Abstract
Objective
Behçet’s disease (BD) is a rare vasculitis that results in multi-organ inflammatory disease. At-risk populations are most prevalent in the Middle East and East Asia. Clinical data on BD in Western countries, especially in the United States, are scarce. We have compared clinical patterning of BD vasculitis in two geographically defined patient cohorts in the Western United States and Iran.
Methods
Comparative analysis of a retrospective cohort of 56 patients with BD evaluated at Stanford University Hospital between 2000 and 2016 and a cohort of 163 patients from the BD Registry at Tehran University of Medical Sciences. Clinical, demographic, laboratory, and treatment data were available. Comparisons were performed using descriptive statistics, Student’s t-test, and χ2-test.
Results
The Stanford patients with BD were significantly younger at disease onset, had a higher proportion of females, and had longer disease duration than Iranian patients with BD. Genital ulcers, skin, joint, neurological, vascular, cardiopulmonary manifestations were all significantly more common in the Stanford cohort and 38% of Stanford patients had four or more organ systems involved compared with approximately 10% of Iranian patients. In contrast, Stanford patients had fewer ocular lesions (Stanford 21.4% vs. Iran 53.4% p<0.05), with the biggest difference seen for retinal vasculitis.
Conclusion
Patients with BD from the Western US have a more severe disease course when compared to Iranian patients with BD, as demonstrated by earlier onset and a higher rate of multi-organ involvement. The high risk of Iranian patients with BD developing vasculitis of ocular structures suggests distinct pathomechanisms driving ocular versus extra-ocular BD.
Keywords: Vasculitis, inflammation, Behçet’s disease, uveitis, retinal vasculitis, eye disease
Introduction
Behçet’s disease (BD) is a complex systemic disease, which is classified as a vasculitis, and like other vasculitic syndromes is associated with increased morbidity and mortality (1–3). BD typically causes oral and genital ulcerations along with eye manifestations; however, it may affect a range of different organ systems requiring different pharmacological interventions and a multidisciplinary approach (3–5). Early diagnosis is considered important to prevent irreversible organ damage (6, 7). Hurdles to diagnosing BD include its heterogenous presentation, the lack of a pathognomonic diagnostic test, and its low prevalence in Western Europe and North America (8, 9). Although the diagnosis of BD is made on clinical grounds, several classification criteria exist, which may aid in the diagnosis, with the two most commonly used being the International Study Group (ISG) criteria, and the International Criteria for Bechet’s Disease (ICBD) criteria (10, 11). BD has been reported worldwide, but there are considerable geographical differences in both prevalence rates and disease presentation. BD is most common in countries along the Silk Road, with high rates found in Turkey (80–420/100,000), Iran (80–100/100,000), and Japan (2, 3, 12, 13). In the United States (US), BD is considered a rare disease, with one study from Olmsted County, Minnesota, reporting an overall incidence rate of 0.38 and a point prevalence rate of 5.2 per 100,000 (14). Studies comparing cohorts of patients between countries give insights into the presentation and heterogeneity of the disease in different populations. Despite the lower incidence of BD in the US, some studies reported a higher than expected prevalence of central nervous system (CNS) and gastrointestinal (GI) manifestations compared with other endemic regions (15, 16). There are few or no studies from the US reporting prevalence of pulmonary and cardiac manifestations and frequency of constitutional symptoms (15).
In the current study, we compare the clinical features of patients with BD from a tertiary referral center located on the West Coast of the US with those from a tertiary referral center in Iran. We also review the literature for further comparison of disease heterogeneity and course in different geographic regions of the US.
Methods
This was a retrospective comparative cohort study where patients with a diagnosis of BD and evaluated at Stanford University Hospital were compared with a sample of Iranian patients with BD recruited from the BD registry established at Tehran University of Medical Sciences.
Medical records were reviewed for all Stanford patients (age ≥18 y) that fulfilled the International Classification of Diseases, Ninth Revision (ICD-9) diagnostic code of BD (ICD-9: 136.1). The study period spanned from January 1, 2000 to December 31, 2016. The diagnosis of BD was made based on clinical manifestations by experienced treating specialists (rheumatologists, dermatologists, ophthalmologists, and internists), and the diagnosis of BD was confirmed by the expertise of the reviewing rheumatologists for final inclusion into the cohort of patients with BD. We also reviewed the medical records of a representative sample of Iranian patients with BD, recruited from the BD registry at the Shariati Hospital, Rheumatology Research Center (RRC), Tehran University of Medical Sciences. Iranian patients were selected by the stratified randomization method (with the follow-up time as the main covariate) among patients that had their first visit during the same time period (between years 2000 and 2016). Both hospitals function as tertiary referral medical centers.
All demographic, clinical, laboratory, and treatment data were collected. Demographic information included: gender, age at onset of the first BD symptoms, race/ethnicity, date of disease onset, diagnosis, first and last visits, and family history of BD. Clinical characteristics included the presenting manifestation, the presence of oral aphthosis, genital ulcers (GU), skin lesions (pseudofolliculitis/acneiform lesions, erythema nodosum, or other lesions), ocular involvement (uveitis, retinal vasculitis), constitutional signs and symptoms (fatigue, fever, weight loss, malaise), joint manifestations (arthralgia, arthritis, inflammatory back pain), vascular involvement (superficial and deep venous thrombosis, arterial aneurysm and thrombosis), headache, neurological manifestations, including CNS involvement (parenchymal, vascular, meningoencephalitis) and peripheral nervous system, GI involvement, epididymitis, pulmonary, and cardiac involvement. Laboratory tests included pathergy test, erythrocyte sedimentation rate (ESR), C-reactive protein, urinalysis, and the presence of HLA-B5/51 and HLA-B27. All the medications prescribed during the disease course were recorded.
Descriptive statistics, including mean (±standard deviation), numbers or percentages, were calculated to describe the data. The two cohorts were compared using either independent t-tests for continuous (normally distributed) variables, Chi square test, or Fischer’s exact test when appropriate for dichotomous variables. The level for statistical significance was set at p<0.05. Data were analyzed using Statistical Package for Social Sciences software version 18 (SPSS Inc.; Chicago, IL, USA).
Ethical consideration
The study protocol was approved by the Stanford Institutional Review Board and the Research Committee of RRC and the Ethics Committee of TUMS. It was conducted in accordance with the principles of the Helsinki Declaration of 1975/83 and Good Clinical Practice guidelines.
Results
Demographics and clinical characteristics
The demographics of 163 Iranian patients with BD were compared with 56 American patients with BD from Stanford (Table 1). Patients in the Stanford cohort were of mixed ethnicity, while in the Iranian cohort all patients were of Iranian decent. There was a considerable gender difference between the two cohorts, with 69.6% females in the Stanford cohort compared with 38.7% females in the Iranian cohort (p<0.001). Stanford patients had significantly earlier disease onset (21.9 vs. 26.5 y, p<0.05) and longer disease duration (16.8 vs. 12.0 y, p<0.05) than the Iranian cohort, and had similar follow-up time. Similar proportions of patients fulfilled the ISG (57%) and ICBD criteria (approximately 94%–98%) in the two cohorts.
Table 1.
Demographics and criteria fulfillment in the Stanford and Iranian Behçet’s disease cohorts
| Stanford | Iran | p | |
|---|---|---|---|
| Total (%) | 56 (100) | 163 100 | |
| Gender (Female) (%) | 39 (69.6) | 63 (38.7) | 0.00006 |
| ISG criteria (%) | 32 (57.1) | 93 (57.1) | 0.99 |
| ICBD revised (%) | 53 (94.6) | 159 (97.5) | 0.29 |
| Age at first symptom (SD) | 21.9 (11.6) | 26.5 (10.1) | 0.006 |
| Age at diagnosis (SD) | 35.3 (13.3) | 34.3 (9.7) | 0.60 |
| Follow-up years (SD) | 3.4 (3.8) | 4.1 (4.2) | 0.22 |
| Disease duration; years (SD) | 16.8 (11.7) | 12.0 (7.5) | 0.0006 |
| Family history of BD (%) | 3 (5.4) | 12 (7.6) | 0.76 |
| Positive HLA-B5/51 [tested] (%) | 12/[17] (71) | 87/[159] (53) | 0.21 |
| Positive pathergy [tested] (%) | 12/[23] (52) | 63/[161] (39) | 0.23 |
| Ethnicity (%) | |||
| White | 35 (62.5) | 163 (100)# | |
| Asian | 5 (9.8) | ||
| Hispanic/Latino | 5 (9.8) | ||
| African-American | 5 (9.8) | ||
| Mixed/Other | 4 (7.1) | ||
| Unknown | 2 (3.6) | ||
Means with standard deviation (SD) are reported for continuous data, with n (%) for dichotomous data. Group comparisons were performed using Chi square test/Fischer’s exact test or Students t-test/Welch’s unequal variances t-test according to distribution of data.
All patients were from Iran.
ISG: International Study Group clinical diagnostic criteria; ICBD: International Criteria for Behçet’s Disease; p: p-value; HLA: human leukocyte antigen.
Presenting symptoms for both cohorts are shown in Table 2. The most common presenting symptom was oral ulcer(s), which was present in >80% of both cohorts. The proportion of patients presenting with skin manifestations (erythema nodosum, pustular folliculitis) and ocular manifestations (anterior/posterior uveitis, retinal vasculitis) were comparable between the two cohorts. GU were a significantly more common presenting symptom in the Stanford cohort (19.6% vs. 8.6%, p=0.025) while a borderline difference was seen for joint manifestations (p=0.05). One Stanford patient presented with CNS manifestations (brainstem lesions and meningoencephalitis). Presenting manifestations classified as “other” were more frequent in Stanford patients (12.5% vs. 1.2%, p<0.0001).
Table 2.
Presenting symptoms in the Stanford and Iranian Behçet’s disease cohorts
| Stanford n (%) | Iran n (%) | p | |
|---|---|---|---|
| Total | 56 (100) | 163 (100) | |
| Oral ulcer | 47 (83.9) | 146 (89.6) | 0.26 |
| Genital ulcer | 11 (19.6) | 14 (8.6) | 0.025 |
| Skin manifestations | 4 (7.1) | 9 (5.5) | 0.74 |
| Ocular manifestations | 4 (7.1) | 10 (6.1) | 0.76 |
| Joint manifestations | 5 (8.9) | 4 (2.5) | 0.05 |
| CNS manifestations | 1 (1.8) | 0 (0) | 0.26 |
| Others | 7 (12.5) | 1 (1.2) | <0.0001 |
| Two or more presenting symptoms | 19 (33.9) | 18 (11.0) | <0.0001 |
Groups were compared using Chi square test or Fischer’s exact test when appropriate. Skin manifestations: erythema nodosum, and/ or pseudofolliculitis; Ocular manifestations: uveitis and/or vascular retinitis; Joint manifestations: arthralgia and/or arthritis; CNS manifestations: central nervous system; n: sample size; Others: gastrointestinal, constitutional symptoms, or headache.
There were several striking differences seen in the accumulative disease manifestations in the two cohorts (Table 3). For mucosal involvement, similar frequencies were seen for oral ulcers, but GU was more common in Stanford patients. Overall, skin manifestations occurred more frequently in Stanford patients, though no statistical difference was seen in the subgroup analyses regarding type of lesion (pseudofolliculitis, erythema nodosum, and other skin lesions).
Table 3.
Accumulative disease manifestations in the Stanford and Iranian Behçet’s disease cohorts
| Stanford n (%) | Iran n (%) | p | |
|---|---|---|---|
| Total | 56 (100) | 163 (100) | |
| Mucosal involvement | |||
| Oral ulcer | 56 (100) | 162 (99.4) | 1 |
| Genital ulcer | 48 (85.7) | 102 (62.6) | 0.001 |
| Skin | 33 (58.9) | 65 (39.9) | 0.013 |
| Pseudofolliculitis | 23 (41.1) | 49 (30.1) | 0.13 |
| Erythema nodosum | 10 (17.9) | 26 (16.0) | 0.74 |
| Other lesions | 5 (8.9) | 15 (9.2) | 0.95 |
| Ocular | 12 (21.4) | 87 (53.4) | 0.00003 |
| Anterior uveitis | 7 (12.5) | 64 (39.3) | 0.0002 |
| Posterior uveitis | 6 (10.7) | 73 (44.8) | <0.00001 |
| Retinal vasculitis | 1 (1.8) | 53 (32.5) | <0.00001 |
| Joint | 33 (58.9) | 62 (38.0) | 0.006 |
| Arthralgia only | 18 (32.1) | 27 (16.6) | 0.013 |
| Arthritis | 14 (25.0) | 39 (23.9) | 0.87 |
| Inflammatory back pain | 4 (7.1) | 5 (3.1) | 0.24 |
| Vascular | 15 (26.8) | 8 (4.9) | <0.0001 |
| Superficial phlebitis | 4 (7.1) | 3 (1.8) | 0.73 |
| Lower limb thrombosis | 5 (8.9) | 6 (3.7) | 0.15 |
| Other large vein thrombosis | 8 (14.3) | 0 (0) | 0.00001 |
| Arterial thrombosis | 0 | 0 | - |
| Arterial aneurysm | 6 (10.7) | 0 (0) | <0.0002 |
| Headache | 18 (32.1) | 7 (4.3) | <0.00001 |
| Neurological* | 10 (17.9) | 6 (3.7) | 0.001 |
| Parenchymal | 4 (7.1) | 3 (1.8) | 0.07 |
| Meningoencephalitis | 2 (3.6) | 2 (1.2) | 0.28 |
| CNS vascular lesions | 5 (8.9) | 0 | 0.001 |
| Gastrointestinal** | 15 (26.8) | 3 (1.8) | <0.00001 |
| Terminal ileitis | 4 (7.1) | 1 (0.6) | 0.016 |
| Colitis | 4 (7.1) | 0 (0) | 0.004 |
| Rectal bleeding | 4 (7.1) | 2 (1.2) | 0.038 |
| Other GI# | 5 (8.9) | 0 (0) | 0.001 |
| Epididymo-orchitis | 1 (5.9) | 5 (5) | 1 |
| Renal | 1 (1.8) | 0 (0) | 0.25 |
| Pulmonary | 6 (10.7) | 0 (0) | <0.0002 |
| Cardiac | 4 (7.1) | 1 (0.6) | 0.016 |
Groups were compared using Chi square test or Fischer’s exact test when appropriate.
1 Iranian patient had PNS lesion.
We did not consider diarrhea, nonspecific dyspepsia, and/or GERD/peptic ulcer disease as gastrointestinal manifestations. Data on aphthous ulcers were available for the Stanford cohort only (n=3, 5.4%), and therefore not included.
Other GI manifestations: pancreatitis, eosinophilic esophagitis, gastroparesis/malabsorption, idiopathic gastroparesis, and intestinal obstruction. Pulmonary involvement; embolism (n=3), fibrosis (n=2), and pulmonary artery aneurysm (n=1).
Epididymo-orchitis: only present in male patients; Renal: 1 case of interstitial nephritis; Pulmonary involvement: embolism (n=3), fibrosis (n=2), and pulmonary artery aneurysm; Cardiac: pericarditis (n=0), ischemic heart disease (n=4), valvular disease (n=1), cardiomyopathy (n=1), and ventricular aneurysm (n=1).
Stanford patients were more affected by joint manifestations, with arthralgia (without arthritis) being nearly twice as common (32.1% vs. 16.6%, p=0.013) as in the Iranian patients. No significant differences were seen for arthritis or inflammatory back pain. Vascular involvement was five times more frequent in the Stanford cohort (26.8% vs. 4.9%, p=0.0002), with the biggest differences between groups with “other large vein thrombosis” [(i.e., not deep vein thrombosis (DVT)] and arterial aneurysms (p=0.00001, p<0.0002). No cases of arterial thrombosis were encountered in either cohort. Neurological manifestations were more common in the Stanford cohort. Vascular intracranial lesions accounted for the biggest difference, with no lesions in Iranian patients, but 5 (approximately 9%) Stanford patients affected (p=0.001). Cases of meningoencephalitis and parenchymal lesions were found in both cohorts, with numerically higher rates in Stanford patients. Iranian patients developed ocular manifestations more frequently, with significantly higher occurrence of anterior uveitis, posterior uveitis and retinal vasculitis (Table 3).
Gastrointestinal involvement was nearly seven times more frequent in Stanford patients with BD (Stanford 26.8% vs. Iran 1.8% p<0.00001). Subgroup analyses showed significant differences for all GI conditions, including terminal ileitis, colitis, rectal bleeding, and “other GI lesions.” There was significantly greater pulmonary involvement (fibrosis, emboli, and pulmonary artery aneurysm) in Stanford patients (10.7%) compared with the Iranian ones (0% p=0.001). Similarly, there was significantly greater cardiac involvement in Stanford patients (7.1%) compared with Iranian patients (<1% p=0.016). No difference was seen for epididymo-orchitis, renal involvement, or presence of hematuria/proteinuria (Table 3 and Supplementary Table S1).
Similar rates (approximately 42%–45%) of patients had increased ESR (Supplementary Table S1). There were no significant differences in the proportion of patients with a positive pathergy test or positive testing for HLA-B5/51 alleles (Table 1). However, the pathergy test and HLA-B5/51 genotyping had only been performed in 23 (41%) and 17 (30%) of the Stanford BD cohort. With regards to treatment, the two most frequently prescribed drugs in both cohorts were prednisolone and colchicine. Prednisolone was prescribed significantly more often in Stanford patients (Table 4). A range of immunosuppressant drugs were prescribed in both cohorts, with significant differences seen for mycophenolate, cyclophosphamide, and levamisole. Biological drugs, primarily tumor necrosis factor inhibitors, were utilized in 32% of Stanford patients, but less than 1% of Iranian patients. Total sixty percent of the Iranian cohort and 73% of the Stanford cohort had received at least one systemic disease-modifying antirheumatic drug or biological drug. Data on constitutional symptoms were only available for the Stanford cohort, with 53% (n=30) of patients affected. Constitutional symptoms encompassed fever (28.6%), fatigue (41.1%), or other symptoms (19.6%), including anorexia, weight loss, or night sweats.
Table 4.
Systemic medications used in the Stanford and Iranian Behçet’s disease cohorts
| Stanford n (%) | Iran n (%) | p | |
|---|---|---|---|
| Total | 56 (100) | 163 (100) | |
| Colchicine | 42 (75) | 108 (66.3) | 0.22 |
| Prednisolone | 46 (82.1) | 110 (67.5) | 0.04 |
| Azathioprine | 23 (41.1) | 48 (29.4) | 0.11 |
| Methotrexate | 14 (25) | 51 (31.3) | 0.37 |
| Cyclosporine | 4 (7.1) | 13 (8.0) | 0.80 |
| Cyclophosphamide | 6 (10.7) | 39 (23.9) | 0.04 |
| Mycophenolate | 6 (10.7) | 0 (0) | <0.0001 |
| Sulphasalazine | 3 (1.8) | 3 (5.4) | 0.16 |
| Levamizole | 0 (0) | 20 (12.3) | 0.006 |
| Biologic drugs | 18 (32) | 1 (0.6) | <0.0001 |
Groups were compared using Chi square test or Fischer’s exact test when appropriate.
n: sample size.
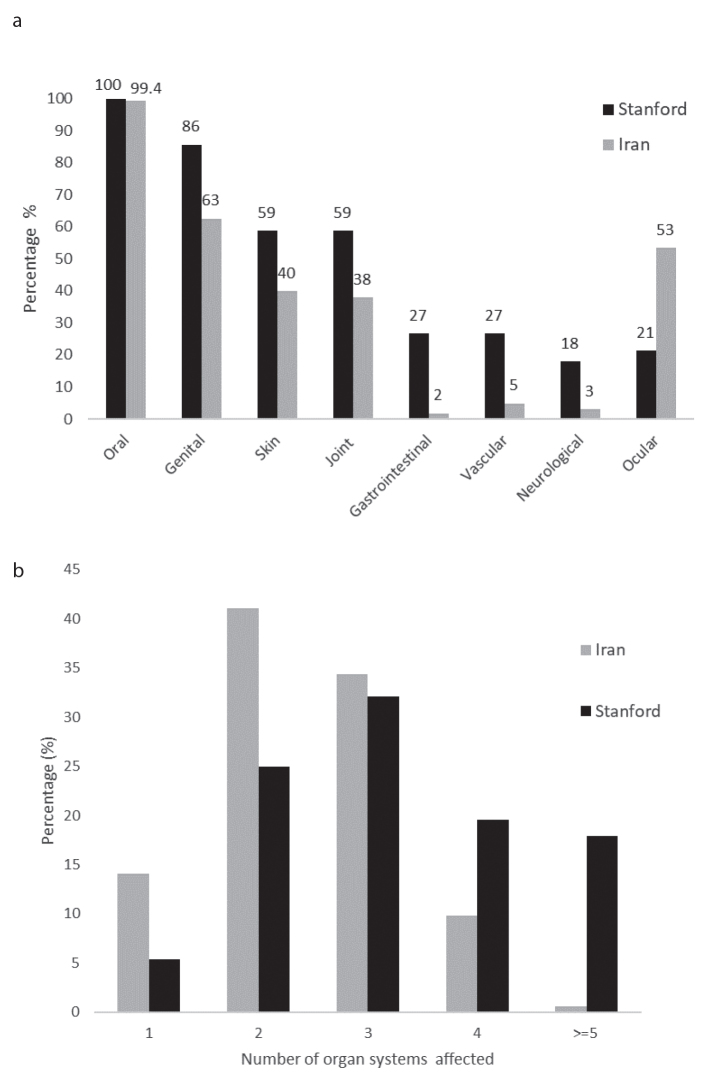
There were marked differences between organ manifestations between the Iranian and Stanford BD cohorts, with higher frequencies for skin, joint, GI, CNS, and vascular manifestations in the Stanford BD cohort (Figure 1a). Only ocular manifestations were more frequent in the Iranian cohort. Overall, Stanford patients had a higher burden of disease reflected by a higher number of organ systems affected by the vasculitis. A total of 38% of Stanford patients had disease manifestations in four or more organ systems, while approximately only 10% of the Iranian patients had a multi-organ involvement pattern (Figure 1b).
Figure 1. a, b.
Comparison of BD organ involvement patterns in American and Iranian patients. Oral and genital ulcers. Skin: pseudofolliculitis, erythema nodosum, and other skin manifestations. Joint: arthritis, arthralgia, and/or axial affection. Gastrointestinal: colitis, ileitis, rectal bleeding, diarrhea, and other. Vascular: venous and arterial thrombosis and aneurysms. Neurological manifestations: parenchymal lesions, meningoencephalitis, vascular lesions, and PNS lesions. Ocular lesions: uveitis, vascular retinitis, and episcleritis (n=1) (a); Number of organ systems involved in Iranian and American patients with Behçet’s disease. Organ systems includes: mucocutaneous (oral and genital ulcers), ocular, skin, joint, gastrointestinal, vascular, neurological, cardiac, pulmonary, and/or renal (b).
As the two BD cohorts had different gender ratios, explorative analyses were performed to investigate whether disease manifestations were associated with gender. In the Iranian BD cohort, ocular and skin manifestations occurred more frequently in male patients (p=0.03, p=0.003), while in the American BD cohort, GU were more common in women (p=0.007). However, the rate of GU in American men was comparable to Iranians of both genders, thus the significant difference between Iranian and American patients was primarily due to the high rates of GU in Stanford women. Interestingly, with regards to vascular events, male patients were twice as often affected as women in both the Iranian (6% vs. 3%) and American cohorts (41% vs. 21%), though these differences were not statistically significant.
We compared the Stanford cohort with four other American BD cohorts from New York University Hospital (NYU I n=634), Michigan (n=114), and two cohorts from NIH and NYU II (n=35 and n=77, respectively) (15–17). The latter two (NIH/NYUII) were reported in the same study (Table 5) (16). As selection criteria varied between cohorts, statistical tests were not performed and differences were interpreted with caution. The proportion of patients fulfilling the ISG criteria was similar between the cohorts assembled at Stanford, NYU, and Michigan; however, all NIH/NYU patients fulfilled the ISG criteria. Consistently, all American cohorts had a predominance of female patients (64%–80%). Ethnic and racial backgrounds differed between cohorts, with Stanford having the most diverse population and the lowest percentage of white patients. We found a lower age of onset in the Stanford cohort. The rates of superficial phlebitis, lower limb thrombosis/DVT, and pulmonary aneurysms were comparable among Stanford, NYU I, and Michigan. CNS and GI involvement were similar among Stanford, NYU I, and NIH/NYU II (CNS, 16%–20%; GI, 27%–42%), with Michigan having lower rates for both GI and CNS (5.3%, respectively). In general, the Stanford cohort was more similar to the NYU I cohort except for considerably higher rates of HLA-B51 and positive pathergy test in the Stanford cohort.
Table 5.
Comparison of American Behçet’s disease cohorts
| Stanford n=56 |
NYU I n=634 |
Michigan n=114 |
NIH n=35 |
NYU II n=77 |
|
|---|---|---|---|---|---|
| Diagnosis | Clinical | Clinical | ICBD | ISG | ISG |
| ICBD criteria (%) | 52 (92.9) | 114 (100) | |||
| ISG (%) | 32 (57.1) | 390 (61.5) | 76 (67) | 35 (100) | 77 (100) |
| Female (%) | 39 (69.6) | 488 (76.8) | 91 (79.8) | 28 (80.0) | 71 (92.2) |
| Age at onset† | 22.3 (11.7) | 35.2 (13.7) | |||
| Age | 35.3 (13.3) | 41.4 (14.5) | 36.1 (13.4) | 36.8 (13.5) | |
| Disease duration | 16.8 (11.7) | 10.7 (11.5) | 8.5 (5.7) | ||
| Ethnic background | |||||
| White/caucasian | 35 (62.5) | 475 (74.9) | 99 (86.8) | ||
| Asian | 6 (10.7) | 34 (5.3) | 3 (2.6) | ||
| Hispanic | 6 (10.7) | 42 (6,7) | |||
| African-American | 5 (8.9) | 23 (3.7) | 6 (5.3) | ||
| Unknown/other | 6 (5.3) | ||||
| Oral ulcer | 56 (100) | 574 (90.5) | 114 (100) | 35 (100) | 71 (92.2) |
| Genital ulcer | 48 (85.7) | 463 (73) | 109(95.6) | 31 (88.6) | 59 (78.7) |
| Skin lesions | 33 (58.9) | 423 (66.7) | 61(53.5) | 32 (91.4) | 63 (84.0) |
| Ocular lesions | 12 (21.4) | 169 (26.7) | 40 (35.1) | 14 (40.0) | 30 (42.2) |
| Uveitis | 11 (19.6) | 38 (33.3) | |||
| Retinal vascultitis | 1 (1.8) | 14 (12.3) | |||
| Arthralgia | 31 (55.4) | 81(71.7) | 30 (85.7) | 59 (77.6) | |
| Arthritis | 14 (25) | 326 (51.4) | 58 (50.9) | ||
| Superficial phlebitis | 4 (7.1) | 23 (3.6) | 11 (9.6) | ||
| DVT | 5 (8.9) | 32 (5) | 12 (10.5) | ||
| Arterial/venous thrombosis | 11 (19.6) | 8 (22.9)* | 8 (10.7)* | ||
| CNS | 10 (17.9) | 100 (15.8) | 6 (5.3)# | 7 (20.0) | 13 (17.3) |
| GI involvement | 15 (26.8) | 217 (34.2) | 6 (5.3)# | 15 (42.9) | 27 (36.9) |
| Pulmonary aneurysm | 1 (1.8) | 9 (1.4) | |||
| Pulmonary involvement$ | 5 (8.9) | 3 (0.5) | |||
| Epididymo-orchitis (men) | 1/17 (5.8) | 9/146 (6.1) | |||
| Pathergy positive [tested] (%) | 12 [23] (52.2) | 59 (9.3) | 16 (14) | ||
| HLA-B5/51 positive [tested] (%) | 12 [17] (70.6) | 68 [211] (32.2) | |||
| Colchicine | 42 (75) | 295 (46.5) | 59 (52) | 8 (22.9) | 16 (20.8) |
| Prednisone | 46 (82.1) | 408 (64.4) | 39 (34) | 17 (48.6) | 37 (48.1) |
| Azathioprine | 23 (41.1) | 126 (19.9) | 17 (15) | ||
| Methotrexate | 14 (25) | 101 (15.9) | 7 (6) | ||
| Mycophenolate | 6 (10.7) | 27 (4.3) | 7 | ||
| Cyclosporine | 4 (7.1) | 40 (6.3) | 0 | ||
| Cyclophosphamide | 6 (10.7) | 17 (2.7) | 0 | ||
| Hydroxychloroquine | 6 (10.7) | 51 (8.0) | NA | ||
| Dapsone | 7 (12.5) | 29 (4.6) | NA | ||
| DMARD | 39 (69.6) | 20 (57.1) | 43 (55.8) | ||
| TNF Inhibitor | 16 (28.6) | 132 (20.8) | 21 (18) | ||
Pulmonary involvement does not include pulmonary artery aneurysm.
Colonoscopy/MRI findings needed for diagnoses of GI or neuro Behçet’s in the Michigan cohort.
Numbers given as reported in studies. Discrepancies between studies regarding definition of organ manifestations may exist. For NIH and NYU II no information is given regarding race, except that 2/35 patients (NIH), and 28/77 patients (NYU) were of “typical Ethnic background for BD.”
ISG: International Study Group clinical diagnostic criteria; ICBD International Criteria for Behçet’s Disease; DVT: deep vein thrombosis; CNS: central nervous system; GI: gastrointestinal; HLA: human leukocyte antigen; DMARD: disease-modifying antirheumatic drug; TNF: tumor necrosis factor; n: sample size.
Discussion
Our findings indicate that American patients with BD have more severe disease than Iranian patients as demonstrated by higher prevalence of disease manifestations in the Stanford BD cohort. The differences were marked, with considerably higher rates seen for almost all organ systems, including the vascular tree, the CNS, the GI tract, and the cardiopulmonary system. Almost 40% of the Stanford patients had four or more organ systems affected, whereas multi-organ disease appeared to be the exception in Iranian patients with BD (10%). The rule of more aggressive disease in the American patients did not extend to the eye; ocular BD occurred in every second Iran patient, but only in one of five American patients, consistent with the concept that pathomechanisms in ocular and non-ocular BD can diverge.
Although there are no previous studies comparing American patients with BD with Iranian ones, there are several studies, which point toward higher prevalence of organ manifestations in American patients with BD, especially for GI and CNS manifestations (15, 16). Moreover, in a study by Sibley et al. (16) two American BD cohorts had significantly higher disease activity according to disease specific outcome measures (BDCAF and BSAS scores), when compared to a Turkish BD cohort (16).
The task of correctly diagnosing disease manifestations in BD is challenging as there is limited international consensus on what and how to classify disease manifestations (18). For BD, typical gastrointestinal lesions are oval shaped ulcers in the GI tract often found in the terminal ileum, but possibly affecting the whole GI tract (19–21). We considered terminal ileitis, colitis, rectal bleeding, and other GI manifestations (pancreatitis, eosinophilic esophagitis, gastroparesis/malabsorption, idiopathic gastroparesis, and intestinal obstruction) as evidence of GI involvement. All diagnoses of colitis and ileitis were verified by endoscopy. We excluded diarrhea, peptic ulcer disease/gastroesophageal reflux disease, and nonspecific dyspepsia as these symptoms are unspecific with other potential etiology. Thus, it is possible that we have under or overestimated GI manifestations attributable to BD. Still, the rates of GI manifestations in the Stanford cohort were comparable to rates seen in other American cohorts, as well as in Japan and Italy (12, 14, 15).
Vascular manifestations in BD are associated with increased mortality, and arterial aneurysms are one of the most feared complications (7). The rates of vascular manifestations worldwide vary greatly (7%–43%), and accordingly, we found considerably higher rates in the Stanford cohort than in the Iranian cohort (12, 22). The biggest differences between the two cohorts were seen for arterial aneurysms and thrombosis in large veins, exclusive of DVT. Moreover, vascular CNS manifestations were only encountered in American patients, while other CNS lesions were numerically, but not statistically, different. Interestingly, for retinal vasculitis, which involves part of the CNS, the relationship was reversed, with considerably higher rates seen in Iranian patients than American patients. Retinal vasculitis is a quintessential small-vessel vasculitis, and these results may indicate that BD affects medium to large vessels in the American population and small vessels in the Iranian population. Though BD can manifest in both arteries and veins, we found venous involvement to be more common, which is in accordance to previous reports (4, 22). The rates of superficial phlebitis, DVT, and pulmonary aneurysms in the Stanford cohort were comparable to those seen in cohorts from NYU and Michigan (15, 17).
Pulmonary manifestations, which are considered rare in BD, were present in nearly 10% of the Stanford cohort while undetected in the Iranian cohort (23). There is very limited data on pulmonary manifestations in other American cohorts, except for the NYU cohort, where rates were distinctly low (0.5%) (15). Of the five American patients with pulmonary manifestations, only one had a pulmonary artery aneurysm while the other four had fibrosis or pulmonary embolism. One could question whether fibrosis and PE represent true BD-associated manifestations. Considering the limited literature and the wide spectrum of this vasculitis, we included these patients in the analyses.
An intriguing difference between the American and Iranian BD cohorts is the difference in gender composition. BD is known to be more common in men in Turkey and the Middle East/Western Asia, while in Korea and Japan the rates are similar between genders, and in Spain/UK it is more common in women (12, 24). One might hypothesize that the disease etiology differs between populations, affecting men and women at different rates, while giving rise to a similar overall phenotype. Gender has also been shown to be associated with particular disease manifestations, and we find American women are more often affected with GU, which is in accordance to previous studies (25).
Eye manifestations, typically retinal vasculitis and/or nongranulomatous panuveitis, are associated with high morbidity and mortality, and typically occur in men (1, 25–27). Frequencies have been reported to be as high as 50%–70% of patients with BD, with 23% of patients developing poor visual acuity (28). In the current study, the rate of ocular manifestations was 53% in the Iranian cohort and 21% in the Stanford cohort, and a gender difference (male>female) was seen in the Iranian cohort only. Although the rates of ocular BD were slightly lower (21%) in the Stanford cohort in comparison to the other American cohorts (27%–42%), the rates of ocular manifestations in American BD cohorts are generally lower than those reported for other geographic regions; specifically Iran, Turkey, and Japan (15–17, 29). A study from Germany found that German patients of Turkish descent had higher rates of ocular disease than patients of German descent, which may indicate divergent disease pathways in genetically distinct populations (30). Interestingly, posterior uveitis, which involves part of the CNS (the retina and optic nerve), was more common in Iranian patients, despite all other CNS manifestations being markedly more common in Stanford patients.
The Stanford cohort was both similar and different to other American BD cohorts with regards to patient demographics and disease manifestations. As with other American cohorts, our cohort was predominantly female. As some studies indicate that male gender is associated with worse prognosis, our overall findings are unexpected. However, it is challenging to draw any clear conclusions regarding severity in American patients with BD, due to several biases, which could have confounded the analysis. For instance, one could question whether the cohort represents the full spectrum of BD or only those with a very severe disease. As BD is a rare disease in the US, one might suspect that only patients with severe disease are diagnosed. On the other hand, for the same reason, one would expect most patients to be referred to a tertiary center and thus our cohort should be representative of “all” patients with BD. The surprisingly high rates of HLA-B5/51 and positive pathergy tests in the US cohort may indicate overrepresentation of more severe cases, as HLA-B5/51 has been associated to severity (31). Still, as there was only a limited number of Stanford patients who were tested, there may have been selection bias in which patients were tested. Moreover, studies have shown that pathergy test readings may be affected by needle sharpness and sterilization routines (32). Interestingly, we did observe a younger age at onset in the Stanford cohort than other US studies. Previous studies have suggested that age at onset may be associated with a more severe course and higher mortality, which could support the high rates of disease manifestations seen in the present study (33, 34). Furthermore, one could hypothesize whether genetic factors play a greater role in patients with early onset, resulting in a more severe phenotype. We do not consider difference in disease duration a likely explanation for differences in prevalence of clinical features between Iran and Stanford, as the disease duration spanned an extended period for both cohorts.
In the current study, we report for the first time rates of constitutional symptoms in an American BD cohort. We found high rates of fatigue with more than 40% of patients affected. Fatigue is a common symptom in many rheumatological diseases, such as rheumatoid arthritis (RA), but there is limited literature on fatigue in BD (35–37). Interestingly, one study found that patients with BD had more fatigue and functional disability than patients with RA (35). Moreover, in a cross-sectional study by Ilhan et al. (37) disease activity in BD, as well as major organ involvement, were associated with higher fatigue scores. This association could support the findings in the Stanford cohort, where both major organ involvement and fatigue were common.
Taken together, in this retrospective cohort study, we found that American patients with BD are younger, more often female, and have a more severe disease course than those from Iran, where the disease is more common. An interesting discrepancy emerges in the disease patterns typical for American and Iranian patients: Iranian patients are at higher risk for ocular vasculitis, but often present with single-organ disease. Conversely, American patients often have manifestations in multiple organ systems, but are less likely to develop ocular vasculitis. We propose that 1) several pathogenic pathways contribute to BD; 2) ocular and extra-ocular BD follow separable, possibly independently regulated trajectories; and 3) occurrence may vary depending on geographic environment and the genetic background of populations.
Main Points.
Behçet’s disease (BD) has distinct clinical patterns in patient from the United States (US) versus Iranian patients.
US patients often have multi-organ involvement.
Vascular, central nervous system, cardiopulmonary and gastrointestinal complications of BD occur more frequently in American patients.
Ocular manifestations are typical for Iranian BD patients, but less frequent amongst US patients. American patients rarely develop retinal vasculitis.
Distinct pathomechanisms may lead to ocular versus extra-ocular BD.
Supplementary Material
Supplementary Table S1.
Laboratory tests in the Stanford and Iranian cohorts.
| Stanford n (%) | Iran n (%) | p | |
|---|---|---|---|
| Raised ESR | 22 (42.3) | 69 (44.5) | 0.78 |
| Raised CRP | 22 (39.3) | NA | - |
| Proteinuria | 1 (1.8) | 2 (1.3) | 1 |
| Hematuria | 4 (7.1) | 18 (11.8) | 0.34 |
| HLA-B27 positive/tested (%) | 1/12 (8.3) | 7/159 (4.4) | 0.45 |
Groups were compared using Chi square test or Fischer’s Exact Test when appropriate. Patients with available data in the Stanford (total n= 56)/Iranian cohort (total n=163): ESR n=52/155, CRP n= 56/0, Proteinuria n=56/153, Hematuria n=56/153, HLA-B27 n=12/159.
Acknowledgements
We would like to thank Dr. Jamshidi for his contributions.
Footnotes
Ethics Committee Approval: Ethics Committee Approval was received for this study from the Stanford Institutional Review Board and the Research Committee of RRC and the Ethics Committee of TUMS.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.M.W., F.S.; Design - F.S., C.M.W.; Supervision - C.M.W.; Resources - C.M.W.; Data Collection and/or Processing - F.S., M.M., M.A., F.D.; Analysis and/or Interpretation - C.M.W., M.M., F.S., Y.J.L.; Literature Search - C.M.W., M.M., F.S., Y.J.L.; Writing Manuscript - M.M., C.M.W., F.S.; Critical Reviews - C.M.W., F.S., M.M., Y.J.L.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This work was supported by the National Institutes of Health (R01 HL117913 and P01 HL129941 to CMW).
References
- 1.Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, et al. The long-term mortality and morbidity of Behcet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore) 2003;82:60–76. doi: 10.1097/00005792-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Sakane T, Takeno M, Suzuki N, Inaba G. Behcet’s disease. N Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 3.Yazici H, Fresko I, Yurdakul S. Behcet’s syndrome: disease manifestations, management, and advances in treatment. Nat Clin Pract Rheumatol. 2007;3:148–55. doi: 10.1038/ncprheum0436. [DOI] [PubMed] [Google Scholar]
- 4.Davatchi F. Behcet’s disease. Int J Rheum Dis. 2014;17:355–7. doi: 10.1111/1756-185X.12378. [DOI] [PubMed] [Google Scholar]
- 5.Ozguler Y, Leccese P, Christensen R, Esatoglu SN, Bang D, Bodaghi B, et al. Management of major organ involvement of Behcet’s syndrome: a systematic review for update of the EULAR recommendations. Rheumatology (Oxford) 2018;57:2200–12. doi: 10.1093/rheumatology/key242. [DOI] [PubMed] [Google Scholar]
- 6.Desbois AC, Wechsler B, Resche-Rigon M, Piette JC, Huong Dle T, Amoura Z, et al. Immunosuppressants reduce venous thrombosis relapse in Behcet’s disease. Arthritis Rheum. 2012;64:2753–60. doi: 10.1002/art.34450. [DOI] [PubMed] [Google Scholar]
- 7.Saadoun D, Asli B, Wechsler B, Houman H, Geri G, Desseaux K, et al. Long-term outcome of arterial lesions in Behcet disease: a series of 101 patients. Medicine (Baltimore) 2012;91:18–24. doi: 10.1097/MD.0b013e3182428126. [DOI] [PubMed] [Google Scholar]
- 8.Calamia KT, Kaklamanis PG. Behcet’s disease: recent advances in early diagnosis and effective treatment. Curr Rheumatol Rep. 2008;10:349–55. doi: 10.1007/s11926-008-0057-y. [DOI] [PubMed] [Google Scholar]
- 9.Davatchi F, Chams-Davatchi C, Shams H, Shahram F, Nadji A, Akhlaghi M, et al. Behcet’s disease: epidemiology, clinical manifestations, and diagnosis. Expert Rev Clin Immunol. 2017;13:57–65. doi: 10.1080/1744666X.2016.1205486. [DOI] [PubMed] [Google Scholar]
- 10.Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 11.International Team for the Revision of the International Criteria for Behcet’s D. The International Criteria for Behcet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28:338–47. doi: 10.1111/jdv.12107. [DOI] [PubMed] [Google Scholar]
- 12.Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Nadji A, Akhlaghi M, et al. Behcet’s disease: from East to West. Clin Rheumatol. 2010;29:823–33. doi: 10.1007/s10067-010-1430-6. [DOI] [PubMed] [Google Scholar]
- 13.Davatchi F, Jamshidi AR, Banihashemi-Tehrani A, Gholami J, Forouzanfar MH, Moradi M, et al. Prevalence of Behcet’s disease in Iran: a WHO-ILAR COPCORD stage I study. APLAR J Rheumatol. 2007;10:239–43. doi: 10.1111/j.1479-8077.2007.00295.x. [DOI] [Google Scholar]
- 14.Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behcet’s disease in the US: a population-based study. Arthritis Rheum. 2009;61:600–4. doi: 10.1002/art.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi T, Kishimoto M, Swearingen CJ, Filopoulos MT, Ohara Y, Tokuda Y, et al. Differences in clinical manifestations, treatment, and concordance rates with two major sets of criteria for Behcet’s syndrome for patients in the US and Japan: data from a large, three-center cohort study. Mod Rheumatol. 2013;23:547–53. doi: 10.3109/s10165-012-0696-8. [DOI] [PubMed] [Google Scholar]
- 16.Sibley C, Yazici Y, Tascilar K, Khan N, Bata Y, Yazici H, et al. Behcet syndrome manifestations and activity in the United States versus Turkey-a cross-sectional cohort comparison. J Rheumatol. 2014;41:1379–84. doi: 10.3899/jrheum.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilian NC, Sawalha AH. Behcet’s disease in the United States: A single center descriptive and comparative study. Eur J Rheumatol. 2017;4:239–44. doi: 10.5152/eurjrheum.2017.17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, et al. Diagnosis and management of Neuro-Behcet’s disease: international consensus recommendations. J Neurol. 2014;261:1662–76. doi: 10.1007/s00415-013-7209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi K, Ueno F, Bito S, Iwao Y, Fukushima T, Hiwatashi N, et al. Development of consensus statements for the diagnosis and management of intestinal Behcet’s disease using a modified Delphi approach. J Gastroenterol. 2007;42:737–45. doi: 10.1007/s00535-007-2090-4. [DOI] [PubMed] [Google Scholar]
- 20.Hisamatsu T, Naganuma M, Matsuoka K, Kanai T. Diagnosis and management of intestinal Behcet’s disease. Clin J Gastroenterol. 2014;7:205–12. doi: 10.1007/s12328-014-0488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greco A, De Virgilio A, Ralli M, Ciofalo A, Mancini P, Attanasio G, et al. Behcet’s disease: New insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018;17:567–75. doi: 10.1016/j.autrev.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Duzgun N, Ates A, Aydintug OT, Demir O, Olmez U. Characteristics of vascular involvement in Behcet’s disease. Scand J Rheumatol. 2006;35:65–8. doi: 10.1080/03009740500255761. [DOI] [PubMed] [Google Scholar]
- 23.Erkan F, Gul A, Tasali E. Pulmonary manifestations of Behcet’s disease. Thorax. 2001;56:572–8. doi: 10.1136/thorax.56.7.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeidan MJ, Saadoun D, Garrido M, Klatzmann D, Six A, Cacoub P. Behcet’s disease physiopathology: a contemporary review. Auto Immun Highlights. 2016;7:4. doi: 10.1007/s13317-016-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishido T, Horita N, Takeuchi M, Kawagoe T, Shibuya E, Yamane T, et al. Clinical manifestations of Behcet’s disease depending on sex and age: results from Japanese nationwide registration. Rheumatology (Oxford) 2017;56:1918–27. doi: 10.1093/rheumatology/kex285. [DOI] [PubMed] [Google Scholar]
- 26.Davatchi F, Shahram F, Chams-Davatchi C, Sadeghi Abdollahi B, Shams H, Nadji A, et al. Behcet’s disease: is there a gender influence on clinical manifestations? Int J Rheum Dis. 2012;15:306–14. doi: 10.1111/j.1756-185X.2011.01696.x. [DOI] [PubMed] [Google Scholar]
- 27.Yesilirmak N, Lee WH, Gur Gungor S, Yaman Pinarci E, Akkoyun I, Yilmaz G. Enhanced depth imaging optical coherence tomography in patients with different phases of Behcet’s panuveitis. Can J Ophthalmol. 2017;52:48–53. doi: 10.1016/j.jcjo.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Kitaichi N, Miyazaki A, Iwata D, Ohno S, Stanford MR, Chams H. Ocular features of Behcet’s disease: an international collaborative study. Br J Ophthalmol. 2007;91:1579–82. doi: 10.1136/bjo.2007.123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammad A, Mandl T, Sturfelt G, Segelmark M. Incidence, prevalence and clinical characteristics of Behcet’s disease in southern Sweden. Rheumatology (Oxford) 2013;52:304–10. doi: 10.1093/rheumatology/kes249. [DOI] [PubMed] [Google Scholar]
- 30.Zouboulis CC, Kotter I, Djawari D, Kirch W, Kohl PK, Ochsendorf FR, et al. Epidemiological features of Adamantiades-Behcet’s disease in Germany and in Europe. Yonsei Med J. 1997;38:411–22. doi: 10.3349/ymj.1997.38.6.411. [DOI] [PubMed] [Google Scholar]
- 31.Bonitsis NG, Luong Nguyen LB, LaValley MP, Papoutsis N, Altenburg A, Kotter I, et al. Gender-specific differences in Adamantiades-Behcet’s disease manifestations: an analysis of the German registry and meta-analysis of data from the literature. Rheumatology (Oxford) 2015;54:121–33. doi: 10.1093/rheumatology/keu247. [DOI] [PubMed] [Google Scholar]
- 32.Dilsen N, Konice M, Aral O, Ocal L, Inanc M, Gul A. Comparative study of the skin pathergy test with blunt and sharp needles in Behcet’s disease: confirmed specificity but decreased sensitivity with sharp needles. Ann Rheum Dis. 1993;52:823–5. doi: 10.1136/ard.52.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yazici H, Tuzun Y, Pazarli H, Yurdakul S, Ozyazgan Y, Ozdogan H, et al. Influence of age of onset and patient’s sex on the prevalence and severity of manifestations of Behcet’s syndrome. Ann Rheum Dis. 1984;43:783–9. doi: 10.1136/ard.43.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saadoun D, Wechsler B, Desseaux K, Le Thi Huong D, Amoura Z, Resche-Rigon M, et al. Mortality in Behcet’s disease. Arthritis Rheum. 2010;62:2806–12. doi: 10.1002/art.27568. [DOI] [PubMed] [Google Scholar]
- 35.Moses Alder N, Fisher M, Yazici Y. Behcet’s syndrome patients have high levels of functional disability, fatigue and pain as measured by a Multi-dimensional Health Assessment Questionnaire (MDHAQ) Clin Exp Rheumatol. 2008;26:S110–3. [PubMed] [Google Scholar]
- 36.Tascilar NF, Tekin NS, Ankarali H, Sezer T, Atik L, Emre U, et al. Sleep disorders in Behcet’s disease, and their relationship with fatigue and quality of life. J Sleep Res. 2012;21:281–8. doi: 10.1111/j.1365-2869.2011.00976.x. [DOI] [PubMed] [Google Scholar]
- 37.Ilhan B, Can M, Alibaz-Oner F, Yilmaz-Oner S, Polat-Korkmaz O, Ozen G, et al. Fatigue in patients with Behcet’s syndrome: relationship with quality of life, depression, anxiety, disability and disease activity. Int J Rheum Dis. 2016 doi: 10.1111/1756-185X.12839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1.
Laboratory tests in the Stanford and Iranian cohorts.
| Stanford n (%) | Iran n (%) | p | |
|---|---|---|---|
| Raised ESR | 22 (42.3) | 69 (44.5) | 0.78 |
| Raised CRP | 22 (39.3) | NA | - |
| Proteinuria | 1 (1.8) | 2 (1.3) | 1 |
| Hematuria | 4 (7.1) | 18 (11.8) | 0.34 |
| HLA-B27 positive/tested (%) | 1/12 (8.3) | 7/159 (4.4) | 0.45 |
Groups were compared using Chi square test or Fischer’s Exact Test when appropriate. Patients with available data in the Stanford (total n= 56)/Iranian cohort (total n=163): ESR n=52/155, CRP n= 56/0, Proteinuria n=56/153, Hematuria n=56/153, HLA-B27 n=12/159.