 951 (76.2) | 4061 (73.7) | 6385 (74.4) |
| Total, n (% of all patients) | 1830 (21.3) | 1247 (14.5) | 5503 (64.1) | 8580 (100.0) |
Values are for EAS.
Information on pubertal status was available for >82% of patients in all indications in both studies, with one exception (ISS in NordiNet IOS –75%) (numbers are shown in Table 2). In both studies, most patients for whom information was available were prepubertal (generally >80% in both studies, and >65% for GHD and ISS in ANSWER) (Table 2). For all indications, treatment started at an older age in ANSWER compared with NordiNet IOS (Table 2; Fig. 3b). Excluding PWS, mean age at treatment start ranged from 8 to 10 years in NordiNet IOS and 9 to 11 years in ANSWER. In both studies, patients with ISS were the oldest and patients with PWS were youngest when compared with other indications, followed by SGA in NordiNet IOS and TS in ANSWER.
Across indications, children were shorter at treatment start in NordiNet IOS than in ANSWER. In NordiNet IOS, patients born SGA were the shortest at baseline (height SDS almost −3) (Table 2; Fig. 3c). In ANSWER, patients with TS or NS were the shortest. With the exception of PWS, where treatment is based on body composition rather than height, patients with GHD were the tallest of those starting GH treatment in both studies. In ANSWER, the mean height SDS for patients with GHD was only just within the cut-off for short stature (height SDS less than −2).
Mean IGF-I SDS was <0 at baseline for all indications in both studies, and lower in NordiNet IOS than in ANSWER (Fig. 3d).
Exposure to GH and duration of treatment follow-up
For all indications, doses of GH at treatment start were higher in the United States than in Europe (Table 2; Fig. 3e). In both studies, mean starting doses at baseline were well within the approved doses for approved indications [Table 4 (18, 19)].
Table 4.
Approved Doses of Norditropin (mg/kg/d)
| Europe | United States | |
|---|---|---|
| GHD | 0.025–0.035 mg/kg/d or 0.7–1.0 mg/m2 BSA | 0.024–0.034 mg/kg/d |
| SGA | 0.035 mg/kg/d or 1.0 mg/m2 BSA | Up to 0.067 mg/kg/d |
| TS | 0.045–0.067 mg/kg/d or 1.3–2.0 mg/m2 BSA | Up to 0.067 mg/kg/d |
| ISS | Not approveda | Up to 0.067 mg/kg/d |
| NS | Up to 0.066 mg/kg/d (Switzerland and Japan) | Up to 0.066 mg/kg/d |
| PWS | 0.025–0.035 mg/d (Switzerland) | 0.034 mg/kg/d |
| CRD | 0.050 mg/kg/d or 1.4 mg/m2 BSA | Not approved |
For most indications, mean overall dose during follow-up was similar to mean dose at baseline; however, mean dose increased slightly during follow-up for patients with NS and ISS in NordiNet IOS (Table 2).
In all indications, duration of treatment follow-up was longer in NordiNet IOS than in ANSWER (Table 2), reflecting the fact that many patients in ANSWER were enrolled near the end of the study. Patients in ANSWER may also have been switched to other GH brands due to reimbursement issues.
Concomitant medications
Information on concomitant medications did not have to be reported and was available for only 39.1% (7757/19,847) of patients in the FAS in ANSWER and 10.6% (1869/17,711) of patients in the FAS in NordiNet IOS. Among these patients, treatments for hypothyroidism (mainly levothyroxine) were commonly prescribed. The proportions of patients prescribed treatments for hypothyroidism, as a percentage of all patients in the FAS, were as follows in ANSWER: GHD 14.5%, SGA 6.5%, TS 18.9%, ISS 6.9%, NS 6.6%, and PWS 20.1%. In NordiNet IOS, proportions were as follows: GHD 7.7%, SGA 1.9%, TS 7.9%, ISS 3.5%, NS 2.6%, and PWS 1.5%.
In the United States, methylphenidate and dexmethylphenidate were prescribed for attention deficit hyperactivity disorder (ADHD) as follows: for patients with GHD 9.1%, SGA 6.7%, TS 6.9%, ISS 8.3%, NS 13.2%, and PWS 4.3%. These two drugs were prescribed to 8.6% of patients in the whole cohort (all indications). No prescriptions for methylphenidate were recorded in Europe, but other ADHD drugs may have been prescribed.
Safety endpoints
Patient-years of exposure (PYE), based on the FAS, are shown in Table 2. Total PYE were 70,436 (NordiNet IOS) and 51,070 (ANSWER).
Detailed safety results for pediatric patients from NordiNet IOS and ANSWER will be reported in a separate publication, but are summarized here. The most frequent events across indications were those commonly observed in children treated with GH and included headache, injection-site reactions, edema, and arthralgia. For each indication, the safety profile was consistent with data in the approved labeling for Norditropin; no new safety signals of concern were observed.
In NordiNet IOS, 421 ARs were reported in 334 pediatric patients: 288 NSARs in 249 patients and 133 SARs in 90 patients. There were also 352 SAEs reported in 224 patients. There were 11 deaths among pediatric patients; causality was considered “unlikely” by both the reporter and the study sponsor in all but two cases (Table 5). For two patients, both with multiple comorbidities, the reporter considered a causal link with GH was “possible” whereas the study sponsor considered a causal link unlikely; narratives are provided in Table 6.
Table 5.
Overview of Fatal Cases by Indication in NordiNet IOS and ANSWER
| Indication | Age Group at Onset (y)a | Sex | Duration of GH Treatment (y) | Event Onset (Years After Start of GH Treatment)b | Event Reported as Fatal | Causalityc |
|---|---|---|---|---|---|---|
| NordiNet IOS | ||||||
| GHD | Adolescent | M | Unknown | 3.02 | Cardiac failure | Unlikely |
| GHD | Infant | F | Unknown | 0.87 | Cardiac failure | Unlikely |
| GHD | Child | F | 0.9 | 0.89 | Acute myeloid leukemia | Unlikely |
| GHD | Adolescent | M | 7.9 | 8.9 | Metastases to meninges | Reporter: possible; Novo Nordisk: unlikely |
| SGA | Child | F | 7.0 | 7.00 | Cardiac failure | Unlikely |
| CRD | Adult | M | Unknown | 8.79 | Cerebral hemorrhage; subdural hematoma; brain edema; cerebral venous thrombosis; transverse sinus thrombosis; depressed level of consciousness | Unlikely |
| CRD | Child | M | Unknown | 2.28 | Pulmonary edema | Unlikely |
| PWS | Adult | M | 0.7 | 0.70 | Sudden unexpected death | Unlikely |
| Other diagnoses | Child | F | Unknown | 1.91 | Sepsis; bone marrow depression bleeding (hemorrhage); bone marrow depression bleeding (bone marrow failure) | Unlikely |
| Other diagnoses | Child | M | 0.6 | 0.53 | Medulloblastoma recurrent | Unlikely |
| Other diagnoses | Adolescent | M | 0.3, then discontinued for 0.8, then treated for 0.8 | 0.25 | Primitive neuroectodermal tumor; condition aggravated | Unlikely |
| ANSWER | ||||||
| GHD | Infant | F | Unknown | 0.5 | Respiratory distress | Unlikely |
| GHD | Adolescent | M | 5.8 | 5.8 | Cause unknown | Unlikely |
| GHD | Child | F | 1.7 (approx.) | 1.7 (approx..) | Child abuse | Unlikely |
| PWS | Child | F | 2.0 | 2.0 | Cardiac arrest | Unlikely |
Abbreviation: CRD, chronic renal disease.
Defined as: infant, aged <2 y; child, aged 2–12 y; adolescent, aged 13–19 y; adult, aged ≥ 20 y.
Time is shown to the onset of the event. Deaths occurred on the same date or later.
Causality is shown for reporter and Novo Nordisk. Causality was the same for both unless listed in the table.
Table 6.
Narratives for Two Fatal Cases in NordiNet IOS
| Event | Narrative |
|---|---|
| Event reported as fatal: metastases to meninges | An adolescent male in the GHD group, treated with GH for 8 y. Medical history included: suprasellar cyst, hydrocephalus, short stature, congenital neurocutaneous melanosis, and known hydrocephalus. He became increasingly tired, shaky, and complained about abdominal pain; GH treatment was withdrawn. One year after the last GH injection, the patient was diagnosed with leptomeningeal melanocytosis and neurologic symptoms due to leptomeningeal tumor. The patient died 9.6 mo later. |
| Events reported as fatal: brain edema, cerebral hemorrhage, cerebral venous thrombosis, depressed level of consciousness, subdural hematoma, and transverse sinus thrombosis | An adult male, treated from childhood with GH due to childhood-onset GHD and CRD for an unknown length of time. Medical history included: panhypopituitarism due to empty sella syndrome, GHD, perinatal asphyxia, CRD with peritoneal dialysis, and epilepsy. He was admitted to the emergency department with reduced consciousness, with subsequent collapse and shock requiring endotracheal intubation, and died 2 wk later. |
For both cases, causality was considered as “possible” by the reporter and “unlikely” by Novo Nordisk.
Abbreviation: CRD, chronic renal disease.
In ANSWER, 396 ARs overall were reported in 284 pediatric patients: 328 NSARs in 240 patients and 68 SARs in 49 patients. Among 81 patients, 128 SAEs were reported after 9 November 2011. Death was reported as an SAE in four patients; causality was judged to be “unlikely” in all cases (Table 5).
IGF-I SDS during follow-up
In both studies, for most indications, mean IGF-I SDS increased to >0 by year 1 (EAS and FAS). For all indications, mean values remained >0 at each year of follow-up in NordiNet IOS, and at most years in ANSWER.
Discussion
These long-term data from NordiNet IOS and ANSWER, based on 70,436 and 51,070 PYE, respectively, provide information on characteristics of patients prescribed Norditropin across six different indications. In the United States compared with Europe, the proportion of boys was larger in all indications except PWS, patients were generally older and taller at treatment start, and doses of GH were higher at baseline and throughout follow-up.
Our data confirm previous reports that more males than females are treated with GH in GHD, ISS, and SGA (1–4). The question of whether biology or sociocultural differences underlie the sex imbalance has been discussed extensively, notably recently by Ranke et al. (4). Our data suggest that the tendency to refer more boys than girls is more common after the age of 3 years. No impact of baseline GH peak (<7 or ≥7 ng/mL) on referral patterns by sex was observed, suggesting that boys were not more or less GH deficient at referral than girls. Limitations of these data are that only a small proportion of our cohort was aged <3 years, and that stimulation test results were available for only 57% of GHD patients in NordiNet IOS and 36% in ANSWER.
In all indications except PWS, the sex difference was more highly skewed toward males in the United States compared with Europe, suggesting that at least some of the sex imbalance in the United States arises from factors other than biology. Similar United States–European differences for GHD and SGA were reported in recent analyses from the Kabi International Growth Study (KIGS) (4) and from GeNeSiS (2).
Across all indications, patients were generally older at treatment start in ANSWER than in NordiNet IOS, in line with findings from KIGS (4) for GHD, ISS, and SGA. In contrast, Pfäffle et al. (2) reported that age at treatment start was similar between the United States and Germany, but higher across indications in France. Overall, data from these different analyses suggest that, in general, mean age at treatment start was higher than desired. Several studies, including previous subanalyses of ANSWER and NordiNet IOS (12, 14, 20, 21), have reported that starting treatment earlier rather than later is associated with better growth response.
National surveillance systems, algorithm-based data mining of electronic medical records, and provision of tools for parents to monitor their children’s growth could help to detect growth failure earlier. For example, earlier diagnosis and referrals to specialist care increased in Finland after an automated growth-monitoring strategy was integrated into the primary care electronic health record system (22). Educational programs should specifically target the need for physicians and families to be aware of short stature or decreased growth in girls.
For all indications, children were taller at treatment start in ANSWER compared with NordiNet IOS, possibly because, in ANSWER, children could already have had up to 6 months of GH treatment before enrollment, or because children were generally older at treatment start compared with NordiNet IOS. Furthermore, childhood GHD is a multifaceted condition that may require treatment even in the absence of short stature (23).
A limitation of all international observational studies is that local differences in the diagnosis of conditions and access to treatment could confound the results. Marked United States–European differences were observed in the sizes of the study populations in some indications. The smaller SGA population and larger ISS population in the United States compared with Europe could result from the later approval of Norditropin in the United States for SGA (2008) vs Europe (2004). Alternatively, children in Europe may have been classified as SGA rather than ISS to qualify for treatment, as Norditropin is not licensed for ISS in Europe. However, as concerns regarding safety of GH in short children born SGA (24) have been raised by some but not by others, it is important to classify SGA correctly, as was done by Sävendahl et al. (15), and to analyze safety outcomes carefully (25).
In all indications, and as expected due to differences in prescribing practice, GH doses at baseline and during treatment were higher in the United States compared with Europe. Mean GH starting doses were expected to fall within the approved range, and this was the case for all indications except for GHD in the United States. Low mean starting doses for PWS reflect the young ages of the children, the approved dose in the United States, and the fact that, as PWS is not an approved indication in many countries in Europe, it may have been dosed in accordance with GHD dosing.
In Europe, doses were noticeably below approved doses for TS and NS. The low dose for NS may reflect possible prescription by clinicians at GHD-approved doses, as within Europe NS is only approved in Switzerland, which is not under European Medicines Agency jurisdiction. Previous reports from NordiNet IOS have noted that GH dosing varied considerably across indications and countries, with patients with TS frequently receiving doses below the approved level (10, 13).
It is reassuring that no new safety signals of concern were noted in any of the indications. Data from a number of large-scale studies of GH treatment, covering all indications reported in the individual studies, were recently reviewed (26). The authors concluded that the data provide reassurance on the long-term safety of GH treatment, but that patients with preexisting risk factors for diabetes and malignancy should be treated with caution and monitored regularly during follow-up.
The limited data available on concomitant medications showed that stimulant medications were prescribed in the United States at a frequency (8.6%) similar to that in children without growth disorders (reported as 5.1% based on the 2016 National Survey of Children’s Health) (27). A subanalysis of ANSWER data reported that the linear growth response to GH treatment was not modified by ADHD stimulant medication (28); however, in a subanalysis of KIGS data, Miller et al. concluded that the ADHD phenotype, alone or in conjunction with stimulant therapy, may impair the short-term growth response to GH treatment (29).
As mentioned above, NordiNet IOS and ANSWER are subject to the limitations of large, multicenter, observational studies. During the period covered (2002 to 2016), there were changes in diagnostic practices and definitions of eligibility for GH treatment, in particular with respect to GHD diagnosis, and changes in approvals for indications and recommended doses. Changes in prescribing practices due to financial constraints or external influences are likely to have occurred and to have differed by country. Comorbidities, concomitant medications, and AEs may have been underreported due to protocol amendments or reported differently according to local practice. Indeed, possible underreporting of AEs has been acknowledged as a limitation in similar registries such as GeNeSIS (6) and KIGS (30).
However, the two similar studies in different geographic regions provide a wealth of real-world data, enabling comparison of treatment practices between the United States and Europe in NS and PWS, in addition to indications previously compared in other studies (2, 4, 5). The data describe the characteristics of patients that clinicians could expect to meet in everyday clinical practice, and they highlight areas where improvements could be considered, such as earlier identification of patients and, in some cases, adjustments to GH dosing. The results also provide reassurance regarding the safety of GH, especially because no increased safety risks were evident in data from the United States, where consistently higher doses of GH were used. The populations are more heterogeneous than those from single-region studies, and the larger numbers available will facilitate analyses of the safety of GH therapy in indications such as NS, where reports to date have been limited to small numbers.
In conclusion, results from ANSWER and NordiNet IOS show that, at treatment start, across all indications, mean age was higher and children were taller, and mean doses of GH at baseline and during treatment were higher, in the United States than in Europe. In both regions, diagnoses, and therefore treatment start, occurred later than optimal to improve growth. In most indications, more boys were treated than girls, with the sex difference greater in the United States than in Europe for GHD, ISS, and SGA. Overall, these data accrued from 37,558 pediatric patients and 121,506 PYE support the favorable benefit–risk profile of treatment with Norditropin across the indications analyzed.
Acknowledgments
The authors thank all the investigators, as well as patients and their families, who participated in the two studies. We would like to warmly thank Birgitte Tønnes Pedersen for valuable input to the manuscript. Data analysis was performed by Birgitte Tønnes Pedersen, employee of Novo Nordisk A/S at the time, and Jean-Marc Ferran (Qualiance ApS) under contract to Novo Nordisk A/S. We also thank Jean-Marc Ferran and Navid Nedjatian (Novo Nordisk) for review and input to the manuscript. Medical writing and submission support were provided by Grace Townshend, Penny Butcher, Germanicus Hansa-Wilkinson, and Helen Marshall of Watermeadow Medical, funded by Novo Nordisk Health Care AG. These results have been partially presented at the 20th European Congress of Endocrinology, Barcelona, Spain, 19–22 May 2018; 57th Annual Meeting of the European Society for Paediatric Endocrinology, Athens, Greece, 27–29 September 2018; XXVII Latin American Congress of Pediatric Endocrinology, Cusco, Peru, 24–27 October 2018; and 10th Asia Pacific Paediatric Endocrine Society (APPES) Biennial Scientific Meeting, Chiang Mai, Thailand, 7–10 November 2018.
Financial Support: This work was supported by Novo Nordisk.
Clinical Trial Information: ClinicalTrials.gov nos. NCT00960128 (registered 1 April 2006) and NCT01009905 (registered 24 June 2002).
Additional Information
Disclosure Summary: L.S. is President of the NordiNet IOS Committee and has received consultation fees from Ascendis, Novo Nordisk, Merck, Pfizer, and Sandoz. M.P. consulted for, and received honoraria, travel grants, and unrestricted research grants from Novo Nordisk; was on the Advisory Board for the Ipsen’s Increlex registry, on an Advisory Board for Pfizer, and advised for Novo Nordisk GNAP; and has received research grants from Novo Nordisk, Merck, Ipsen, Pfizer, and Sandoz. P.B. has received research funding from Novo Nordisk and has been a consultant for Novo Nordisk, including advisory board participation. J.B. has received payment for roles on the NordiNet IOS Steering Committee and Publication Steering Committee; has received honoraria from Novo Nordisk and Sandoz for presentations at academic meetings; and has received financial support in the form of registration fees from Novo Nordisk to attend academic meetings. B.S.M. is a consultant for AbbVie, Ascendis, Ferring, Novo Nordisk, Pfizer, Sandoz, Soleno, and Tolmar and has received research support from Alexion, Ascendis, BioMarin, Endo Pharmaceuticals, Genentech, Genzyme, Novo Nordisk, Opko, Sandoz, Sangamo, Shire, Tolmar, and Versartis. T.R.R. has acted as a consultant for Novo Nordisk and received speaker honoraria from Novo Nordisk. A.P. and V.O. are employees of Novo Nordisk. J.R. is a consultant for Novo Nordisk and Opko and receives research funding from Novo Nordisk.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. The study protocol and redacted clinical study reports are available according to Novo Nordisk data sharing commitments. Individual participant data have been shared with the study investigators in a pseudonymized format. The data will be available as redacted study reports permanently after research completion and approval of product use in both the EU and United States with no end date. Data will be shared with bona fide researchers submitting a research proposal requesting access to data, for analyses in line with the aims of the study protocol and approved by the Independent Review Board according to the Independent Review Board charter (see novonordisk-trials.com). Request proposal forms and the access criteria can be found at novonordisk-trials.com. The data will be made available on a specialized SAS data platform.
Glossary
Abbreviations:
- ADHD
attention deficit hyperactivity disorder
- AE
adverse event
- ANSWER
American Norditropin Studies: Web-Enabled Research Program
- AR
adverse reaction
- EAS
effectiveness analysis set
- FAS
full analysis set
- GHD
GH deficiency
- ISS
idiopathic short stature
- KIGS
Kabi International Growth Study
- NordiNet IOS
NordiNet International Outcome Study
- NS
Noonan syndrome
- NSAR
nonserious AR
- PYE
patient-years of exposure
- PWS
Prader–Willi syndrome
- SAE
serious AE
- SAR
serious AR
- SDS
SD score
- SGA
small for gestational age
- TS
Turner syndrome
References and Notes
- 1. Kaplowitz PB, Shulman DI, Frane JW, Jacobs J, Lippe B. Characteristics of children with the best and poorest first- and second-year growth during rhGH therapy: data from 25 years of the Genentech national cooperative growth study (NCGS). Int J Pediatr Endocrinol. 2013;2013(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfäffle R, Land C, Schönau E, Holterhus PM, Ross JL, Piras de Oliveira C, Child CJ, Benabbad I, Jia N, Jung H, Blum WF. Growth hormone treatment for short stature in the USA, Germany and France: 15 years of surveillance in the Genetics and Neuroendocrinology of Short-Stature International Study (GeNeSIS). Horm Res Paediatr. 2018;90(3):169–180. [DOI] [PubMed] [Google Scholar]
- 3. Plotnick L, Rapaport R, Desrosiers P, Fuqua JS. Update from the GHMonitorSM observational registry in children treated with recombinant human growth hormone (Saizen). Pediatr Endocrinol Rev. 2009;6(Suppl 2):278–282. [PubMed] [Google Scholar]
- 4. Ranke MB, Lindberg A, Tanaka T, Camacho-Hübner C, Dunger DB, Geffner ME. Baseline characteristics and gender differences in prepubertal children treated with growth hormone in Europe, USA, and Japan: 25 Years’ KIGS® experience (1987–2012) and review. Horm Res Paediatr. 2017;87(1):30–41. [DOI] [PubMed] [Google Scholar]
- 5. Wyatt D. Lessons from the national cooperative growth study. Eur J Endocrinol. 2004;151(Suppl 1):S55–S59. [DOI] [PubMed] [Google Scholar]
- 6. Child CJ, Zimmermann AG, Chrousos GP, Cummings E, Deal CL, Hasegawa T, Jia N, Lawrence S, Linglart A, Loche S, Maghnie M, Pérez Sánchez J, Polak M, Predieri B, Richter-Unruh A, Rosenfeld RG, Yeste D, Yorifuji T, Blum WF. Safety outcomes during pediatric GH therapy: final results from the prospective GeNeSIS observational program. J Clin Endocrinol Metab. 2019;104(2):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ranke MB, Lindberg A; KIGS International Board. Prediction models for short children born small for gestational age (SGA) covering the total growth phase. Analyses based on data from KIGS (Pfizer International Growth Database). BMC Med Inform Decis Mak. 2011;11(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ranke MB, Lindberg A, Chatelain P, Wilton P, Cutfield W, Albertsson-Wikland K, Price DA. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999;84(4):1174–1183. [DOI] [PubMed] [Google Scholar]
- 9. Blankenstein O, Pedersen BT, Schlumpf M, Andreasen AH, Júlíusson PB. Management and interpretation of heterogeneous observational data: using insulin-like growth factor-I data from the NordiNet® International Outcome Study. Growth Horm IGF Res. 2015;25(1):41–46. [DOI] [PubMed] [Google Scholar]
- 10. Blankenstein O, Snajderova M, Blair J, Pournara E, Pedersen BT, Petit IO. Real-life GH dosing patterns in children with GHD, TS or born SGA: a report from the NordiNet® International Outcome Study. Eur J Endocrinol. 2017;177(2):145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee PA, Ross JL, Pedersen BT, Kotnik P, Germak JA, Christesen HT. Noonan syndrome and Turner syndrome patients respond similarly to 4 years’ growth-hormone therapy: longitudinal analysis of growth-hormone-naïve patients enrolled in the NordiNet® International Outcome Study and the ANSWER Program. Int J Pediatr Endocrinol. 2015;2015(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polak M, Blair J, Kotnik P, Pournara E, Pedersen BT, Rohrer TR. Early growth hormone treatment start in childhood growth hormone deficiency improves near adult height: analysis from NordiNet® International Outcome Study. Eur J Endocrinol. 2017;177(5):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polak M, Konrad D, Tønnes Pedersen B, Puras G, Šnajderová M. Still too little, too late? Ten years of growth hormone therapy baseline data from the NordiNet® International Outcome Study. J Pediatr Endocrinol Metab. 2018;31(5):521–532. [DOI] [PubMed] [Google Scholar]
- 14. Ross J, Lee PA, Gut R, Germak J. Factors influencing the one- and two-year growth response in children treated with growth hormone: analysis from an observational study. Int J Pediatr Endocrinol. 2010;2010(1):494656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sävendahl L, Pournara E, Pedersen BT, Blankenstein O. Is safety of childhood growth hormone therapy related to dose? Data from a large observational study. Eur J Endocrinol. 2016;174(5):681–691. [DOI] [PubMed] [Google Scholar]
- 16. Höybye C, Sävendahl L, Christesen HT, Lee P, Pedersen BT, Schlumpf M, Germak J, Ross J. The NordiNet® International Outcome Study and NovoNet® ANSWER Program®: rationale, design, and methodology of two international pharmacoepidemiological registry-based studies monitoring long-term clinical and safety outcomes of growth hormone therapy (Norditropin®). Clin Epidemiol. 2013;5:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brabant G, von zur Mühlen A, Wüster C, Ranke MB, Kratzsch J, Kiess W, Ketelslegers JM, Wilhelmsen L, Hulthén L, Saller B, Mattsson A, Wilde J, Schemer R, Kann P; German KIMS Board. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res. 2003;60(2):53–60. [DOI] [PubMed] [Google Scholar]
- 18. Novo Nordisk. Norditropin prescribing information. Available at: www.novo-pi.com/norditropin.pdf. Accessed 15 November 2018.
- 19. Novo Nordisk. Norditropin summary of product characteristics. Available at: www.medicines.org.uk/emc/medicine/2760. Accessed 15 November 2018.
- 20. Ross J, Lee PA, Gut R, Germak J. Impact of age and duration of growth hormone therapy in children with Turner syndrome. Horm Res Paediatr. 2011;76(6):392–399. [DOI] [PubMed] [Google Scholar]
- 21. Ross JL, Lee PA, Gut R, Germak J. Increased height standard deviation scores in response to growth hormone therapy to near-adult height in older children with delayed skeletal maturation: results from the ANSWER Program. Int J Pediatr Endocrinol. 2015;2015(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sankilampi U, Saari A, Laine T, Miettinen PJ, Dunkel L. Use of electronic health records for automated screening of growth disorders in primary care. JAMA. 2013;310(10):1071–1072. [DOI] [PubMed] [Google Scholar]
- 23. Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990–3993. [DOI] [PubMed] [Google Scholar]
- 24. Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, Coste J. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97(2):416–425. [DOI] [PubMed] [Google Scholar]
- 25. Albertsson-Wikland K, Mårtensson A, Sävendahl L, Niklasson A, Bang P, Dahlgren J, Gustafsson J, Kriström B, Norgren S, Pehrsson NG, Odén A. Mortality is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab. 2016;101(5):2149–2159. [DOI] [PubMed] [Google Scholar]
- 26. Stochholm K, Kiess W. Long-term safety of growth hormone-A combined registry analysis. Clin Endocrinol (Oxf). 2018;88(4):515–528. [DOI] [PubMed] [Google Scholar]
- 27. Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rose SR, Reeves G, Gut R, Germak J. Attention-deficit/hyperactivity disorder medication treatment impact on response to growth hormone therapy: results from the ANSWER program, a non-interventional study. J Pediatr. 2015;167(6):1389–1396. [DOI] [PubMed] [Google Scholar]
- 29. Miller BS, Aydin F, Lundgren F, Lindberg A, Geffner ME. Stimulant use and its impact on growth in children receiving growth hormone therapy: an analysis of the KIGS International Growth Database. Horm Res Paediatr. 2014;82(1):31–37. [DOI] [PubMed] [Google Scholar]
- 30. Darendeliler F, Karagiannis G, Wilton P. Headache, idiopathic intracranial hypertension and slipped capital femoral epiphysis during growth hormone treatment: a safety update from the KIGS database. Horm Res. 2007;68(Suppl 5):41–47. [DOI] [PubMed] [Google Scholar]