An Arabidopsis farnesyl transferase loss-of-function mutant exhibited a permeable cuticle and enhanced immunity to Botrytis. This immunity was fully suppressed by loss of function of the cell death regulator BOTRYTIS SENSITIVE1.
Keywords: BOS1, Botrytis cinerea, cell death, cuticle permeable, ERA1, farnesyl transferase, immunity, RNA sequencing
Abstract
Prevailing evidence indicates that abscisic acid (ABA) negatively influences immunity to the fungal pathogen Botrytis cinerea in most but not all cases. ABA is required for cuticle biosynthesis, and cuticle permeability enhances immunity to Botrytis via unknown mechanisms. This complex web of responses obscures the role of ABA in Botrytis immunity. Here, we addressed the relationships between ABA sensitivity, cuticle permeability, and Botrytis immunity in the Arabidopsis thaliana ABA-hypersensitive mutants protein phosphatase2c quadruple mutant (pp2c-q) and enhanced response to aba1 (era1-2). Neither pp2c-q nor era1-2 exhibited phenotypes predicted by the known roles of ABA; conversely, era1-2 had a permeable cuticle and was Botrytis resistant. We employed RNA-seq analysis in cuticle-permeable mutants of differing ABA sensitivities and identified a core set of constitutively activated genes involved in Botrytis immunity and susceptibility to biotrophs, independent of ABA signaling. Furthermore, botrytis susceptible1 (bos1), a mutant with deregulated cell death and enhanced ABA sensitivity, suppressed the Botrytis immunity of cuticle permeable mutants, and this effect was linearly correlated with the extent of spread of wound-induced cell death in bos1. Overall, our data demonstrate that Botrytis immunity conferred by cuticle permeability can be genetically uncoupled from PP2C-regulated ABA sensitivity, but requires negative regulation of a parallel ABA-dependent cell-death pathway.
Introduction
The necrotrophic fungal pathogen Botrytis cinerea is considered to be among the most important plant pathogens, both economically and as a model for research (Dean et al., 2012). Multiple mechanisms determine the outcome of plant–Botrytis interactions, among which stress-hormone-regulated responses have been extensively studied (Mengiste, 2012; AbuQamar et al., 2017). Jasmonic acid (JA) and ethylene signaling are essential and often function together in the activation of immunity to Botrytis (Mengiste, 2012), while salicylic acid (SA) signaling plays a lesser role (AbuQamar et al., 2017). The function of abscisic acid (ABA) signaling in defense against Botrytis is generally considered to be negative (Mengiste, 2012; AbuQamar et al., 2017). Exogenous ABA application enhances Botrytis pathogenicity in a dose-dependent manner (Kettner and Dörffling, 1995; Shaul et al., 1996; Audenaert et al., 2002). Genetic evidence for the role of ABA is primarily based on loss-of-function (ABA-deficient or -insensitive) mutants, which exhibit enhanced immunity to Botrytis (Audenaert et al., 2002; Asselbergh et al., 2007; L’Haridon et al., 2011). However, the evidence is not entirely consistent. We previously tested the Botrytis sensitivity of the ABA hyperaccumulation double mutant cyp707a1 cyp707a3 in Arabidopsis, which is deficient in ABA inactivation, and did not find significantly increased Botrytis susceptibility compared with wild type (Okamoto et al., 2006; Liu et al., 2010c; Cui et al., 2016). Furthermore, a recent study reported that exogenous ABA, applied 24 hours prior to infection, could prime the plant defense response and increase Botrytis immunity (Liao et al., 2016). The resolution of this apparent contradiction may lie in the complexity of ABA signaling pathways. ABA signaling branches into many divergent downstream responses, which may differentially participate in regulating immunity to Botrytis.
ABA is required for cuticle formation (Curvers et al., 2010; Cui et al., 2016). Plants with impaired ABA signaling pathways are cuticle permeable. These mutants include the ABA biosynthesis mutants aba deficient2 (aba2) and aba3; the ABA receptor mutants pyrabactin resistance1 (pyr1)/pyr1-like (pyl)/regulatory components of aba receptors (rcar); and the triple mutant of the three core kinases in ABA signaling, snf1-related protein kinase (snrk)2.2 snrk2.3 snrk2.6 (here abbreviated to snrk2.236) (Curvers et al., 2010; L’Haridon et al., 2011; Cui et al., 2016). These ABA mutants are also more resistant to Botrytis (L’Haridon et al., 2011; Cui et al., 2016). Although the cuticle acts as a barrier to exclude pathogens (Riederer, 2007), a defective cuticle confers strong immunity to Botrytis (Bessire et al., 2007) that is independent of the canonical antifungal defense signaling pathways, JA, ethylene, SA, and camalexin (Chassot et al., 2007; Serrano et al., 2014). Although the mechanism for this phenomenon remains unknown, it is thought that a permeable cuticle could facilitate early or enhanced perception of Botrytis (Asselbergh et al., 2007; Ziv et al., 2018). Other biological processes may also be involved, including defense activation by cuticle damage-associated molecular patterns, secretion of antifungal compounds, generation of reactive oxygen species (ROS), control of cell death, altered metabolism, and altered foliar microbiome composition (Kliebenstein et al., 2005; Asselbergh et al., 2007; Bessire et al., 2007; Chassot et al., 2007; Curvers et al., 2010; L’Haridon et al., 2011; Seifi et al., 2013; Ritpitakphong et al., 2016). Thus, this strong enhanced Botrytis immunity may be multilayered, involving several of the processes listed above. Remarkably, no genetic suppressors of this phenotype have been reported.
ABA is also an important regulator of abiotic stresses and cell death. Treating plants with ABA leads to leaf chlorosis and cell death (Fan et al., 1997; Jiang and Zhang, 2001; Takasaki et al., 2015; Zhao et al., 2016). Thus, it is possible that high levels of ABA trigger cell death to promote plant susceptibility to Botrytis. Indeed, mutants with enhanced cell death after ABA treatment were reported to be susceptible to Botrytis. BOTRYTIS SUSCEPTIBLE1 (BOS1) is a MYB-type transcription factor (MYB108; Mengiste et al., 2003). The bos1 mutant exhibits enhanced cell-death spread in an ABA-dependent manner (Cui et al., 2013). Accordingly, bos1 is hypersensitive to Botrytis (Mengiste et al., 2003). HOOKLESS1 (HLS1) encodes a putative histone acetyltransferase, and the hls1 mutant exhibits increased cell death upon ABA application and enhanced susceptibility to Botrytis (Liao et al., 2016). Interestingly, a germination assay showed that hls1 is ABA insensitive (Liao et al., 2016). Thus, testing different mutants with altered ABA sensitivities would help to clarify the relationship between ABA sensitivity and cell death control in plant–Botrytis interactions.
To further address these issues, we examined two additional ABA-hypersensitive mutants, enhanced response to aba1 (era1) and the PROTEIN PHOSPHATASE TYPE 2C (PP2C) quadruple knockout mutant, aba insensitive1-2 (abi1-2) abi2-2 hypersensitive to aba1-1 (hab1-1) pp2ca-1 (Rubio et al., 2009) (here abbreviated to pp2c-q). ERA1 encodes the beta subunit of farnesyl-trans-transferase; this enzyme transfers farnesyl groups to target proteins at the consensus sequence CaaX (Galichet and Gruissem, 2003). Only two confirmed ERA1 substrates are known, ALTERED SEED GERMINATION2 (ASG2) and the cytochrome P450 CYP85A2. Loss of function in these loci results in alterations in different aspects of ABA signaling (Dutilleul et al., 2016; Northey et al., 2016). Predicted targets potentially subject to ERA-dependent farnesylation include 700 proteins in Arabidopsis (Northey et al., 2016). Accordingly, the era1 mutant is pleiotropic, with altered responses in multiple biological processes, including enhanced ABA sensitivity, late flowering, enlarged organs, and increased susceptibility to Hyaloperonospora parasitica and Pseudomonas syringae pv. maculicola (Cutler et al., 1996; Pei et al., 1998; Galichet and Gruissem, 2003, 2006; Goritschnig et al., 2008). Whether farnesyl-trans-transferase is involved in cuticle formation has not been reported. PP2Cs directly interact with the PYR/PYL ABA receptors and negatively regulate ABA signaling (Park et al., 2009). Hence, the pp2c-q mutant exhibits constitutively activated ABA signaling (Antoni et al., 2013). Using these two ABA-hypersensitive mutants, we dissected the relationships between ABA sensitivity, cuticle permeability, cell death, and sensitivity to Botrytis.
Materials and methods
Growth conditions and plant material
Seeds were germinated at high density on a mixture of peat and vermiculite (2:1) after stratification at 4 °C for 2 days. One-week-old seedlings were transplanted, two per pot, in the same soil mixture. Plants were grown in a growth chamber with conditions of 150–200 μmol m–2 s–2 light intensity, 12/12 h (light/dark) photoperiod, 60% humidity, and 23/18 °C (day/night) temperature. For double mutant construction, lacs2.3 and bos1 were used as pollen donors to pollinate era1-2. In the F2 generation, seedlings with era1 phenotypes were genotyped with primers of each of the other genotypes. To circumvent the sterility of the bos1 era1-2 double mutant, segregating populations of F2 plants were phenotyped and genotyped to identify plants for use in experiments. The era1-like plants were selected for experiments and then genotyped with bos1 primers. Only the data of true bos1 era1-2 double mutants were recorded.
ABA-related mutants used in this study were aba3 (aba deficient3); abi1-1 (aba insensitive1); snrk2.236 (snf1-related protein kinase2.2 snrk2.3 snrk2.6 triple mutant); pp2c-q (protein phosphatase2c quadruple mutant abi1-2 abi2-2 hab1-1 pp2ca-1); era1 (enhanced response to aba1); and 112458 [sextuple mutant of pyrabactin resistance1 (pyr1) pyr1-like1 (pyl1) pyl2 pyl4 pyl5 pyl8]. Putative ERA1 target mutants were cyp85a2, asg2-1, and asg2-2, and are described in Jalakas et al. (2017).
Arabidopsis thaliana Columbia (Col-0) was used in all experiments. All mutant alleles used were confirmed by PCR genotyping. Mutants were obtained from the European Arabidopsis Stock Centre (http://arabidopsis.info/) or were gifts (see Acknowledgements).
Fungal cultivation and disease assays
Botrytis cinerea strain BO5.10 was grown on potato dextrose agar. For conidia production, spores with mycelium were collected using forceps into 1/3 strength potato dextrose broth, mixed, filtered, and diluted to 2×106 spores ml–1. For lesion size assays, 3 μl drops were inoculated on to leaves of 24-day-old plants, closed in a moist tray at 100% humidity, and transferred to a growth chamber with conditions of 180 μmol m–2 s–2, 12/12 h light/day at 21 °C. Lesions were photographed at 3 days post infection (dpi) and their diameters were measured by using ImageJ (http://rsb.info.nih.gov/ij/). Spray infections were done using conidia suspension as described above with sprayers on the whole rosettes of 24-day-old plants.
Tissue staining and wounding assays
Dye exclusion experiments were performed with fully expanded leaves from 24-day-old plants, for 20 min with immersion treatment or for 2 h with 5 µl droplets of a 0.05% solution of toluidine blue stain as described by Tanaka et al. (2004). The stained areas were measured with ImageJ according to Cui et al. (2016). Wounding-induced cell death was determined from leaves punctured with a needle. Wounded leaves at 6 days post wounding (dpw) were subjected to trypan blue staining to visualize the cell death. Samples were photographed with a stereomicroscope (Olympus SZX16, Japan) and measured with ImageJ. Each lesion was measured four times through its center. The mean of the four lengths of each wound was taken as the length of spread of cell death. For assessment of H2O2 production and Botrytis-induced cell death, spray-infected rosette leaves were stained with 3,3′-diaminobenzidine (DAB; D8001, Sigma-Aldrich) at 16 hours post infection (hpi) and trypan blue (T6146, Sigma) at 36 hpi. The stained leaves were mounted in water to eliminate reflection before being photographed with a stereomicroscope (Olympus SZX16, Japan). The DAB- and trypan blue-stained area and whole leaf area in each sample were measured with ImageJ. The percentage stained area was calculated by dividing the stained area by the whole leaf area.
RNA-seq and data analysis
Botrytis-infected and mock-sprayed plants (five rosettes of each genotype) were collected at 20 and 44 hpi. Three biological replicates were used. All samples were collected at 15.00 h to eliminate the influence of circadian-regulated genes. RNA was extracted with the MiniBEST Universal RNA Extraction Kit (TaKaRa). The total RNA of each sample ranged from 13.8 to 41 μg, with an RNA quality score (RQS) value from 6.7 to 8.5. The RNA-seq was executed by using an Illumina HiSeq 4000 in 150 bp paired-end sequencing. By filtering out adaptors and low-quality reads using Trimmomatic-0.38 (Bolger et al., 2014), we obtained at least 8 Gb clean reads for each sample. The clean data have been uploaded to the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra, accession number PRJNA495475). The clean reads were then aligned to the reference A. thaliana genome (release: TAIR10.v32 download from Ensembl Plants) using hisat2 v2.1.0 (Kim et al., 2015) with a modification of intron length for plants (--min-intronlen 20 --max-intronlen 5000). StringTie v1.3.4d (Pertea et al., 2015) was used to construct the new transcripts and generate the merged gene annotations. Finally, to obtain a high confidence of differentially expressed genes (DEGs), at least two of three programs, cuffdiff (Trapnell et al., 2013), edgeR (Robinson et al., 2010), and DESeq2 (Love et al., 2014), were used to determine the DEGs with P≤0.05 and absolute (log2 fold change) ≥ 1. DEGs were used to create Venn diagrams using Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny). Gene Ontology (GO) enrichment was analyzed with the online tools of the Gene Ontology Consortium (http://geneontology.org/page/go-enrichment-analysis) and then the exported data were used to render the GO enrichment pictures in R. For heatmap construction, the heatmap2 package in R was used and then manually adjusted with CorelDRAW (X4) software.
qPCR assay
For confirming gene expression of the RNA-seq data, RNA isolation was performed with the same plant material used for RNA-seq and then treated with DNase I. Real-time quantitative PCR (qPCR) was performed as described by Cui et al. (2016) using YLS8 (AT5G08290), TIP41 (AT4G34270), and PP2AA3 (AT1G13320) as reference genes. Primer sequences are given in Supplementary Table S6 at JXB online. For Botrytis growth assays, fungal DNA was extracted from 10 leaf discs (7 mm diameter) from infected plants and qPCR was performed according to Gachon and Saindrenan (2004), using specific primers for cutinase A. The raw cycle threshold values were analyzed with Qbase (Hellemans et al., 2007).
Statistical analysis
Statistical analysis of lesion sizes and cell death spread were carried out with scripts in R (version 3.0.3). Using the nlme package, a linear mixed model with fixed effects for genotype, treatment, and their interaction was fitted to the data, plus a random effect for biological repeat. The model contrasts were estimated with the multcomp package, and the estimated P-values were subjected to single-step P-value correction. A logarithm of the data was taken before modeling to improve the model fit. The qPCR data were log10-transformed and significance was estimated with a two-tailed Student’s t-test using equal variance. The significance of overlaps between two gene sets was evaluated with Fisher’s exact test in R.
Results
Enhanced immunity to Botrytis in era1-2
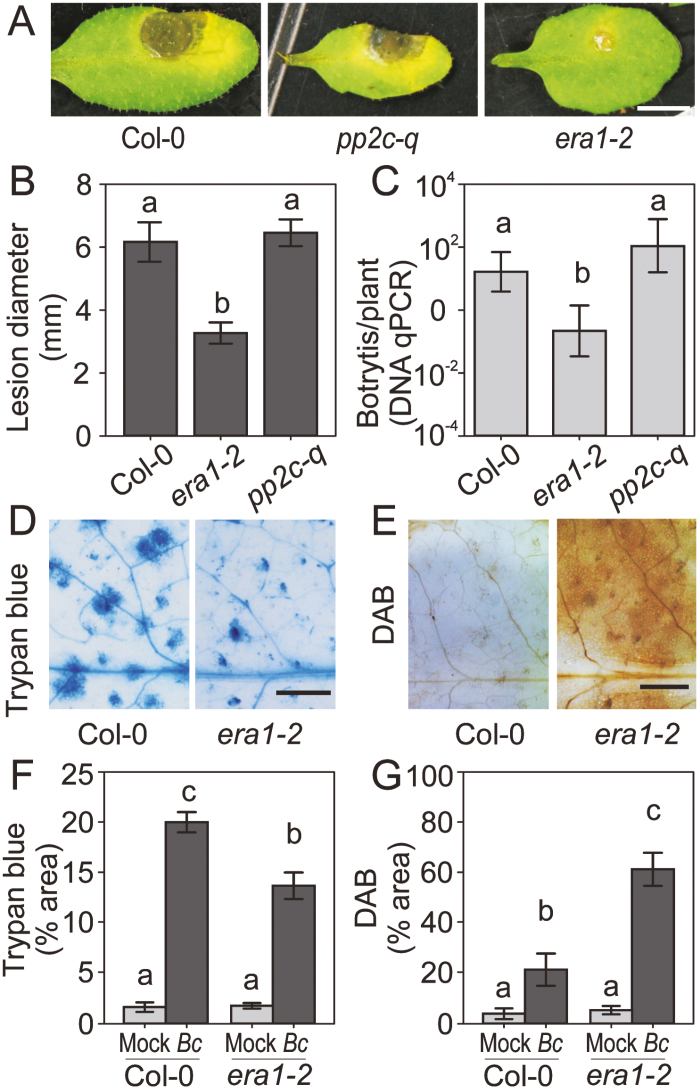
Botrytis infections of two ABA-hypersensitive mutants, enhanced response to aba1-2 (era1-2) and pp2c-quadruple (pp2c-q), revealed phenotypes inconsistent with the enhanced susceptibility that would be predicted based on the assumption that ABA acts solely as a negative regulator of Botrytis immunity (Fig. 1A). Disease progression in era1-2 was reduced, measured as smaller lesion size (Fig. 1B) and less Botrytis DNA accumulation (measured with real-time qPCR; Fig. 1C). The extent of cell death was also reduced in era1-2 (Fig. 1D, F). The Botrytis immunity associated with two additional era1 alleles, era1-7 and era1-8, was also enhanced compared with the wild type (Supplementary Fig. S1).
Fig. 1.
Enhanced Botrytis immunity in the ABA hypersensitive era1-2 mutant. (A) Botrytis infection of Col-0, pp2c-quad, and era1-2. Droplets of Botrytis conidia suspensions (3 μl, 2×106 spores ml–1) were applied to 24-day-old fully expanded leaves. Photographs were taken at 3 days post infection (dpi). Scale bar=5 mm. (B) Quantitative data of lesion sizes in (A). The lesion diameters were measured with ImageJ. Combined results of four experiments (n=10 in each independent biological repeat) were analyzed in a linear mixed model with single-step P-value adjustment. Error bars represent the SE of means. Different letters above the bars indicate significant differences (P<0.05). (C) DNA of Botrytis on leaves at 3 dpi was quantified with real-time qPCR using the Arabidopsis actin gene as a control. Different letters indicate significant differences (two-tailed t-test, P<0.05). (D–G) Leaves were stained at 36 hours post infection (hpi) for cell death with trypan blue (D, F), and at 16 hpi for H2O2 accumulation with 3,3′-diaminobenzidine (E, G). The percentage stained area was used for comparisons between Col-0 and era1-2. Two biological repeats with seven leaves in each repeat were analyzed with a linear model. Different letters above the bars indicate significant differences (P<0.05). Scale bar=2 mm.
To identify potential mechanisms of enhanced immunity in era1-2, we monitored ROS accumulation during Botrytis infection. Strong H2O2 accumulation in Botrytis-infected era1-2 was documented via DAB staining at 16 hpi (Fig. 1E, G). This suggests that the enhanced immunity phenotype of era1-2 may be due to early and/or enhanced ROS production.
ERA1 and cuticle development
An early Botrytis-induced H2O2 burst is associated with cuticle permeability (Asselbergh et al., 2007; L’Haridon et al., 2011), prompting us to assay for this phenotype. Toluidine blue staining is a classical cuticle-permeability assay: cuticle-defective leaves fail to exclude the dye, resulting in dark blue staining (O’Brien et al., 1964; Tanaka et al., 2004). The era1-2 mutant stained dark blue, while the wild type and pp2c-q did not (Fig. 2A). To exclude the possibility of second site mutations independent of era1, we confirmed enhanced permeability in mutants in two additional alleles, era1-7 and era1-8 (Goritschnig et al., 2008), using the ABA-insensitive snrk2.236 as a positive control (Fig. 2B, C). The enhanced permeability of the era1-2 mutant was further tested with an immersive staining assay, in which the era1-2 leaf stained mostly dark while the wild-type leaf remained unstained (Fig. 2D). Knockout mutants of ASG2 and CYP85A2, the two known ERA1 substrates that are involved in modulating the ABA response, exhibited normal cuticle permeability (Supplementary Fig. S2), suggesting that the role of ERA1 in cuticle formation is independent of these loci.
Fig. 2.
Enhanced cuticle permeability in the ABA hypersensitive era1-2 mutant. (A, B) The cuticle of era1-2 was more permeable than other mutants or the wild type Col-0 under toluidine blue staining. Leaves of 24-day-old plants were stained with 5 μl droplets of 0.05% toluidine blue solution for 2 h. The ABA-insensitive snrk2.236 was used as a positive control. (C) Quantification of the toluidine blue-stained areas. Combined results of three experiments (n=12 in each independent biological repeat) were analyzed in a linear mixed model with single-step P-value adjustment. Error bars represent the SE of means. Different letters above the bars indicate significant differences (P<0.05). (D) Leaves immersed in toluidine blue solution for 20 min. Bar=5 mm.
Overall, our findings genetically uncoupled ABA sensitivity from Botrytis susceptibility and revealed that ERA1 is required for cuticle formation and negatively regulates Botrytis immunity.
ABA-independent genes deregulated in cuticle-defective mutants
As era1-2 displayed Botrytis immunity, enhanced ABA sensitivity, and a permeable cuticle (Figs 1 and 2), it was utilized as a tool to further explore the relationships between these phenotypes. We used era1-2, snrk2.236, and the cuticle biosynthesis mutant lacs2.3 (Bessire et al., 2007; Tang et al., 2007), to examine global transcriptional changes under Botrytis infection and control conditions. All three mutants are cuticle permeable and Botrytis resistant, whereas their ABA sensitivities are largely different: era1-2 is hypersensitive, lacs2.3 is moderately sensitive, and snrk2.236 is strongly insensitive (Bessire et al., 2007; Fujii and Zhu, 2009; Cui et al., 2016). These three mutants allowed us to identify the effects of ABA sensitivity and reveal the core genes involved in the enhanced Botrytis immunity conditioned by cuticle deficiency.
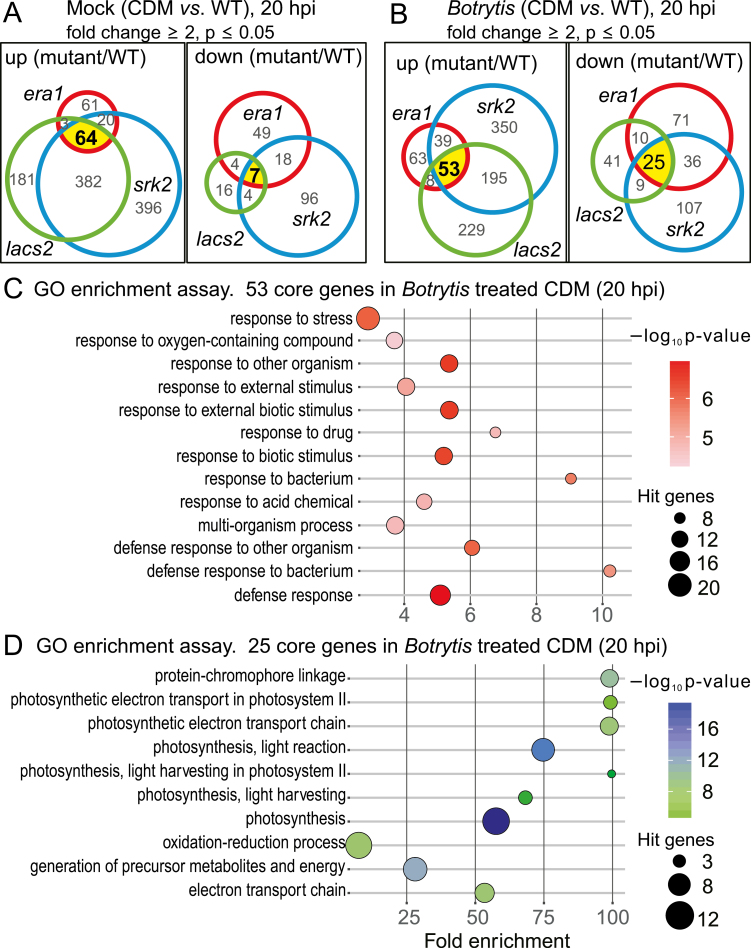
Mock-treated and Botrytis spore-suspension-infected plants were sampled at 20 hpi. To control for genes under circadian regulation, the second time point was 24 hours later (44 hpi). Samples of three biological repeats were subjected to RNA-seq analysis. To identify the core genes potentially involved in immunity to Botrytis in cuticle-defective mutants (CDMs), we first defined the significantly up-/down-regulated genes in each of the CDMs compared with the wild type. The genes that were mis-regulated in each CDM with fold change ≥2 and P≤0.05 in comparison to the wild type were chosen for further analysis (Supplementary Table S1). The mock-treated CDMs shared a common set of 64 genes with increased expression and seven with decreased expression (Fig. 3A; Supplementary Table S2A, B). Additionally, each mutant had its own unique set of DEGs, but generally lacs2 and snrk2.236 were more similar to each other than to era1-2 (Fig. 3A). Under Botrytis treatment, the three CDMs had in common 53 genes with increased expression and 25 genes with decreased expression (Fig. 3B; Supplementary Table S2C, D). As in the mock-treated condition, each single mutant had its own unique set of mis-regulated genes (Fig. 3B). Further analysis focused on the genes that were commonly regulated in all three CDMs, as these could be considered as ABA-independent genes and may function as CDM-specific components that regulate plant immune responses to Botrytis.
Fig. 3.
Analysis of the differentially expressed genes (DEGs) in cuticle-defective mutants (CDMs) compared with Col-0 in mock and Botrytis treatments. Plants at 24 days old were sprayed with 2×106 ml–1Botrytis spores. Samples were collected at 20 and 44 hours post infection (hpi) for RNA-seq analysis. (A) DEGs in the CDMs under mock treatment. DEGs were identified in comparison between each CDM and wild type (fold change ≥2 and P≤0.05; see also Supplementary Table S2). There were 64 genes commonly up-regulated (left) and seven genes down-regulated (right) in the CDMs (genes are listed in Supplementary Table S2A, B). Mutant names are abbreviated: era1-2 to era1, lacs2.3 to lacs2, snrk2.236 to srk2. (B) DEGs under Botrytis treatment in the CDMs. There were 53 genes commonly up-regulated (left) and 25 genes down-regulated (right) in the CDMs (genes are listed in Supplementary Table S2C, D). The DEGs at 44 hpi are presented in Supplementary Fig. S3B and Supplementary Table S2E, F. (C, D) GO enrichment analysis of the core genes in (B). The 53 up-regulated genes were enriched in defense-related terms (C). The 25 down-regulated genes were enriched exclusively in photosynthesis-related terms (D). The GO enrichment analysis of the core genes of mock-treated CDMs at 20 hpi is presented in Supplementary Fig. S3A.
In mock-treated CDMs, multiple receptor-like kinase genes related to defense responses and wounding-responsive genes were up-regulated. (Supplementary Table S2A). In addition, genes required for the pathogenicity of biotrophic or hemibiotrophic pathogens or with negative roles in SA-regulated defense responses were up-regulated (Supplementary Table S2A). For selected genes, RNA-seq data were confirmed with real-time qPCR (Supplementary Fig. S4).
In the Botrytis-treated CDMs, the core up-regulated genes were quite similar to the mock-treated CDMs, as seen in the GO enrichment analysis (Fig. 3C; Supplementary Fig. S3A). The term ‘defense response to bacterium’ was enriched (Fig. 3C). A common set of genes was up-regulated in the mock-treated CDMs and Botrytis-treated CDMs; these included Cysteine-rich receptor-like protein kinase (CRK) family and other kinase genes, salicylic acid signaling genes, and other pathogen-defense-related genes, including the metacaspase gene MC2 (Supplementary Table S2C; Supplementary Fig. S4).
There were also genes specifically up-regulated only in the Botrytis-treated CDMs (Supplementary Table S2C; Supplementary Fig. S4); these included Enhanced Disease Resistance4 and Downy Mildew Resistant6, two SA-regulated genes that are required for plant susceptibility to the biotrophic powdery mildew pathogen (van Damme et al., 2008; Wu et al., 2015). Only a few genes possibly related to Botrytis immunity were up-regulated in the Botrytis-treated CDMs, such as the P450 family member CYP82C2, which is required for the activation of JA signaling and the synthesis of a cyanogenic metabolite to defend against Botrytis (Liu et al., 2010a; Rajniak et al., 2015). In the Botrytis-treated CDMs, 25 genes were identified as the core down-regulated genes (Fig. 3B). Most of these genes are related to functions in the chloroplast and photosynthesis (Fig. 3D; Supplementary Table S2D).
Overall, the core genes mis-regulated in CDMs were mainly genes previously implicated in pathogen defense responses or SA signaling. This finding indicated that a signal derived from the defective cuticle may lead to the activation of defense responses, ultimately resulting in Botrytis immunity.
ABA signaling in the transcriptional response to Botrytis
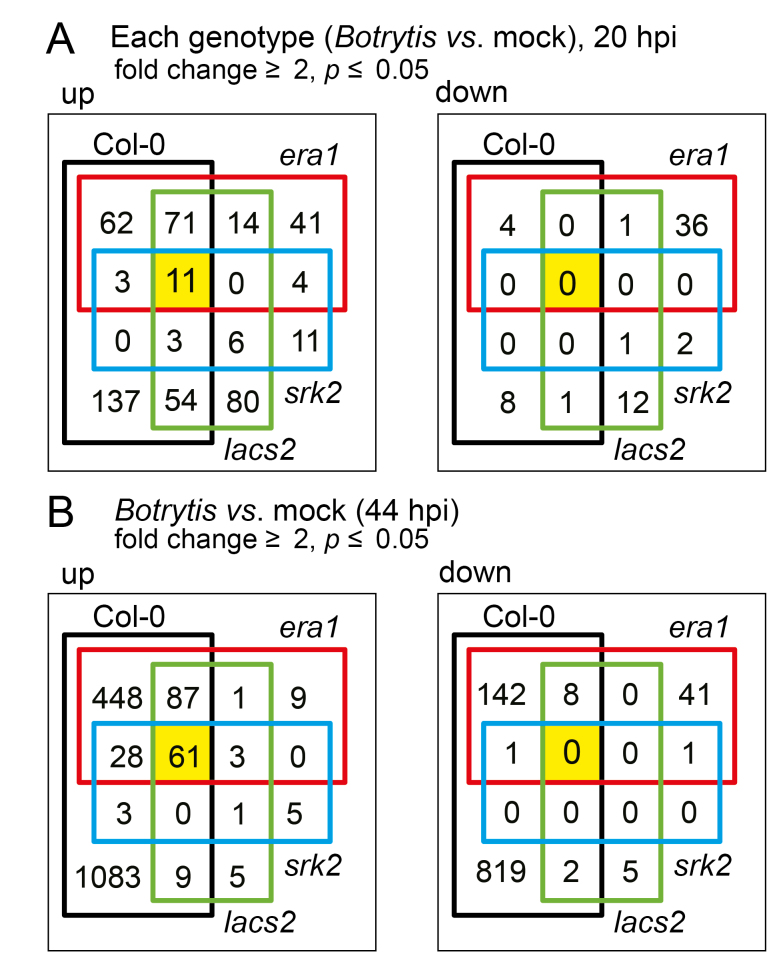
We further compared the Botrytis-regulated genes at 20 and 44 hpi between the wild type and CDMs. DEGs were identified in each genotype and were compared between genotypes (Fig. 4). At 44 hpi, substantially fewer DEGs were observed in all three CDMs compared with the wild type. This was likely due to the strong immunity in these mutants; that is, there was less dead tissue in the mutants compared with the wild type (Fig. 1; Bessire et al., 2007; Cui et al., 2016).
Fig. 4.
Identification of Botrytis-induced differentially expressed genes (DEGs). DEGs were identified in comparisons between Botrytis-treated CDMs and Col-0 to their corresponding genotype under mock treatment (fold change ≥2 and P≤0.05; see also Supplementary Table S3). (A) At 20 h post infection (hpi), there were 11 genes commonly up-regulated (left) and no common genes down-regulated (right) in the Botrytis-treated genotypes. The genes are listed in Supplementary Table S3E. Mutant names are abbreviated: era1-2 to era1, lacs2.3 to lacs2, snrk2.236 to srk2. (B) At 44 hpi, there were 61 genes commonly up-regulated (left) and no common genes down-regulated (right) in the Botrytis treated genotypes. The genes are listed in Supplementary Table S3J.
Col-0 had the most Botrytis-regulated genes, followed by era1-2, lasc2.3, and then snrk2.236 (Fig. 4). The number of Botrytis-regulated genes in the mutants correlated with their ABA sensitivity. This indicated that in the CDMs, the extent of ABA sensitivity still influenced transcriptional responses to Botrytis. To further explore the relation between ABA and Botrytis in transcriptional regulation, we made a comparison between Botrytis-regulated and ABA-regulated genes (Supplementary Fig. S5; Supplementary Table S4) using publicly available data from an ABA RNA-seq experiment (50 µM ABA, 3h; Zhu et al., 2017). Significant overlap was observed between up-regulated genes at both 20 and 44 hpi, and for down-regulated genes at 44 hpi (Supplementary Fig. S5A). The genes commonly regulated by both ABA and Botrytis were subjected to a GO enrichment analysis (Supplementary Table S4; Supplementary Fig. S6). The GO terms ‘toxin catabolic process’, ‘glutathione metabolic process’, ‘response to oxidative stress’, and ‘response to hydrogen peroxide’ were enriched in the overlap of ABA- and Botrytis-induced genes (Supplementary Fig. S6A, B). Genes down-regulated by ABA and Botrytis at 44 hpi were enriched for ‘syncytium formation’ and ‘plant-type cell wall loosening and modification’ (Supplementary Fig. S6C). Taken together, these data indicate that exogenous ABA likely activates signaling required for a subset of plant responses to Botrytis.
Expression of previously identified Botrytis-response genes
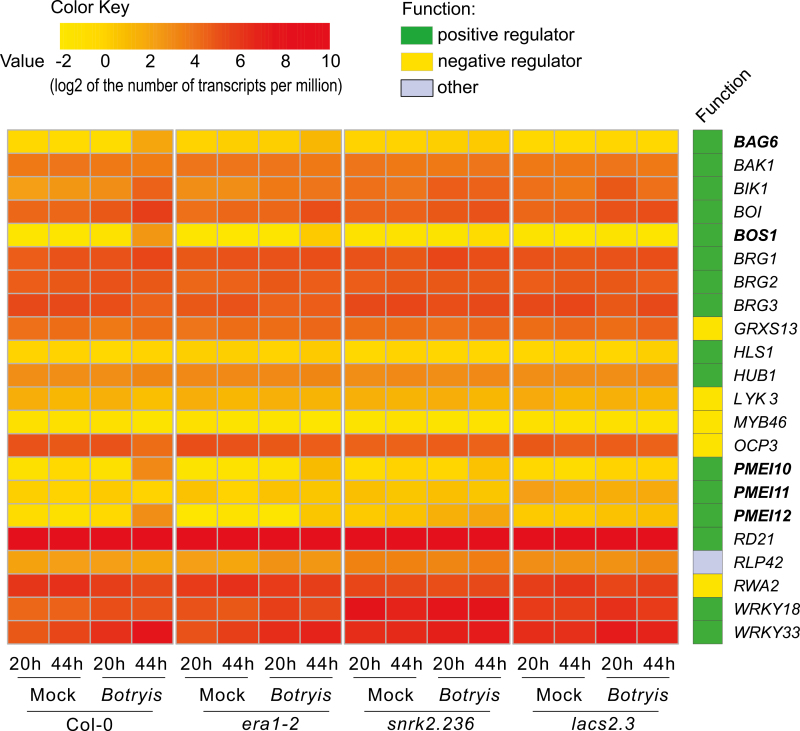
Extensive research on the interactions between Botrytis and Arabidopsis has identified several positive and negative regulators. We constructed a list of these genes through keyword searches of TAIR (https://www.arabidopsis.org; Supplementary Table S5). The genes related to the JA, ethylene, and PAD3 pathways were excluded, as the Botrytis immunity mediated by cuticle permeability was previously shown to be independent of these signaling pathways (Chassot et al., 2007). Expression values (log2 of the number of transcripts per million) for each gene were used to build a heatmap (Fig. 5). The expression of most genes was similar among genotypes and treatments (Fig. 5), indicating that these genes were not Botrytis inducible or cuticle dependent. However, the expression of some genes, including the autophagy inducer BAG6 (Li et al., 2016), the cell-death regulator BOS1 (Mengiste et al., 2003; Cui et al., 2013), and several pectin methylesterase inhibitors (PMEIs; Lionetti et al., 2017), had increased expression at 44 hpi (Fig. 5). The expression of these genes was genotype dependent; the wild type showed the highest expression, era1-2 showed moderate expression, while snrk2.236 and lacs2.3 showed the lowest expression (Fig. 5). We propose that the expression of these genes may be correlated to cell-death control and lesion development, as Botrytis extracts nutrients from dead and dying tissue. Hence, less expression of these genes would be seen in highly Botrytis-tolerant genotypes. Furthermore, cell-death control is a key factor that determines lesion development in cuticle-defective plants (Asselbergh et al., 2007; Curvers et al., 2010; Seifi et al., 2013). Thus, we chose the bos1 mutant, which exhibited mis-regulated cell-death development (Cui et al., 2013), for further analysis in relation to the other mutants used in this study.
Fig. 5.
Relative expression of genes known to regulate Botrytis immunity. The gene list was obtained from https://www.arabidopsis.org via the gene searching tool with the keywords ‘Botrytis’ or ‘B. cinerea’. Genes related to the jasmonic acid, ethylene, and PAD3 pathways were excluded, as they are not required for Botrytis immunity conferred by cuticle permeability (Chassot et al., 2007). The expression values (log2 of the number of transcripts per million) for each gene were used to build a heatmap. The genes are listed in alphabetical order. The treatments were mock and Botrytis spray infection at the indicated time points and are grouped according to genotypes.
The enhanced Botrytis immunity of era1-2 is BOS1 dependent
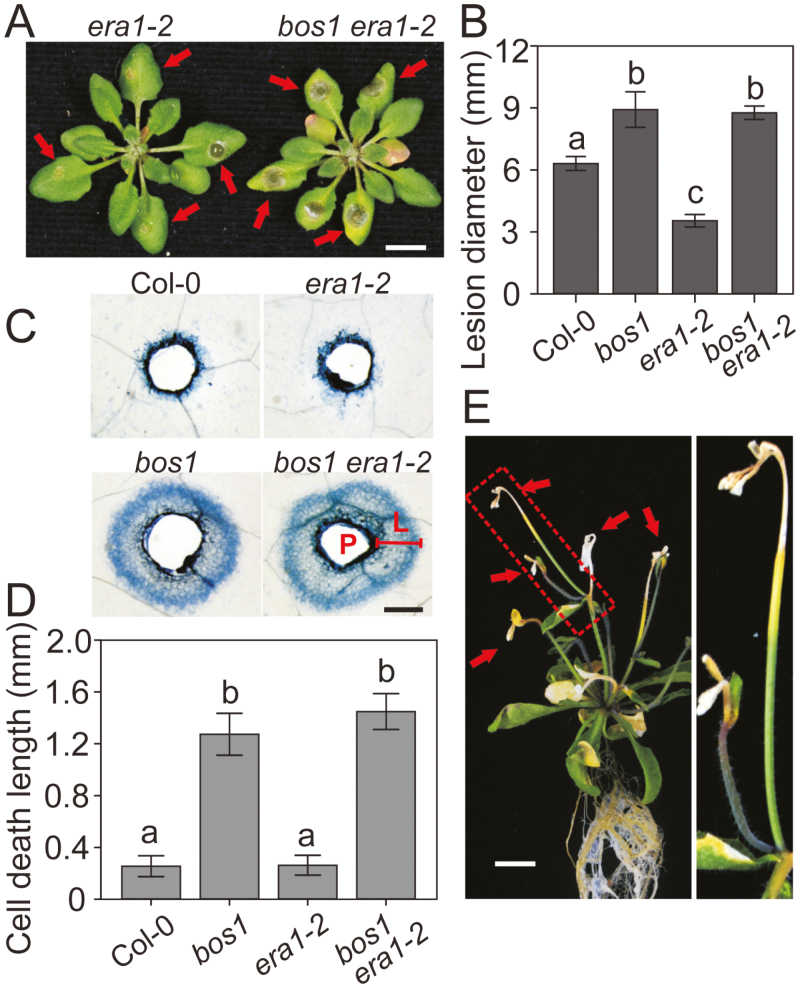
We constructed the bos1 era1-2 double mutant to test whether the BOS1-regulated control of cell death was required for the immunity to Botrytis associated with cuticle-defective plants. Interestingly, the enhanced immunity of era1-2 was fully suppressed in bos1 era1-2 (Fig 6A, B). The lesion sizes of Botrytis-infected bos1 era1-2 leaves were markedly larger than those of era1-2 (Fig. 6A) and were very similar to those of the bos1 single mutant (Fig. 6B). We previously demonstrated that bos1 exhibited uncontrolled runaway cell death after wounding (Cui et al., 2013). Thus, we assessed cell death initiated from needle-puncture wounds in bos1, era1-2, and the bos1 era1-2 double mutant. Dead tissue was visualized by trypan blue staining and quantified by measuring the length from the wound edge to the frontier of the spreading dead tissue (Fig. 6C). We found that the spread of cell death in bos1 era1-2 was slightly but not significantly greater than in bos1 (Fig. 6C, D). This indicated that the cell-death control conferred by BOS1 is required for the Botrytis immunity of the era1-2 mutant.
Fig. 6.
The Botrytis immunity of era1-2 was fully suppressed by bos1. (A) Typical lesion symptoms of era1-2 and bos1 era1-2. The arrows indicate Botrytis-infected lesions. Droplets of Botrytis conidia suspensions (3 μl, 2×106 spores ml–1) were applied to 24-day-old fully expanded leaves. Photographs were taken at 3 dpi. Scale bar=1 cm. (B) Quantitative lesion size data. The lesion diameters were measured with ImageJ. Combined results of three biological experiments (n=36 in total) were analyzed in a linear mixed model with single-step P-value adjustment. Error bars represent the SE of means. Different letters above the bars indicate significant differences (P<0.05). (C) Representative wound-induced cell death symptoms stained with trypan blue. Needle-punctured leaves were stained with trypan blue at 6 days post wounding to detect cell death. L, Length of cell death spread; P, puncture site. Scale bar=1 mm. (B) Quantitative spreading cell death data. The length of spread of cell death was measured as indicated in (C) around each wound four times in four directions (up, down, left, and right), and the mean value was used. Data of three repeats were analyzed in a linear mixed model with single-step P-value adjustment. Error bars represent the SE of means. Different letters above the bars indicate significant differences (P<0.05). (E) Spreading cell-death symptoms were enhanced in bos1 era1-2. Four-week-old in vitro-grown plants are shown. Once buds started opening, cell death initiated in the buds and then spread along the shoots, eventually causing the death of the whole plant. Right panel, close-up of the area indicated by the dashed box in the left panel. Arrows indicate dead buds. Scale bar=1 cm.
Compared with bos1, cell death in bos1 era1-2 was developmentally enhanced in older plants, as bos1 era1-2 exhibited spontaneous cell death during flowering (Fig. 6E). We observed that once buds started opening, cell death initiated in the buds and then spread along the shoots (Fig. 6E); this led to a sterility phenotype, with no bos1 era1-2 seeds obtained.
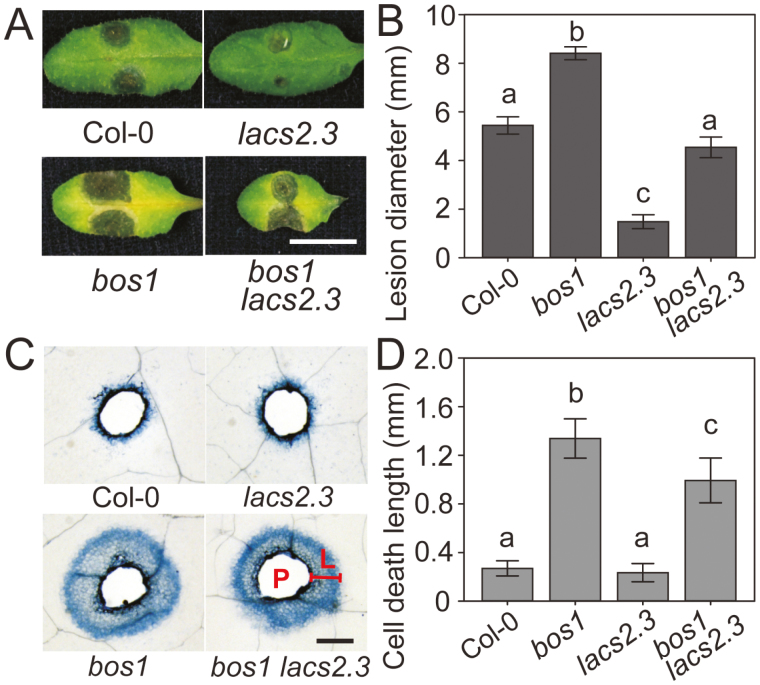
To test whether loss of BOS1 function could suppress the enhanced Botrytis immunity of other CDMs, we made the bos1 lacs2.3 double mutant. The bos1 mutation restored the Botrytis susceptibility of lacs2.3 to wild-type levels (Fig. 7A, B). Furthermore, runaway cell death in bos1 lacs2.3 was more extensive than in the wild type, but significantly less extensive than in the bos1 single mutant (Fig. 7C, D). The early ROS burst present in cuticle-permeable mutants under Botrytis treatment was assessed in bos1 era1-2 and bos1 lacs2.3, but was unaltered in both double mutants compared with the respective era1-2 and lacs2.3 single mutants (Supplementary Fig. S7). Taken together, these findings indicate that cell-death control is required, but the early ROS burst is not sufficient for BOS1-regulated Botrytis resistance.
Fig. 7.
Botrytis immunity in lacs2.3 was attenuated in the bos1 lacs2.3 double mutant. (A) Typical lesion symptoms of lacs2.3 and bos1 lacs2.3. Droplets of Botrytis conidia suspensions (3 μl, 2×106 spores ml–1) were applied to 24-day-old fully expanded leaves. Photographs were taken at 3 dpi. Scale bar=1 cm. (B) Quantitative lesion size data. Data of three biological repeats (n=36 in total) were analyzed in a linear mixed model with single-step P-value adjustment. Error bars represent the SE of means. Different letters above the bars indicate significant differences (P<0.05). (C) Representative wound-induced cell death symptoms stained with trypan blue. Needle-punctured leaves were stained at 6 days post wounding to detect cell death. L, Length of cell death spread; P, puncture site. Scale bar=1 mm. (D) Wound-induced cell death of bos1 was reduced in the bos1 lacs2.3 double mutant. Quantitative spreading cell death data. The length of spread of cell death around each wound was measured four times in four directions (up, down, left, and right), and the mean value was used. Data of three repeats were analyzed in a linear mixed model with single-step P-value adjustment. Error bars represent the SE of means. Different letters above the bars indicate significant differences (P<0.05).
ABA sensitivity can be uncoupled from Botrytis immunity
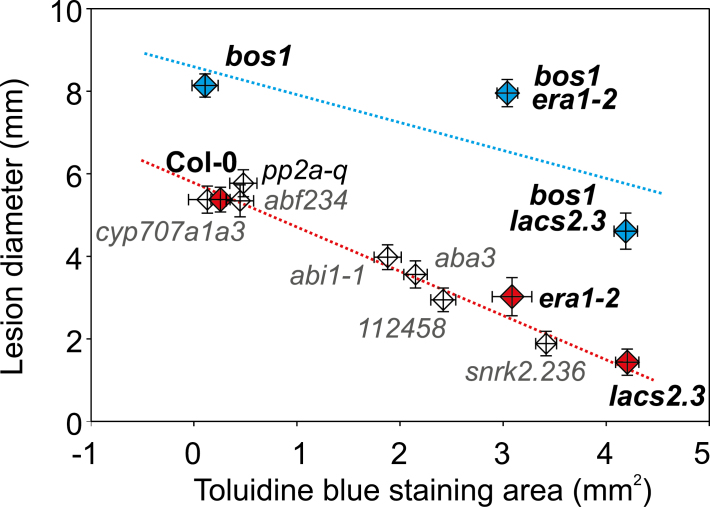
To explore the relationship between cuticle deficiency, ABA sensitivity, and Botrytis resistance, we analyzed the lesion sizes and cuticle permeability of multiple ABA-related mutants used in this study (Figs. 1, 2, 8) and included normalized data from our previous work (Cui et al., 2016). In these mutants, a linear relationship was observed between cuticle permeability and Botrytis immunity (Fig. 8; R2=0.96, P≤0.01). However, there was no correlation between ABA sensitivity and immunity. The most ABA-insensitive mutant, 112458, which is impaired in six ABA receptors (Gonzalez-Guzman et al., 2012), was more Botrytis-susceptible than the ABA-hypersensitive mutant era1-2 (Fig. 8). The lacs2.3 mutant, which was moderately impaired in ABA signaling (Wang et al., 2011), was more resistant than the snrk2.236 mutant, which is severely impaired in ABA signaling (Fig. 8). These data genetically demonstrate that Botrytis resistance associated with cuticle permeability could be uncoupled from ABA sensitivity.
Fig. 8.
ABA sensitivity is uncoupled from Botrytis resistance in cuticle-deficient plants. The relationship between Botrytis resistance and cuticle permeability of the indicated genotypes was examined. The red line was calculated with all single-mutant genotypes (not in the bos1 background), which showed a linear correlation between cuticle permeability and lesion size (R2=0.96, P≤0.001). The blue line was calculated with genotypes of the bos1 background (R2=0.52, P=0.49). The genotypes in black were examined in this study and the genotypes in grey are from previously published data (Cui et al., 2016). All experiments were repeated three to six times. All the data were normalized, pooled, and analyzed with a linear model.
In the bos1 background, Botrytis resistance was not dependent on cuticle permeability (Fig. 8; R2=0.52, P=0.49). Cuticle permeability in era1-2 and bos1 era1-2 was similar, as was the case for lacs2.3 and bos1 lacs2.3 (Fig. 8), indicating that BOS1 was not involved in cuticle formation. The lesion sizes of bos1, bos1 era1-2, and bos1 lacs2.3 were proportional to the severity of their wound-induced spreading cell death phenotypes (Fig. 6C; Fig. 7C). This suggested that BOS1-regulated cell death was genetically required for cuticle-related Botrytis immunity.
Role of cell-death control in enhanced Botrytis immunity
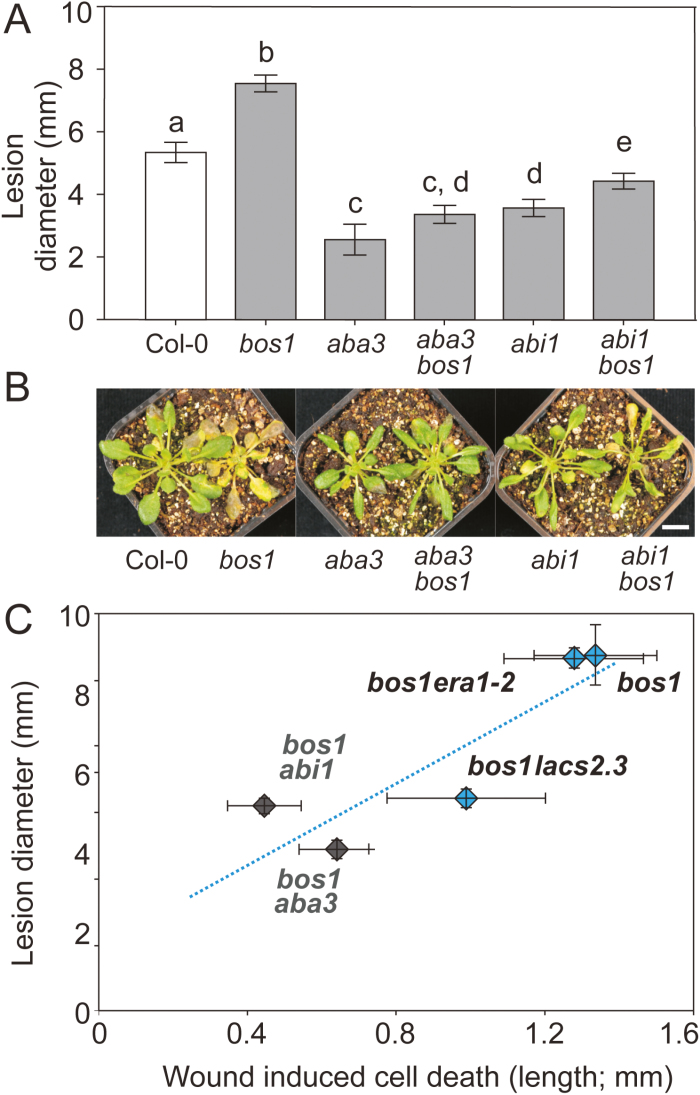
We previously demonstrated that impaired ABA signaling or biosynthesis could largely suppress the runaway cell-death phenotype of bos1 (Cui et al., 2013). To test the correlation between wound-induced cell death and Botrytis lesion size, the aba3 bos1 and abi1-1 bos1 double mutants were further examined with Botrytis droplet infection. Similar to wound-induced cell death (Cui et al., 2013), Botrytis-induced lesions in aba3 bos1 and abi1-1 bos1 were significantly smaller than in bos1 (Fig. 9A). Spray infection with Botrytis caused enhanced necrosis in bos1, which was also significantly reduced in the aba3 bos1 and abi1-1 bos1 double mutants (Fig. 9B). We plotted Botrytis-induced lesion sizes against the extent of wounding-induced cell death (Fig. 9C; this includes meta-analysis of normalized wound-induced cell death data from Cui et al., 2013), and found a significant correlation (R2=0.78, P≤0.05; Fig. 9C). Thus, the extent of cell death regulated by BOS1 plays a determinate role in the regulation of plant Botrytis sensitivity.
Fig. 9.
Botrytis susceptibility of bos1 double mutants was positively correlated with the extent of wound-induced cell death. (A) Suppressors of spreading cell death in bos1 (aba3-1 and abi1-1) also suppressed the Botrytis susceptibility of bos1. Lesion sizes from four independent experiments were combined and analyzed in a linear mixed model with a single step P-value adjustment. Error bars represent the SE of means (n=48 in total). Different letters above the bars indicate significant differences (P<0.05). (B) Representative symptoms of plants sprayed with Botrytis spore suspensions at 3 days post infection. Scale bar=1 cm. (C) Botrytis sensitivity correlated with the extent of wound-induced cell death in bos1 double mutants (R2=0.78, P=0.047). The blue dotted line shows a linear correlation between the extent of wound-induced cell death and the Botrytis-induced lesion sizes. The genotypes indicated with blue squares were examined in this study. The spread of cell death of the genotypes indicated with grey squares are from previously published data (Cui et al., 2013), which were calculated with normalization to the reference values of the wild type and bos1. All experiments were repeated three to six times.
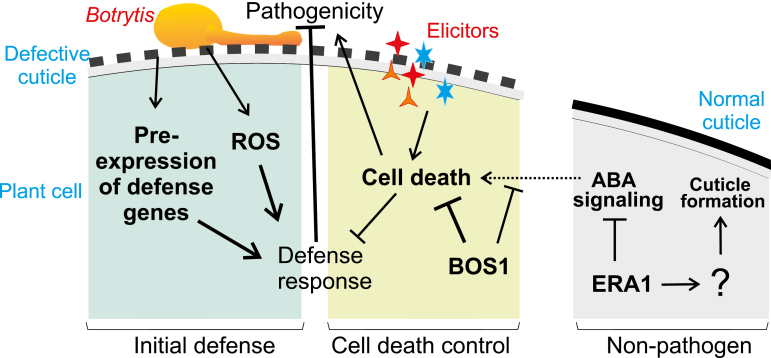
Taken together, our data illustrate that the Botrytis resistance in CDMs likely consists of several layered biological mechanisms. Initial resistance is conferred by pre-activated defense signaling, including increased expression of defense-related genes (Fig. 3C; Supplementary Table S2; Fig. 10). During early infection, enhanced ROS production (Fig. 1E; L’Haridon et al., 2011) may augment plant defenses. At a later stage of infection, control of cell death becomes more important and determines the development of Botrytis-induced necrosis (Fig. 10). On the leaves of Botrytis-infected CDMs, cell death is dramatically attenuated at the frontier of the lesions, resulting in smaller lesion sizes compared with the wild type (Curvers et al., 2010; AbuQamar et al., 2017). This control of Botrytis-induced cell death requires BOS1 (Figs. 5–7, 10).
Fig. 10.
Model of layered defense responses to Botrytis in cuticle-deficient plants. Cuticle permeability itself leads to increased expression of defense genes related to pathogen perception and salicylic acid signaling. In addition, a permeable cuticle enhances the production of reactive oxygen species (ROS), which act as signaling molecules and regulators of cell death (see also L’Haridon et al., 2011). BOS1 is required to maintain cell viability and contain Botrytis-induced lesion expansion. The left panel represents the initial resistance and the middle panel represents a later stage of infection. The right panel illustrates signaling relationships under normal conditions, with an intact cuticle and the absence of pathogens, in which ERA1 attenuates ABA signaling and independently promotes cuticle formation via an unknown mechanism.
Discussion
Understanding the complexities of ABA signaling at the intersection between cuticle deficiency and Botrytis responses is challenging: ABA-deficient and -insensitive mutants are generally cuticle defective (Cui et al., 2016; Martin et al., 2017), while cuticle biosynthesis mutants, such as bgd/ced1, lcr/cyp86a8, gpat4 gpat8, and lacs2-1, exhibit altered stress-induced accumulation of ABA-biosynthesis transcripts and enhanced sensitivity to osmotic stress (Wang et al., 2011). Here we provide new genetic data that refines the roles of pathways downstream of ABA in plant–Botrytis interactions.
The role of ABA signaling in plant–Botrytis interactions
Our comparisons between mutants with different levels of ABA sensitivity, cuticle permeability, and response to Botrytis (Fig. 8) demonstrated that cuticle permeability, but not ABA sensitivity, determines Botrytis resistance in CDMs. However, the role of ABA in wild-type plants is complicated during Botrytis infection. The application of ABA to plants results in enhanced Botrytis susceptibility (Audenaert et al., 2002), while Botrytis also secretes ABA (Siewers et al., 2004, 2006; Amselem et al., 2011), which could be one mechanism used by the pathogen to promote virulence. These data would suggest ABA as a negative regulator of plant Botrytis immunity. In contrast, increasing ABA levels in vivo via drought, which doubled the ABA content in plants (Achuo et al., 2006), or knocking out the ABA-degrading enzymes CYP707a1 and CYP707a3, did not alter Botrytis immunity (Cui et al., 2016). Accordingly, the enhanced ABA sensitivities in pp2c-q mutants did not alter their Botrytis resistance either (Fig. 1A). Overexpression of the transcription factor WRKY33 led to increased ABA sensitivity and also enhanced Botrytis resistance (Liu et al., 2015; Liao et al., 2016). Thus, exogenous ABA is likely to have a different role from the overactivated ABA signaling in vivo in the course of Botrytis infection. ABA signaling also plays a fundamental role in several plant development processes, including determination of cell wall thickness and the number of stomata (Asselbergh et al., 2007; Lake and Woodward, 2008). The long-term effects of altered ABA signaling in vivo (i.e. using strongly ABA-insensitive mutants) could be expected to have a stronger influence on several biological processes compared with the response to exogenous ABA application. Upon infection, ABA signaling is precisely controlled by the plant during plant–Botrytis interactions. A high-resolution transcriptional time-course series of Botrytis infection showed that repression of ABA signaling takes place at 20–22 hpi in wild type plants (Windram et al., 2012). Thus, the timing and location of initiating ABA signaling should also be considered to influence the initiation or extent of cell death, to influence responses to Botrytis in plants.
Exogenous ABA application enhances the development of cell death symptoms (Fan et al., 1997; Cui et al., 2013; Takasaki et al., 2015; Zhao et al., 2016). The GO categories ‘response to oxidative stress’ and ‘response to hydrogen peroxide’ were enriched in the genes regulated by both ABA treatment and Botrytis infection at both the early and later time points (Supplementary Fig. S6). These categories are integrated in the process of cell death (Overmyer et al., 2003; Van Breusegem and Dat, 2006). Thus, one role for ABA in responses to Botrytis could be to alter the extent of cell death. We explored this further utilizing the bos1 mutant, which displays enhanced cell death upon ABA application and increased ROS production after both Botrytis infection and wounding (Mengiste et al., 2003; Kraepiel et al., 2011; Cui et al., 2013). The abi1-1 and aba3 mutants suppressed the severe Botrytis susceptibility of bos1. Proportionally, these mutants also suppressed the extent of wound-induced spreading cell death in bos1 (Fig. 9C). Thus, a linear correlation between Botrytis sensitivity and ABA-regulated cell death was demonstrated. This was also supported by the symptoms shown by lacs2.3 bos1 and era1 bos1. The lacs2 mutant is deficient in one step of cuticle biosynthesis, but also exhibits a secondary phenotype of deficient ABA biosynthesis (Wang et al., 2011). The lacs2.3 mutant attenuated both the runaway cell death and Botrytis susceptibility phenotypes of bos1 (Fig. 7). However, era1-2 had no effect on either of these phenotypes in era1-2 bos1 (Fig. 6). This genetic evidence supports the hypothesis that ABA can influence Botrytis sensitivity through the regulation of cell death. Since ABA sensitivity had little effect in the Col-0 background (Fig. 8) but was linearly correlated with Botrytis susceptibility in the bos1 background (Fig. 9C), we propose that ABA signaling could affect plant sensitivity to Botrytis only when it is activated at levels high enough to trigger cell death.
Resistance trade-offs in cuticle-permeable mutants
Cuticle permeability confers effective resistance to certain necrotrophic pathogens, such as Botrytis, but also enhances susceptibility to other pathogens (Łaźniewska et al., 2012; Serrano et al., 2014; Ziv et al., 2018). This topic is attracting increasing interest, especially due to its potential use in practical breeding of economically important species (Ziv et al., 2018). Our transcription data showed increased expression of many important pathogen-related genes in the CDMs even under control conditions (Supplementary Fig. S3A and S4; Supplementary Table S2A). These included genes encoding several proposed receptors that detect the presence of pathogens, such as FLG22-induced Receptor-like Kinase 1 (FRK1), Receptor Kinase 3 (RK3), Cysteine-rich receptor-like protein kinase family, and Leucine-rich repeat protein kinase family. Increased expression of these genes could potentially prime CDMs for responses to certain pathogens. The wounding responsive gene Wound-Responsive 3 (WR3) and NRT1/PTR Family 5.2 (NPF5.2) were also up-regulated. Wounding induces plant immunity to Botrytis (Chassot et al., 2008), and pre-activation of wound-regulated signaling might facilitate the restriction of Botrytis pathogenicity in CDMs (Chassot et al., 2008). Remarkably, the mechanism for the enhanced Botrytis immunity of CDMs remains unknown. The activation of defense transcripts observed here under control (Botrytis-free) conditions in CDMs is consistent with the proposed idea that unincorporated cuticle-building substrates that are present in CMDs may act as damage-associated molecular patterns to pre-activate immune signaling (Serrano et al., 2014).
Other genes displaying higher expression in CDMs act as negative regulators in SA signaling. These included genes required for susceptibility to hemibiotrophic or biotrophic pathogens (Supplementary Table S2A, C; Supplementary Fig. S4). For example, the hemibiotrophic pathogen P. syringae requires UDP-Dependent Glycosyltransferase 76B1 (UGT76B1; von Saint Paul et al., 2011), Lysine Histidine Transporter 1 (LHT1; Liu et al., 2010b), and WRKY DNA-Binding Protein 38 (WRKY38; Kim et al., 2008) for pathogenicity. The biotrophic powdery mildew Erysiphe cruciferarum requires Mildew Resistance Locus O12 (MLO12; Consonni et al., 2006), LHT1, and Impaired Oomycete Susceptibility 1 (IOS1; Hok et al., 2014). The biotrophic oomycete downy mildew pathogen H. parasitica requires IOS1 and Late Upregulated in Response to Hyaloperonospora parasitica 1 (LURP1; Knoth and Eulgem, 2008). Finally, the hemibiotrophic species Colletotrichum higginsianum and Phytophthora parasitica require LHT1 and IOS1, respectively, for pathogenicity (Consonni et al., 2006; Kim et al., 2008; Knoth and Eulgem, 2008; Liu et al., 2010b; von Saint Paul et al., 2011; Hok et al., 2014). Differential expression of these genes might explain why cuticle-defective mutants have an altered balance between Botrytis resistance and biotrophic pathogen resistance, resulting in susceptibility to certain pathogens.
ERA1 in defense responses
ERA1 is the β subunit of the plant farnesyl trans-transferase, which was isolated and extensively studied for its functions related to development and abiotic stresses (Galichet and Gruissem, 2003). A few studies have also explored its role in responses to pathogens including the hemibiotrophic bacterial pathogen P. syringae pv. maculicola and the biotrophic oomycete pathogen H. parasitica (Goritschnig et al., 2008). It was suggested that loss of function of ERA1 resulted in a mis-regulation of the interaction between ABA and defense signaling, such as SA (Goritschnig et al., 2008). ERA1 adds a farnesyl to its typical substrate proteins, which contain a CaaX motif at the C-terminal (Galichet and Gruissem, 2003). ERA1 has over 700 potential targets for farnesylation; this presents a considerable challenge to identifying the relevant protein that acts as a regulator of Botrytis defense and/or cuticle formation (Goritschnig et al., 2008; Northey et al., 2016). At the same time, the existence of over 700 potential targets makes era1 a pleiotropic mutant, as is also illustrated by its many other different phenotypes, such as aberrant flower development (Ziegelhoffer et al., 2000). Thus, some caution should be taken in the interpretation of era1 phenotypes compared with other permeability mutants. In the future, identification of the ERA1 substrate that regulates cuticle formation, for example, with a protein purification approach (Dutilleul et al., 2016), could lead to the identification of the mechanisms that regulate cuticle formation. However, even without knowledge of this ERA1 target, the robust ABA hypersensitivity and cuticle permeability of era1 make it a useful tool to explore the interactions between ABA, cell death, cuticle permeability, and responses to Botrytis.
In this study, we reported the functions of ERA1 in cuticle formation and Botrytis resistance, genetically uncoupled plant ABA sensitivity from Botrytis sensitivity, and identified bos1 as the first suppressor of Botrytis immunity in CDMs. These results support the hypothesis that ABA can promote susceptibility to Botrytis infection via the regulation of plant cell death, and provide a framework for future work on the role of ABA in the regulation of plant–Botrytis interactions.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Increased Botrytis immunity in era1-7 and era1-8.
Fig. S2. Cuticle permeability of known ERA1 substrates.
Fig. S3. DEGs in cuticle-defective mutants compared with Col-0 under mock and Botrytis treatments.
Fig. S4. Real-time quantitative reverse transcription–PCR data.
Fig. S5. Significant overlaps between ABA- and Botrytis-regulated genes.
Fig. S6. GO enrichment analysis of genes regulated by both ABA and Botrytis.
Fig. S7. Early Botrytis-induced ROS in era1-2 and lacs2.3 were not attenuated by bos1.
Table S1. DEGs identified in comparisons between genotypes (CDMs versus Col-0) and treatments (Botrytis versus mock).
Table S2. Core genes common to the DEGs of each CDM.
Table S3. Botrytis-responsive genes of each genotype.
Table S4. Common genes regulated by both ABA and Botrytis.
Table S5. Gene information and expression values used for Fig. 5.
Table S6. Primers used in this work.
Acknowledgements
We gratefully acknowledge the following for providing seeds: Dr Pedro L. Rodriguez for pp2c-q; Dr Xin Li for era1-7 and era1-8; Dr Tesfaye Mengiste for bos1; and Dr Christiane Nawrath for lacs2.3. We gratefully acknowledge Jarkko Salojärvi (University of Helsinki) for assistance in statistical analysis; Xiaoxue Ye and Yanmei Yang for help with uploading data and exporting figures; and Tuomas Puukko, Airi Lamminmäki, and Leena Grönholm for excellent technical support. This work supported by the National Natural Science Foundation of China (grant no. 31700224 to FC and YZ, and 31871233 to WW); the Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding (grant no. 2016C02056-1); the Program for Changjiang Scholars and Innovative Research Team in University (grant no. IRT_17R99 to SL); and the Academy of Finland Center of Excellence in Molecular Biology of Primary Producers 2014–2019 (Decisions no. 307335 and 271832).
Data deposition
The RNA-seq data presented in this paper have been deposited in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) under accession number PRJNA495475.
References
- AbuQamar S, Moustafa K, Tran LS. 2017. Mechanisms and strategies of plant defense against Botrytis cinerea. Critical Reviews in Biotechnology 37, 262–274. [DOI] [PubMed] [Google Scholar]
- Achuo EA, Prinsen E, Höfte M. 2006. Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathology 55, 178–186. [Google Scholar]
- Amselem J, Cuomo CA, van Kan JA, et al. . 2011. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genetics 7, e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni R, Gonzalez-Guzman M, Rodriguez L, et al. . 2013. PYRABACTIN RESISTANCE1-LIKE8 plays an important role for the regulation of abscisic acid signaling in root. Plant Physiology 161, 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergh B, Curvers K, Franca SC, Audenaert K, Vuylsteke M, Van Breusegem F, Höfte M. 2007. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiology 144, 1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, De Meyer GB, Höfte MM. 2002. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiology 128, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, Petétot JM, Métraux JP, Nawrath C. 2007. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. The EMBO Journal 26, 2158–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot C, Buchala A, Schoonbeek HJ, Métraux JP, Lamotte O. 2008. Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. The Plant Journal 55, 555–567. [DOI] [PubMed] [Google Scholar]
- Chassot C, Nawrath C, Métraux JP. 2007. Cuticular defects lead to full immunity to a major plant pathogen. The Plant Journal 49, 972–980. [DOI] [PubMed] [Google Scholar]
- Consonni C, Humphry ME, Hartmann HA, et al. . 2006. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nature Genetics 38, 716–720. [DOI] [PubMed] [Google Scholar]
- Cui F, Brosché M, Lehtonen MT, Amiryousefi A, Xu E, Punkkinen M, Valkonen JP, Fujii H, Overmyer K. 2016. Dissecting abscisic acid signaling pathways involved in cuticle formation. Molecular Plant 9, 926–938. [DOI] [PubMed] [Google Scholar]
- Cui F, Brosché M, Sipari N, Tang S, Overmyer K. 2013. Regulation of ABA dependent wound induced spreading cell death by MYB108. New Phytologist 200, 634–640. [DOI] [PubMed] [Google Scholar]
- Curvers K, Seifi H, Mouille G, et al. . 2010. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiology 154, 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. 1996. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Dean R, Van Kan JA, Pretorius ZA, et al. . 2012. The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilleul C, Ribeiro I, Blanc N, et al. . 2016. ASG2 is a farnesylated DWD protein that acts as ABA negative regulator in Arabidopsis. Plant, Cell & Environment 39, 185–198. [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng S, Wang X. 1997. Antisense suppression of phospholipase D alpha retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. The Plant Cell 9, 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu J-K. 2009. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proceedings of the National Academy of Sciences, USA 106, 8380–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon C, Saindrenan P. 2004. Real-time PCR monitoring of fungal development in Arabidopsis thaliana infected by Alternaria brassicicola and Botrytis cinerea. Plant Physiology and Biochemistry 42, 367–371. [DOI] [PubMed] [Google Scholar]
- Galichet A, Gruissem W. 2003. Protein farnesylation in plants–conserved mechanisms but different targets. Current Opinion in Plant Biology 6, 530–535. [DOI] [PubMed] [Google Scholar]
- Galichet A, Gruissem W. 2006. Developmentally controlled farnesylation modulates AtNAP1;1 function in cell proliferation and cell expansion during Arabidopsis leaf development. Plant Physiology 142, 1412–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Pizzio GA, Antoni R, et al. . 2012. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. The Plant Cell 24, 2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritschnig S, Weihmann T, Zhang Y, Fobert P, McCourt P, Li X. 2008. A novel role for protein farnesylation in plant innate immunity. Plant Physiology 148, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology 8, R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok S, Allasia V, Andrio E, et al. . 2014. The receptor kinase IMPAIRED OOMYCETE SUSCEPTIBILITY1 attenuates abscisic acid responses in Arabidopsis. Plant Physiology 166, 1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2001. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant & Cell Physiology 42, 1265–1273. [DOI] [PubMed] [Google Scholar]
- Jalakas P, Huang YC, Yeh YH, Zimmerli L, Merilo E, Kollist H, Brosché M. 2017. The role of ENHANCED RESPONSES TO ABA1 (ERA1) in Arabidopsis stomatal responses is beyond ABA signaling. Plant Physiology 174, 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner J, Dörffling K. 1995. Biosynthesis and metabolism of abscisic acid in tomato leaves infected with Botrytis cinerea. Planta 196, 627–634. [Google Scholar]
- Kim KC, Lai Z, Fan B, Chen Z. 2008. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. The Plant Cell 20, 2357–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nature Methods 12, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Rowe HC, Denby KJ. 2005. Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. The Plant Journal 44, 25–36. [DOI] [PubMed] [Google Scholar]
- Knoth C, Eulgem T. 2008. The oomycete response gene LURP1 is required for defense against Hyaloperonospora parasitica in Arabidopsis thaliana. The Plant Journal 55, 53–64. [DOI] [PubMed] [Google Scholar]
- Kraepiel Y, Pédron J, Patrit O, Simond-Côte E, Hermand V, Van Gijsegem F. 2011. Analysis of the plant bos1 mutant highlights necrosis as an efficient defence mechanism during D. dadantii/Arabidospis thaliana interaction. PLoS One 6, e18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łaźniewska J, Macioszek VK, Kononowicz AK. 2012. Plant-fungus interface: the role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiological and Molecular Plant Pathology 78, 24–30. [Google Scholar]
- L’Haridon F, Besson-Bard A, Binda M, et al. . 2011. A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS Pathogens 7, e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JA, Woodward FI. 2008. Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytologist 179, 397–404. [DOI] [PubMed] [Google Scholar]
- Li Y, Kabbage M, Liu W, Dickman MB. 2016. Aspartyl protease-mediated cleavage of BAG6 is necessary for autophagy and fungal resistance in plants. The Plant Cell 28, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CJ, Lai Z, Lee S, Yun DJ, Mengiste T. 2016. Arabidopsis HOOKLESS1 regulates responses to pathogens and abscisic acid through interaction with MED18 and acetylation of WRKY33 and ABI5 chromatin. The Plant Cell 28, 1662–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Fabri E, De Caroli M, Hansen AR, Willats WG, Piro G, Bellincampi D. 2017. Three pectin methylesterase inhibitors protect cell wall integrity for Arabidopsis immunity to Botrytis. Plant Physiology 173, 1844–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Jiang H, Ye S, et al. . 2010c. The Arabidopsis P450 protein CYP82C2 modulates jasmonate-induced root growth inhibition, defense gene expression and indole glucosinolate biosynthesis. Cell Research 20, 539–552. [DOI] [PubMed] [Google Scholar]
- Liu G, Ji Y, Bhuiyan NH, Pilot G, Selvaraj G, Zou J, Wei Y. 2010b. Amino acid homeostasis modulates salicylic acid-associated redox status and defense responses in Arabidopsis. The Plant Cell 22, 3845–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Kracher B, Ziegler J, Birkenbihl RP, Somssich IE. 2015. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife 4, e07295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, He J, Chen Z, Ren X, Hong X, Gong Z. 2010a. ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. The Plant Journal 63, 749–765. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LBB, Romero P, Fich EA, Domozych DS, Rose JKC. 2017. Cuticle biosynthesis in tomato leaves is developmentally regulated by abscisic acid. Plant Physiology 174, 1384–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T. 2012. Plant immunity to necrotrophs. Annual Review of Phytopathology 50, 267–294. [DOI] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R. 2003. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. The Plant Cell 15, 2551–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey JG, Liang S, Jamshed M, Deb S, Foo E, Reid JB, McCourt P, Samuel MA. 2016. Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nature Plants 2, 16114. [DOI] [PubMed] [Google Scholar]
- O’Brien TP, Feder N, McCully ME. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59, 368–373. [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. 2006. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiology 141, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Brosché M, Kangasjärvi J. 2003. Reactive oxygen species and hormonal control of cell death. Trends in Plant Science 8, 335–342. [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. . 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. 1998. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. 2015. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature Biotechnology 33, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajniak J, Barco B, Clay NK, Sattely ES. 2015. A new cyanogenic metabolite in Arabidopsis required for inducible pathogen defence. Nature 525, 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer M. 2007. Introduction: biology of the plant cuticle. In: Riederer M, Müller C, eds. Annual Plant Reviews. Volume 23: Biology of the plant cuticle. Oxford: Blackwell, 1–10. [Google Scholar]
- Ritpitakphong U, Falquet L, Vimoltust A, Berger A, Métraux JP, L’Haridon F. 2016. The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytologist 210, 1033–1043. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio S, Rodrigues A, Saez A, Dizon MB, Galle A, Kim TH, Santiago J, Flexas J, Schroeder JI, Rodriguez PL. 2009. Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiology 150, 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi HS, Curvers K, De Vleesschauwer D, Delaere I, Aziz A, Höfte M. 2013. Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytologist 199, 490–504. [DOI] [PubMed] [Google Scholar]
- Serrano M, Coluccia F, Torres M, L’Haridon F, Métraux JP. 2014. The cuticle and plant defense to pathogens. Frontiers in Plant Science 5, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O, Elad Y, Zieslin N. 1996. Suppression of Botrytis blight in cut rose flowers with gibberellic acid. Effects of exogenous application of abscisic acid and paclobutrazol. Postharvest Biology and Technology 7, 145–150. [Google Scholar]
- Siewers V, Kokkelink L, Smedsgaard J, Tudzynski P. 2006. Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Applied and Environmental Microbiology 72, 4619–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewers V, Smedsgaard J, Tudzynski P. 2004. The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Applied and Environmental Microbiology 70, 3868–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki H, Maruyama K, Takahashi F, Fujita M, Yoshida T, Nakashima K, Myouga F, Toyooka K, Yamaguchi-Shinozaki K, Shinozaki K. 2015. SNAC-As, stress-responsive NAC transcription factors, mediate ABA-inducible leaf senescence. The Plant Journal 84, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y. 2004. A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. The Plant Journal 37, 139–146. [DOI] [PubMed] [Google Scholar]
- Tang D, Simonich MT, Innes RW. 2007. Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiology 144, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. 2013. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nature Biotechnology 31, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. 2006. Reactive oxygen species in plant cell death. Plant Physiology 141, 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Damme M, Huibers RP, Elberse J, Van den Ackerveken G. 2008. Arabidopsis DMR6 encodes a putative 2OG-Fe(II) oxygenase that is defense-associated but required for susceptibility to downy mildew. The Plant Journal 54, 785–793. [DOI] [PubMed] [Google Scholar]
- von Saint Paul V, Zhang W, Kanawati B, Geist B, Faus-Kessler T, Schmitt-Kopplin P, Schäffner AR. 2011. The Arabidopsis glucosyltransferase UGT76B1 conjugates isoleucic acid and modulates plant defense and senescence. The Plant Cell 23, 4124–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Xiong L, Li W, Zhu JK, Zhu J. 2011. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. The Plant Cell 23, 1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windram O, Madhou P, McHattie S, et al. . 2012. Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. The Plant Cell 24, 3530–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Liu S, Zhao Y, Wang W, Kong Z, Tang D. 2015. ENHANCED DISEASE RESISTANCE4 associates with CLATHRIN HEAVY CHAIN2 and modulates plant immunity by regulating relocation of EDR1 in Arabidopsis. The Plant Cell 27, 857–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chan Z, Gao J, et al. . 2016. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proceedings of the National Academy of Sciences 113, 1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang B, Tang K, Hsu CC, Xie S, Du H, Yang Y, Tao WA, Zhu JK. 2017. An Arabidopsis Nucleoporin NUP85 modulates plant responses to ABA and salt stress. PLoS Genetics 13, e1007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelhoffer EC, Medrano LJ, Meyerowitz EM. 2000. Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proceedings of the National Academy of Sciences, USA 97, 7633–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv C, Zhao Z, Gao YG, Xia Y. 2018. Multifunctional roles of plant cuticle during plant-pathogen interactions. Frontiers in Plant Science 9, 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.