Abstract
Retinal vasculitis is a sight-threatening condition that can occur as an isolated ocular disorder or in association with a number of systemic diseases. Parry–Romberg syndrome, also known as progressive hemifacial atrophy (PHA), is a rare disorder of unknown etiology characterized by unilateral facial atrophy and is associated with multiple ophthalmologic and neurologic manifestations. Here we report the case of a 17-year-old man with no prior diagnosis of PHA, who presented with a sudden onset of floaters and decreased vision in the right eye; he was found to have retinal vasculitis and uveitis in the right eye. Routine workup did not reveal the cause of retinal vasculitis. However, thorough physical examination demonstrated features of PHA overlapping with linear scleroderma en coup de sabre. The patient was started on treatment with systemic steroids with a later addition of methotrexate; he responded to treatment with considerable improvement in his symptoms and ophthalmologic examination.
Keywords: Retinal vasculitis, progressive hemifacial atrophy, Parry–Romberg syndrome
Introduction
Retinal vasculitis is a sight-threatening condition that can occur as an isolated ocular disorder or in association with various systemic diseases such as Behçet’s syndrome, sarcoidosis, systemic lupus erythematosus, and several infectious and neoplastic disorders. Parry–Romberg syndrome (PRS), also known as progressive hemifacial atrophy (PHA), is a rare disorder with unknown etiology characterized by unilateral facial atrophy involving the skin, underlying soft tissues, and bony structures. Several ocular complications have been reported previously in patients with PHA. Here we report on a case of PHA, in which retinal vasculitis was the first noticeable sign of the disease.
Case Presentation
A 17-year-old male with newly diagnosed retinal vasculitis was referred to the rheumatology clinic for evaluation of an underlying systemic inflammatory disorder. A few weeks earlier, he had presented to the ophthalmology clinic with the chief complaint of sudden onset of floaters in the right eye.
The patient did not present with any fever or weight loss and a rheumatologic review of systems showed negative results for arthralgias, inflammatory back pain, skin rashes, oral or genital ulcers, Raynaud’s phenomenon, epistaxis, hemoptysis, cough, and shortness of breath. Upon obtaining a further collateral history from his mother, she recalled the presence of “a triangular pink birthmark” over the right side of his forehead, which disappeared with age. Family history was significant for ulcerative colitis in his mother.
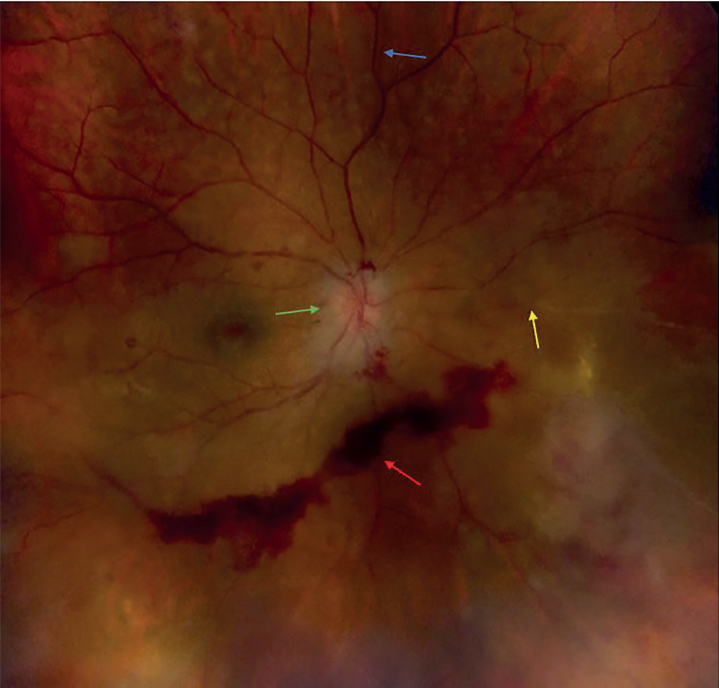
Physical examination was significant for enophthalmos of the right eye, minimal deviation of face to the right side, and presence of a small bald patch on the right side of the scalp. His visual acuity was 20/40 in the right eye and 20/20 in the left; dilated fundus examination showed marked dilation of blood vessels on and around the right optic nerve disc, mild disc hemorrhage, scattered cells in the vitreous body, and multiple areas of segmental sheathing of retinal vessels associated with irregular areas of diffuse retinal whitening and lipid exudates (Figure 1). Anterior chamber examination of the right eye showed trace pigmented cells with trace flare. Dilated fundus examination of the left eye was unremarkable.
Figure 1.
Dilated fundus examination showing intraretinal hemorrhage (red arrow), occluded vessel (yellow arrow), optic disc edema (green arrow), and perivascular sheathing (blue arrow). Retinal hemorrhage was owing to proliferative retinopathy and it improved with intravitreal steroid injection (164×157 mm (120×120 DPI))
A comprehensive workup for infectious etiologies for intermediate uveitis and retinal vasculitis was obtained and was unremarkable. This included negative Treponema pallidum antibody, VZV IgM and PCR, HSV, CMV antibodies, and QuantiFERON Gold as well as serological tests for toxoplasmosis, Lyme disease, HIV, hepatitis B and C, and West Nile encephalitis. The immunological workup was notable for negative antinuclear antibody test, antineutrophil cytoplasmic autoantibody, rheumatoid factor, and HLA B27 and B51. The ACE level was within the normal limit and CXR was unremarkable.
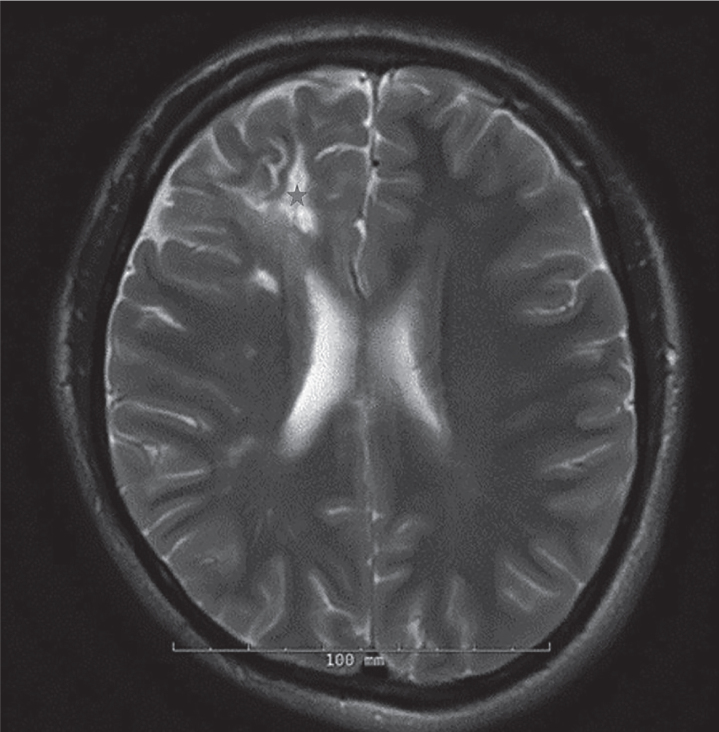
An MRI of the brain was obtained, which demonstrated a linear band-like atrophy/scarring of the scalp soft tissue in the right frontoparietal region and multiple small foci of contrast enhancement in the cortical and subcortical regions in the frontal lobe along with areas of gliosis and encephalomalacia in the subcortical white matter indicating active and chronic inflammatory lesions (Figure 2). MRI of the orbits, optic nerves, optic chiasma, and optic tract were unremarkable.
Figure 2.
Areas of gliosis in the right frontal lobe (asterisk) (160×163 mm (120×120 DPI))
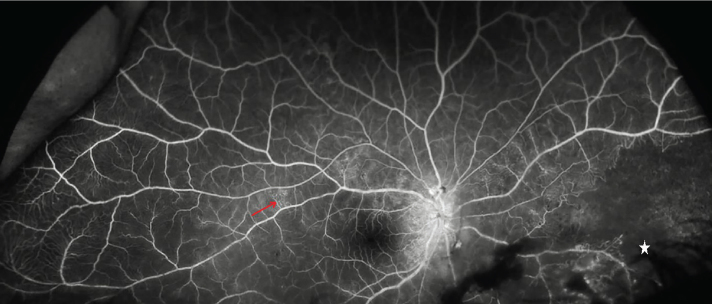
Optical coherence tomography showed optic nerve edema with mild retinal elevation consistent with central subretinal fluid in the right eye. Visual field testing on the right side revealed an enlarged blind spot with a superior arcuate defect. Fluorescein angiography showed an extensive area of capillary non-perfusion and multiple microaneurysms (Figure 3).
Figure 3.
Fluorescein angiography, an extensive area of capillary non-perfusion (asterisk), suggestive of obliteration of blood flow. The red arrow shows telangiectatic changes (164×69 mm (220×220 DPI))
Based on these symptoms, imaging findings, and exclusion of other causes, the patient was diagnosed with retinal vasculitis associated with PHA overlapping with linear scleroderma en coup de sabre (LSCS). He was initially started on treatment with 60 mg prednisone per day. Methotrexate was added later at 10 mg weekly and prednisone was gradually tapered off. He is currently on methotrexate 25 mg weekly as maintenance therapy, and the latest visual acuity improved to 20/25 in the right eye. Patient has been managed collaboratively by ophthalmology, neurology, and rheumatology.
Literature Review
Retinal vasculitis is a sight-threatening inflammatory eye disease affecting the retinal vasculature, which can present as periphlebitis, periarteritis, and angiitis, and is associated with various isolated ocular and systemic diseases such as rheumatologic, infectious, and neoplastic disorders. The common features of retinal vasculitis include cotton wool spots, intraretinal hemorrhage, vascular occlusion, and perivascular sheathing.
Progressive hemifacial atrophy is characterized by progressive atrophy of one side of the face involving the skin and underlying subcutaneous and bony structures (1). Although PHA and LSCS belong to the same disease spectrum, there are some characteristics suggested to be valuable in differentiating these two conditions. The presence of preceding inflammation and induration and less profound scarring restricted to the area above the eyebrow are more likely to be seen in LSCS. Comparable neurologic and ophthalmologic findings were reported in both disorders (2).
Currently, the etiology of PHA is not clear and seems to be heterogeneous. Possible etiologies include focal facial trauma, infectious causes, autoimmune processes, sympathetic nervous system disorders, trigeminal neuritis, and hereditary disorders (3).
Progressive hemifacial atrophy has been associated with multiple ophthalmologic and neurologic manifestations occurring in up to 46% and 60% of cases, respectively (4). Notably, there could be a considerable delay between the diagnosis of PHA and onset of these complications (5). The most common neurological abnormalities described in association with PHA include seizures, headaches, movement disorders, neuropsychological symptoms, and focal neurological symptoms (5). Brain MRI of our patient revealed some active and chronic inflammatory lesions; however, patient’s history and physical exam revealed no neurological findings. The most common ophthalmologic manifestations are enophthalmos, eyelid atrophy, ptosis, ectropion, corneal changes, heterochromia of the iris, strabismus, uveitis, retinal vasculitis, ipsilateral and contralateral third nerve paresis, glaucoma, neuroretinitis, and macular edema (6). Retinal vasculitis in patients with PHA has been described in several case reports, and this association raises the possibility of an underlying immune-mediated inflammatory process as the pathogenesis of this syndrome. Furthermore, frequent association of PHA with autoimmune diseases can support this hypothesis (7).
There is no cure for PHA; however, several case reports have demonstrated a partial response to anti-inflammatory or cytotoxic agents in the neurologic and ophthalmologic complication setting (8). In addition, results of emerging studies, which demonstrate the promising effects of new biologic drugs in the treatment of retinal vasculitis secondary to Behçet’s syndrome and primary systemic vasculitis (9) (10), indicate that biological agents could be a strong consideration in the treatment of retinal vasculitis in PHA. Resolution and improvement of symptoms with immunosuppressive therapy is another fact that indicates the possibility of an autoimmune etiology for PHA. It is evident that further investigations into the efficacy of biological agents must be conducted. We initially treated our patient with systemic steroids and added methotrexate later as a steroid-sparing agent. Upon the last follow-up examination, a considerable improvement in retinal vasculitis was seen.
Conclusion
Ophthalmic complications leading to vision loss, such as retinal vasculitis, may occur in patients with even minimal features of PHA and can occur several years after initial presentation. High clinical suspicion and a multidisciplinary approach, including ophthalmologists and rheumatologists, are required. This case also demonstrates the improvement of complications of PHA, such as vasculitis, with immunosuppressive drugs. The rarity of this entity precludes the availability of a large series of controlled trials, and isolated case reports may improve the understanding and guide the treatment of this condition.
Main Points.
Progressive hemifacial atrophy has been associated with multiple ophthalmologic and neurologic manifestations.
Ophthalmic complications leading to vision loss, such as retinal vasculitis, may occur in patients with even minimal features of progressive hemifacial atrophy.
These complications can occur several years after initial presentation.
A thorough physical exam remains the main important factor in making a correct diagnosis.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.H.; Design - O.G.; Supervision - S.H.; Data Collection and/or Processing - A.V., O.G.; Analysis and/or Interpretation - A.V., O.G.; Literature Search - A.V., O.G.; Writing Manuscript - A.V., D.D.; Critical Review - S.H., O.G.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Duymaz A, Karabekmez FE, Keskin M, Tosun Z. Parry-Romberg syndrome: facial atrophy and its relationship with other regions of the body. Ann Plast Surg. 2009;63:457–61. doi: 10.1097/SAP.0b013e31818bed6d. [DOI] [PubMed] [Google Scholar]
- 2.Lehman TJ. The Parry Romberg syndrome of progressive facial hemiatrophy and linear scleroderma en coup de sabre. Mistaken diagnosis or overlapping conditions? J Rheumatol. 1992;19:844–5. [PubMed] [Google Scholar]
- 3.El-Kehdy J, Abbas O, Rubeiz N. A review of Parry-Romberg syndrome. J Am Acad Dermatol. 2012;67:769–84. doi: 10.1016/j.jaad.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Stone J. Parry-Romberg syndrome A global survey of 205 patients using the Internet. Neurology. 2003;61:674–6. doi: 10.1212/WNL.61.5.674. [DOI] [PubMed] [Google Scholar]
- 5.Vix J, Mathis S, Lacoste M, Guillevin R, Neau JP. Neurological manifestations in Parry-Romberg syndrome: 2 case reports. Medicine (Baltimore) 2015;94:e1147. doi: 10.1097/MD.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fea AM, Aragno V, Briamonte C, Franzone M, Putignano D, Grignolo FM. Parry Romberg syndrome with a wide range of ocular manifestations: a case report. BMC Ophthalmol. 2015;15:119. doi: 10.1186/s12886-015-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-de la Torre I, Castello-Sendra J, Esgleyes-Ribot T, Martinez-Bonilla G, Guerrerosantos J, Fritzler MJ. Autoantibodies in Parry-Romberg syndrome: a serologic study of 14 patients. J Rheumatol. 1995;22:73–7. [PubMed] [Google Scholar]
- 8.Goldberg-Stern H, Passo M, Ball WS., Jr Parry-Romberg syndrome: follow-up imaging during suppressive therapy. Neuroradiology. 1997;39:873–6. doi: 10.1007/s002340050525. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa-Cobos RE, Recillas-Gispert C, Arellanes-García L. Ocular manifestations of primary systemic vasculitis. Reumatol Clin. 2011;7:S12–7. doi: 10.1016/j.reuma.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Pato E, Muñoz-Fernández S, Francisco F, Abad MA, Maese J, Ortiz A, et al. Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis. Semin Arthritis Rheum. 2011;40:314–23. doi: 10.1016/j.semarthrit.2010.05.008. [DOI] [PubMed] [Google Scholar]