Abstract
X-linked hypohidrotic ectodermal dysplasia (XLHED) is caused by defects in the EDA gene that inactivate the function of ectodysplasin A1 (EDA1). This leads to abnormal development of eccrine glands, hair follicles, and teeth, and to frequent respiratory infections. Previous studies in the naturally occurring dog model demonstrated partial prevention of the XLHED phenotype by postnatal administration of recombinant EDA1. The results suggested that a single or two temporally spaced injections of EDI200 prenatally might improve the clinical outcome in the dog model. Fetuses received ultrasound-guided EDI200 intra-amniotically at gestational days 32 and 45, or 45 or 55 alone (of a 65-day pregnancy). Growth rates, lacrimation, hair growth, meibomian glands, sweating, dentition, and mucociliary clearance were compared in treated and untreated XLHED-affected dogs, and in heterozygous and wild-type control dogs. Improved phenotypic outcomes were noted in the earlier and more frequently treated animals. All animals treated prenatally showed positive responses compared with untreated dogs with XLHED, most notably in the transfer of moisture through paw pads, suggesting improved onset of sweating ability and restored meibomian gland development. These results exemplify the feasibility of ultrasound-guided intra-amniotic injections for the treatment of developmental disorders, with improved formation of specific EDA1-dependent structures in dogs with XLHED.
Introduction
X-linked hypohidrotic ectodermal dysplasia [XLHED; OMIM (Online Mendelian Inheritance in Man) #305100] is an inherited disorder in humans characterized by sparse or absent hair, missing and/or malformed dentition, and hypoplastic or absent eccrine glands (lacrimal, meibomian, bronchial, sweat) (Clarke et al., 1987). Morbidity and mortality in children is mostly attributed to their inability to sweat resulting in severe hyperthermic episodes (Clarke et al., 1987; Blüschke et al., 2010), which continues to affect health throughout life (Hammersen et al., 2011). In addition, decreased lacrimation and reduced numbers of meibomian and tracheobronchial glands may result in dry eye and recurrent respiratory problems (Dietz et al., 2013). The XLHED phenotype is caused by variants in the EDA gene leading to disruption of the signaling molecule ectodysplasin A1 (EDA1), which is normally required for continued placode differentiation and development of various glands, skin appendages, and dentition (Mikkola, 2009; Chu and Loomis, 2017). The EDA1 protein features an intracellular domain, a transmembrane domain, a stalk region, a furin cleavage site, a proteoglycan binding domain, a collagen motif, and a tumor necrosis factor homology domain, which trimerizes and contains the receptor binding sites (Schneider et al., 2001; Bodmer et al., 2002; Swee et al., 2009). To exert its activity, EDA1 must be cleaved at the furin site, allowing the trimerized protein to bind to the EDA receptor.
The signaling pathway for EDA1 is highly conserved among vertebrates (Pantalacci et al., 2008), suggesting that results stemming from animal studies in both murine and canine models are directly translatable to human XLHED patients. In previous studies, human recombinant EDA1 (Fc:EDA1) was administered intravenously to pregnant Tabby (Eda-deficient) mice or intraperitoneally to neonatal Tabby mice, the mouse homolog of humans and dogs with XLHED. The recombinant protein contains the Fc portion of human IgG1, allowing for placental transfer into mouse fetuses (Gaide and Schneider, 2003). These studies demonstrated variable rescue of body size, hair coverage, meibomian and sweat glands, and dentition, depending on the time of administration. Due to the lack of Ig transfer across the placenta in the dog model, initial experiments involved the administration of the recombinant protein IV to neonatal XLHED dogs. This resulted in improved weight gain, lacrimation, and adult dentition in both number and appearance, and the ability to sweat by 28 weeks of age (Casal et al., 2007; Mauldin et al., 2009). Mucociliary clearance (MCC) was an indirect measure of tracheobronchial secretions and improvement correlated with partial restoration of tracheobronchial glands (Mauldin et al., 2009) and the absence of pulmonary disease in almost all XLHED dogs within each of the treated groups (Casal et al., 2007).
With the partial rescue achieved by postnatal intravenous administration in dogs, along with the complete reversion of disease in the prenatal mice experiments (Gaide and Schneider, 2003; Hermes et al., 2014), we chose to administer the protein during gestation via ultrasound-guided intra-amniotic injection. The expression of a homologous neonatal Fc receptor for IgG is present in the fetal gut of both rodents and humans (Shah et al., 2003). Because Fc:EDA1 contains a human IgG1 moiety, and swallowing amniotic fluid by the fetus is the primary method for the clearance of proteins from the amniotic fluid (Pitkin and Reynolds, 1975; Underwood and Sherman, 2006; Dasgupta et al., 2016), Fc:EDA1 delivered into the amniotic cavity is expected to be absorbed in the fetal gut. Indeed, Fc:EDA1 administered to Tabby mice intra-amniotically was demonstrated to be present in the circulation and resulted in reversal of the XLHED phenotype with a single injection (Hermes et al., 2014).
The aims of this study were to demonstrate safety and feasibility of prenatal administration of the recombinant protein results in XLHED dogs, and to determine how the timing of Fc:EDA1 administration into the amniotic fluid of developing canine fetuses impacts the clinical and phenotypic outcome.
Materials and Methods
Dogs and Recombinant Protein.
As XLHED is an X-linked disorder, affected and heterozygous females used in the study were produced by breeding XLHED (hemizygous) males to heterozygous females, which resulted in heterozygous and XLHED females and wild-type (WT) and XLHED males (Casal et al, 1997). Five pregnant dams (three heterozygous and two homozygous XLHED) received ultrasound-guided intra-amniotic injections (see below) to 22 fetuses. Age-matched WT (N = 5), heterozygous (N = 4), and XLHED (N = 3) puppies were produced by breeding heterozygous females to XLHED males and served as controls for the in utero–treated dogs. All dogs were cared for in accordance with the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the International Guiding Principles for Biomedical Research Involving Animals.
Fc:EDA1 (EDI200), the recombinant protein that binds to the EDA1 receptor to initiate the developmental pathway, is a fully human variant of Fc:EDA1 used in initial studies (Gaide and Schneider, 2003) and was provided by Edimer Pharmaceuticals (Cambridge, MA). Mature Fc:EDA1 contains amino acid residues 105–330 of human IgG1 (UniProt accession number P01857) followed by amino acid residues 239–391 of human EDA1 (UniProt accession number Q92838).
In Utero Treatment Timing and Injections.
The gestational ages of the fetuses were based on ovulation timing in the female dogs. Gestation length in the domestic dogs is 65 ± 1 days from the surge of luteinizing hormone (LH). Briefly, serial serum LH concentrations were determined. In the dog, ovulation will take place 48–72 hours after the LH surge, and the ova will be mature enough to be fertilized in another 2–4 days (Johnston et al., 2001). Serum LH concentrations were determined using a commercially available kit (Witness LH Ovulation Timing; Synbiotics Corporation, San Diego, CA). Gestational ages were then verified by the ultrasonographic appearance of the fetal structures and their developmental stages (Lopate, 2008).
Three different time points for the intra-amniotic injections were chosen based on published developmental studies of canine dentition (Williams and Evans, 1978), murine sweat gland development (Li et al., 2017), and the results of EDI200 treatment in the mouse XLHED model (Gaide and Schneider, 2003; Hermes et al., 2014). Single injections into the amniotic sac at 32, 45, or 55 days of gestation or two injections at 32 and 45 days of gestation (Table 1) were performed transabdominally using a 22-gauge 40-mm-long needle attached to a 40-cm intravenous extension tubing and a 1-ml syringe, under ultrasound guidance using aseptic techniques. After the protein injection, an approximately 0.05-ml bubble of air provided a short-term label of fetuses that had already received the injection.
TABLE 1.
Experimental protocol
Canine fetuses received intra-amniotic injections at the specified embryonic gestational time points at a dose of 100 mg/kg EDI200. The injections were given only during the gestational period (groups IUTxE32/45, IUTxE45, and IUTxE55) and were not repeated postnatally.
| Name of group |
|||||
|---|---|---|---|---|---|
| IUTXE32/45 | IUTxE45.1 | IUTx45.2 | IUTxE55 | IUTxE32 | |
| Genotype of dam | XLHED | HET | HET | XLHED | HET |
| Time of in utero injection | 32/45 | 45 | 45 | 55 | 32 |
| Genotype of offspring | XLHED | XLHED | 2 WT | XLHED | Unknown |
| 4 XLHED | |||||
| Number of offspring born | 5 | 3 | 6 | 7 | 0 |
| Number of fetuses treated | 5 | 3 | 5 | 4 | 6 |
Dosing.
The dose of EDI200 (100 mg/kg) per fetal unit was derived from previous mouse studies with EDI200 and the calculation of the percentage of injected EDI200 that is expected to go into circulation via the ingestion of amniotic fluid (Hermes et al., 2014). Fetal sac volume was estimated by measuring ultrasonographically the long axis (L) of the sac and the diameter (2r). From these measurements, the volume of a prolate ellipsoid was calculated:
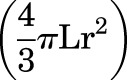 |
.
The fetal unit consists of fluids and the fetus, and because body water content in fetuses is over 80%, 1 ml roughly was set to weigh 1 g for dosing calculations. All females except for the first one were given oral amoxicillin/clavulonic acid at a dose of 12.5 mg/kg beginning at the time of in utero injections for a duration of 10 days.
Clinical Assessment.
Daily weight gain, lacrimation, hair development, dentition, and MCC were evaluated as previously described (Casal et al., 2007). Briefly, weight gain was recorded daily during the first 2 weeks of life, three times weekly from 2 to 4 weeks, biweekly from 4 to 8 weeks, and then monthly for the duration of the study. Any litters produced by XLHED dams were fostered by 4 days of age if milk production was inadequate, as affected dams often have underdeveloped mammary glands as part of their phenotype.
Lacrimation was determined using Schirmer tear test strips (Schering-Plough Animal Health, Kenilworth, NJ). Schirmer tear test strips were placed into the medial canthus of each eye, and the amount of fluid produced was determined by measuring the length of the resulting blue dye after 1 minute. XLHED dogs produce approximately 25% less lacrimal fluid than normal dogs. When clinical signs of dry eye were first noted, typically by the presence of mucoid ocular discharge, ophthalmic lubricants were administered and recorded. Medical management with ophthalmic antibiotics was initiated if indicated by corneal ulceration.
Photographs of each litter and the control animals were taken at 8 weeks of age and again at 5 to 6 months of age. The scale of hair coverage was subjectively assessed by comparing the photos to those taken of untreated XLHED dogs and WT age-matched controls.
Deciduous and adult dentition was evaluated at the typical dental developmental milestones in dogs (2, 4, and 6 months) by visual oral examination.
MCC studies were performed as an indirect measure of respiratory gland function. Briefly, one radioactive marker was taped to the lateral chest wall at the level of the carina (fifth intercostal space), and a second 15- to 20-cm cranial marker was placed at the level of the trachea depending on the size of the dog. Under general anesthesia and using a sleeved polystyrene catheter, a droplet (25–50 µl) of 99mTc-macroaggregated albumin containing 200–300 µCi of radioactivity was placed through the intubation tube onto the tracheal mucosa at the level of the distal (carina) marker. Static images were acquired with the Techni-Care Omega 500 Camera (Techni-Care, Cleveland, OH) interfaced with a NuQuest Veterinary nuclear medicine computer (NuQuest, Arlington Heights, IL) every 5 minutes for 20–30 minutes, and the tracheal mucociliary velocity (in millimeters per minute) was calculated using these images. The dogs were clinically healthy, and there was no indication of respiratory disease at the time of the study. Meibomian glands were enumerated along a 1-cm stretch of the upper eyelid.
To assess sweat gland function in the dogs, a capacitive sensor and an optoelectric sensor for imaging were placed on the paw 10 minutes after oral administration of 4 mg of pilocarpine HCl 1% (Alcon Laboratories, Fort Worth, TX). Pilocarpine was given to standardize sweat production. Capacitance imaging was performed using the Moisture Map MM 100 and accompanying software (Moisture Map MM100 version 1.2.9.0) (Houser et al., 2018). The Moisture Map was calibrated per the manufacturer instructions, which includes white and dark imaging to adjust the functional range. The functional range is automatically set during calibration based on imaging of a dry surface (white) and a wet surface (dark) and cannot be changed manually. Optoelectronic imaging was performed using the prototype Paw Print Sensor and accompanying software (Houser et al., 2018). There is currently no calibration procedure for this device. Images were captured over a 30-second interval with the sensor held in place as a dynamic assessment of sweat production over that period. Changes in capacitance and optoelectric imaging over that period are due to sweating and evaporative water loss through the skin. Image analysis was performed using Image Pro Premiere 9.0 software (Media Cybernetics, Rockville, MD) at approximately 5 months of age. A region of interest within the boundaries of the image (to avoid measuring noise at the edges) was selected. Analysis of average intensity on a 0–255 scale was performed, excluding the range of 245–255 (white), which are areas where contact is not being made with the sensor. This is a more sophisticated analysis than that which was performed during the initial development process (Houser et al., 2018) and is beneficial as it eliminates noise from changes in contact area as the subjects move their paws during acquisition. Analysis was performed on the first image in an acquisition with good paw-pad contact as well as an image 30 seconds later. Active sweating will result in a significant decrease in average intensity over this period.
Statistical Analysis.
Data distribution was analyzed with the Shapiro-Wilk normality test. If passed, multiple groups were compared by the one-way analysis of variance (ANOVA) followed by Dunnett’s multiple-comparison test, comparing the group of untreated XLHED dogs to other groups. If not passed, multiple groups were compared by the nonparametric Kruskal-Wallis test, followed by Dunn’s multiple-comparison test, comparing the group of untreated XLHED dogs to others. For the particular case of the teeth, where unaffected WT and heterozygous dogs have an invariant number of teeth (28 deciduous and 42 adult teeth), it was first evaluated whether the average number of teeth was different from the normal number of teeth by one-sample t test. Then, multiple groups of affected dogs (excluding WT and heterozygous groups) were compared by one-way ANOVA, followed by Dunnett’s multiple-comparison test, comparing the group of untreated XLHED dogs to others. All statistical tests were performed using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA).
Results
In Utero Treatment.
Twenty-three fetuses in five pregnancies were treated by intra-amniotic injections of the recombinant protein (Table 1). In the first experiment, all six fetuses that received amniotic injections were aborted after the bitch contracted an infection. Thereafter, as a precautionary measure, all pregnant bitches received a course of amoxicillin-clavulonic acid at 12.5 mg/kg from the time of injection until whelping. Of the remaining four pregnancies, 17 fetuses were injected, 4 were not, and all 21 survived to term.
Briefly, five fetuses were visualized on ultrasound for the IUTxE32/45 group, and each was injected with EDI200 first at embryonic day 32 (E32) with 0.5 mg (0.1 ml) and then at E45 with 2.2 mg (0.44 ml). Five XLHED pups were born to this XLHED female (Table 1). For the IUTxE45 group, three fetuses received 2.5 mg (0.5 ml) of EDI200 and three affected fetuses were born to the heterozygous dam. In the second heterozygous female of the IUTxE45 group, five fetuses were seen ultrasonographically and were injected with 1.5 mg (0.3 ml) EDI200. However, six pups (two WT and four XLHED) were born in this litter, indicating that one of the fetuses was not identified and did not receive the protein. The two normal males were not used as untreated WT controls, as they may have received EDI200. In the last experiment (IUTxE55), an XLHED female was bred to an XLHED male to produce an entire litter of affected offspring. Seven fetuses were visualized ultrasonographically in the IUTxE55 litter, but only four were injected with 10 mg (2 ml) of EDI200 due to the difficulty in puncturing the amniotic sac and subsequent administration of the protein into the small volume of amniotic fluid during this late stage of pregnancy. One neonate was found dead within 24 hours of birth with condensed lung lobes on gross pathology. A second neonate from this litter had failure to gain weight for 7 days and was euthanized. At the time of necropsy, lung consolidation, a small thymus, and abnormal hemorrhagic streaking of the heart were noted grossly. A definitive cause of death was not confirmed.
Clinical Assessment.
The birth weights of all offspring ranged from 200 to 330 g. As birth weights vary considerably between litters due to the differing sizes of the breeding males and females, direct comparisons of weight gain cannot be assessed. However, the trajectories of weight gain during the first 6 weeks of life provided a better measure of growth (Table 2). Weight gain trajectories in WT dogs were very similar to those in the first IUTx45 puppies, but the trajectory in the second IUTx45 litter was much lower. The weight gain trajectories in the IUTx32/45 and IUTx55 litters were similar to or less than those seen in the XLHED puppies. However, both litters were born to XLHED dams, which tend to produce little or no milk. Once the puppies were fostered, their weight gain trajectories increased substantially (y = 44.2x − 121.3 and y = 56.0x − 274.1, respectively).
TABLE 2.
Weight gain
As growth is fairly linear during the first 6 weeks of life, trajectories of weight gain were calculated as a measure of growth. Each trajectory represents the mean of a group. As birth weights are dissimilar, statistics were not performed. The table is only intended to show trends.
| Name of group | Average weight gain trajectory |
|---|---|
| WT | y = 63.9x + 152.9 |
| IUTx32/45 | y = 34.9x + 154.1 |
| IUTx45.1 | y = 58.6x + 165.9 |
| IUTx.45.2 | y = 36.6x + 99.6 |
| IUTx55 | y = 48.2x − 50.2 |
| XLHED | y = 47.2x + 67.2 |
IUTx45.1, first litter of this group; IUTx45.2, second litter of this group.
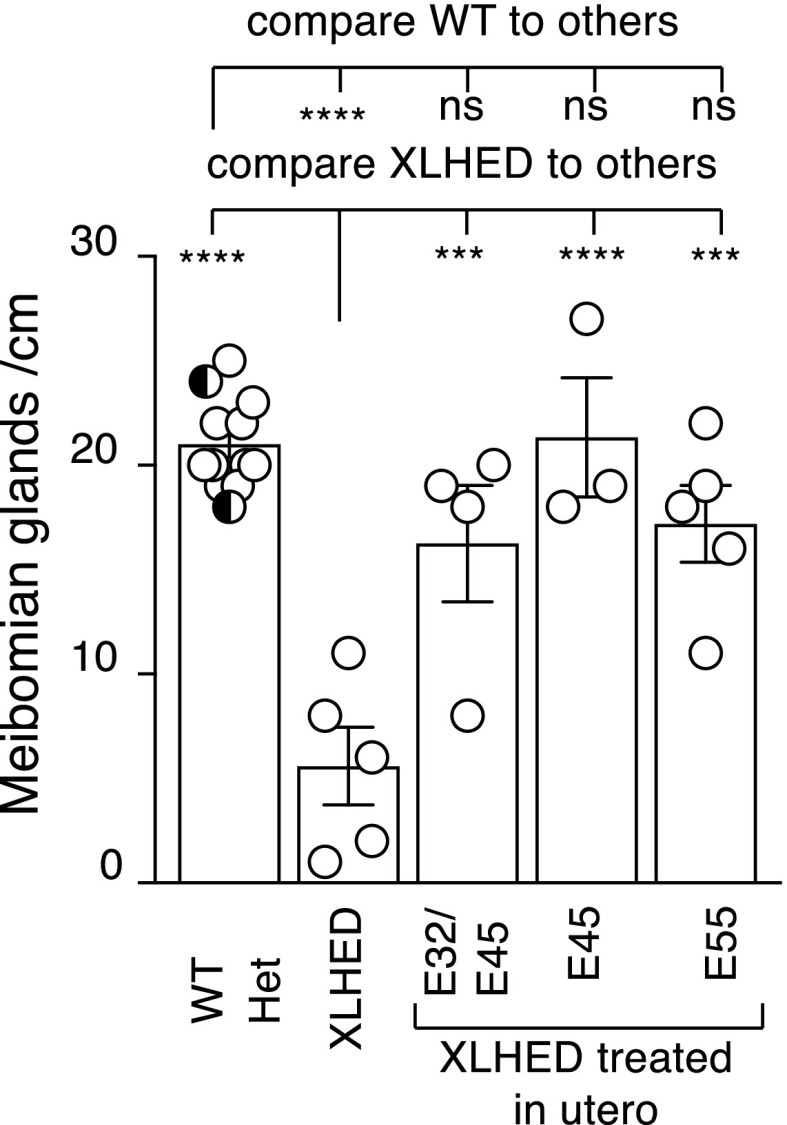
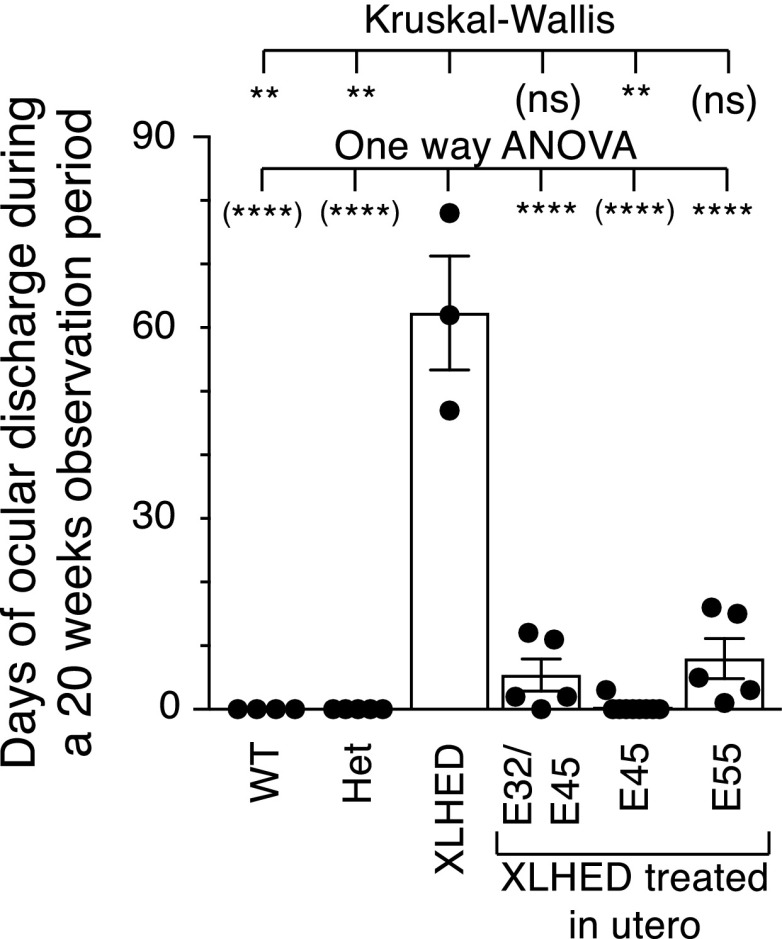
All prenatally treated offspring had significantly more meibomian glands compared with XLHED controls and were not significantly different from WT dogs (Fig. 1). Clear mucoid ocular discharge is a common finding in affected dogs (Casal et al., 2005a). Only one of the IUTxE45 group had a single incidence of mucopurulent ocular discharge lasting 3 days at the time of palpebral separation (10 days of age), which was managed medically with no recurrence. No mucopurulent discharge was recorded in the other IUTxE45 treated dogs. The reduction in the average frequency of mucoid ocular discharge was statistically significant (P < 0.01) in all treatment groups compared with untreated XLHED dogs (Fig. 2). At 25 days of age, two dogs of the E55 group developed corneal ulceration, which was managed with topical neomycin-polymyxin-bacitracin and ophthalmic lubricant twice daily for 5 days with no recurrence.
Fig. 1.
Numbers of meibomian glands per centimeter of eyelid in WT, untreated heterozygous (Het), and XLHED dogs, and XLHED dogs treated in utero with Fc:EDA1 at the indicated embryonic developmental days (E32/45, E45, E55). Untreated WT (white circles) and heterozygous (black and white circles) dogs were pooled for the analysis. Values are the mean ± S.E.M. One-way ANOVA assuming Gaussian distribution, and comparing conditions to a control (WT or XLHED) with Dunnett’s multiple comparison test. nsP > 0.05; ***P < 0.001; ****P < 0.0001.
Fig. 2.
Mean frequency of ocular discharge requiring the administration of ophthalmic lubricants in WT, treated, and untreated XLHED dogs over a 20-week observation period. Values are the mean ± S.E.M. One-way ANOVA assuming Gaussian distribution, and comparing conditions to untreated XLHED with Dunnett’s multiple-comparison test, and nonparametric Kruskal-Wallis test with Dunn’s multiple-comparison test. Both sets of statistical results are displayed, with those believed to be less relevant shown in brackets. nsP > 0.05; **P < 0.1; *****P < 0.0001. Het, heterozygous.
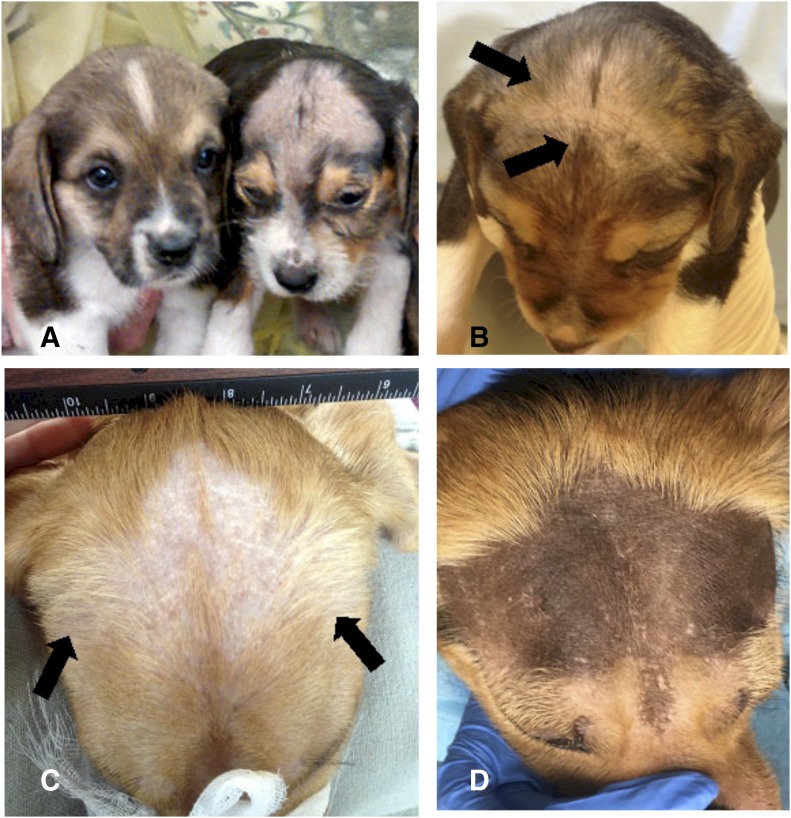
Subjectively, the greatest improvement in hair coat coverage was seen in the IUTxE32/45 group and the first IUTx45 litter when compared with XLHED dogs, with three of eight having good hair growth at 8 weeks and continued improvement by 5–6 months (Fig. 3), but it was never normal. The IUTxE55 treatment group did not show any improvement in hair coat coverage at either time point compared with untreated XLHED puppies. In the second IUTxE45 litter, zero of four early time point images showed improved hair coverage compared with XLHED dogs. However, at 5–6 months of age, two of four dogs showed subjectively increased hair coat compared with untreated XLHED dogs. All WT, untreated, and treated XLHED dogs developed normal whiskers.
Fig. 3.
Hair coverage over the dorsal cranium. Each dog depicted here is representative of all of the dogs in each treatment group. (A) In the WT dog (left), there is complete hair coverage over the forehead, whereas most is missing in the XLHED dog. (B) There is subjectively more hair coverage (arrows) on the forehead of this 8-week-old XLHED puppy from the IUTxE32/45 group when compared with the XLHED control in (A). (C) At 6 months of age, the increased hair coverage (arrows) shown in this XLHED dog from the IUTxE45 was present to the same degree as when the dog was 8 weeks old (data not shown). (D) There was little to no improvement of hair coverage in any of the XLHED dogs from the IUTxE55. The forehead of a 6-month-old dog from this group is shown here.
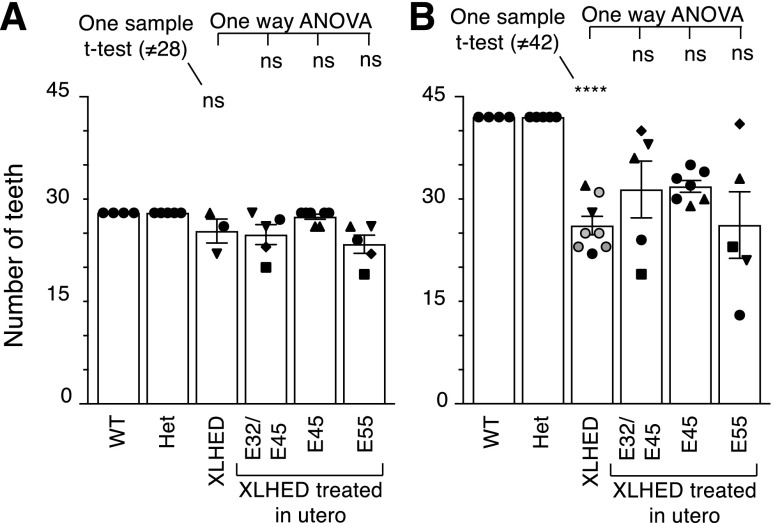
WT and heterozygous dogs have 28 deciduous teeth, whereas three untreated XLHED dogs had 28, 26, and 22 deciduous teeth, respectively. This variation in the XLHED group, combined with the small sample size, limited the interpretation of results from XLHED-treated dogs. Dogs with incomplete sets of deciduous teeth were identified in all treated groups, indicating that treatment did not restore the WT anatomy. However, there was a nonsignificant trend toward an improvement in deciduous dentition in the E45 group where five of seven dogs developed a full set of deciduous teeth (Fig. 4A). For adult teeth, full sets of teeth were never found in dogs in any of the treatment groups (Fig. 4B). Although the mean number of adult teeth was higher in the IUTx32/45 and IUTx45 groups than in the untreated XLHED control group, there was no significant difference (Fig. 4B). Repetition of the statistical analysis after the addition of results obtained with five more adult XLHED dogs (from a previous experiment) supported the conclusion that treatment had no significant outcome on the adult dentition (Fig. 4B).
Fig. 4.
Average number of teeth in WT, heterozygous (Het), treated, and untreated XLHED dogs. (A) Deciduous teeth. (B) Adult teeth. Gray circles in the XLHED group indicate dogs coming from an independent experiment and included in the statistical analysis. In the untreated and treated XLHED groups, identical symbols (black upright triangles, inverted triangles, circles, diamonds, or squares) indicate the same animal in (A and B). Values are the mean ± S.E.M. A one-sample t test first assessed whether the average number of teeth in XLHED dogs differed from normal (28 in pups, 42 in adults). Thereafter, groups of treated dogs were compared with untreated XLHED dogs by one-way ANOVA with Dunnett’s multiple-comparison test. nsP > 0.05; ****P < 0.0001.
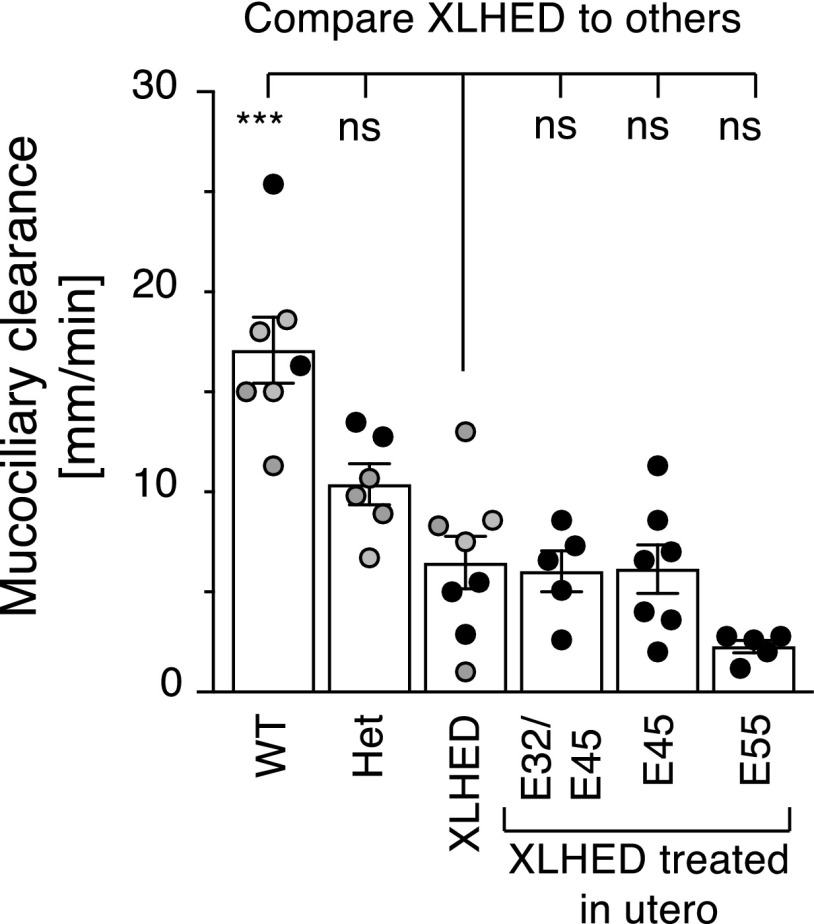
MCC studies were performed as an indirect measure of the presence of tracheal glands (Fig. 5). Although the MCC in affected untreated dogs was significantly different from that in WT dogs, none of the treatment groups was significantly different from untreated XLHED dogs. None of the treated or untreated XLHED offspring showed any clinical signs of respiratory disease during the 6-month study period.
Fig. 5.
MCC in WT, heterozygous (Het), treated, and untreated XLHED dogs. Data indicated with gray circles originate from untreated animals analyzed in an independent experiment (Casal et al., 2007) but were included in the statistical analysis. Values are the mean ± S.E.M. One-way ANOVA assuming Gaussian distribution and comparing conditions to a control (XLHED) with Dunnett’s multiple comparison test: nsP > 0.05; ***P < 0.001.
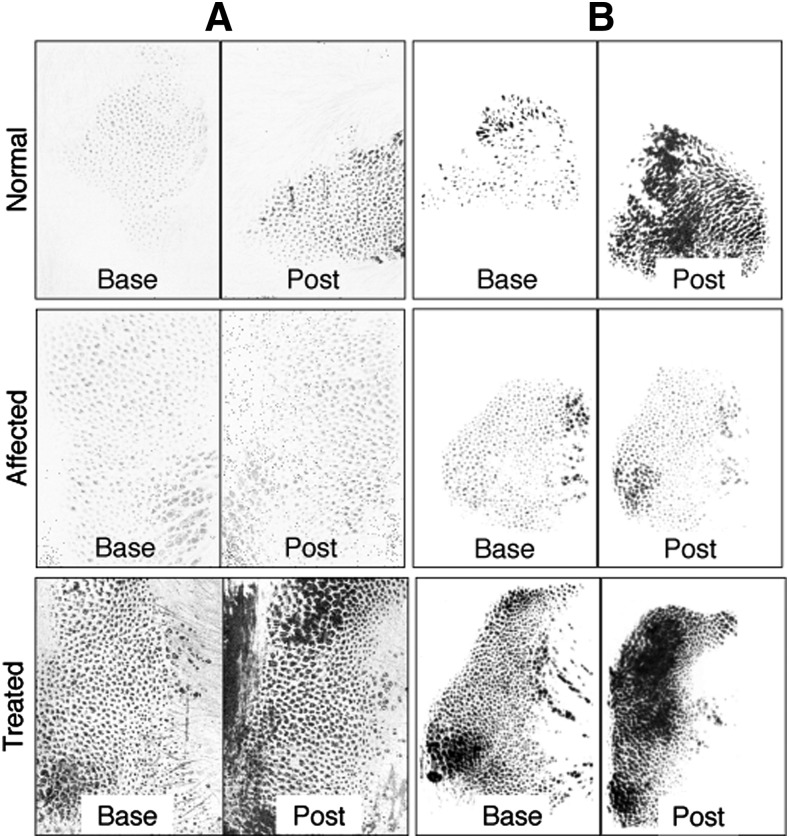
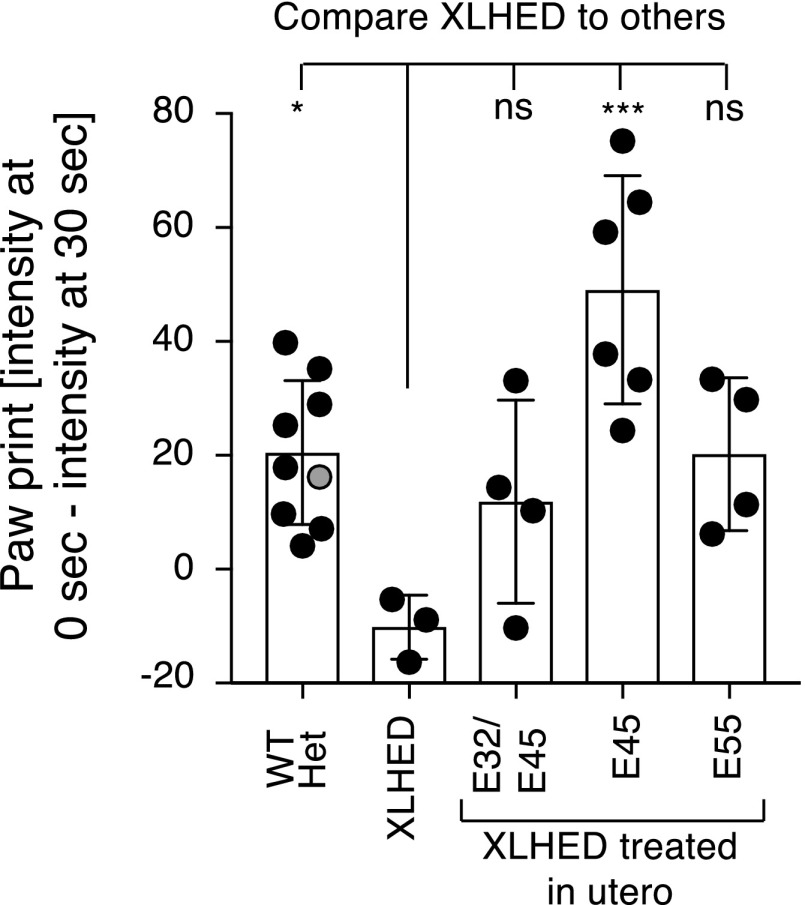
The Moisture Map and the Paw Print Sensor were both able to visualize moisture transfer through the footpads of WT dogs and the lack thereof in the untreated XLHED dogs (Fig. 6). Paw Print Sensor analyses (Fig. 7) showed significantly increased transfer of moisture through the paw pad after pilocarpine administration in the IUTx45 EDI200-treated XLHED litter when compared with untreated XLHED dogs, and nonsignificant trends in other treated pups. No effects after pilocarpine administration were observed with either the Moisture Map or the Paw Print Sensor in untreated XLHED dogs (Fig. 6).
Fig. 6.
Representative images preadministration and postadministration (Post) of pilocarpine with the Moisture Map (A) and the Paw Print Sensor (B) assays for normal dogs, XLHED dogs, and treated XLHED dogs from top to bottom, respectively. There is an appreciable difference between preadministration and postadministration of pilocarpine with the normal group and the treated group, which does not exist with the affected group. Primarily, there are more spots, spots may be larger, and spots may be darker. More spots are interpreted as an increased number of sweat glands or more productive sweat glands.
Fig. 7.
Difference in paw print intensity after administering pilocarpine, as an indirect measure to assess sweat function (mean ± S.D.). The gray circle indicates one heterozygote animal. One-way ANOVA assuming Gaussian distribution, and comparing conditions to a control (XLHED) with Dunnett’s multiple-comparison test. nsP > 0.05; *P < 0.05; ***P < 0.001.
Discussion
The main goals in treating patients with XLHED are to prevent hyperthermia and dry eye and to improve respiratory health and dentition, as these clinical issues have the largest impact on quality of life. In human XLHED, the inability to sweat results in increased morbidity and mortality due to the increased risk of hyperpyrexia (Blüschke et al., 2010). Unlike in mice (Gaide and Schneider, 2003), postnatal treatment with EDI200 did not normalize sweating in dogs (Casal et al., 2007). However, just as in mice, our studies showed that prenatal treatment with the recombinant protein resulted in water transfer through the paw pads, suggesting that they could sweat to a similar degree as WT dogs and that the Moisture Map and the Paw Print Sensor can differentiate between normal and affected dogs and thus assess the efficacy of EDI200 treatment noninvasively (Houser et al., 2018).
Meibomian glands produce the lipid portion of the tear film that slows down evaporation and subsequently prevents dry eye (Arita et al., 2017). These glands are greatly reduced or absent in humans (Kaercher et al., 2015), mice (Gaide and Schneider, 2003), and dogs (shown here) with XLHED. Therefore, dry eye requiring daily lubrication is a significant problem found in affected patients, with development of corneal lesions and scarring if left untreated in humans (Dietz et al., 2013) and dogs (Casal et al., 2007). In the present study, the development of meibomian glands was significantly improved in all prenatal treatment groups compared with untreated affected dogs. This nearly eliminated ocular pathology in all groups and suggests that the increase in meibomian gland total count is functionally relevant for the reduction of clinical manifestations involving the eye.
The absence of respiratory glands contributes to frequent respiratory tract infections in humans and dogs with XLHED (Clarke et al., 1987; Casal et al., 2005a; Mauldin et al., 2009). None of the treated or untreated XLHED dogs had clinical signs of respiratory disease during the study, which is likely due to careful husbandry and standardized colony settings. The improvement of MCC in the postnatal studies compared with minimal to absent improvement with prenatal treatment may be due to the developmental timing and specific placode activity. The canine respiratory tract develops a pseudoglandular phase and goblet cell (mucoid) secretion between days 32 and 56 of gestation, with the final saccular phase beginning between 57 and 60 days of gestation (Bolt et al., 2001; Sipriani et al., 2009; Vannucchi et al., 2012), suggesting that ectodysplasin is required late during gestation or early in the postnatal phase in the dog.
Dogs (Lewis et al., 2010) and human patients with XLHED have a greatly reduced number of teeth, which are of abnormal conical shape (Clarke et al., 1987). Conventional prosthodontic rehabilitation in young human patients is often difficult because of anatomic abnormalities, which result in poor retention and stability of dentures (Montanari et al., 2012). Murine studies demonstrated complete rescue of molars 1 and 2 when EDI200 was administered prenatally compared with no rescue with postnatal administration (Gaide and Schneider, 2003). In contrast, there was no significant improvement in deciduous or adult dentition in any XLHED dogs treated in utero, as opposed to complete rescue in those treated postnatally with five doses of the recombinant protein given 3 days apart beginning on day 2 of life (Casal et al., 2007). The basic pattern of tooth development is homologous in man and dogs. Dental lamina begins to form in humans between weeks 5 and 6 of the 38 weeks of gestation (13% and 16% into the course of pregnancy), as opposed to dogs in which it begins later at 25 of the 63 days of gestation (39% of gestation).
Tooth buds arise from the dental lamina in humans from 17 weeks of gestation until about 5 years of life. In dogs, molar tooth buds appear at a gestational age of 42 days (Williams and Evans, 1978). The molars did not change in size or appearance in any of the prenatally treated dogs. The molar is the only tooth that is not preceded by a deciduous tooth. It is conceivable that recombinant protein would have to be given both earlier than 32 days of gestation during tooth bud formation and postnatally at the time of final tooth development to induce proper molar formation as seen in the postnatal XLHED dog experiments. Interestingly, most of the E45-treated dogs developed a full set of deciduous teeth, suggesting that a transition between dental lamina and the formation of tooth buds for teeth other than molars takes place around this time in gestation. This also suggests that ectodysplasin plays a role in dental development not just at a single time point but both at the stage of dental lamina formation and at tooth bud formation, suggesting that a single administration of EDI200 is insufficient for complete dental development.
Subjectively, there was more hair coverage in the E32/E45- and E45-treated groups than in the E55-treated XLHED dogs. In dogs, the first tactile hair follicles are recognized at 30–32 days of gestation, and they develop into whiskers by 38 days (Evans and Sack, 1973). Dogs in all treated and untreated groups developed whiskers, suggesting that they form independently of EDA effects. At 45 days, body hair begins to grow in normal canine fetuses, and coverage is complete by 53 days of gestation (Al-Bagdadi, 2013), which may explain why earlier prenatal treatment resulted in better hair coverage than in the IUTxE55 group and postnatally treated dogs (Casal et al., 2007). These results suggest that to rescue hair coverage, protein administration should occur at the time of hair follicle formation, which may depend on hair type and species.
Ultrasound-guided injection of recombinant EDI200 into amniotic fluid of affected fetuses was a safe and efficient therapy. In mice, complete phenotypic correction via intra-amniotic injection with EDI200 was reported, with no maternal losses and 93% post-treatment fetal survival (Hermes et al., 2014). There were also no maternal losses and an 80% litter survival rate (four of five) in our study. In sheep undergoing ultrasound-guided or fetoscopic techniques, there were 3%–15% maternal and fetal losses due to trauma of the procedure or iatrogenic bacterial infections (Abi-Nader et al., 2012). In our study, further losses after the first litter coincided with the implementation of the prophylactic administration of antibiotics to the remaining dams at the time of the fetal injections. However, prophylactic administration of antibiotics after amniocentesis in humans has not led to any significant reduction in postprocedural spontaneous abortion (Gramellini et al., 2007).
Overall, the findings of these studies suggest that prenatal administration of EDI200 resulted in an improved ability to sweat, slightly more hair, and improved the development of deciduous dentition (E45 group) when compared with postnatal treatment. The failure of EDI200 to completely rescue and normalize the phenotype may be attributed to 1) differing windows of EDA1 expression and responsiveness of placodes based on organ-specific developmental timelines, 2) an insufficient dose, or 3) insufficient duration of exposure in the amniotic cavity after administration. In our experiments, the relatively poor response to therapy in the E55 group could also be due to the decreased volume of amniotic fluid at this later stage of development, which created more difficulty with precise administration. Therefore, it is possible that some of the EDI200 deposited into the amniotic cavity leaked from there into the allantois, lowering the total ingested dose over time for the E55 group.
Due to differences in developmental time points between humans and dogs, the phenotype in humans may still be corrected by administering recombinant protein at later time points in utero. A single higher prenatal dose than that used in this study may result in improved phenotypic reversion, as it might lead to longer exposure to the protein based on pharmacokinetic clearance rate (Hermes et al., 2014). Finally, although the fetuses were identified by a microbubble of air after injection, it cannot be guaranteed that each fetus was treated as intended. This should not pose difficulty in human patients, as they tend to carry singletons or twins.
The variable statistical significance of many parameters examined in this study can be attributed to small sample size, which is typical when working with canine models where generation times are much longer and the expenses much higher than when using mice. Our findings suggest that EDI200 can be given safely as protein replacement therapy to human patients with XLHED and that intra-amniotic administration is likely to result in measurable efficacy. It was recently shown in two human case studies that Fc:EDA1 administration in the amniotic fluid at weeks 26 and 31 of gestation (twins) or at week 26 only (single fetus) can correct the development of sweat, salivary, and meibomian glands, with a potential partial improvement in permanent dentition (Schneider et al., 2018). Results of the previous study will provide useful interspecies comparison data that may help with the design of upcoming clinical trials on XLHED patients.
Acknowledgments
The authors thank Patricia ODonnell, the Medical Genetics residents, and the dedicated veterinary students for their expert care of the dogs.
Abbreviations
- ANOVA
analysis of variance
- E
embryonic day
- EDA1
ectodysplasin A1
- Fc:EDA1
recombinant ectodysplasin
- IUTx
in utero therapy
- LH
luteinizing hormone
- MCC
mucociliary clearance
- WT
wild-type
- XLHED
X-linked hypohidrotic ectodermal dysplasia
Authorship Contributions
Participated in research design: P. Schneider, Huttner, Kirby, H. Schneider, and Casal.
Conducted experiments: Margolis, Houser, Wildman, Grove, and Casal.
Contributed new reagents or analytical tools: P. Schneider, Kirby, Houser, Wildman, and Grove.
Performed data analysis: P. Schneider, Houser, and Casal.
Wrote or contributed to the writing of the manuscript: Margolis, P. Schneider, Huttner, Houser, Grove, H. Schneider, and Casal.
Footnotes
This work was supported and funded by the National Foundation for Ectodermal Dysplasias, Edimer Pharmaceuticals, and the National Institutes of Health, Office of the Director [OD 010939]. P.S. is supported by grants from the Swiss National Science Foundation [310030_156961; 310030A_176256]. P.S. and N.K. are shareholders of Edimer Pharmaceuticals. N.K. was an employee of Edimer at the time the experiments were performed. H.S. has received research funding from Edimer Pharmaceuticals and EspeRare foundation. P.S. and H.S. are holders of patents related to the topic.
References
- Abi-Nader KN, Boyd M, Flake AW, Mehta V, Peebles D, David AL. (2012) Animal models for prenatal gene therapy: the sheep model, in Methods in Molecular Biology (Coutelle C, Waddington SN. eds) pp 219–248, Humana Press, New York. [DOI] [PubMed] [Google Scholar]
- Al-Bagdadi F. (2013) The integument, in Miller’s Anatomy of the Dog (Evans HE, Miller ME, De Lahunta A. eds) pp 61–79, Elsevier, St. Louis, MO. [Google Scholar]
- Arita R, Fukuoka S, Morishige N. (2017) Functional morphology of the lipid layer of the tear film. Cornea 36 (Suppl 1):S60–S66. [DOI] [PubMed] [Google Scholar]
- Blüschke G, Nüsken KD, Schneider H. (2010) Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev 86:397–399. [DOI] [PubMed] [Google Scholar]
- Bodmer JL, Schneider P, Tschopp J. (2002) The molecular architecture of the TNF superfamily. Trends Biochem Sci 27:19–26. [DOI] [PubMed] [Google Scholar]
- Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. (2001) Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol 32:76–91. [DOI] [PubMed] [Google Scholar]
- Casal ML, Jezyk PF, Greek JM, Goldschmidt MH, Patterson DF. (1997) X-linked ectodermal dysplasia in the dog. J Hered 88:513–517. [DOI] [PubMed] [Google Scholar]
- Casal ML, Lewis JR, Mauldin EA, Tardivel A, Ingold K, Favre M, Paradies F, Demotz S, Gaide O, Schneider P. (2007) Significant correction of disease after postnatal administration of recombinant ectodysplasin A in canine X-linked ectodermal dysplasia. Am J Hum Genet 81:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal ML, Mauldin EA, Ryan S, Scheidt JL, Kennedy J, Moore PF, Felsburg PJ. (2005a) Frequent respiratory tract infections in the canine model of X-linked ectodermal dysplasia are not caused by an immune deficiency. Vet Immunol Immunopathol 107:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal ML, Scheidt JL, Rhodes JL, Henthorn PS, Werner P. (2005b) Mutation identification in a canine model of X-linked ectodermal dysplasia. Mamm Genome 16:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DH, Loomis CA. (2017) Structure and Development of Skin and Cutaneous Appendages in Fetal and Neonatal Physiology (Polin RA, Abman SH, Rowitch DH, Benitz WE, Fox WW. eds) Chapter 48, pp 490–498, Elselvier, Philadelphia. [Google Scholar]
- Clarke A, Phillips DI, Brown R, Harper PS. (1987) Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child 62:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Arya S, Choudhary S, Jain SK. (2016) Amniotic fluid: source of trophic factors for the developing intestine. World J Gastrointest Pathophysiol 7:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz J, Kaercher T, Schneider AT, Zimmermann T, Huttner K, Johnson R, Schneider H. (2013) Early respiratory and ocular involvement in X-linked hypohidrotic ectodermal dysplasia. Eur J Pediatr 172:1023–1031. [DOI] [PubMed] [Google Scholar]
- Evans HE, Sack WO. (1973) Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Zentralbl Veterinarmed [C] 2:11–45. [DOI] [PubMed] [Google Scholar]
- Gaide O, Schneider P. (2003) Permanent correction of an inherited ectodermal dysplasia with recombinant EDA. Nat Med 9:614–618. [DOI] [PubMed] [Google Scholar]
- Gramellini D, Fieni S, Casilla G, Raboni S, Nardelli GB. (2007) Mid-trimester amniocentesis and antibiotic prophylaxis. Prenat Diagn 27:956–959. [DOI] [PubMed] [Google Scholar]
- Hammersen JE, Neukam V, Nüsken KD, Schneider H. (2011) Systematic evaluation of exertional hyperthermia in children and adolescents with hypohidrotic ectodermal dysplasia: an observational study. Pediatr Res 70:297–301. [DOI] [PubMed] [Google Scholar]
- Hermes K, Schneider P, Krieg P, Dang A, Huttner K, Schneider H. (2014) Prenatal therapy in developmental disorders: drug targeting via intra-amniotic injection to treat X-linked hypohidrotic ectodermal dysplasia. J Invest Dermatol 134:2985–2987. [DOI] [PubMed] [Google Scholar]
- Houser T, Wildman L, Grove G, Casal ML, and Margolis CA (2018) Diminished sweat gland activity is associated with X-linked hypohidrotic ectodermal dysplasia in dogs. 2018 World Congress of The International Society For Biophysics And Imaging of the Skin (ISBS); 2018 May 1–4; San Diego, CA. Abstract 7, The International Society For Biophysics And Imaging of the Skin, Zurich, Switzerland.
- Johnston SD, Root Kustritz MV, Olson PS. (2001) Breeding management and AI of the dam, in Canine and Feline Theriogenology (Johnston SD, Root Kustritz MV, Olson PS. eds) pp 41–65, WB Saunders, Philadelphia. [Google Scholar]
- Kaercher T, Dietz J, Jacobi C, Berz R, Schneider H. (2015) Diagnosis of X-linked hypohidrotic ectodermal dysplasia by meibography and infrared thermography of the eye. Curr Eye Res 40:884–890. [DOI] [PubMed] [Google Scholar]
- Lewis JR, Reiter AM, Mauldin EA, Casal ML. (2010) Dental abnormalities associated with X-linked hypohidrotic ectodermal dysplasia in dogs. Orthod Craniofac Res 13:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen L, Zhang M, Zhang B. (2017) Foxa1 gene and protein in developing rat eccrine sweat glands. J Mol Histol 48:1–7. [DOI] [PubMed] [Google Scholar]
- Lopate C. (2008) Estimation of gestational age and assessment of canine fetal maturation using radiology and ultrasonography: a review. Theriogenology 70:397–402. [DOI] [PubMed] [Google Scholar]
- Mauldin EA, Gaide O, Schneider P, Casal ML. (2009) Neonatal treatment with recombinant ectodysplasin prevents respiratory disease in dogs with X-linked ectodermal dysplasia. Am J Med Genet A 149A:2045–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML. (2009) Molecular aspects of hypohidrotic ectodermal dysplasia. Am J Med Genet A 149A:2031–2036. [DOI] [PubMed] [Google Scholar]
- Montanari M, Callea M, Battelli F, Piana G. (2012) Oral rehabilitation of children with ectodermal dysplasia. BMJ Case Rep 2012:bcr0120125652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalacci S, Chaumot A, Benoît G, Sadier A, Delsuc F, Douzery EJ, Laudet V. (2008) Conserved features and evolutionary shifts of the EDA signaling pathway involved in vertebrate skin appendage development. Mol Biol Evol 25:912–928. [DOI] [PubMed] [Google Scholar]
- Pitkin RM, Reynolds WA. (1975) Fetal ingestion and metabolism of amniotic fluid protein. Am J Obstet Gynecol 123:356–363. [DOI] [PubMed] [Google Scholar]
- Schneider H, Faschingbauer F, Schuepbach-Mallepell S, Körber I, Wohlfart S, Dick A, Wahlbuhl M, Kowalczyk-Quintas C, Vigolo M, Kirby N, et al. (2018) Prenatal correction of X-linked hypohidrotic ectodermal dysplasia. N Engl J Med 378:1604–1610. [DOI] [PubMed] [Google Scholar]
- Schneider P, Street SL, Gaide O, Hertig S, Tardivel A, Tschopp J, Runkel L, Alevizopoulos K, Ferguson BM, Zonana J. (2001) Mutations leading to X-linked hypohidrotic ectodermal dysplasia affect three major functional domains in the tumor necrosis factor family member ectodysplasin-A. J Biol Chem 276:18819–18827. [DOI] [PubMed] [Google Scholar]
- Shah U, Dickinson BL, Blumberg RS, Simister NE, Lencer WI, Walker WA. (2003) Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res 53:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipriani TM, Grandi F, da Silva LC, Maiorka PC, Vannucchi CI. (2009) Pulmonary maturation in canine foetuses from early pregnancy to parturition. Reprod Domest Anim 44 (Suppl 2):137–140. [DOI] [PubMed] [Google Scholar]
- Swee LK, Ingold-Salamin K, Tardivel A, Willen L, Gaide O, Favre M, Demotz S, Mikkola M, Schneider P. (2009) Biological activity of ectodysplasin A is conditioned by its collagen and heparan sulfate proteoglycan-binding domains. J Biol Chem 284:27567–27576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MA, Sherman MP. (2006) Nutritional characteristics of amniotic fluid. Neoreviews 7:e310–e316. [Google Scholar]
- Vannucchi CI, Silva LC, Lúcio CF, Regazzi FM, Veiga GA, Angrimani DS. (2012) Prenatal and neonatal adaptations with a focus on the respiratory system. Reprod Domest Anim 47 (Suppl 6):177–181. [DOI] [PubMed] [Google Scholar]
- Williams RC, Evans HE. (1978) Prenatal dental development in the dog, Canis familiaris: chronology of tooth germ formation and calcification of deciduous teeth. Anat Histol Embryol 7:152–163. [DOI] [PubMed] [Google Scholar]